The Terebrantia (Insecta: Thysanoptera) of the Maltese Islands
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Key to the Terebrantia of the Maltese Islands
- 1
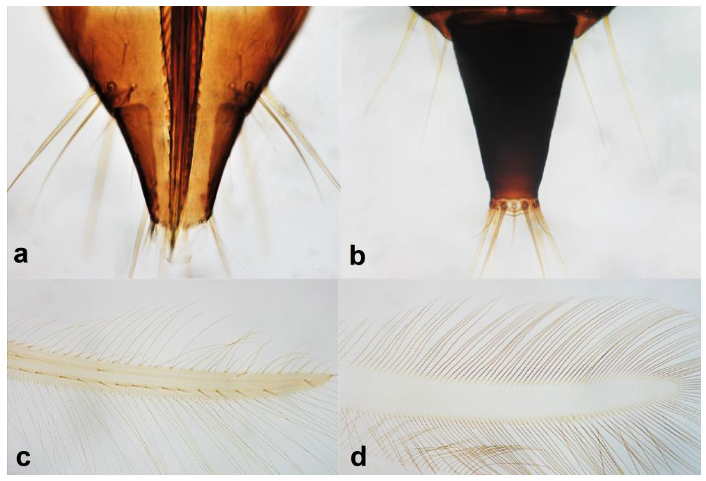
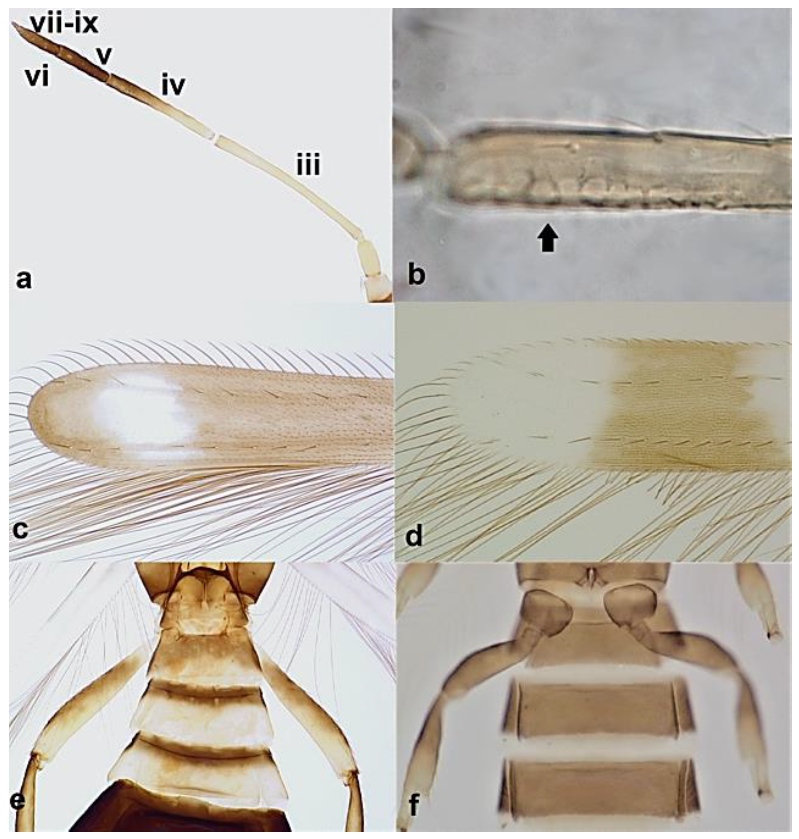
- Abdominal segment X conical in females (Figure 2a) and rounded posteriorly in males; fore wings with one or two longitudinal veins (Figure 2c); females with external ovipositor (Figure 3e,f) ………………………………………… Terebrantia .. 2
- 2
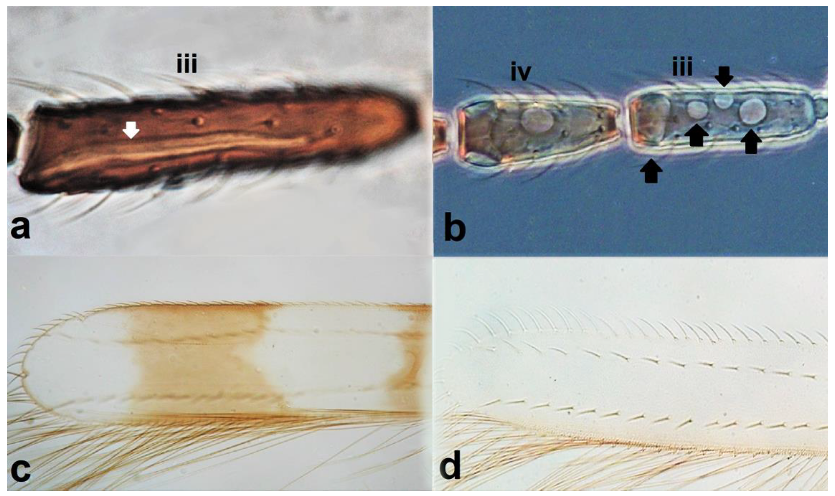
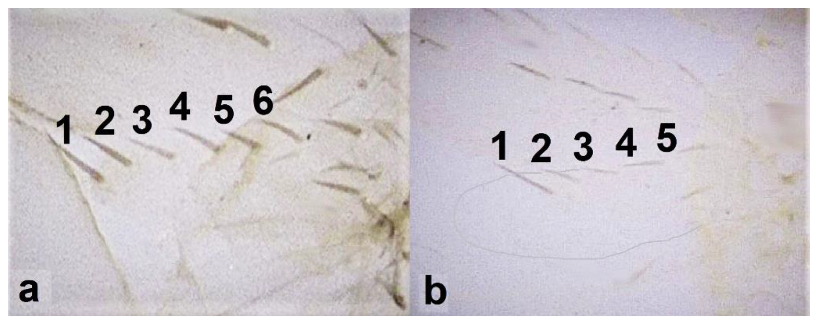
- Antennal segments III and IV with sensoria consisting of a ridge-like structure set in parallel (Figure 3a) or perpendicular (or oblique) to each segment (Figure 3b), never produced as trichomes; fore wing broad, being five to ten times as long as broad (Figure 3c); females with ovipositor curving upwards towards abdominal segments (Figure 3e) ………………………………………………………………………………….. 3Antennal segments III and IV with emergent sensoria, consisting of an acorn-shaped structure (Figure 3g), or a simple (Figure 3h) or forked trichome (Figure 3i); fore wing narrow, being 11–18 times as long as broad and often tapering apically (Figure 3d); females with ovipositor curving downwards (Figure 3f) ……………………………. 15

- 3
- Antennal segments III and IV with sensoria set in parallel to length of segment (Figure 3a); antennal segments VI–IX broadly joined basally (Figure 4a) Aeolothripidae .. 4
- 4
- Antennal segment III about 15 times as long as wide (Figure 5a); antennal segments III and IV with sensoria consisting of a multitude of dot-like structures lined up parallel to length of segment (Figure 5b); fore wings with dark band sub-apically (Figure 5c); abdominal segments I–III significantly narrower than other segments, giving the appearance of a wasp-like waist (Figure 5e) ………………………………………………………………………………………….. Franklinothrips megalops TrybomAntennal segments III 3.3–5 times as long as wide; antennal segments III and IV with sensoria consisting of one or a small number of continuous ridge shapes lined up parallel to length of segment (Figure 5b); fore wings with light coloured sub-apical region (Figure 5d); abdominal segments I–III only slightly narrower than other segments (Figure 5f) ……………………………………………………………………….. 5
- 5
- Antennal segments III and IV with sensoria consisting of one continuous ridge-like structure lined up parallel to length of segment (Figure 6a); fore wings broad, being less than six times as long as broad and with two dark bands (Figure 6c); pronotum postero-marginal setae as long as discal setae ……………….. Aeolothrips Haliday .. 6Antennal segments III and IV with sensoria consisting of lens-shaped structures lined up parallel to length of segment (Figure 6b); fore wings narrow, being more than six times as long as broad and of a uniformly pale brown hue (Figure 6d); pronotum bearing a pair of postero-marginal setae that are longer than discal setae ……………… ……………………………………………………………………… Rhipidothrips Uzel .. 9
- 6
- Fore wing margin at tip as light as wing membrane (Figure 7a) …………………….. 7Fore wing margin at tip darker than wing membrane (Figure 7b) …………………... 8
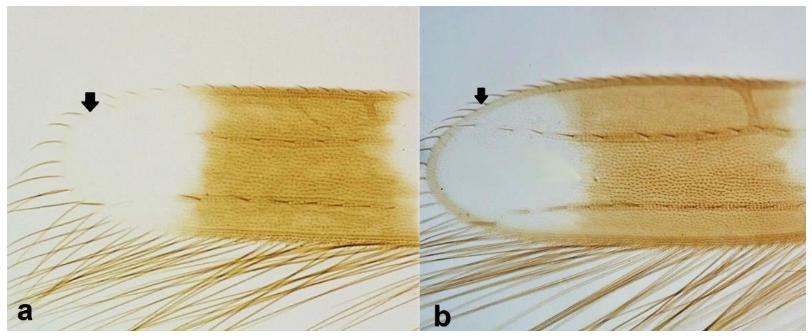
- 7
- Body bicoloured, with head and thorax yellow with brown areas medially (Figure 8a); antennal segment III bicoloured, with the basal half of segment pale yellow, while the apical half dark brown (Figure 8c) …………………….. Aeolothrips gloriosus Bagnall

- 8
- Antennal segment III with sensorium being one third the length of the segment (Figure 9a); antennal segment IV with sensorium two thirds the length of the segment (Figure 9a); pronotum pale, often yellow (Figure 9c) …….. Aeolothrips melisi PriesnerAntennal segment III and IV with sensoria about half the length of the segment (Figure 9b); pronotum dark, usually brown (Figure 9d) ……………………………………………………………………………………………… Aeolothrips tenuicornis Bagnall
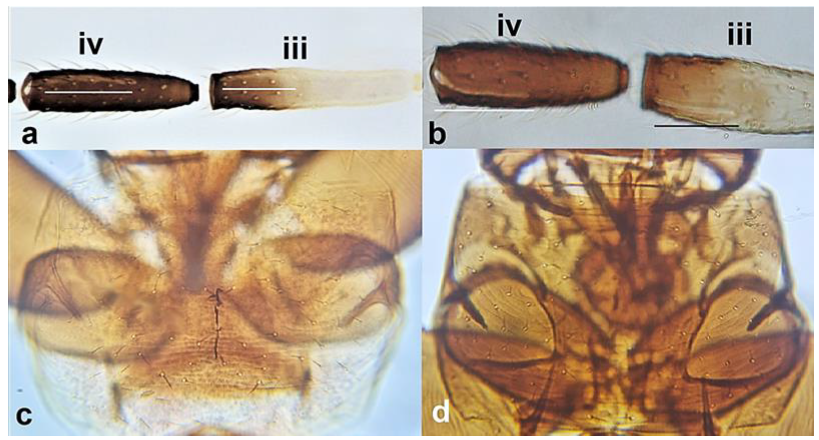
- 9
- Antennal segment II yellow (Figure 10c); pronotum yellow to light brown and with three to four long pairs of postero-marginal setae (Figure 10a); females always macropterous (Figure 10f); abdominal tergites with little or no sculpture or microtrichia (Figure 10g) ………………………………… Rhipidothrips gratiosus Uzel.
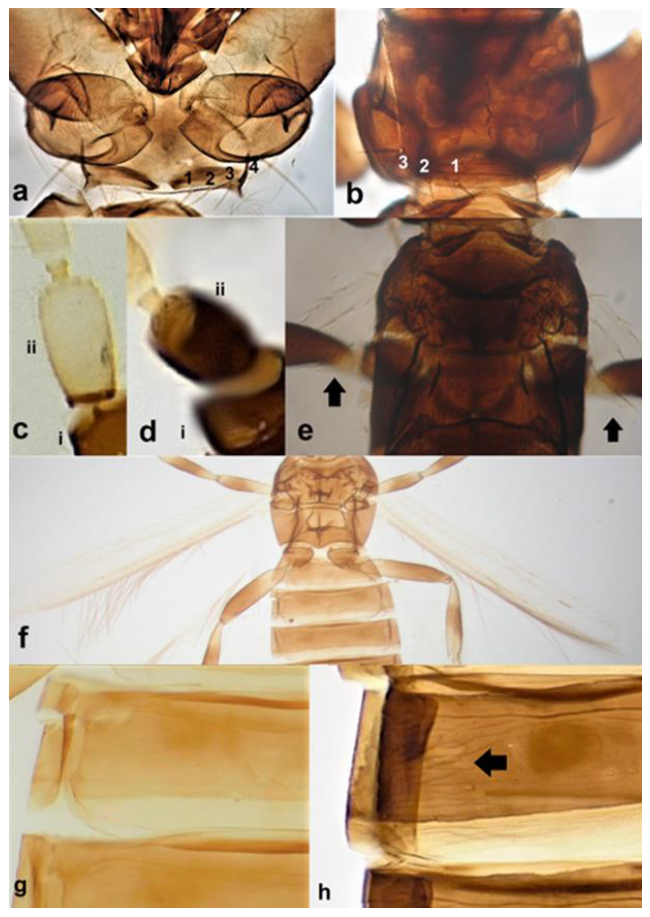
- 10
- Internal body colour orange brown (Figure 11a); females macropterous. On Stipa capensis …………………………………………… Rhipidothrips unicolor zur Strassen.

