Description of Neochlorella semenenkoi gen. et. sp. nov. (Chlorophyta, Trebouxiophyceae), a Novel Chlorella-like Alga with High Biotechnological Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Algal Strain
2.2. Light and Electron Microscopy
2.3. Grazing Test
2.4. DNA Isolation and Sequencing
2.5. Phylogenetic Analysis
2.6. Physiological Tests
2.7. Statistics
3. Results
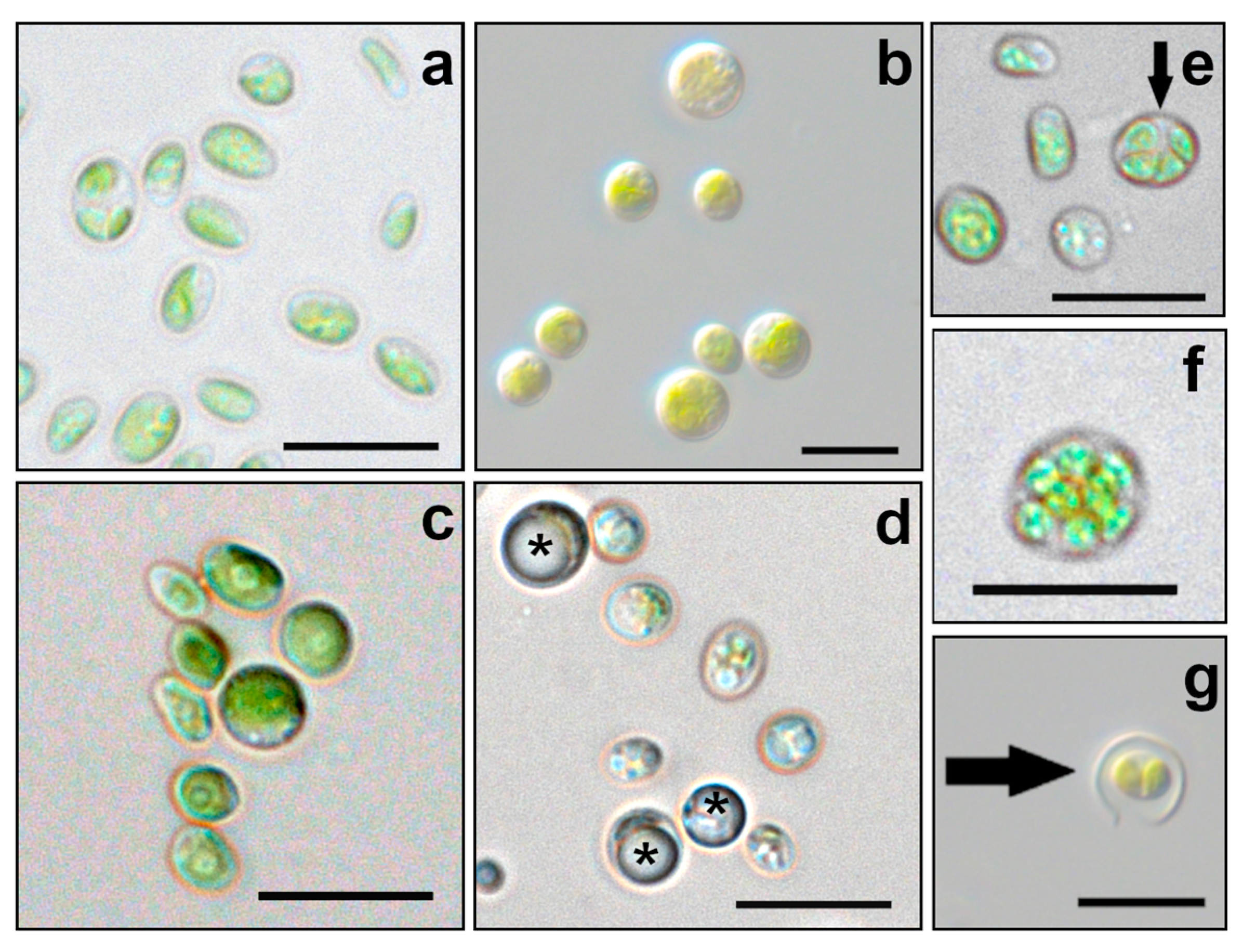
3.1. Morphology and Ultrastructure
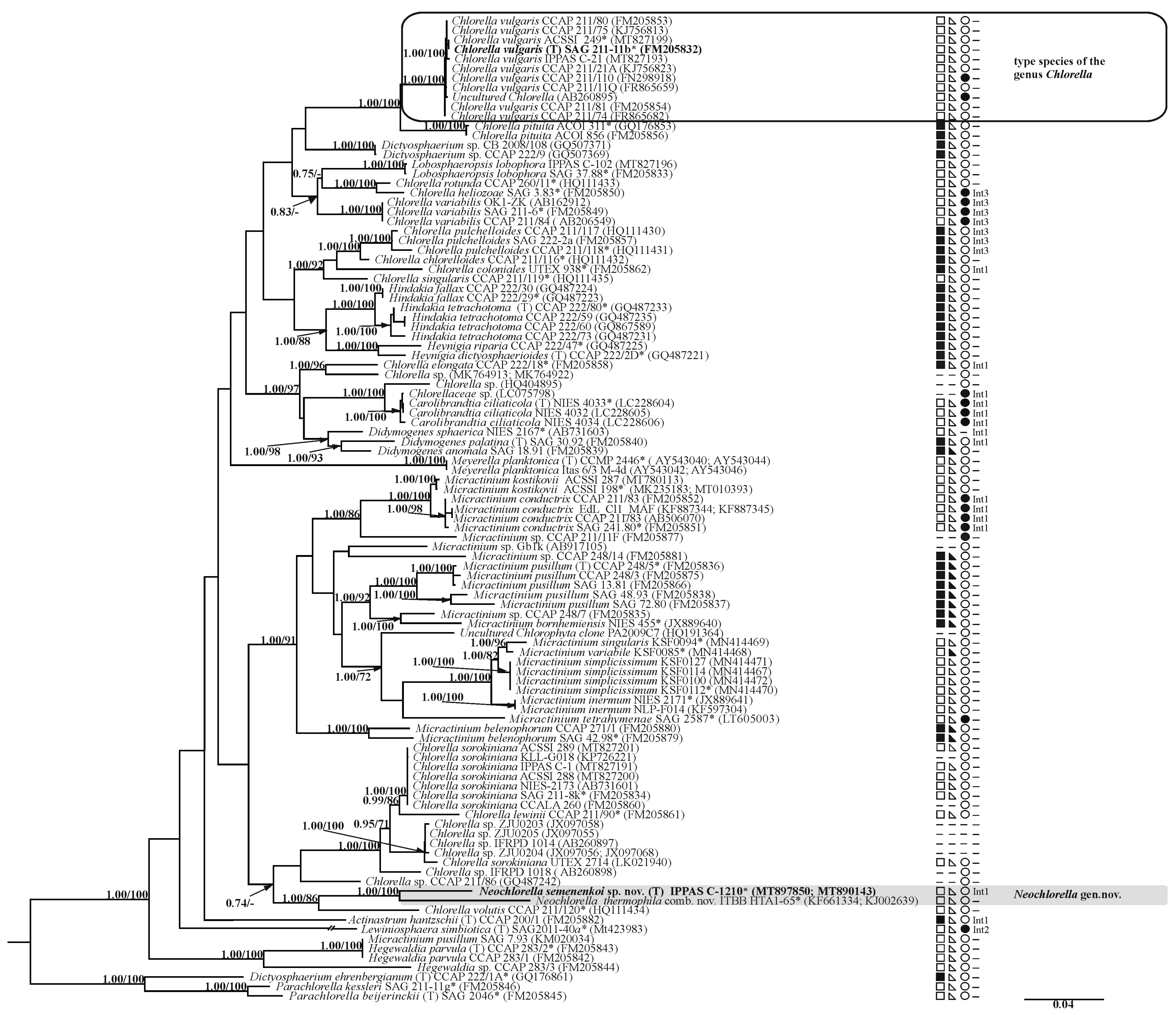
3.2. Phylogenetic Analysis
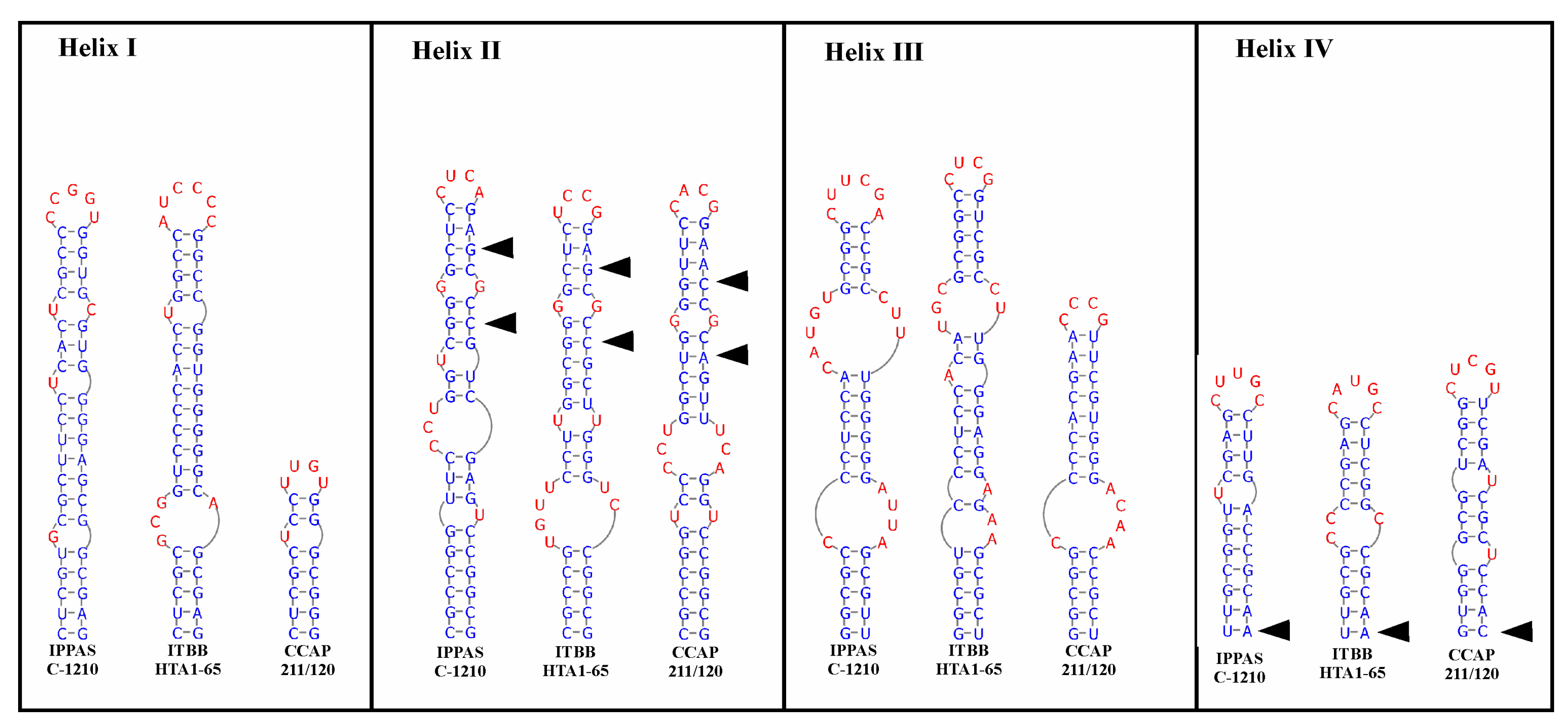
3.3. ITS1 and ITS2 Secondary Structures
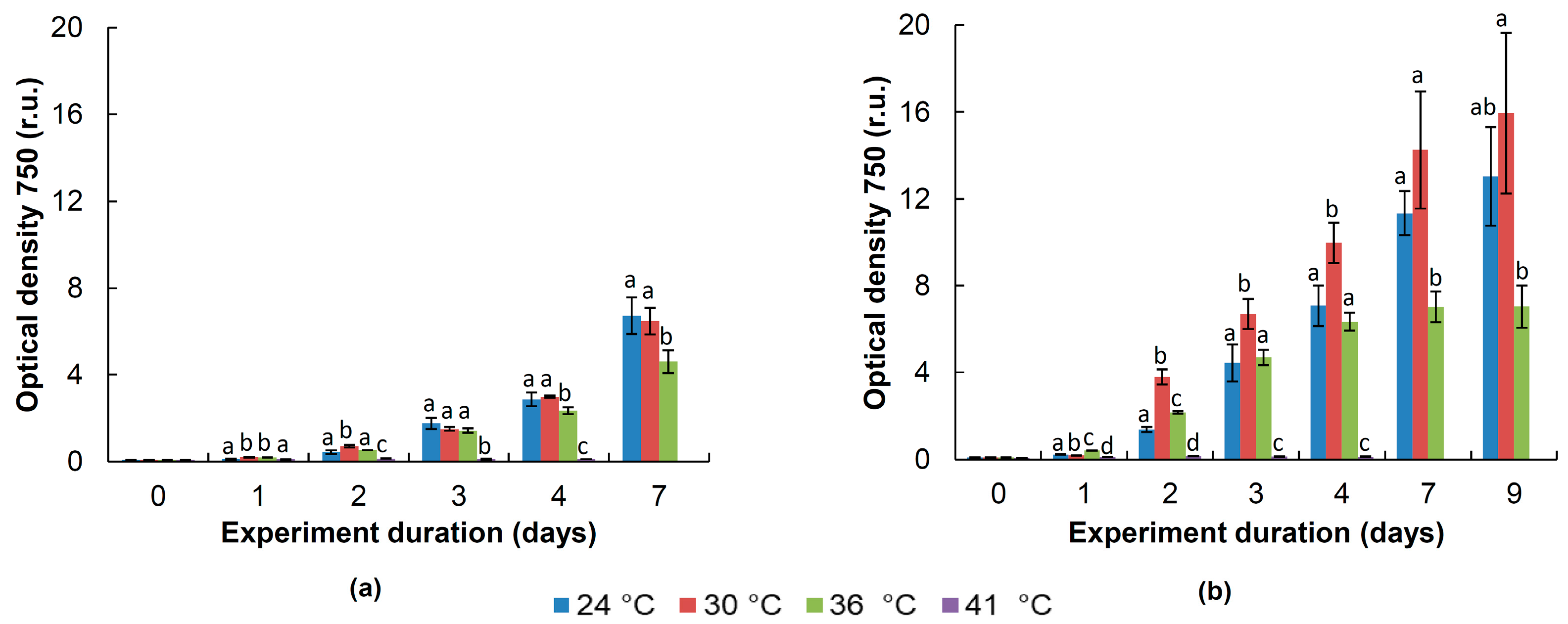
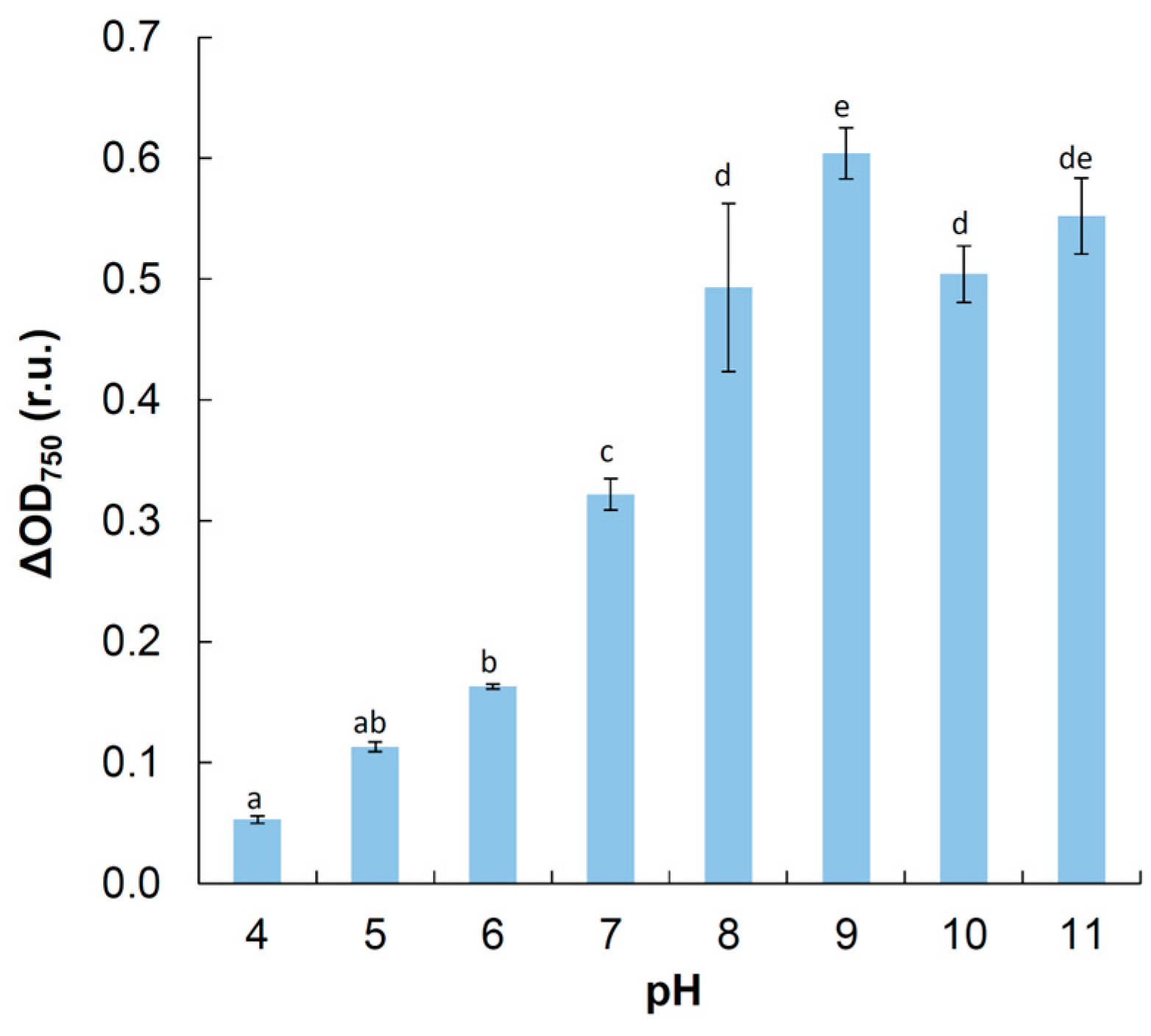
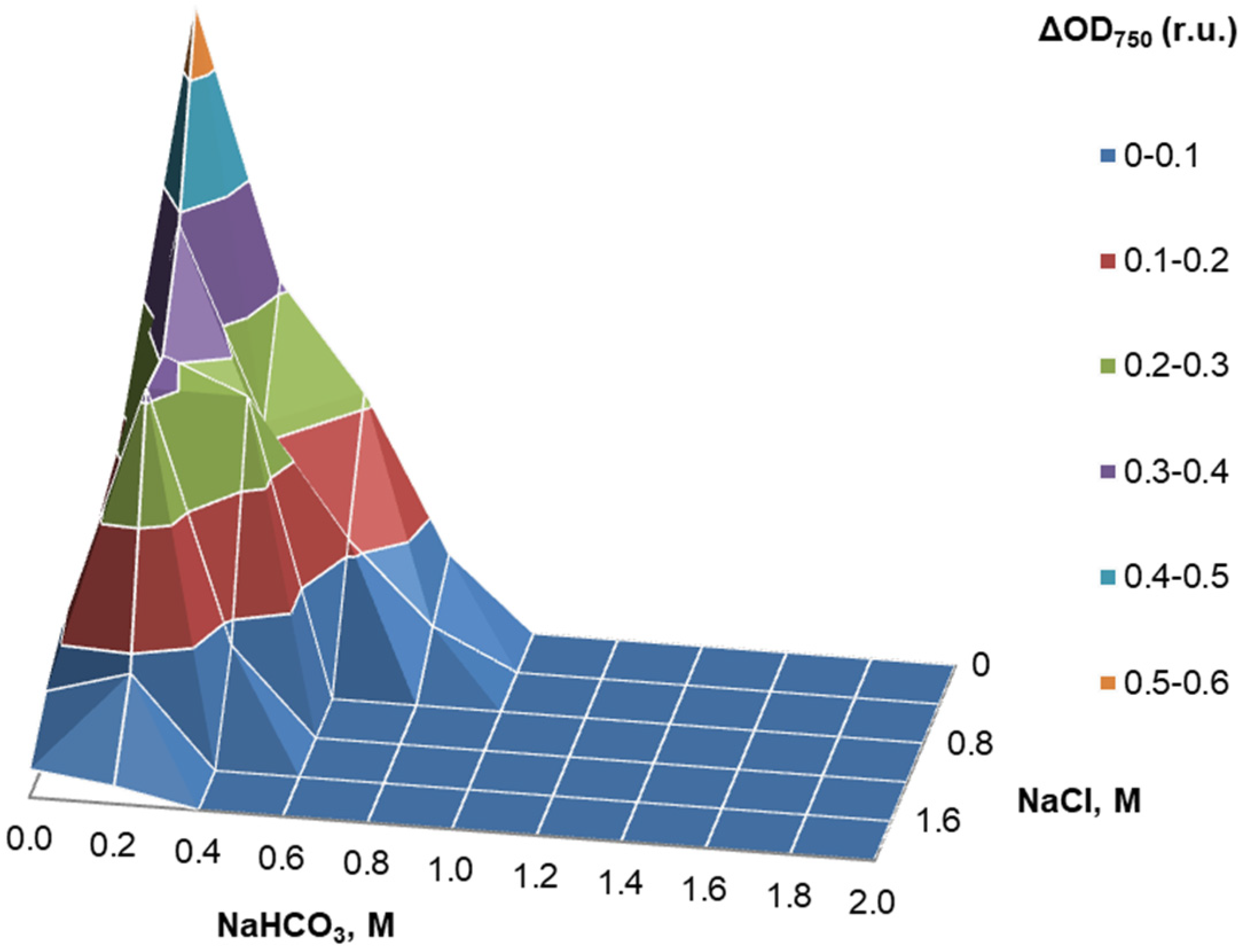
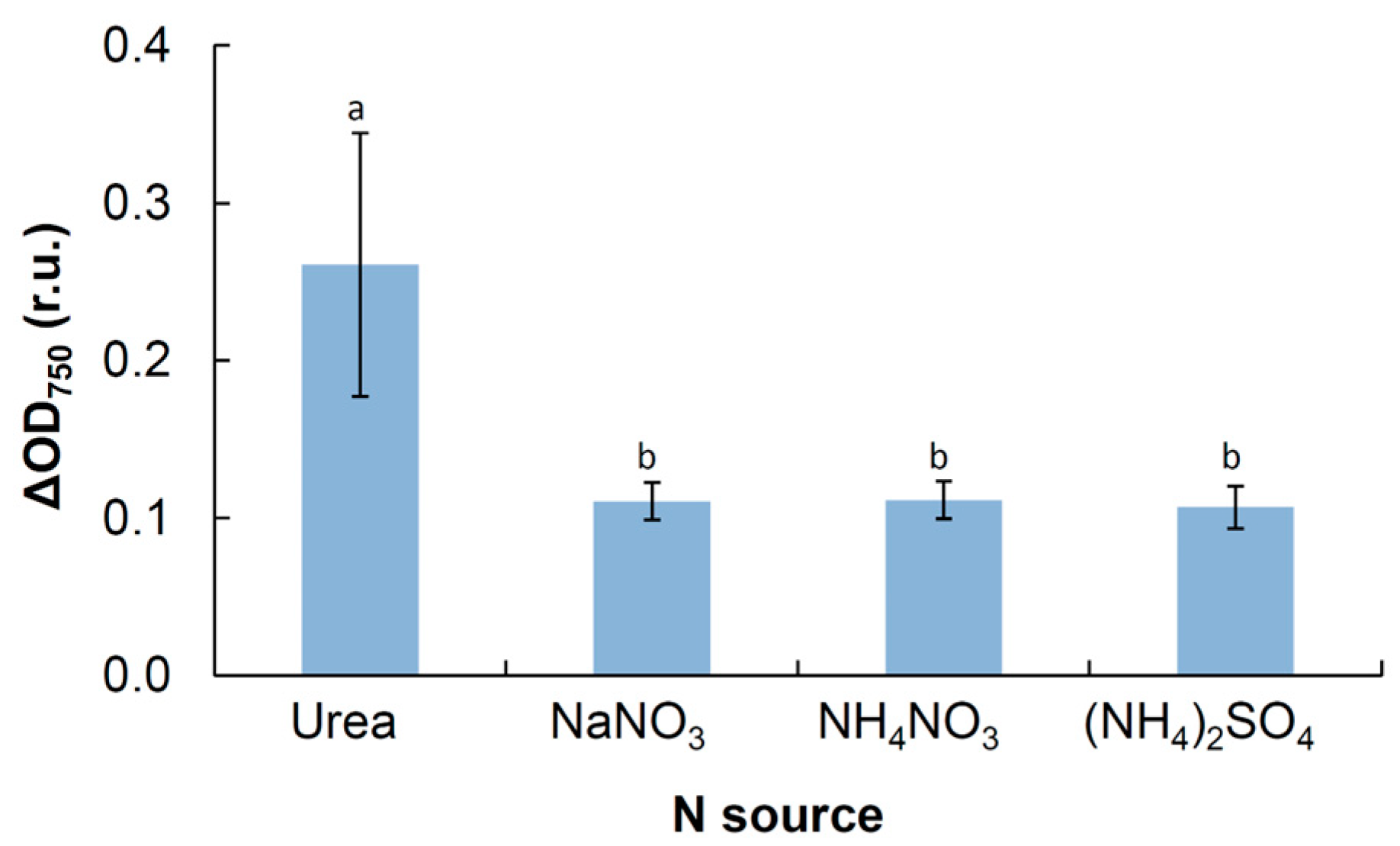
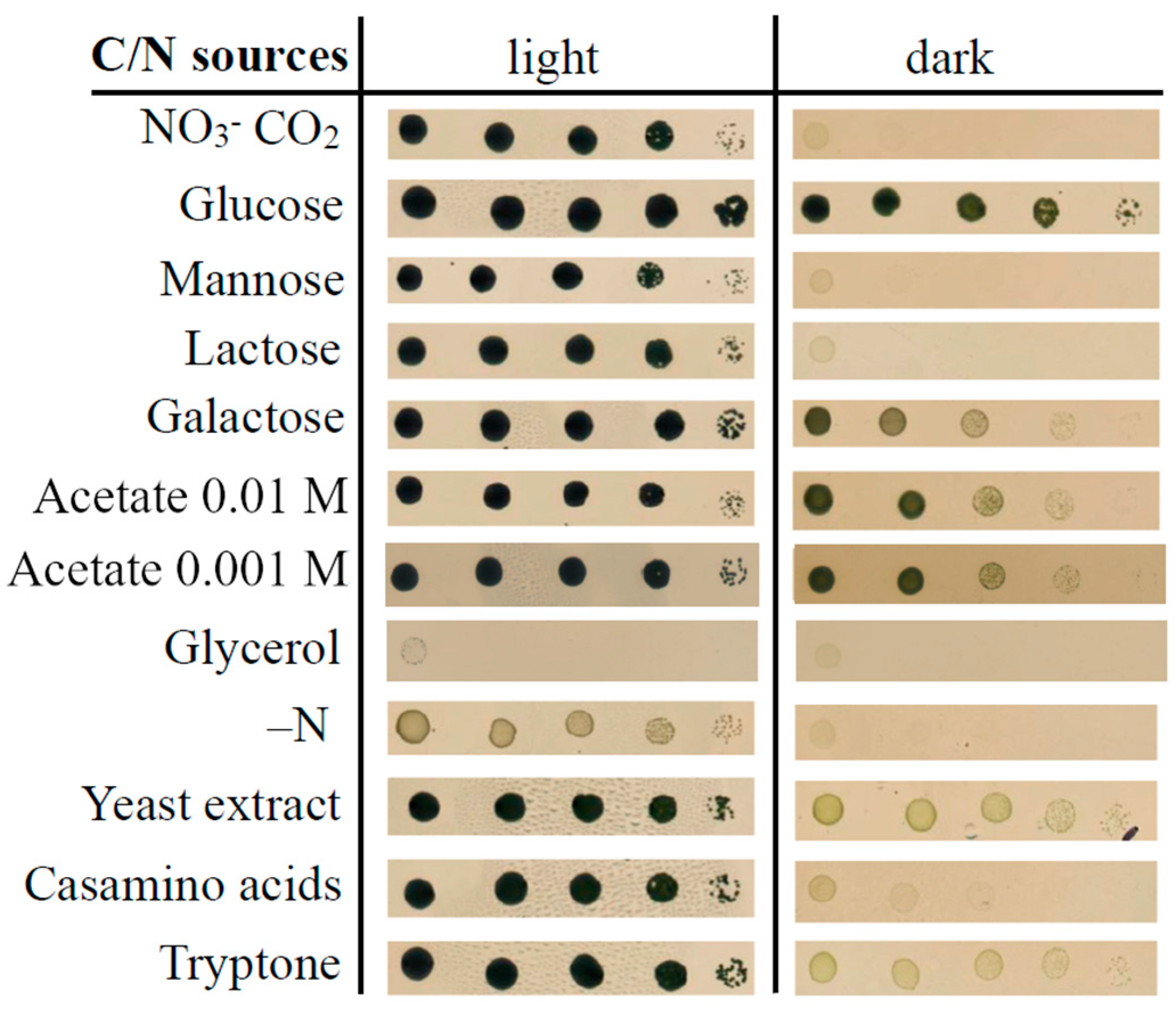
3.4. Physiological Tests
3.5. Formal Description
4. Discussion
4.1. Morphology and Ultrastructure
4.2. Phylogenetic Analysis
4.3. ITS1 and ITS2 Secondary Structures
4.4. Physiology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beijerinck, M. Culturversuche mit Zoochlorellen, Lichenengonidien und anderen niederen Algen. Bot. Ztg. 1890, 48, 781–788. [Google Scholar]
- Krienitz, L.; Hegewald, E.H.; Hepperle, D.; Huss, V.A.R.; Rohr, T.; Wolf, M. Phylogenetic relationship of Chlorella and Parachlorella gen. nov. (Chlorophyta, Trebouxiophyceae). Phycologia 2004, 43, 529–542. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kurihara, I.; Kawano, S. Late type of daughter cell wall synthesis in one of the Chlorellaceae, Parachlorella kessleri (Chlorophyta, Trebouxiophyceae). Planta 2005, 221, 766–775. [Google Scholar] [CrossRef]