- 11
- Abdominal tergites with microtrichia on sculpture lines (Figure 12a); females usually macropterous ………………………………….. Rhipidothrips niveipennis O.M. ReuterAbdominal tergites with no microtrichia on sculpture (Figure 12b); females usually micropterous ………………………………………… Rhipidothrips brunneus Williams

- 12
- Fore wing banded (Figure 13a) …………………………….. Melanthrips ficalbii BuffaFore wing evenly brown, not banded (Figure 13b) and with a small pale sub-basal area ………………………………………………………………………………………… 13

- 13
- Anterior margin of fore wing between transverse veins with one row of setae (Figure 14a) ……………………………………………………….. Melanthrips knechteli PriesnerAnterior margin of fore wing between transverse veins with two rows of setae (Figure 14b) ……………………………………………………………………………………….. 14
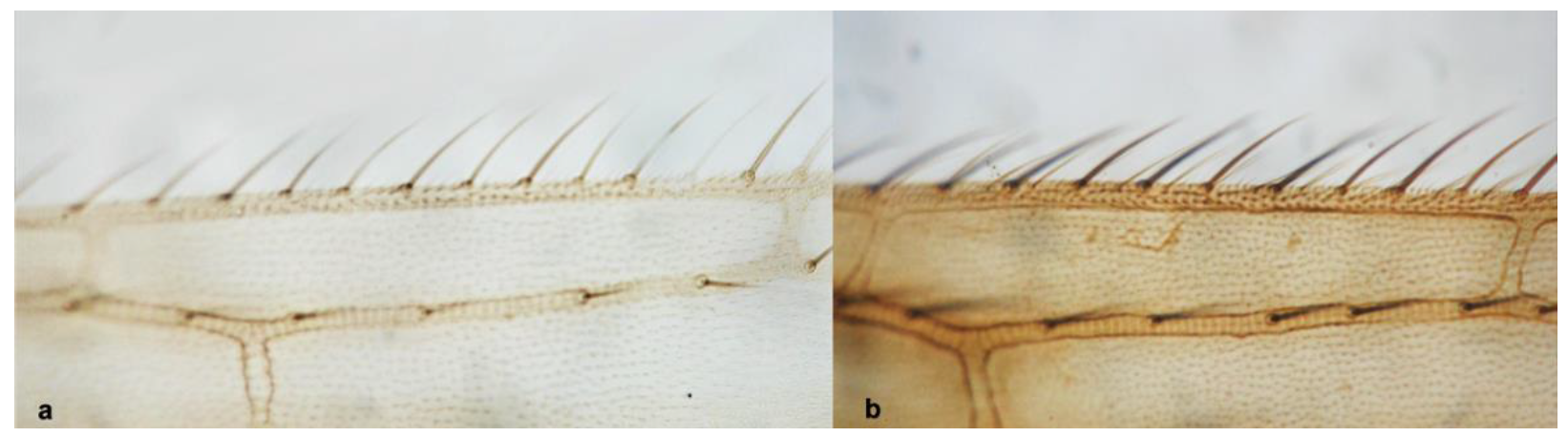
- 14
- Hind tibia with one long seta (Figure 15a) ……………… Melanthrips fuscus (Sulzer)Hind tibia with two long setae (Figure 15b) …………… Melanthrips lybicus Priesner
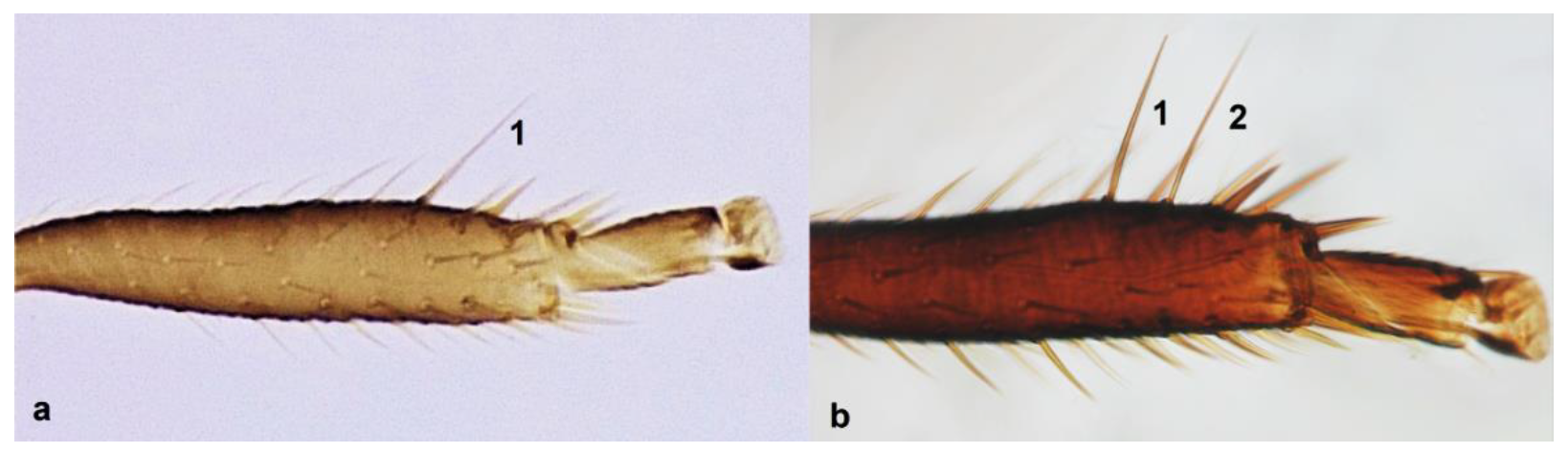
- 15
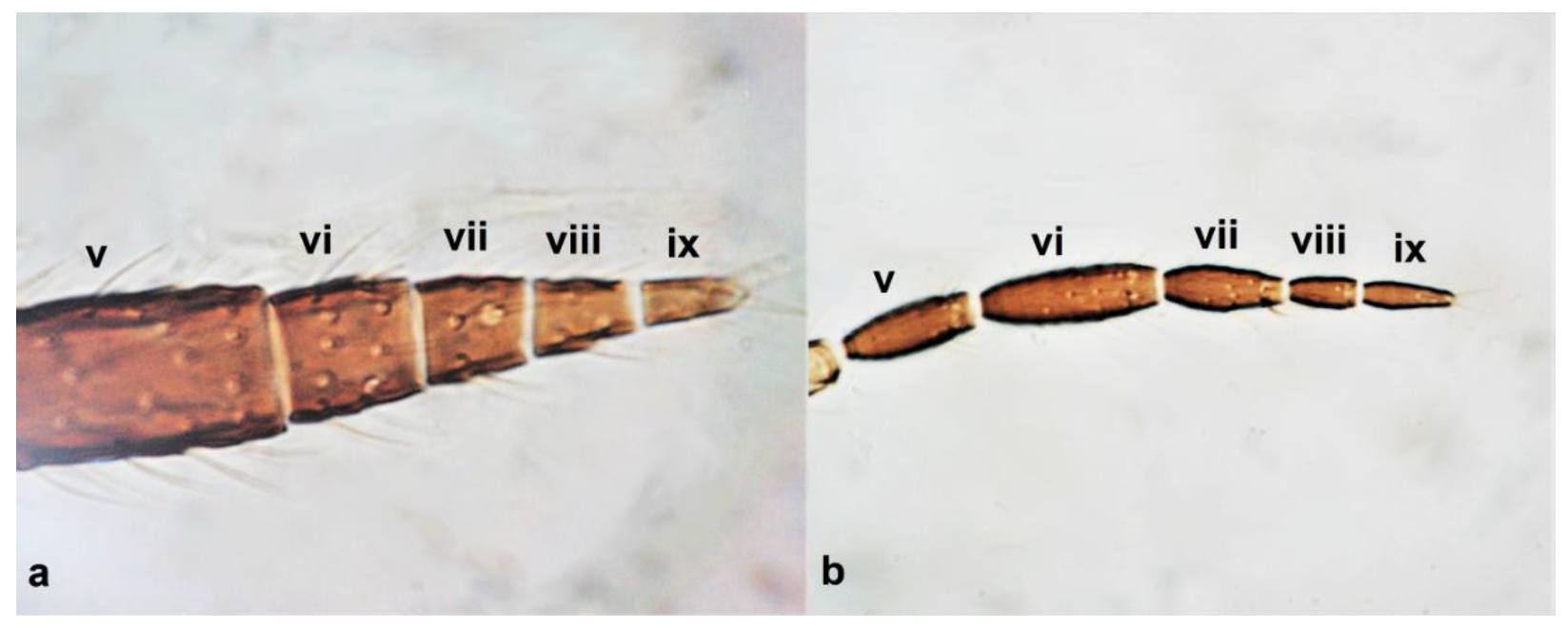
- Antennae nine-segmented (Figure 16a); antennal segments III and IV with cone-shaped sensoria (Figure 3g) ………………………….Stenurothripidae ……………………………………………………..Holarthrothrips tenuicornis Priesner
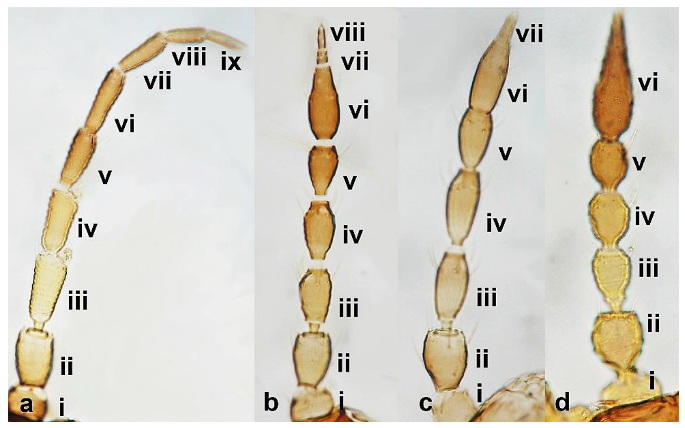
- 16
- Metathoracic endofurca lyre-shaped, with arms extending well beyond length of segment (Figure 17a); median tergal setae on abdomen arising close to each other (Figure 17c) ………………………………………………………….. Dendrothripinae …………………………………………………………………. Dendrothrips saltator Uzel

- 17
- Fore wing with one row of postero-marginal cilia (Figure 18a); antennal segments VI–VIII long and slender when compared with basal segments. (Figure 18c) ………………………………… ………………………………… Panchaetothripinae .. 18
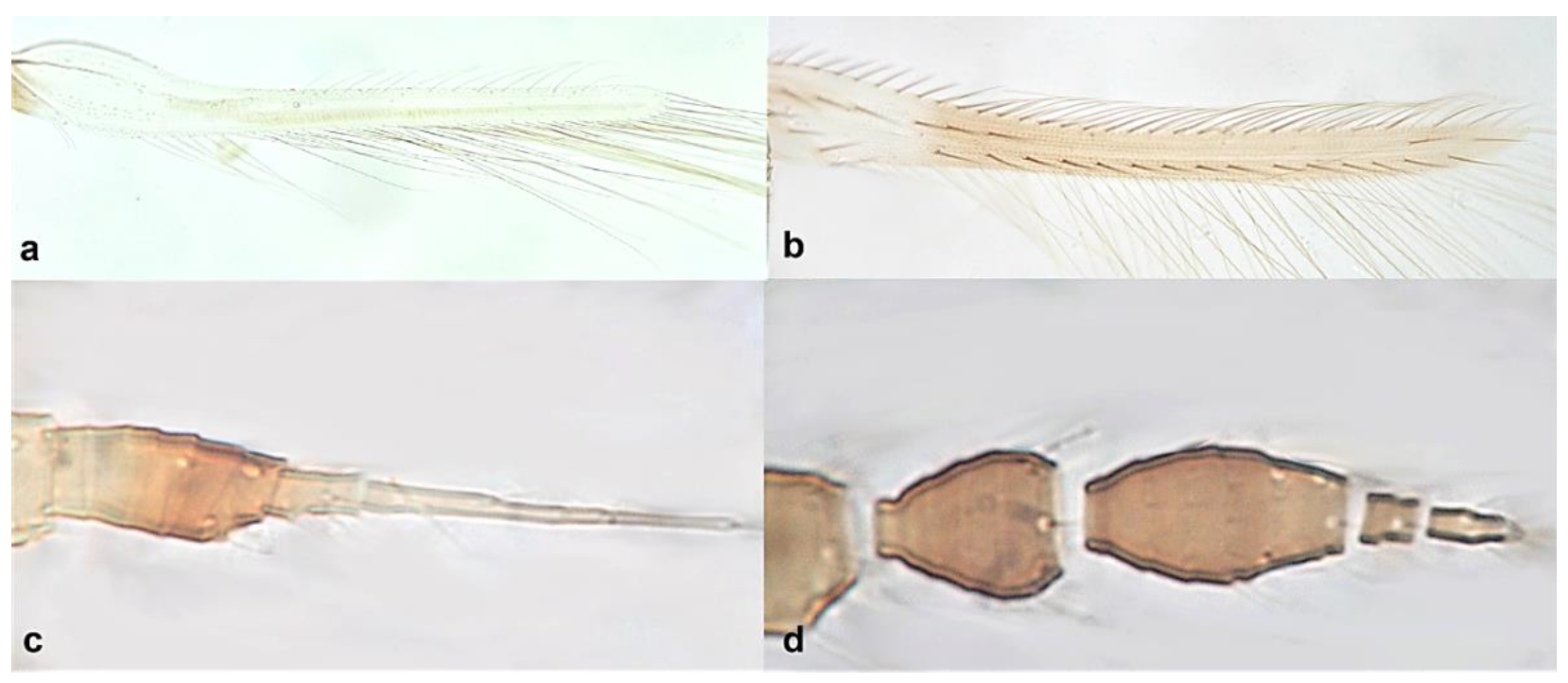
- 18
- Fore tarsi one-segmented (Figure 19a); fore wings uniformly light brown; postero-marginal cilia straight (Figure 19c); fore wing apex rounded (Figure 19c) …………………………………………………… Heliothrips haemorrhoidalis (Bouchè)
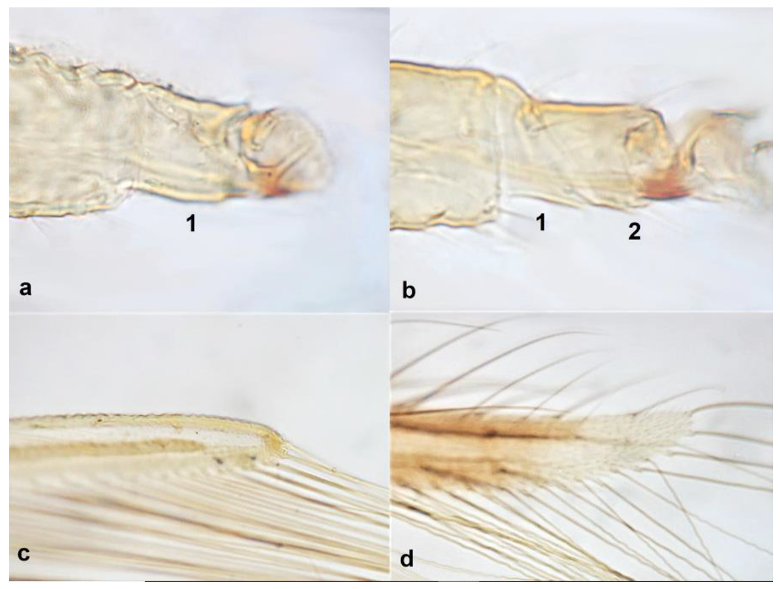
- 19
- Pronotum considerably narrower at anterior margin than at posterior margin (Figure 20a) ………………………………………………………………. Chirothrips Haliday .. 20Pronotum having the same width at both margins (Figure 20b) ……………………. 22

- 20
- Antennal segment II symmetrical ……………………….. Chirothrips hamatus TrybomAntennal segment II asymmetrical, projecting laterally (Figure 21a,b) ……………. 21
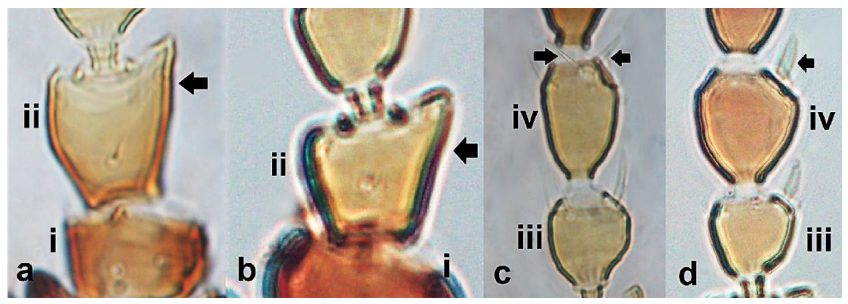
- 21
- Antennal segment II with the outer edge nearly straight (Figure 21a); antennal segment IV with forked sense cone (Figure 21c); antennal segment VII longer than VIII; males usually macropterous ………………….. Chirothrips meridionalis BagnallAntennal segment II with the outer edge at an obtuse angle from the base of segment, creating a tip with bulge (Figure 21b); antennal segment IV with simple sense cone (Figure 21d); antennal segment VII shorter than VIII; males micropterous …………………………………………………………… Chirothrips manicatus Haliday
- 22
- Abdominal segment X bearing a pair of thorn-shaped setae (Figure 22a) …………………………………………………………………… Limothrips Haliday .. 23Abdominal segment X without thorn-shaped setae (Figure 22b) …………………… 24
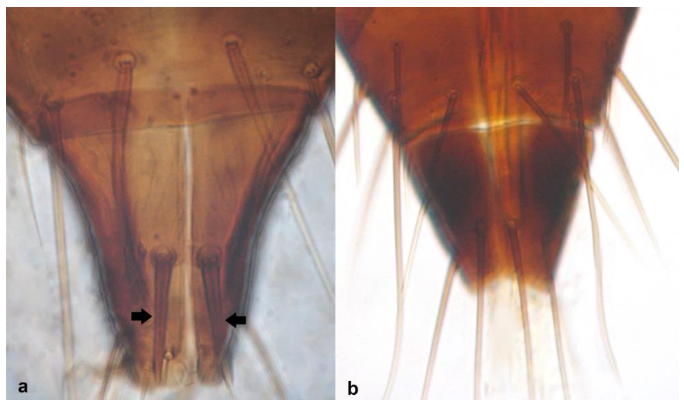
- 23
- Antennal segment II asymmetrical, with elongated extremity at outer side of segment (Figure 23a); antennal segments III and IV with forked sense cones (Figure 23c) …………………………………………………….. Limothrips angulicornis Jablonowski

- 24
- Antennal segments III and IV with simple sense cones (Figure 3h) …………..…… 25Antennal segments III and IV with forked sense cones (Figure 3i) ………………… 28
- 25
- Antennae six-segmented (Figure 16d); head and body having a distinct bright yellow colour (Figure 24a) …………………………………………. Aptinothrips rufus Haliday

- 26
- Fore wing with setae on vein always long, dark and capitate (blunt tipped) (Figure 25b); second vein with no setae (Figure 25a); antennal segment VIII ending in a sharp point (Figure 18c); males macropterous ……………. Echinothrips americanus Morgan
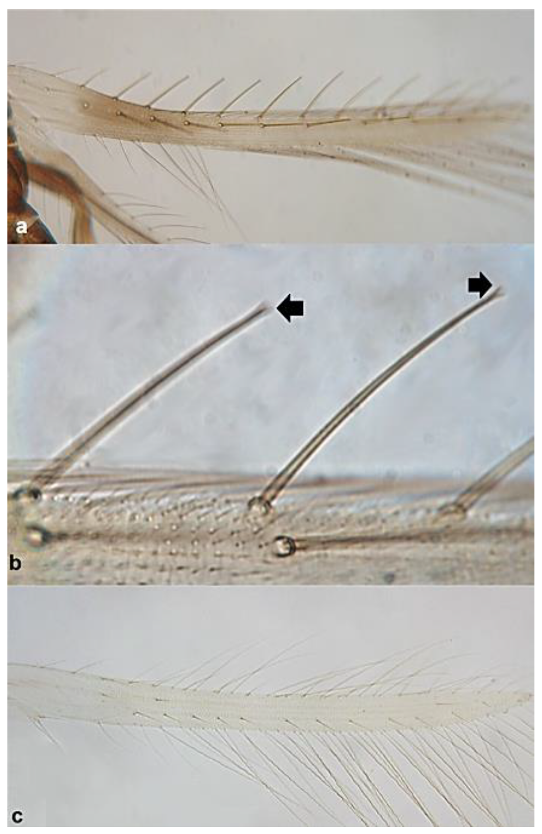
- 27
- Females micropterous; head with reticulate (network-like) sculpture; ridge present between antennal sockets (Figure 26a) ……………… Prosopothrips nigriceps BagnallFemales macropterous; head with no distinct sculpture; ridge lacking between antennal sockets (Figure 26b) ………………… Bregmatothrips dimorphus (Priesner)
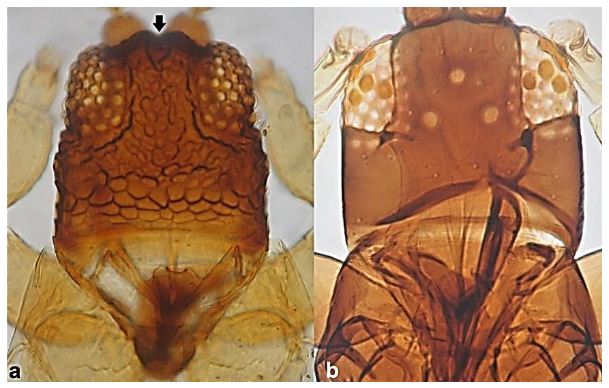
- 28
- Pronotum with no long postero-angular setae (Figure 27a); fore wing with dark band at proximal region (Figure 27c) …………………….. Anaphothrips sudanensis Trybom
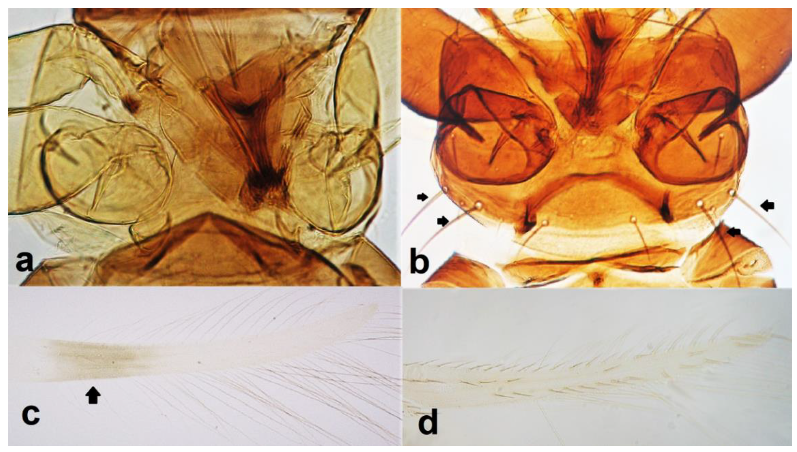
- 29
- Both sexes micropterous; antennal segment V yellow with very little brown on distal margin (Figure 28a) ……………………………… Asphodelothrips croceicollis (Karny)
- 30
- Pronotum with five (rarely 4) pairs of prominent postero-marginal setae, with submedian pair more than twice as long as the discal setae (Figure 29a); male antennal segment VI as long as segments IV and V together (Figure 29c); male with a row of around 12 pore plates on each of abdominal sternites II–VII (Figure 29e) …………………………………………………………….. Pezothrips kellyanus (Bagnall)Pronotum with variable number of prominent postero-marginal setae, usually fewer than five and submedian pair never twice as long as discal setae (Figure 29b); male antennal segment VI shorter than segments IV and V together (Figure 29d); male with only one or no pore plate on each of abdominal sternites II–VII (Figure 29f) ……… 31

- 31
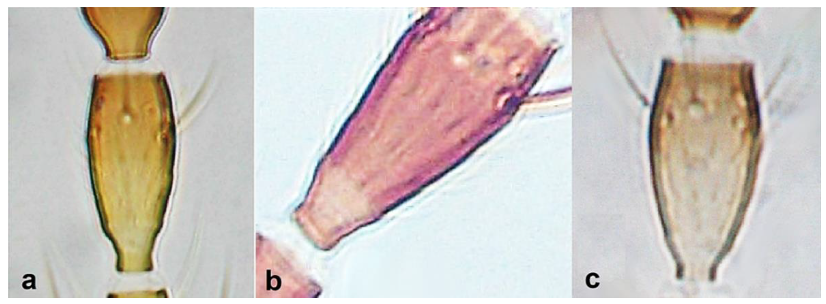
- Antennal segment VI with a sensorium of which the length of the base (insertion) of the sense cone is longer than the width of segment VII (Figure 30a) …………………………………………………………….. Odontothrips meliloti PriesnerAntennal segment VI with sensorium of which base is shorter than the width of segment VII (Figure 30b) …………………………………………………………………. 32
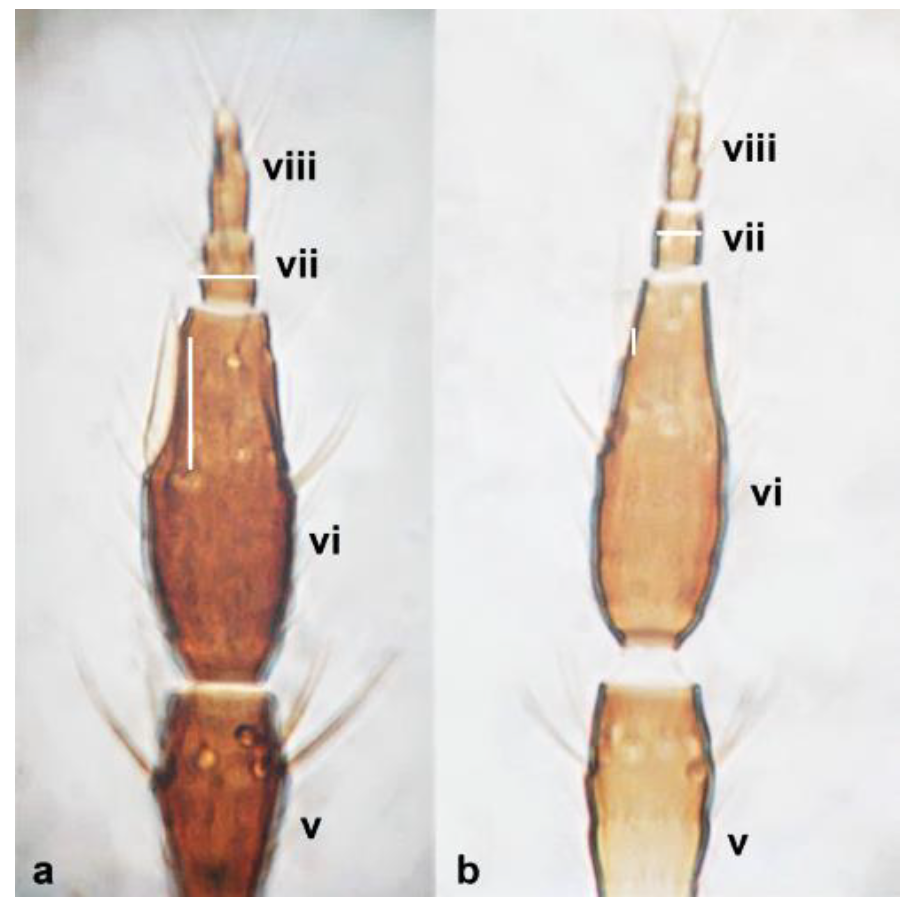
- 32
- Abdominal tergite VIII without paired lateral ctenidia (Figure 31a) often replaced by rows of microthrichia …………………………………………………………………….. 33Abdominal tergite VIII with paired lateral ctenidia (Figure 31b) sometimes with only few and sparse (not arranged in a single row) ………………………………………… 35

- 33
- Fore wing clavus with five marginal setae (Figure 32a); second vein of fore wing with 8–10 setae ……………………………………………………. Tenothrips discolor (Karny)Fore wing clavus with six marginal setae (Figure 32b); second vein of fore wing with 11 or more setae …………………………………………………………………………… 34
- 34
- Abdominal sternites bearing discal setae; ocellar setae iii arising just antero-lateral to ocellar triangle (Figure 33a); pronotum with one pair of postero-angular setae longer than discal setae (Figure 33c) …………………………………… Oxythrips ajugae Uzel
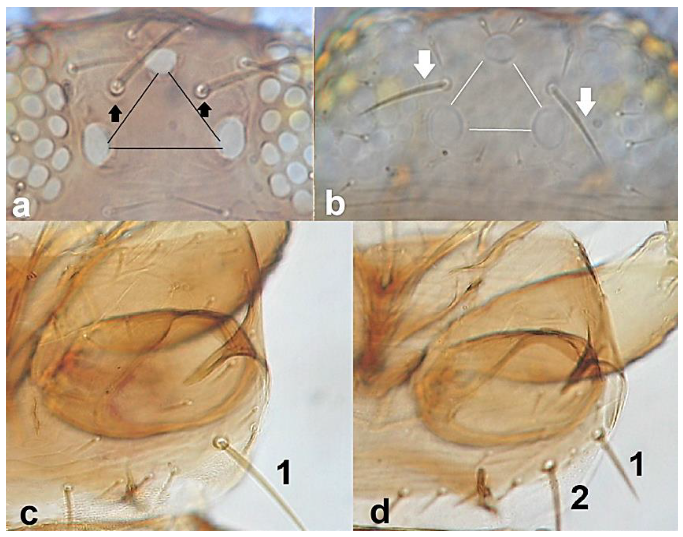
- 35
- Ctenidia on abdominal segment VIII situated antero-laterally to spiracle (Figure 34a); anterior margin of pronotum bearing at least one pair of long setae …………………………………………………………………….. Frankliniella Karny .. 36Ctenidia on abdominal segment VIII situated postero-mesad to spiracles (Figure 34b). anterior margin of pronotum with no long setae ………………….. Thrips Linnaeus 37
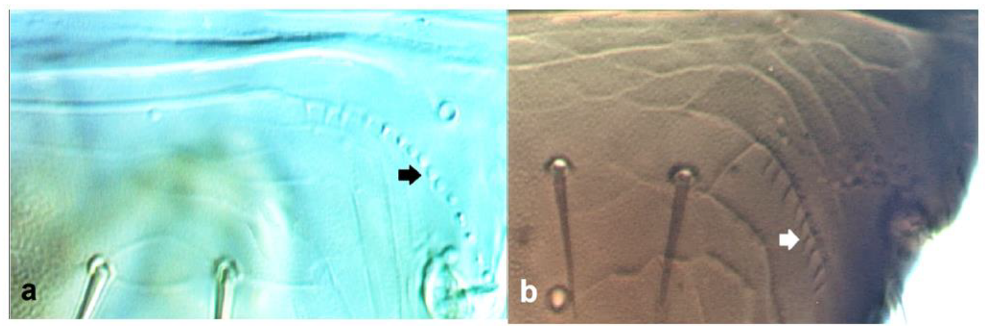
- 36
- Ocellar setae iii arising on anterior margins of ocellar triangle (Figure 35a); meta-thoracic campaniform sensilla present (Figure 35e); microtrichial comb at the posterior margin of tergite VIII fully developed (Figure 35d) ………………………………………………………. Frankliniella occidentalis PergandeOcellar setae iii arising close together between hind ocelli (Figure 35b); meta-thoracic campaniform sensilla absent (Figure 35d); microtrichial comb at the posterior margin of tergite VIII not developed or with few short teeth on lateral margins (Figure 35f) …………………………………………………………….. Frankliniella schultzei Trybom
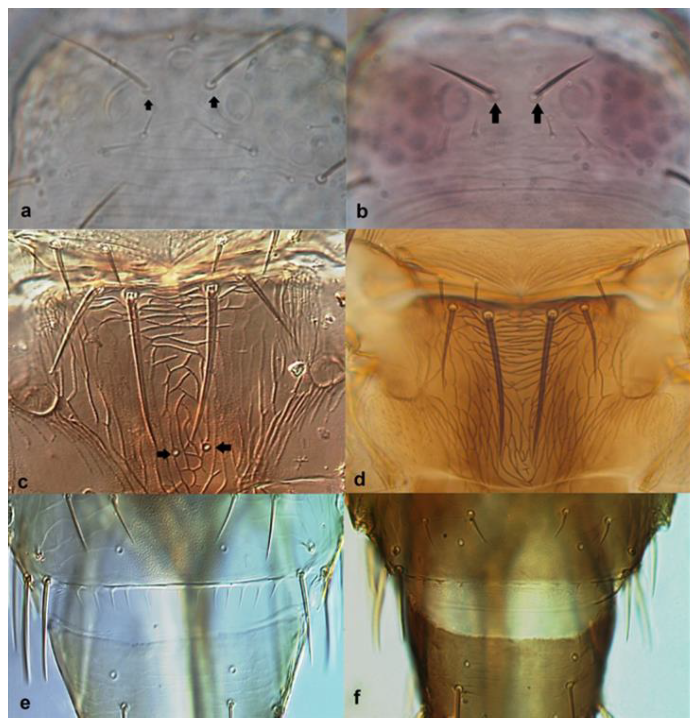
- 37
- Antennae eight-segmented (Figure 16b) ………………………. Thrips simplex MorisonAntennae seven-segmented (Figure 16c) ………………………………………………. 38
- 38
- Abdominal tergite II with four lateral setae (Figure 36a); fore wing clavus with six marginal setae (Figure 32a) ………………………………….. Thrips australis (Bagnall)
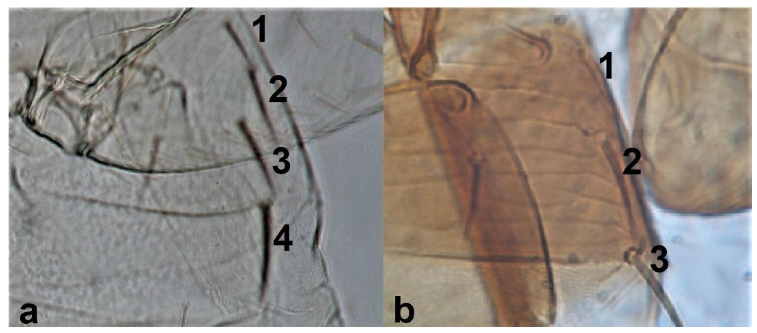
- 39
- Abdominal tergite VIII with microtrichial comb medially incomplete (Figure 37a) ……………………………………………………………………………. Thrips major UzelAbdominal tergite VIII with microtrichial comb complete (Figure 37b) …………………………………………………………………….. Thrips tabaci Lindeman
3.2. Species Catalogue
- FAMILY AEOLOTHRIPIDAE
- Aeolothrips gloriosus Bagnall, 1914 †
- Material examined: MALTA: Buskett, 19.iv.2016, 2 ♀♀ (sm) on Laurus nobilis, GD; Buskett, 21.ii.2018, 1 ♀ (sm) on Rhamnus alaternus, GD; Xemxija, 3.iii.2018, 2 ♀♀ (sm) on Pistacea lentiscus, GD
- Body length: ♀: 1760–1940 µm; ♂: no records.