- Luo, W.; Proschold, T.; Bock, C.; Krienitz, L. Generic concept in Chlorella-related coccoid green algae (Chlorophyta, Trebouxiophyceae). Plant Biol. 2010, 12, 545–553. [Google Scholar] [CrossRef]
- Pröschold, T.; Bock, C.; Luo, W.; Krienitz, L. Polyphyletic distribution of bristle formation in Chlorellaceae: Micractinium, Diacanthos, Didymogenes and Hegewaldia gen. nov. (Trebouxiophyceae, Chlorophyta). Phycol. Res. 2010, 58, 1–8. [Google Scholar] [CrossRef]
- Bock, C.; Krienitz, L.; Proeschold, T. Taxonomic reassessment of the genus Chlorella (Trebouxiophyceae) using molecular signatures (barcodes), including description of seven new species. Fottea 2011, 11, 293–312. [Google Scholar] [CrossRef]
- Ma, S.; Han, B.; Huss, V.A.R.; Hu, X.; Sun, X.; Zhang, J. Chlorella thermophila (Trebouxiophyceae, Chlorophyta), a novel thermo-tolerant Chlorella species isolated from an occupied rooftop incubator. Hydrobiologia 2015, 760, 81–89. [Google Scholar] [CrossRef]
- Hoshina, R.; Kobayashi, M.; Suzaki, T.; Kusuoka, Y. Brandtia ciliaticola gen. et sp. nov. (Chlorellaceae, Trebouxiophyceae) a common symbiotic green coccoid of various ciliate species. Phycol. Res. 2018, 66, 76–81. [Google Scholar] [CrossRef]
- Hoshina, R.; Nakada, T. Carolibrandtia nom. nov. as a replacement name for Brandtia Hoshina (Chlorellaceae, Trebouxiophyceae). Phycol. Res. 2018, 66, 82–83. [Google Scholar] [CrossRef]
- Chae, H.; Lim, S.; Kim, H.S.; Choi, H.-G.; Kim, J.H. Morphology and phylogenetic relationships of Micractinium (Chlorellaceae, Trebouxiophyceae) taxa, including three new species from Antarctica. Algae 2019, 34, 267–275. [Google Scholar] [CrossRef]
- Krivina, E.; Temraleeva, A.; Sinetova, M. New species Micractinium kostikovii (Chlorellaceae, Trebouxiophyceae) from Russia. Phycol. Res. 2022, 70, 22–34. [Google Scholar] [CrossRef]