- Wing type: Both sexes are macropterous.
- Aeolothrips intermedius Bagnall, 1934 †
- Material examined: MALTA: 03.iv.1959, 1 ♀ and instar larva (sm) on Gladiolus sp., ERS (BMNH); Wied Qirda, 06.iv.2016, 1 ♂ (sm) on Glebionis coronaria, GD; Wied Ħesri, 22.iv.2016, 2 ♀♀ (sm) on Avena sp., GD (BMNH); Wied Qirda, 17.iii.2017, 1 ♀ (sm) on Asphodelus ramosus, GD; Manikata, 29.iv.2017, 1 ♀ (sm) on Pallenis spinosa, GD; Fawwara, 22.v.2017, 1 ♀ (sm) from Malaise trap, DM; Lapsi, 09.x.2018, 1 ♀ (sm) on Hyparrhenia hirta, GD.
- Body length: ♀: 1700–1840 µm; ♂: 1300 µm.
- Wing type: Both sexes are macropterous.
- Aeolothrips melisi Priesner, 1936 †
- Material examined: MALTA: Wied Ħesri, 22.iv.2016, 1 ♀ (sm) on Capparis orientalis, GD; Popeye Village, 29.iv.2017, 1 ♀ (sm) on Tamarix africana, GD. GOZO: Ramla Bay, 18.iv.2017, 2 ♀♀ and 1 ♂ (sm) on Cakile maritima and 2 ♀♀ (sm) on Medicago marina, 18.iv.2017, GD.
- Body length: ♀: 1960–2550 µm; ♂: 1780 µm
- Wing type: Both sexes are macropterous.
- Aeolothrips tenuicornis Bagnall, 1926
- Material examined: MALTA: Għammieri, 19.xii.1996, on Galium sp., 2 ♀♀ (sm), DM; M’Scala, 02.ii.1997, on Hedysarum coronarium, 2 ♀♀ (sm), DM; Siġġiewi (private garden), 04.xi.2015, 2 ♀♀ (sm) on Rosa sp., GD; Siġġiewi (private garden), 28.x.2015, 1 ♀ (sm) on Rosa sp., GD; Wied Ħesri, 15.i.2016, 1 ♂ (sm) on Silene colorata, GD; Siġġiewi (private garden), 29.iii.2016, 4 ♀♀ (sm) and 2 ♂♂ (sm) on Ranunculus asiaticus, GD; Siġġiewi (road), 04.iv.2016, 1 ♀ and 1 ♂ (sm) copula pair on Glebionis coronaria, GD; Wied Ħesri, 15.iv.2016, 1 ♀ (sm) on Convolvulus arvensis, GD; Wied Qirda, 06.iv.2016, 2 ♀♀ (sm) on Glebionis coronaria, GD; Wied Ħesri, 22.iv.2016, 1 ♀ (sm) on Capparis orientalis, GD; Kunċizzjoni, 22.iv.2016, 1 ♂ (sm) on Reichardia picroides, GD; Siġġiewi (road), 23.v.2016, 1 ♀ (sm) on Glebionis coronaria, GD; Siġġiewi (road), 21.vi.2016, 3 ♀♀ (sm, aga) on Glebionis coronaria, GD; Msida, Junior College grounds, 08.x.2016, 1 ♀ (sm) on Cynodon dactylon, GD; Lapsi 09.i.2017, 1 ♀ (sm) on Periploca angustifolia, GD; Siġġiewi (road), 21.iv.2017, 1 ♀ (sm) on Argyranthemum frutescens, GD; Mellieħa road l/o Popeye Village, 29.iv.2017, 1 ♂ (sm) on Convolvulus althoides, GD; Wied Ħesri, 30.iv.2017, 1 ♀ (sm) on Gladiolus communis, GD; Pembroke, 05.xi.2018, 1 ♀ (sm) on Reichardia picroides, GD. GOZO: Ramla Bay, 18.iv.2017, 1 ♀ (sm) on Malva arborea, GD.
- Body length: ♀: 1900–2400 µm; ♂: 1200–1680 µm.
- Wing type: Both sexes are macropterous.
- Franklinothrips megalops (Trybom, 1912) †
- Material examined: MALTA: Msida, University of Malta grounds, 04.xi.2016, 1 ♀ (sm) on Rosmarinus officinalis, DM; Fawwara, 22.v.2017, 1♀ (sm) from Malaise trap, DM.
- Body length: ♀: 2380–2480 µm, ♂: no records.
- Wing type: ♀: macropterous; ♂: no records.
- Rhipidothrips brunneus Williams, 1913 †
- Material examined: MALTA: Wied Ħesri, 24.ii.2017, 2 ♀♀ (micropterous) (sm) on Bromus diandrus, GD.
- Body length: ♀: 1880–2060 µm; ♂: no records.
- Wing type: ♀: micropterous; ♂: no records.
- Rhipidothrips gratiosus Uzel, 1895 †
- Material examined: MALTA: Wied Ħesri, 03.iv.2016, 1 ♀ (sm) on Avena sterilis, GD; Wied Ħesri, 22.iv.2016, 2 ♀♀ (sm) on Avena sterilis, GD; Fiddien, 14.iv.2016, 1 ♀ (sm) on Avena sp., GD; Wied Għollieqa 02.xii.2016, 1 ♂ (micropterous–sm) on Cynodon dactylon, GD; Wied Ħesri, 03.iv.2017, 4 ♀♀ (sm, aga) on Gladiolus communis, GD; Fiddien, 24.iv.2017, 1 ♀ (sm) on Avena sp., 1 ♀ (sm) on Medicago sp., GD; Wied Qirda, 17.iii.2017, 1 ♀ (sm) on Medicago sp., GD.
- Body size: ♀: 1900–2120 µm; ♂: 1240 µm.
- Wing type: ♀: macropterous; ♂: micropterous.
- Rhipidothrips niveipennis Reuter, 1899 †
- Material examined: MALTA: Wied Ħesri, 04.iv.2016, 1 ♀ (sm) on Avena sterilis, GD; Wied Ħesri, 22.iv.2016, 1 ♀ (sm) on Avena sterilis, GD; Gudja, l/o Malta International Airport, 26.ix.2016, 1 ♀ (sm) on Ficus microcarpa, GD.
- Body size: ♀: 2000–2014 µm; ♂: no records
- Wing type: ♀: macropterous; ♂: no records
- Rhipidothrips unicolor zur Strassen, 1965 †
- Material examined: MALTA: Għajn Tuffieħa, 29.iv.2017, 7 ♀♀ (sm, aga) and instar larva (sm) on Stipa capensis, GD.
- Body size: ♀: 1760–2250 µm; ♂: n/a.
- Wing type: Both sexes are macropterous.
- FAMILY MELANTHRIPIDAE
- Melanthrips ficalbii Buffa, 1907 †
- Material examined: GOZO: Victoria, (road), 20.iv.2019, 2 ♀♀ (sm) on Galium aparine, GD.
- Body length: ♀: 1840–1960 µm; ♂: n/a.
- Wing type: Both sexes are macropterous.