- Heeg, J.S.; Wolf, M. ITS2 and 18S rDNA sequence-structure phylogeny of Chlorella and allies (Chlorophyta, Trebouxiophyceae, Chlorellaceae). Plant Gene 2015, 4, 20–28. [Google Scholar] [CrossRef]
- Krivina, E.; Temraleeva, A. Identification problems and cryptic diversity of Chlorella-clade microalgae (Chlorophyta). Microbiology 2020, 89, 720–732. [Google Scholar] [CrossRef]
- Hoshina, R.; Tsukii, Y.; Harumoto, T.; Suzaki, T. Characterization of a green Stentor with symbiotic algae growing in an extremely oligotrophic environment and storing large amounts of starch granules in its cytoplasm. Sci. Rep. 2021, 11, 2865. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. Über die Geschwindigkeit der photochemischen Kohlensäurezersetzung in lebenden Zellen. In Über die Katalytischen Wirkungen der Lebendigen Substanz: Arbeiten aus dem Kaiser Wilhelm-Institut für Biologie, Berlin-Dahlem; Warburg, O., Ed.; Springer: Berlin/Heidelberg, Germany, 1919; pp. 308–340. [Google Scholar]
- Yamada, T.; Sakaguchi, K. Comparative studies on Chlorella cell walls: Induction of protoplast formation. Arch. Microbiol. 1982, 132, 10–13. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Energy from Microalgae: A Short History. In Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 1–15. [Google Scholar]
- Masojídek, J.; Torzillo, G. Mass Cultivation of Freshwater Microalgae. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 2226–2235. [Google Scholar]
- Semenenko, V.E.; Rudova, T.S. On the mechanism of biosynthesis reorientation in Chlorella under the influence of factors limiting cellular division. Arch. Hydrobiol. Suppl. 1976, 49, 185–198. [Google Scholar]
- Sinetova, M.A.; Sidorov, R.A.; Starikov, A.Y.; Voronkov, A.S.; Medvedeva, A.S.; Krivova, Z.V.; Pakholkova, M.S.; Bachin, D.V.; Bedbenov, V.S.; Gabrielyan, D.A.; et al. Assessment of biotechnological potential of cyanobacteria and microalgae strains from the IPPAS culture collection. Appl. Biochem. Microbiol. 2020, 56, 36–50. [Google Scholar] [CrossRef]
- Andersen, R.A. (Ed.) Algal Culturing Techniques; Elsevier Academic Press: London, UK, 2005. [Google Scholar]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Gorman, D.S.; Levine, R.P. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1965, 54, 1665–1669. [Google Scholar] [CrossRef]
- Komárek, J.; Fott, B. Chlorococcales. Das Phytoplankton des Süßwassers, Vol. 7; Schweizerbart: Stuttgart, Germany, 1983; 1043 p. [Google Scholar]
- Andreyeva, V.M. Terrestrial and Aerophilic Green algae (Chlorophyta: Tetrasporales, Chlorococcales, Chlorosarcinales); NAUKA: St. Petersburg, Russia, 1998; 349 p. [Google Scholar]
- Červený, J.; Sinetova, M.A.; Zavřel, T.; Los, D.A. Mechanisms of high temperature resistance of Synechocystis sp. PCC 6803: An impact of histidine kinase 34. Life 2015, 5, 676–699. [Google Scholar] [CrossRef]
- Adar, O.; Kaplan-Levy, R.N.; Banet, G. High temperature Chlorellaceae (Chlorophyta) strains from the Syrian-African Rift Valley: The effect of salinity and temperature on growth, morphology and sporulation mode. Eur. J. Phycol. 2016, 51, 387–400. [Google Scholar] [CrossRef]
- Chen, M.; Chen, F.; Yu, Y.; Ji, J.; Kong, F. Genetic diversity of eukaryotic microorganisms in Lake Taihu, a large shallow subtropical lake in China. Microb. Ecol. 2008, 56, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Friedl, T. Evolution of the polyphyletic genus Pleurastrum (Chlorophyta): Inferences from nuclear-encoded ribosomal DNA sequences and motile cell ultrastructure. Phycologia 1996, 35, 456–469. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S. Taylor, Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 27 April 2022).