- Melanthrips fuscus Sulzer, 1776
- Material examined: MALTA: Siġġiewi (private garden), 04.xi.2015, 3 ♂♂ (sm) on Rosa sp., GD; Siġġiewi (private garden), 12.i.2016, 3 ♀♀ and 3 ♂♂ (sm) on Rosa sp., GD; Siġġiewi (private garden), 15.i.2016, 2 ♀♀ (sm) and 2 ♂♂ (sm) on Mercurialis annua, GD; Wied Ħesri, 15.i.2016, 1 ♀ (sm) on Silene colorata, GD; Wied Qirda, 29.i.2016, 2 instar larvae (aga) and 1 ♂ (sm) on Brassica rapa, GD; Siġġiewi (private garden), 29.iii.2016, 1 ♀ (sm) and 1 ♂ (sm) on Ranunculus asiaticus, GD; Siġġiewi (private garden), 16.x.2016, 1 ♀ (sm) on Lobularia maritima, GD; Wied Qirda, 17.iii.2017, 1 ♂ (sm) on Asphodelus ramosus, GD; Wied Ħesri, 03.iv.2017, 2 ♀♀ (sm) and 1 ♂ (sm) on Acacia saligna, GD; Dingli Cliffs, 27.iv.2017, 1 ♂ (sm) on Brassica rapa, GD; Lapsi, 09.x.2017, 1 ♂ (sm) on Potentilla reptans, GD; Wied Baqqiegħa, 22.i.2018, 1 ♀ (sm) on Cerinthe major, GD.
- Body length: ♀: 1540–2140 µm; ♂: 1360–1560 µm.
- Wing type: Both sexes are macropterous.
- Melanthrips knechteli Priesner, 1936 †
- Material examined: MALTA: Siġġiewi (private garden), 15.i.2016, 1 ♀ (sm) on Mercurialis annua, GD; Siġġiewi (private garden), 29.iii.2016, 1 ♂ (sm) on Ranunculus asiaticus, GD; Wied Xkora, 30.x.2017, 1 ♂ (sm) on Mercurialis annua, GD; Wied Baqqiegħa, 22.i.2018, 1 ♀ (sm) on Cerinthe major, GD.
- Body length: ♀: 1580 µm; ♂: 1160–1240 µm.
- Wing type: Both sexes are macropterous.
- Melanthrips libycus Priesner, 1936
- Material examined: MALTA: Siġġiewi (private garden), 12.i.2016, 1 ♀ (sm) and 1 ♂ (sm) on Rosa sp., GD; Wied Ħesri, 15.i.2016, 1 ♀ (sm) and 2 ♂♂ (sm) on Silene colorata, GD; Wied Qirda, 29.i.2016, 1 ♀ (sm) on Trifolium nigrescens, 1 ♀ (sm) on Brassica sp., GD; Qormi (private farm), 07.ii.2016, 1 ♀ (sm) on Brassica oleracea var. botrytis, GD; Wied Qirda, 24.ii.2016, 1 ♀ (sm) on Bromus diandrus, GD; Ta’ Qali, 27.ii.2016, 5 instar larvae (aga) and 2 ♀♀ (sm) on Diplotaxis tenuifolia, SF; Siġġiewi (private garden), 29.ii.2016, 1 ♀ (sm) on Ranunculus asiaticus, GD; Wied Qirda, 17.iii.2017, 1 ♀ (sm) on Medicago sp., GD; Wied Ħesri, 03.iv.2017, 1 ♀ (sm) on Acacia saligna, GD; Dingli Cliffs, 29.x.2017, 1 ♀ (sm) on Brassica rapa, GD. GOZO: Ramla Bay, 18.iv.2017, 1 ♂ (sm) on Cakile maritima, GD.
- Body length: ♀: 1600–2300 µm; ♂: 1400–1725 µm.
- Wing type: Both sexes are macropterous.
- FAMILY STENUROTHRIPIDAE
- Holarthrothrips tenuicornis Bagnall, 1927 †
- Material examined: MALTA: Qormi (roundabout), 14.ix.2017, 13 ♀♀ (sm, aga) on male Phoenix dactylifera, GD; Qormi (roundabout), 03.x.2017, 13 ♀♀ (sm, aga) on male flowers of Phoenix dactylifera, GD; Msida, Junior College grounds, 10.x.2017, 1 ♀ on male flowers of Phoenix dactylifera, GD; Siġġiewi (private garden), 30.vii.2018, 2 ♀♀ (sm), 10 ♀♀ (aga) and 1 ♂ (sm), 1 ♂ (aga) on male flowers of Phoenix dactylifera, GD.
- Body length: ♀: 1550–1820 µm; ♂: 1260 µm.
- Wing type: Both sexes are macropterous.
- FAMILY THRIPIDAE
- Subfamily Dendrothripinae
- Dendrothrips saltator Uzel, 1895 †
- Material examined: MALTA: Wied Babu, 15.xii.1996, 7 ♀♀ (sm) on Ferula melitensis, DM; Wied Qirda, 06.iv.2016, 13 instar larvae (aga) on Foeniculum vulgare, GD; Maqluba, l/o Qrendi, 08.iv.2016, 9 ♀♀ (sm, aga) on Foeniculum vulgare, GD; Qormi (road), 19.iv,2016, 1 instar larva (aga) on Ferula melitensis, GD; 09.vii.2018, 6 ♀♀ (sm, aga) and 1 ♂ (sm) on Foeniculum vulgare, GD.
- Body length: ♀: 1260–1440 µm; ♂: 860 µm.
- Wing type: Both sexes are macropterous.
- Subfamily Panchaetothripinae
- Heliothrips haemorrhoidalis (Bouchè, 1883)
- Material examined: MALTA: Żabbar, 06.v.1996, 1 ♀ (sm) on Viburnum sp., CF; Msida, University of Malta grounds, 28.iv.2017, 1 ♀ (sm) on Apium graveolens, GD.
- Body length: ♀: 1660–1900 µm; ♂: no records.
- Wing type: ♀: macropterous; ♂: no records.
- Hercinothrips femoralis (Reuter, 1881) †
- Material examined: MALTA: Msida, University of Malta grounds, 04.v.2016, 1 ♀ (sm) on Origanum majorana, GD; Siġġiewi (private garden), 05.xi.2016, 14 ♀♀ (sm, aga) and 1 instar larva (aga) on Hippeastrum sp., GD; Birguma, 20.viii.2020, 13 ♀♀ (sm, aga) on Ocimum basilicum, NY; Siġġiewi private garden, 04.xi.2020, 8 ♀♀ (sm, aga) on Calendula officinalis, GD.
- Body length: ♀: 1320–1580 µm: ♂: no records.
- Wing type: ♀: macropterous; ♂: no records.
- Subfamily Thripinae
- Anaphothrips sudanensis Trybom, 1911 †
- Material examined: MALTA: Wied Ħesri, 04.xi.2016, 1 ♀ (sm) on Cynodon dactylon, GD.
- Body length: ♀: 1340 µm; ♂: no records.
- Wing type: the locally collected female specimen is macropterous.
- Aptinothrips rufus (Haliday, 1836) †
- Material examined. MALTA: Wied Ħesri, 04.iv.2016, 1 ♀ (sm) and 1 instar larva (aga) on Hyparrhenia hirta and 4 ♀♀ (sm) and 1 instar larva (aga) on Avena sterilis, GD; Wied Qirda, 06.iv.2016, 1 ♀ (sm) on Plantago major, GD; Maqluba l/o Qrendi, 15.iv.2016, 8 ♀♀ (sm) on Triticum aestivum, GD; Wied Ħesri, 15.iv.2016, 1 ♂ (sm) on Convolvulus arvensis, GD; Msida, Junior College grounds, 27.iv.2016, 1 ♀ (sm) and 2 instar larvae (aga) on Hordeum leporinum, GD; Kunċizzjoni, 22.i.2017, 1 ♀ (sm) on Hordeum leporinum, GD; Msida, Junior College grounds, 03.iv.2017, 2 ♀♀ (sm) on Hordeum leporinum, GD; Għajn Tuffieħa, 29.iv.2017, 1 ♀ (sm) on Stipa capensis, GD. GOZO: Ramla Bay, 18.iv.2017, 1 ♀ (sm) on Medicago marina, GD.
- Body length: ♀: 1460–1700 µm; ♂: 900 µm.
- Wing type: Both sexes are apterous.
- Asphodelothrips croceicollis (Karny, 1914) †
- Material examined: MALTA: Dingli Cliffs, 27.i.2016, 4 ♂♂ (sm) on Asphodelus ramosus, GD; Buskett, 19.ii.2016, 2 instar larvae (aga) on Asphodelus ramosus, GD; Dingli Cliffs, 24.i.2017, 5 ♀♀ (sm) on Asphodelus ramosus, GD; Kunċizzjoni, 28.i.2017, 2 ♀♀ (sm) and 1 ♂ (sm) on Asphodelus ramosus (ag) and 1 ♀ (sm) on Erica multiflora, GD; Wied Ħesri, 24.ii.2017, 1 ♀ (sm) and 1 ♂ (sm) and on Asphodelus ramosus, GD; Buskett, 23.ii.2018, 2 ♀♀ (sm) on Asphodelus ramosus, GD; Kunċizzjoni, 29.i.2018, 1 ♀ (sm) on Glebionis coronaria, GD.
- Body length: ♀: 1760–2160 µm; ♂: 1000–1580 µm.
- Wing type: Both sexes of locally recorded specimens are micropterous.
- Bregmatothrips dimorphus (Priesner, 1919) †
- Material examined: MALTA: Wied Ħesri, 15.i.2016, 5 ♀♀ (aga) and 4 ♂♂ (sm, aga) on Pipatherum miliaceum, GD; Buskett, 03.ii.2016, 1 ♀ (sm) on Cynodon dactylon, 3 ♀♀ (sm, aga) and 2 ♂♂ (sm, aga) on Pipatherum miliaceum, GD.
- Body length: ♀: 1440–1460 µm; ♂: 1140–1240 µm.
- Wing type: ♀: macropterous; ♂: micropterous.
- Ceratothrips ericae (Haliday, 1836) †
- Material examined: MALTA: Lapsi, 9.x.2017, 1 ♀ (sm) on Limbarda crithmoides, GD. GOZO: Qbajjar, 31.iii.2018, 1 ♀ (sm) on Helychrysum melitense, GD.
- Body length: ♀: 1200–1460 µm; ♂: no records.
- Wing type: ♀: macropterous; ♂: no records.
- Chirothrips hamatus Trybom, 1895 †
- Material examined: GOZO: Xlendi, 21.vii.1956, 1 ♀ (sm) on Lithospermum arvense (JIS & ERS), (BMNH).
- Body length: ♀: 1453 µm; ♂: no records.
- Wing type: ♀: macropterous; ♂: no records.
- Chirothrips manicatus (Haliday, 1836) †
- Material examined: MALTA: Siġġiewi (private farm), 03.v.2016, 1 ♀ and 1 ♂ (sm) on Koeleria cristata, GD; Wied Ħesri, 02.xi.2016, 1 ♂ (sm) on Phragmites australis, GD; Wied Ħesri, 04.xi.2016, 4 ♀♀ (sm, aga) and 5 ♂♂ (sm, aga) on Arundo donax, GD; Naxxar (road), 29.iv.2017 1 ♂ (sm) on Triticum aestivum, GD; Wied Qirda, 31.v.2018, 1 ♂ (sm) on Hyparrhenia hirta, GD; Fiddien 16.vi.2021, 1♀ (sm) on Avena sterilis, GD.
- Body length: ♀: 1400–1700 µm; ♂: 960–1440 µm.
- Wing type: ♀: macropterous; ♂: micropterous.
- Chirothrips meridionalis Bagnall, 1927 †
- Material examined: MALTA: Wied Ħesri, 04.iv.2016, 2 ♂♂ (sm) on Hyparrhenia hirta, GD. Wied Qirda, 04.xi.2016, 2 ♀♀ (sm) on Arundo donax, GD; Wied Qirda, 31.v.2018, 3 ♀♀ (sm) on Hyparrhenia hirta, GD (1 ♀ ag).
- Body length: ♀: 1600–1680 µm; ♂: 1240 µm.
- Wing type: Both sexes are macropterous.
- Echinothrips americanus Morgan, 1913 †
- Material examined: MALTA: Siġġiewi (private farmhouse), 21.v.2017, 1 ♀ (sm) on Salvia officinalis, GD; Siġġiewi (private garden), 14.vii.2017, 13 ♀♀ (sm, aga) on Azalea indica, Siġġiewi (private garden), 16.vii.2017, 8 ♀♀, 3 instar larvae (aga) on Azalea indica, GD.
- Body length: ♀: 1100–1340 µm; ♂: no records.
- Wing type: ♀: macropterous; ♂: no records.
- Frankliniella occidentalis (Pergande, 1895)
- Material examined: MALTA: Żabbar, 11.iii.1994, 2 ♀♀ (sm) (BMNH) on Gerbera sp., DM; St. Paul’s Bay, 14.iii.1994, 2 ♀♀ (sm) (BMNH) on Solanum melanogena, GW; St. Paul’s Bay, 14.iv.1994, 1 ♀ (sm) (BMNH) on Dianthus caryophyllus, JWI; St. Paul’s Bay, 14.iv.1994, 2 ♀♀ (sm) (BMNH) on Chrysanthemum sp., JWI; St. Paul’s Bay (glasshouse), 14.iii.1994, 2 ♀♀ (sm) (BMNH) on Fragraria x ananassa, GW; Żabbar, 13.i.1997, 1 ♀ (sm) (dark morph) on Dianthus caryophyllus, DM; Siġġiewi (road), 14.x.2015, 1 ♀ (sm) (yellow morph) on Diplotaxis tenuifolia, GD; Siġġewi (private garden), 29.i.2016, 1 ♀ (sm) (yellow morph) on Narcissus tazzetta, GD; Wied Qirda, 06.iv.2016, 1 ♂ (sm) on Matricaria chamomilla, GD; Wied Ħesri, 04.iv.2016, 1 ♂ (sm) on Avena sterilis, GD; Siġġiewi (private garden), 13.iv.2016, 1♀ (sm) (yellow morph) on Foeniculum vulgare, GD; Manikata, 21.iv.2017, 1 ♂ (sm) on Pallenis spinosa, GD; Mellieħa (road l/o Popeye Village), 21.iv.2017, 1 ♀ (sm) (yellow morph), 1 ♀ (sm) (dark form) on Tamarix africana, GD; Il-Ballut, l/o M’Xlokk, 05.v.2017, 1 ♀ (sm) (pale morph), 1 ♀ (sm) (dark morph) on Malva arborea, GD; road, l/o Wied Ħesri, 07.viii. 2017, 1 ♀ (sm) (pale morph), 3 ♀♀ (aga) on Ipomoea carnosa, GD; Qormi (road), 18.viii.2017, 1 ♀ (sm) (pale morph), 3 ♀♀ (aga) (pale morph) on Yucca gloriosa, GD; San Ġwann (private farm), 29.x.2018, 2 ♀♀ (sm) (yellow morph), 3 ♀♀ (aga) (pale form) and 1 ♂ on Lactuca sativa, GD; Wied Ħesri, 12.iii.2018, 2 ♀♀ (sm) (dark morph) on Trifolium nigrescens, GD; Fiddien, 16.v.2021, 1 ♀ (sm) on Avena sterilis, GD.