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Darienko, T.; Rad-Menéndez, C.; Campbell, C.; Pröschold, T. Are there any true marine Chlorella species? Molecular phylogenetic assessment and ecology of marine Chlorella-like organisms, including a description of Droopiella gen. nov. Syst. Biodivers. 2019, 17, 811–829. [Google Scholar] [CrossRef]
- Hoshina, R.; Iwataki, M.; Imamura, N. Chlorella variabilis and Micractinium reisseri sp. nov. (Chlorellaceae, Trebouxiophyceae): Redescription of the endosymbiotic green algae of Paramecium bursaria (Peniculia, Oligohymenophorea) in the 120th year. Phycol. Res. 2010, 58, 188–201. [Google Scholar] [CrossRef]
- Luo, W.; Pflugmacher, S.; Proschold, T.; Walz, N.; Krienitz, L. Genotype versus phenotype variability in Chlorella and Micractinium (Chlorophyta, Trebouxiophyceae). Protist 2006, 157, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Pröschold, T.; Darienko, T.; Silva, P.C.; Reisser, W.; Krienitz, L. The systematics of Zoochlorella revisited employing an integrative approach. Environ. Microbiol. 2011, 13, 350–364. [Google Scholar] [CrossRef]
- Pröschold, T.; Pitsch, G.; Darienko, T. Micractinium tetrahymenae (Trebouxiophyceae, Chlorophyta), a new endosymbiont isolated from ciliates. Diversity 2020, 12, 200. [Google Scholar] [CrossRef]
- Coleman, A.W. ITS2 is a double-edged tool for eukaryote evolutionary comparisons. Trends Genet. 2003, 19, 370–375. [Google Scholar] [CrossRef]
- Coleman, A.W. Is there a molecular key to the level of “biological species” in eukaryotes? A DNA guide. Mol. Phylogenet. Evol. 2009, 50, 197–203. [Google Scholar] [CrossRef]
- Coleman, A.W. Nuclear rRNA transcript processing versus internal transcribed spacer secondary structure. Trends Genet. 2015, 31, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Caisova, L.; Marin, B.; Melkonian, M. A consensus secondary structure of ITS2 in the Chlorophyta identified by phylogenetic reconstruction. Protist 2013, 164, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Seibel, P.N.; Müller, T.; Dandekar, T.; Schultz, J.; Wolf, M. 4SALE–a tool for synchronous RNA sequence and secondary structure alignment and editing. BMC Bioinform. 2006, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Seibel, P.N.; Müller, T.; Dandekar, T.; Wolf, M. Synchronous visual analysis and editing of RNA sequence and secondary structure alignments using 4SALE. BMC Res. Notes 2008, 1, 1–7. [Google Scholar] [CrossRef]
- Hoshina, R.; Fujiwara, Y. Molecular characterization of Chlorella cultures of the National Institute for Environmental Studies culture collection with description of Micractinium inermum sp. nov., Didymogenes sphaerica sp. nov., and Didymogenes soliella sp. nov. (Chlorellaceae, Trebouxiophyceae). Phycol. Res. 2013, 61, 124–132. [Google Scholar] [CrossRef]
- Gabrielyan, D.A.; Sinetova, M.A.; Gabrielian, A.K.; Bobrovnikova, L.A.; Bedbenov, V.S.; Starikov, A.Y.; Zorina, A.A.; Gabel, B.V.; Los, D.A. Laboratory system for intensive cultivation of microalgae and cyanobacteria. Rus. J. Plant. Physiol. 2023; in press. [Google Scholar]