- Body length: ♀: 1440–1800 µm; ♂: 1080–1300 µm.
- Wing type: Both sexes are macropterous.
- Frankliniella schultzei (Trybom, 1910) †
- Material examined: MALTA: Siġġiewi (private garden), 04.xi.2015, 1 ♀ (sm) (yellow morph) form) on Rosa sp.
- Body length: ♀: 1400 µm; ♂: no records.
- Wing type: ♀: mactopterous; ♂: no records.
- Limothrips angulicornis Jablonowski, 1894 †
- Material examined: MALTA: Fiddien, 24.iv.2017, 1 ♀ (sm) on Avena sp., GD.
- Body length: ♀: 2080 µm; ♂: no records.
- Wing type: ♀: macropterous; ♂: no records.
- Limothrips cerealium (Haliday, 1836) †
- Material examined: MALTA: Wied Qirda, 10.iv.2016, 2 ♀♀ (sm) on Bromus diandrus, GD; Wied Ħesri, 22.iv.2016, 1 ♀ (sm) on Hyparrhenia hirta, GD; Wied Speranza, 26.iv.2016, 1 ♀ (sm) on Mentha pulegium, GD; Junior College grounds, 27.iv.2016, 1 ♀ (sm) on Hordeum leporinum, GD; Dingli Cliffs, 24.v.2016, 1 ♀ (sm) on Brassica rapa, GD; Dingli Cliffs, 27.v.2016, 1 ♀ (sm) on Glebionis coronaria, GD; Fiddien, 24.iv.2017, 2 ♀♀ (sm) on Avena sp., GD; Naxxar, road, 29.iv.2017, 1 ♀ (sm) on Avena sterilis and 1 ♂ (sm) on Triticum aestivum, GD; Wied Ħesri, 12.iii.2018, 1 ♀ (sm) on Trifolium nigrescens, GD.
- Body length: ♀: 1520–2200 µm; ♂: 1260 µm.
- Wing type: ♀: macropterous; ♂: micropterous.
- Odontothrips meliloti Priesner, 1951 †
- Material examined: MALTA: Wied Qirda, 17.iii.2017, 1 ♀ (sm) on Hedysarum coronarium, GD; Fiddien, 24.iv.2017, 1 ♀ (sm) on Medicago sp., GD; Il-Ballut l/o M’Xlokk, 05.v.2017, 1 ♀ (sm) on Medicago arborea, GD; Siggiewi (private residence), 18.iii.2018, 1 ♀ on glass window pane, GD. GOZO: Xlendi, 31.iii.2016, 2 ♀♀ (sm) on Lotus ornithopodioides, GD; Ramla Bay, 17.iii.2017, 1 ♂ (sm) on Medicago marina, GD.
- Body length: ♀: 1900–2460 µm; ♂: 1500 µm.
- Wing type: Both sexes are macropterous.
- Oxythrips ajugae Uzel, 1895 †
- Material examined: MALTA: Wied Għollieqa, 10.iii.2017, 10 ♀♀ (aga, sm) on Pinus halepensis, GD; Wied Qirda, 17.iii.2017, 6 ♀♀ (aga, sm) on Pinus halepensis, GD; Kunċizzjoni, 29.i.2018, 5 ♀♀ (sm) and 3 ♂♂ (sm) on Pinus halepensis, GD; Xemxija, 03.iii.2018, 5 ♀♀ (sm) on Pinus halepensis, GD.
- Body length: ♀: 1500–1700 µm; ♂: 920 µm.
- Wing type: Both sexes are macropterous.
- Pezothrips kellyanus (Bagnall, 1916) †
- Material examined: MALTA: Siġġiewi (private garden), 04.xi.2015, 3 ♂♂ (sm) on Rosa sp.
- Body length: ♀: no records; ♂: 1460–1660 µm.
- Wing type: ♀: no records; ♂: macropterous.
- Prosopothrips nigriceps Bagnall, 1927 †
- Material examined: MALTA: Pembroke, 05.xi.2018, 1 ♀ (sm) on Reichardia picroides, GD; Xrobb l-Għaġin, 14.iii.2018, 2 ♀♀ (aga) on Hedysarum glomeratum, GD. GOZO: Ramla Bay, 18.iv.2017, 6 ♀♀ (aga, sm) on Silene colorata, GD.
- Body length: ♀: 1340–1500 µm; ♂: no records.
- Wing type: ♀: apterous; ♂: no records.
- Tenothrips discolor (Karny, 1907)
- Material examined: MALTA: St. Paul’s Bay, vii.1956, 1 ♀ (sm) (BMNH) on Limbarda crithmoides, ERS.
- Body length: ♀: 1200 µm; ♂: no records.
- Wing type: ♀: macropterous; ♂: no records.
- Thrips australis (Bagnall, 1915) †
- Material examined: MALTA: Wied Ħesri, 10.x.2015, 6 ♀♀ (sm) and 2 ♂♂ (sm) on Sambucus nigra, GD; Wied Ħesri, 16.iv.2016, 1 ♀ (sm), 5 ♀♀ (aga) and 1 ♂ (sm) on Schinus terebinthifolius, GD; Dingli Cliffs, 27.i.2017, 1 ♂ (sm) on Asphodelus ramosus, GD; Wied Musa l/o Siġġiewi, 30.x.2017, 1 ♀ (sm) on Smilax aspera, GD; Rabat road 2, 23.xi.2017, 2 ♀♀ (sm) on Ceratonia siliqua, GD; Luqa (by-pass), 14.i,2018, 1 ♀ (sm) on Solandra maxima, GD; Siġġiewi (private farm), 29.i.2018, 3 ♀♀ (sm) and 5 ♀♀ (aga) on Eriobotrya japonica, GD.
- Body length: ♀: 1330–1540 µm; ♂: 800–1260 µm.
- Wing type: Both sexes are macropterous.
- Thrips major Uzel, 1895 †
- Material examined: MALTA: Wied Babu, 15.xii.1996, 2 ♀♀ (sm), on Erica multiflora, DM; Siġġiewi (private garden), 04.xi.2015, 4 ♀♀ (sm) on Rosa sp., GD; Siġġiewi (private garden), 12.i.2016, 1 ♀ (sm) on Rosa sp., GD; Siġġiewi (private garden), 01, 29.i.2016, 4 ♀♀ (sm) on Narcissus tazzetta, GD; Siġġiewi (private garden), 21.iii.2016, 2 ♀♀ (sm) on Pittosporum tobira, GD; Buskett, 19.iv.2016, 1 ♀ (sm) on Laurus nobilis, GD; Żabbar (private garden), 05.iv.2016, 1 ♀ (sm) on Stephanotis floribunda, GD; Wied Qirda, 15.iv.2016, 1 ♀ (sm) on Bromus madritensis, GD; Siġġiewi (road), 20.iv.2016, 3 ♀♀ (sm) on Olea europaea, GD; Wied Speranza, 26.iv.2016, 1 ♀ (sm) on Tropaeolum majus, GD; Siġġiewi (private garden), 10.v.2016, 2 ♀♀ (sm) on Lonicera caprifolium, GD; Siġġiewi, 21.viii.2016, 3 ♀♀ (sm) on Glebionis coronaria, GD; Msida, University of Malta grounds, 11.x.2016, 3 ♀♀ (sm) on Quercus robur, GD; Siġġiewi (private garden), 21.x.2016, 4 ♀♀ (sm) on Rosa sp., GD; Kunċizzjoni, 19.i.2017, 1 ♀ (sm) on Erica multiflora, GD; Dingli Cliffs, 27.i.2017, 2 ♀♀ (sm) on Asphodelus ramosus, GD; Msida, University of Malta grounds, 28.iv.2017, 2 ♀♀ (sm) on Olea europaea, GD; Luqa (by-pass), 14.i.2018, 1 ♀ (sm) on Solandra maxima, GD; Siġġiewi (private garden), 14.vii.2017, 1 ♀ (sm) on Azalea indica, GD; Siġġiewi (private farm), 29.i.2018, 4 ♀♀ (sm) on Eriobotrya japonica, GD; Buskett, 20.ii.2018, 1 ♀ (sm) on Rhamnus alaternus, GD; Wied Għollieqa, 11.iv.2018, 2 ♀♀ (sm) on Cercis siliquastrum, GD; Wied Għollieqa, 14.iv.2018, 1 ♀ (sm) on Cercis siliquastrum, GD; Għaxqet l-Għajn, l/o Naxxar, 28.iv.18, 2 ♀ (sm) on Pistacea lentiscus, GD; San Ġwann (private farm), 05.vii. 2019, 2 ♀♀ (sm) on Cucurbita sp., GD; Siġġiewi, (private garden), 22.iv.2020, 1 ♀ (sm) on Citrus limon, GD; Mġiebaħ l/o Xemxija, 2.iii.2018, 2 ♀♀ (sm) on Tamarix africana, GD; Siġġiewi(private garden), 22.iv.2020, 4 ♀♀ (sm) on Prunus persica, GD.
- Body length: ♀: 1300–1580 µm; ♂: no records.
- Wing type: ♀: macropterous; ♂: no records.
- Thrips simplex (Morison, 1930) †
- Material examined: MALTA: 3.vi.1959, 1♀ (sm–BMNH) on Gladiolus sp., ERS; St. Paul’s Bay, 14.iv.1994, 1♀, Gladiolus sp., JWI;
- Body length: ♀: 1697 µm; ♂: no records.
- Wing type: ♀: macropterous; ♂: no records.
- Thrips tabaci Lindeman, 1889
- Material examined: MALTA: St. Paul’s Bay, vii.1956, 1 ♀ (sm—BMNH) on Limbarda crithmoides (JIS & ERS); Żabbar, 11.iii.1994, 2 ♀♀ (sm—BMNH) on Matthiola bicornis, GW; Armier, 14.vi.1994, 1 ♀ (sm—NMNH) on Fragraria x ananassa, GW; Lapsi, 26.i.1997, 1 ♀ (dark morph) on Asphodelus ramosus, DM; Siġġiewi (private garden), 21.iii.2016, 1 ♀ (dark morph) on Pittosporum tobira, GD; Żabbar (private garden), 05.iv.2016, 1 ♀ (dark morph) on Stephanotis floribunda, GD; Siġġiewi (private garden), 13.iv.2016, 2 ♀♀ (dark morph—sm) on Foeniculum vulgare, GD; Wied Ħesri, 16.iv.2016, 1 ♀ (dark morph) on Schinus terebinthifolius, GD; Dingli, Buskett, 19.iv.2016, 1 ♀ (pale form—sm) on Laurus nobilis, GD; Wied Speranza, 26.iv.2016, 2 ♀♀ (dark morph—sm) on Tropaeolum majus, and 3 ♀♀ (dark morph—sm) on Parietaria judaica, GD; Siġġiewi (private garden), 10.v.2016, 1 ♀ (dark morph—sm) on Prunus persica, GD; Wied Ħesri, 15.v.2016, 2 ♀♀ (pale morph–sm) on Hypericum sinuatum, GD; Wied Qirda, 30.v.2016, 1 ♀ on Hyparrhenia hirta, GD; Msida, University of Malta grounds, 11.x.2016, 1 ♀ (dark morph—sm) on Quercus robur, GD; Dingli Cliffs, 27.i.2017, 1 ♀ (dark morph—sm) on Asphodelus ramosus, GD; Fiddien, 24.iv.2017, 2 ♀♀ (dark morph—sm) on Medicago sp., GD; Popeye Village, 29.vii.2017, 1 ♀ (pale morph–sm) on Tamarix africana, GD; Siġġiewi, (road l/o Wied Ħesri), 07.viii.2017, 3 ♀♀ (pale morph–sm) on Ipomoea carnosa, GD; Siġġiewi (private farm), 13.xi.2017, 1 ♀ (pale form–sm) on Eriobotrya japonica, GD; Xemxija, 03.iii.2018, 7 ♀♀ (dark morph—sm) on Anacamptis urvilleana, GD; Qormi (private farm), 7.vii.2018, 1 ♀ (pale morph–sm), 3 ♀♀ (dark morph—sm) on Foeniculum vulgare, GD; Pembroke, 05.xi.2018, 1 ♀ (dark morph—sm) on Reichardia picroides, GD. GOZO: Ramla Bay, 02.vii.2018, 2 ♀♀ (pale form–sm) on Pancratium maritimum, GD.
- Body length: ♀: 1060–1360 µm; ♂: no records.
- Wing type: ♀: macropterous; ♂: no records.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Chorotype | Notes |
|---|---|---|
| Aeolothrips gloriosus | West Palaearctic | |
| Aeolothrips imtermedius | Asiatic European | |
| Aeolothrips melisi | Mediterranean | |
| Aeolothrips tenuicornis | Turano Mediterranean | |
| Franklinothrips megalops | Afrotropico Mediterranean | |
| Rhipidothrips brunneus | Palaearctic | also Australian region |
| Rhipidothrips gratiosus | Palaearctic | |
| Rhipidothrips niveipennis | European | |
| Rhipidothrips unicolor | Turano Mediterranean | |
| Melanthrips ficalbii | Palaearctic | |
| Melanthrips fuscus | Palaearctic | |
| Melanthrips knechteli | Turano European | |
| Melanthrips lybicus | Mediterranean | |
| Holarthropthips tenuicornis | Mediterranean | |
| Dendrothrips saltator | Asiatic European | also Oriental region |
| Heliothrips haemorrhoidalis | Cosmopolitan | |
| Hercinothrips femoralis | Cosmoplolitan | |
| Anaphothrips sudanensis | Subcosmopolitan | |
| Aptinothrips rufus | Cosmopolitan | |
| Asphodelothrips croceicollis | Turano Europo Mediterranean | |
| Bregmatothrips dimorphus | Afrotropico Mediterranean | also East Palaearctic |
| Ceratothrips ericae | Asiatic European | also Australian region |
| Chirothrips hamatus | Sibero European | Also Great Britain. Does not extend beyond Turkey |
| Chirothrips manicatus | Subcosmopolitan | |
| Chirothrips meridionalis | Afrotropico Indo Mediterranean | |
| Echinothrips americanus | Subcosmopolitan | |
| Franklinella occidentalis | Cosmopolitan | |
| Frankliniella schultzei | Subcosmopolitan | |
| Limothrips angulicornis | Subcosmopolitan | |
| Limothrips cerealium | Subcosmopolitan | |
| Odontothrips meliloti | Turano European | |
| Oxythrips ajugae | Holarctic | |
| Pezothrips kellyanus | Turano Mediterranean | also the Netherlands and the Australian region |
| Prosopothrips nigriceps | Turano Mediterranean | |
| Tenothrips discolor | Turano Europeo Mediterranean | |
| Thrips australis | Afrotropico Mediterranean | also Australian region |
| Thrips major | Palaearctic | |
| Thrips simplex | Subcosmopolitan | |
| Thrips tabaci | Cosmopolitan |
References
- Mound, L.A.; Wang, Z.; Lima, É.F.B.; Marullo, R. Problems with the Concept of “Pest” among the Diversity of Pestiferous Thrips. Insects 2022, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, D.; Ouvrard, D.; Queiroz, D.; Percy, D. Psyllid Host-Plants (Hemiptera: Psylloidea): Resolving a Semantic Problem. Fla. Entomol. 2014, 97, 242–246. [Google Scholar] [CrossRef]
- Pedley, M.; Hughes Clarke, M.; Galea, P. Limestone Isles in a Crystal Sea; PEG: San Gwann, Malta, 2002; p. 109. [Google Scholar]
- Chetcuti, D.; Buhagiar, A.; Schembri, P.J.; Ventura, F. The Climate of the Maltese Islands; Malta University Press: Msida, Malta, 1992; p. 108. [Google Scholar]
- FAO. Malta Water Resources Review; FAO: Rome, Italy, 2006. [Google Scholar]
- MEPA Terrestral Habitats. Available online: http://www.mepa.org.mt/biodiversity-habitats-terrestrial##8 (accessed on 25 February 2015).
- Mifsud, S. Malta Wild Plants. Available online: www.maltawildplants.com (accessed on 14 July 2018).
- Saliba, L.J. Insect Pests of Crop Plants in the Maltese Islands; Department of Information: Msida, Malta, 1963. [Google Scholar]
- Farrugia, C. Insect Pests on Cauliflower (Brassica oleracea var. botrytis) in Gozo (Maltese Islands, Central Mediterranean); University of Malta: Msida, Malta, 1997. [Google Scholar]
- Mifsud, D. Biological control in the Maltese Islands—Past initiatives and future programmes. Bull. OEPP 1997, 27, 77–84. [Google Scholar] [CrossRef]
- Mound, L.A.; Palmer, J.M. Notes on Thysanoptera from Israel. Entomol. Mon. Mag. 1973, 109, 102–106. [Google Scholar]
- Mifsud, D.; Watson, G.W. Introduced Sap-Feeding Insect Pests of Crop Plants in the Maltese Islands; University of Malta: Msida, Malta, 1999. [Google Scholar]
- Vierbergen, G. Fauna Europaea—All European animal species on the web: Thysanoptera Section. Biodivers. Data J. 2014, 2, e4034. [Google Scholar]
- Zur Strassen, R. Die Terebrantien Thysanopteren Europas; Die Tierwelt Deutschlands; Goecke & Evers: Keltern, Germany, 2003; p. 277. [Google Scholar]
- Zur Strassen, R.; Kuslitzky, W. An annotated checklist of the thrips of Israel (Thysanoptera). Isr. J. Entomol. 2012, 41, 53–66. [Google Scholar]
- National Statistics Office. Census of Population and Housing 2021; Preliminary Report; National Statistics Office: Valletta, Malta, 2021; p. 100. [Google Scholar]
- Mound, L.A.; Kibby, G. Thysanoptera; CAB International: Wallingford, UK, 1998; p. 70. [Google Scholar]
- Palmer, J.M.; Du Heaume, G.J.; Betts, C. 1959 CIE Guides to Insects of Importance to Man. 2, Thysanoptera. Betts, C.R., Ed.; CAB International: Wallingford, UK, 1989; p. 73. [Google Scholar]
- Marullo, R. I Tisanotteri dell’ Italia meridionale. II Contributo. Le specie italiane del genere Aeolothrips Haliday. Boll. Lab. Entomol. Agrar. Filippo Silvestri 1993, 50, 121–140. [Google Scholar]
- Marullo, R. I Tisanotteri dell’Italia meridionale. III Contributo. La Collezione del Museo Civico di Storia Naturale “G. Doria” di Genova. Boll. Lab. Entomol. Agrar. Filippo Silvestri 1996, 51, 57–65. [Google Scholar]
- Marullo, R. Thysanoptera of southern Italy. V Contribution. Morphological remarks and biological notes on some southern Mediterranean species. Boll. Lab. Entomol. Agrar. Filippo Silvestri Portici 2004, 59, 49–57. [Google Scholar]
- Priesner, H. A Monograph of the Thysanoptera of the Egyptian Deserts; Desert Institute: El Mataria, Egypt, 1960; p. 582. [Google Scholar]
- Priesner, H. Ordnung Thysanoptera (Fransenfluger Thripse); Academie-Verlag: Berlin, Germany, 1964; p. 242. [Google Scholar]
- Mound, L.A.; Morrison, G.D.; Pitkin, B.R.; Palmer, J.M. Thysanoptera. Handbooks for the Identification of British Insects; Royal Entomological Society of London: London, UK, 1976; Volume 1, p. 79. [Google Scholar]
- Zhang, S.M.; Mound, L.A.; Hastings, A. Thysanoptera Chinensis—Thripidae Genera from China. Available online: https://keys.lucidcentral.org/keys/v3/thysanoptera_chinensis/ (accessed on 9 May 2018).
- Moritz, G.; Brandt, S.; Sseruwagi, P.; Waiganjo, M.; Subramanian, S. Pest thrips of Eastern Africa—Identification and Information based on LucID 3.5. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2012, 18, 533–539. [Google Scholar]
- Mound, L.A.; Collins, D.W.; Hastings, A. Thysanoptera Britannica et Hibernica—Thrips of the British Isles. Available online: https://keys.lucidcentral.org/keys/v3/british_thrips/.html (accessed on 9 May 2018).
- Mound, L.A.; Hoddle, M.S.; Hastings, A. Thysanoptera Californica—Thrips of California. Available online: https://keys.lucidcentral.org/keys/v3/thrips_of_california_2019/index.html (accessed on 26 April 2019).
- Marullo, R.; De Grazia, A. Territorial distribution, classification and relationships amongst Italian Thysanoptera. Bull. Insectology 2013, 66, 127–134. [Google Scholar]
- Bhatti, J.S.; Alavi, J.; zur Strassen, R. Thysanoptera in Iran 1938–2007: An Overview. Thrips 2009, 7–8, 373. [Google Scholar]
- Canale, A.; Conti, B.; Petacchi, R.; Rizzi, I. Thysanoptera collected in an olive growing area of the northern Tuscany (Italy). Entomol. Probl. 2003, 33, 105–110. [Google Scholar]
- Moritz, G. Thripse; Meiling Druck: Haldensleben, Germany, 2006; p. 384. [Google Scholar]
- Kirk, W.D.J. Thrips. Naturalist’s Handbook No 25; Richmond Publishing Co., Ltd.: Richmond, UK, 1996; p. 70. [Google Scholar]
- Fallahzedah, M.; Elaheh, A.; Nazila, S.; Hassan, A.; Jalil, A. Faunistic survey of Thysanoptera in Fars province, Iran. Munis Entomol. Zool. 2011, 6, 251–261. [Google Scholar]
- Nickle, D. A Checklist of Commonly Intercepted Thrips (Thysanoptera) from Europe, the Mediterranean, and Africa at U.S. Ports-of Entry (1983–1999). Part 1. Key to Genera. In Proceedings of the Entomological Society of Washington, Washington, DC, USA, 2 May 2003; Volume 105, pp. 80–99. [Google Scholar]
- Tunç, İ.; Bahşi, Ş.Ü.; Göçmen, H. Thysanoptera fauna of the Aegean region, Turkey, in the spring. Turk. J. Zool. 2012, 36, 592–606. [Google Scholar] [CrossRef]
- Mound, L.A.; Reynaud, P. Franklinothrips; a pantropical Thysanoptera genus of ant-mimicking obligate predators (Aeolothripidae). Zootaxa 2005, 864, 1–16. [Google Scholar] [CrossRef]
- Alavi, J.; zur Strassen, R.; Bagherani, N. Thrips (Thysanoptera) species associated with wheat and barley in Golestan province, Iran. J. Entomol. Soc. Iran 2007, 27, 1–28. [Google Scholar]
- Mirab-balou, M.; Chen, X.; Tong, X. Tenothrips bhatti, a newly recorded genus of Thripinae (Thysanoptera: Thripidae) from China. Entomotaxonomia 2012, 34, 162–166. [Google Scholar]
- Razi, S.; Laamari, M. Thysanoptera survey on Vicia faba (broad bean) in the arid Biskra region of Algeria. Agric. Biol. J. North Am. 2013, 4, 268–274. [Google Scholar] [CrossRef]
- Gertsson, C. An annotated checklist of Thysanoptera (thrips) from the Nordic countries. [Provinsförteckning över Nordens tripsar.]. Entomol. Tidskr. 2015, 134, 185–198. [Google Scholar]
- Marullo, R. Un Profilo della Tisanotterofauna italiana: Le Interazioni tra Specie e Piante Ospiti e gli Effetti delle Colture sulla Diversita’ delle Popolazioni Naturali; Congresso Nazionale Italiano di Entomologia: Rome, Italy, 2002; pp. 179–184. [Google Scholar]
- Stoch, F. Fauna Italica. Available online: http://www.faunaitalia.it/ (accessed on 20 February 2015).
- Zur Strassen, R. Thysanoptera on islands of the northern Sporades in the Aegean (Greece) (Insecta: Thysanoptera). Senckenberg. Biol. 1986, 67, 85–129. [Google Scholar]
- Tunc, I. Studies on the Thysanoptera of Antalya I. Aeolothripidae Uzel. Türk. Entomoloji Derg. 1991, 15, 129–141. [Google Scholar]
- Sierka, W.; Fedor, P.; Vasiliu-Oromulu, L.; Jenser, G.; Bărbuceanu, D. The state of knowledge of thrips (Insecta: Thysanoptera) of the Carpathian Mountains. Acta Phytopathol. Entomol. Hung. 2008, 43, 355–366. [Google Scholar] [CrossRef]
- Karadjova, O.; Krumov, V. Thysanoptera of Bulgaria. ZooKeys 2015, 504, 93–131. [Google Scholar]
- Marullo, R. Conoscere i Tisanotteri; Edizioni Agricole de Il Sole 24 ore Edagricole S.r.l. via Goito 13—40126; Università degli Studi Mediterranea di Reggio Calabria: Reggio Calabria, Italy, 2003; p. 75. [Google Scholar]
- ThripsWiki Contributors zur Strassen Distribution Lists. Available online: http://thrips.info/w/index.php?title=Zur_Strassen_distribution_lists&oldid=41628 (accessed on 6 December 2016).
- ThripsWiki. ThripsWiki—Providing Information on the World’s Thrips in the Catalogue of Life; ThripsWiki: Leiden, The Netherlands, 2018. [Google Scholar]
- Nakahara, S.; O’donnell, C.A.; Mound, L.A. Heliothrips haemorrhoidalis and its relatives, with one new species and one new genus (Thysanoptera: Thripidae). Zootaxa 2015, 4021, 578–584. [Google Scholar] [CrossRef]
- Mound, L.A.; Nielsen, M.; Hastings, A. Thysanoptera Aaotearoa—Thrips of New Zealand. Available online: https://keys.lucidcentral.org/keys/v3/nz_thrips/ (accessed on 3 April 2019).