- Mikhodyuk, O.; Gerasimenko, L.; Akimov, V.; Ivanovsky, R.; Zavarzin, G. Ecophysiology and polymorphism of the unicellular extremely natronophilic cyanobacterium Euhalothece sp. Z-M001 from Lake Magadi. Microbiology 2008, 77, 717–725. [Google Scholar] [CrossRef]
- Shihira, I.; Krauss, R.W. Physiology and Taxonomy of Forty-One Isolates; University of Maryland: College Park, MD, USA, 1965; pp. 1–92. [Google Scholar]
- Henley, W.J.; Hironaka, J.L.; Guillou, L.; Buchheim, M.A.; Buchheim, J.A.; Fawley, M.W.; Fawley, K.P. Phylogenetic analysis of the ‘Nannochloris-like’ algae and diagnoses of Picochlorum oklahomensis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta). Phycologia 2004, 43, 641–652. [Google Scholar] [CrossRef]
- Krienitz, L.; Huss, V.A.; Bock, C. Chlorella: 125 years of the green survivalist. Trends Plant. Sci. 2015, 20, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Hodac, L.; Hallmann, C.; Spitzer, K.; Elster, J.; Fasshauer, F.; Brinkmann, N.; Lepka, D.; Diwan, V.; Friedl, T. Widespread green algae Chlorella and Stichococcus exhibit polar-temperate and tropical-temperate biogeography. FEMS Microbiol. Ecol. 2016, 92, fiw122. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, V.; Škvorová, Z.; Němcová, Y.; Škaloud, P. Laetitia sardoa gen. & sp. nov., a new member of the Chlorellales (Trebouxiophyceae, Chlorophyta) isolated from Sardinia Island. Phycologia 2022, 61, 375–383. [Google Scholar] [CrossRef]
- Pröschold, T.; Rieser, D.; Darienko, T.; Nachbaur, L.; Kammerlander, B.; Qian, K.; Pitsch, G.; Bruni, E.P.; Qu, Z.; Forster, D.; et al. An integrative approach sheds new light onto the systematics and ecology of the widespread ciliate genus Coleps (Ciliophora, Prostomatea). Sci. Rep. 2021, 11, 5916. [Google Scholar] [CrossRef]