- Nickle, D.A. Commonly Intercepted Thrips at U.S. Ports-of-Entry from Africa, Europe, and the Mediterranean. IV. Miscellaneous Thripine Genera Excluding Frankliniella, Iridothrips, and Thrips (Thysanoptera: Thripidae). In Proceedings of the Entomological Society of Washington, Washington, DC, USA, 1 January 2009; Volume 111, pp. 215–238. [Google Scholar]
- Raspudić, E.; Ivezić, M.; Brmež, M.; Trdan, S. Distribution of Thysanoptera species and their host plants in Croatia. Acta Agric. Slov. 2009, 93, 275–283. [Google Scholar] [CrossRef]
- Trdan, S. Thrips in Slovenia: Thrips and tospoviruses. In Proceedings of the 7th International Symposium on Thysanoptera, Reggio Calabria, Italy, 2–7 July 2001; pp. 351–356. [Google Scholar]
- Garcia-Fayos, P.; Goldarazena, A. Role of Thrips in Pollination of Arctostaphyllos uva-ursi. Int. J. Plant Sci. 2008, 169, 776–781. [Google Scholar] [CrossRef]
- Jenser, G. Data to the Thysanoptera fauna of Tunisia. Folia Entomol. Hung. 1982, 43, 55–57. [Google Scholar]
- Trdan, S.; Andjus, L.; Raspudić, E.; Kač, M. Distribution of Aeolothrips intermedius Bagnall (Thysanoptera: Aeolothripidae) and its potential prey Thysanoptera species on different cultivated host plants. J. Pest Sci. 2005, 78, 217–226. [Google Scholar] [CrossRef]
- Badieritakis, E.G.; Thanopoulos, R.C.; Fantinou, A.A.; Emmanouel, N.G. Emmanouel qualitative and quantitative study of thrips (Thysanoptera) on alfalfa and records of thrips species on cultivated and wild Medicago species of Greece. Biologia 2015, 70, 504–515. [Google Scholar] [CrossRef]
- Mound, L.A.; Teulon, D.A.J. Thysanoptera as Phytophagous Opportunists; Thrips Biology and Management; Springer: Boston, MA, USA, 1995; pp. 3–19. [Google Scholar]
- Tyagi, K.; Kumar, V. Thrips of Economic importance in India—An Identification guide. Zool. Surv. India 2020, 2020, 96. [Google Scholar]
- Goldarazena, A. Orden Thysanoptera. Rev. SEA 2015, 52, 1–20. [Google Scholar]
- Jenser, G.; Tzanakakis, M.E. Records of Thysanoptera from Northern Greece. Entomol. Hell. 2017, 3, 59. [Google Scholar] [CrossRef]
- Kucharczyk, H.; Zawirska, I. The occurrence of Thysanoptera in Poland; Thrips and Tospoviruses. In Proceedings of the 7th International Symposium on Thysanoptera, Reggio Calabria, Italy, 2–7 July 2001; pp. 341–344. [Google Scholar]
- Vassiliou, V.A. Ecology and Behavior of Pezothrips kellyanus (Thysanoptera: Thripidae) on Citrus. J. Econ. Entomol. 2010, 103, 47–53. [Google Scholar] [CrossRef]
- Conti, F.; Tumminelli, R.; Amico, C.; Fisicaro, R.; Frittitta, C.; Perrotta, G.; Marullo, R. Monitoring Pezothrips kellyanus on citrus in eastern Sicily. Thrips and Tospoviruses. In Proceedings of the 7th International Symposium on Thysanoptera, Reggio Calabria, Italy, 2–7 July 2001; pp. 207–210. [Google Scholar]
- Navarro Campos, C. Pezothrips Kellyanus (Thysanoptera: Thripidae), Nueva Plaga en Cítricos; Comportamiento de sus Poblaciones, Muestreo y Enemigos Naturales. Unpublished. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2013; p. 166. [Google Scholar]
- Varikou, K.; Tsitsipis, I.; Alexandrakis, V.; Hoddle, M. Effect of Temperature on the Development and Longevity of Pezothrips kellyanus (Thysanoptera: Thripidae). Ann. Entomol. Soc. Am. 2009, 102, 835–841. [Google Scholar] [CrossRef]
- Nickle, D.A. Commonly Intercepted Thrips at U.S. Ports-of-Entry from Africa, Europe, and the Mediterranean. III. The Genus Thrips Linnaeus, 1758 (Thysanoptera: Thripidae). Proc. Entomol. Soc. Wash. 2008, 110, 165–185. [Google Scholar] [CrossRef]
- Kucharczyk, H.; Kucharczyk, M. Characteristic and diagnostic features of the most frequently occurring species of the Thripidae family (Insecta, Thysanoptera) in crown canopies of Central European forests. Leśne Pr. Badaw. 2013, 74, 5–11. [Google Scholar]
- Khan, F.; Roy, M.C.; Kim, Y. Thelytokous Reproduction of Onion Thrips, Thrips tabaci Lindeman 1889, Infesting Welsh Onion and Genetic Variation among Their Subpopulations. Insects 2022, 13, 78. [Google Scholar] [CrossRef]
- Mifsud, D. Present knowledge of the Entomofauna of the Maltese Islands. Entomol. Basiliensia 2000, 22, 75–86. [Google Scholar]
- Schembri, P.J. Current state of knowledge of the Maltese non-marine fauna. In Malta Environment and Planning Authority Annual report and accounts. Malta Environ. Plan. Auth. 2003, 2003, 33–65. [Google Scholar]
- Cassar, T.; Mifsud, D. Insects of the Maltese Islands. Entomol. Soc. Malta 2023, 12, 711. [Google Scholar]
- Berzosa, J. Thysanoptera checklist (Insecta, Thysanoptera) of the Canary Islands. Geographical distribution, host·plants and bibliography. Boll. Real Soc. Esp. Hist. Nat. Sec. Biol. 2000, 96, 93–112. [Google Scholar]
- Vigna Taglianti, A.; Audisio, P.A.; Biondi, M.; Bologna, M.A.; Carpaneto, G.M.; De Biase, A.; Fattorini, S.; Piattella, E.; Sindaco, R.; Venchi, A.; et al. A proposal for a chorotype classification of the Near East fauna, in the framework of the Western Palearctic region. Biogeogr. J. Integr. Biogeogr. 1999, 20, 1. [Google Scholar] [CrossRef]
- Carapezza, A.; Mifsud, D. New records of true bugs (Hemiptera, Heteroptera) from the Maltese Islands 2015. Bull. Entomol. Soc. Malta 2015, 7, 27–50. [Google Scholar]
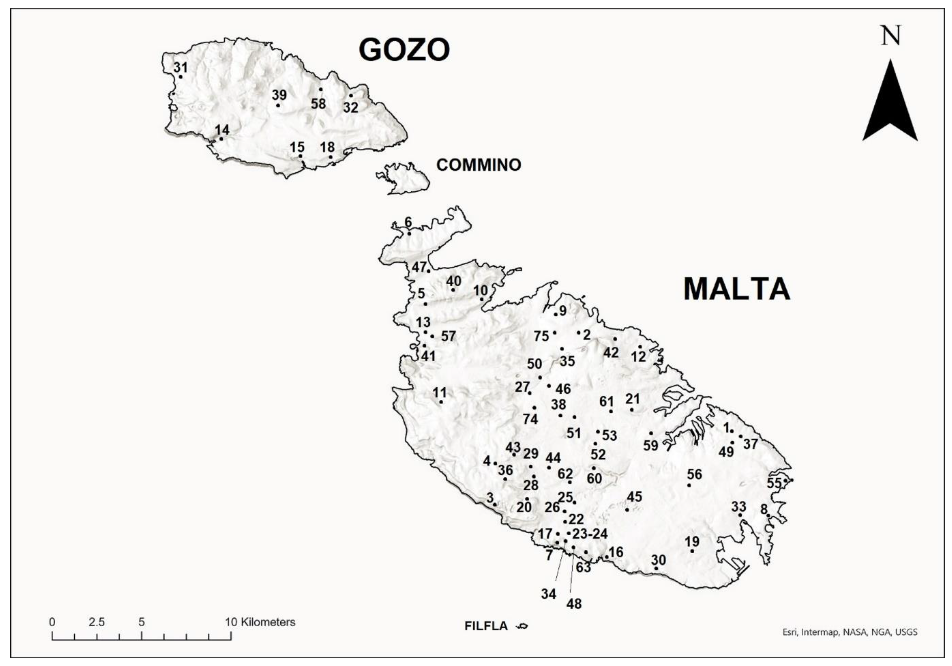
| Site No. | Location | Habitat Type and Description |
|---|---|---|
| 1 | Ta’ Sabbara, Żabbar | Steppe, with typical vegetation including Foeniculum vulgare, Vicia sativa and, especially when in a degraded state, Asphodelus ramosus, Galactites tomentosa and Stipa capensis, and. Most plants in this habitat dry up during the dry summer period [6]. |
| 2 | Wied Anġlu, Birguma | |
| 3 | Dingli Cliffs, Dingli | Garigue, characterised by low lying, often aromatic shrubs adapted to resist drought and strong winds, e.g., Thymbra capitata. Commonly found species include Drimia (=Urginea) maritima, Erica multiflora, and Euphorbia melitensis [6]. |
| 4 | Country road, l/o Rabat | |
| 5 | Manikata | |
| 6 | Ċirkewwa | |
| 7 | Lapsi, Siġġiewi | |
| 8 | Xrobb l-Għaġin | |
| 9 | Għaxqet l-Għajn, Għargħur | |
| 10 | Mġiebaħ, Xemxija | |
| 11 | Lippija Tower, Mġarr | |
| 12 | Pembroke | |
| 13 | l/o Majjistral Park, Għajn Tuffieħa | |
| 14 | Xlendi Tower, Xlendi, Gozo | |
| 15 | Ta Ċenċ, Gozo | |
| 16 | Il-Maqluba, Qrendi | Maquis, with plants consisting largely of evergreen small trees such as Ceratonia siliqua and Olea europaea, large shrubs such as Pistacea lentiscus and climbers such as Hedera helix and Smilax aspera, as well as herbaceous shade loving species such as Acanthus mollis [6]. |
| 17 | Il-Fawwara, Siġġiewi | |
| 18 | Mgarr ix-Xini, Gozo | |
| 19 | Ħas-Saptan, Gudja | Woodland, comprising of Mediterranean sclerophyll forest with trees including Quercus ilex and Pinus halepensis and an undergrowth of smaller shrubs. Tamarix spp. prevails on coastal wood remnants, while Populus alba occurs in riparian woodland remnants [6]. |
| 20 | Buskett, Dingli | |
| 21 | Wied Għollieqa, San Ġwann | Valleys, with slopes having plants typically found in maquis environments and the valley itself. Plants occurring in these habitats include Hyparrhenia hirta, Rubus ulmifolius and Potentilla reptans and, where water courses are present also some hydrophytes such as Ranunculus saniculifolius. |
| 22 | Wied Ħesri, Siġġiewi | |
| 23 | Wied Musa, Siġġiewi | |
| 24 | Wied Xkora, Siġġiewi | |
| 25 | Wied Qirda, Żebbuġ | |
| 26 | Wied il-Baqqiegħa, Żebbuġ | |
| 27 | Wied Speranza, Mosta | |
| 28 | Wied Qlejgħa, Rabat | |
| 29 | Wied tal-Fiddien, Rabat | |
| 30 | Wied Babu, Żurrieq | |
| 31 | Il-Qattara, Dwejra, Gozo | |
| 32 | Ramla l-Ħamra, Gozo. | Sand dune, with vegetation that needs to be suited to harsh conditions including high temperatures, dryness, occasional inundation by seawater and accumulation of sand. These plants include Cakile maritima, Elytrigia juncea, Eryngium maritimum, Lotus cytisoides and Pancratium maritimum. |
| 33 | Il-Ballut, Marsaxlokk | Saltmarshes, with plants that tolerate high salinity such as Athrocnemum macrostachyum, Salsola soda and Salicornia ramosissima. |
| 34 | Siġġiewi (roads) | Town roads. Plants growing along these roads include a variety of both indigenous species (e.g., Diplotaxis tenuifolia) as well as non-indigenous species. These sites yield plants which are cultivated, non-indigenous species. |
| 35 | Naxxar (road) | |
| 36 | Rabat (roads) | |
| 37 | Żabbar (road) | |
| 38 | Attard (road) | |
| 39 | Victoria, Gozo (roads) | |
| 40 | Popeye Village, Mellieħa road | Country roads. These host a number of indigenous plants including Glebionis coronaria and Antirrhinum siculum. |
| 41 | Għajn Tuffieħa road | |
| 42 | Ġebel San Pietru, Madliena | |
| 43 | Il-Kunċizzjoni, l/o Rabat | |
| 44 | Żebbuġ (roads) | By-passes. A number of central strips and roundabouts on the proximity of these roads include cultivated species such as Solandra maxima, Albizia julibrissin and Nerium oleander. |
| 45 | Luqa road | |
| 46 | Mosta road | |
| 47 | Mellieha by-pass | |
| 49 | Zabbar (private gardens) | Private gardens. These include a variety of cultivated herbaceous plants and fruit trees such as Citrus. |
| 50 | Mosta garden | |
| 51 | Attard garden | |
| 52 | Junior College Ringroad, Msida | |
| 53 | University of Malta Grounds, Msida | |
| 54 | Siggiewi plant nursery | |
| 55 | St. Thomas Bay, Marsaskala garden | Public gardens. These feature a combination of cultivated plants, e.g., Yucca gloriosa and Salvia coccinea and indigenous species including Tamarix africana and Atriplex halimus. |
| 56 | Sta. Lucia garden | |
| 57 | Golden Bay garden | |
| 58 | Qbajjar, Gozo garden | |
| 59 | Floriana Car Park | Public cultivated green areas with plants as described in the “Town roads” section. |
| 60 | Qormi (roundabout) | |
| 61 | San Ġwann farm | Private farms, which grow a variety of crop plants. |
| 62 | Qormi farm | |
| 63 | Siġġiewi farm | |
| 64 | Ta’ Qali l/o Attard field | Private open cultivated fields with a variety of plants as described in private farms. |
| 65 | Tal-Ferħa, Għargħur field |
| Chorotype | Number of Species | % |
|---|---|---|
| Subcosmopolitan | 7 | 17.9 |
| Cosmopolitan | 5 | 12.8 |
| Palaearctic | 5 | 12.8 |
| Turano Mediterranean | 4 | 10.3 |
| Asiatic European | 3 | 7.7 |
| Afrotropico Mediterranean | 3 | 7.7 |
| Mediterranean | 3 | 7.7 |
| Turano European | 2 | 5.1 |
| Afrotropico-Indo-Mediterranean | 1 | 2.6 |
| European | 1 | 2.6 |
| Europeo Mediterranean | 1 | 2.6 |
| Holarctic | 1 | 2.6 |
| Sibero European | 1 | 2.6 |
| Turano-Europeo-Mediterranean | 1 | 2.6 |
| West Palaearctic | 1 | 2.6 |
| Total: | 39 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degabriele, G.; Cavalleri, A.; Goldarazena, A.; Mifsud, D. The Terebrantia (Insecta: Thysanoptera) of the Maltese Islands. Diversity 2023, 15, 514. https://doi.org/10.3390/d15040514
Degabriele G, Cavalleri A, Goldarazena A, Mifsud D. The Terebrantia (Insecta: Thysanoptera) of the Maltese Islands. Diversity. 2023; 15(4):514. https://doi.org/10.3390/d15040514
Chicago/Turabian StyleDegabriele, Godwin, Adriano Cavalleri, Arturo Goldarazena, and David Mifsud. 2023. "The Terebrantia (Insecta: Thysanoptera) of the Maltese Islands" Diversity 15, no. 4: 514. https://doi.org/10.3390/d15040514
APA StyleDegabriele, G., Cavalleri, A., Goldarazena, A., & Mifsud, D. (2023). The Terebrantia (Insecta: Thysanoptera) of the Maltese Islands. Diversity, 15(4), 514. https://doi.org/10.3390/d15040514