- Sorokin, C. Tabular comparative data for the low-and high-temperature strains of Chlorella. Nature 1959, 184, 613–614. [Google Scholar] [CrossRef]
- Mizuno, Y.; Sato, A.; Watanabe, K.; Hirata, A.; Takeshita, T.; Ota, S.; Sato, N.; Zachleder, V.; Tsuzuki, M.; Kawano, S. Sequential accumulation of starch and lipid induced by sulfur deficiency in Chlorella and Parachlorella species. Bioresour. Technol. 2013, 129, 150–155. [Google Scholar] [CrossRef]
- Kessler, E.; Huss, V.A.R. Comparative physiology and biochemistry and taxonomic assignment of the Chlorella (Chlorophyceae) strains of the Culture Collection of the University of Texas at Austin. J. Phycol. 1992, 28, 550–553. [Google Scholar] [CrossRef]
- Němcová, Y.; Kalina, T. Cell wall development, microfibril and pyrenoid structure in type strains of Chlorella vulgaris, C. kessleri, C. sorokiniana compared with C. luteoviridis (Trebouxiophyceae, Chlorophyta). Arch. Hydrobiol. Suppl. Bd. Algol. Stud. 2000, 100, 95–105. [Google Scholar] [CrossRef]
- Serra-Maia, R.; Bernard, O.; Gonçalves, A.; Bensalem, S.; Lopes, F. Influence of temperature on Chlorella vulgaris growth and mortality rates in a photobioreactor. Algal. Res. 2016, 18, 352–359. [Google Scholar] [CrossRef]
- Stephenson, A.L.; Dennis, J.S.; Howe, C.J.; Scott, S.A.; Smith, A.G. Influence of nitrogen-limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biofuels 2010, 1, 47–58. [Google Scholar] [CrossRef]
- Sakarika, M.; Kornaros, M. Effect of pH on growth and lipid accumulation kinetics of the microalga Chlorella vulgaris grown heterotrophically under sulfur limitation. Bioresour. Technol. 2016, 219, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Kessler, E. Upper limits of temperature for growth in Chlorella (Chlorophyceae). Plant Syst. Evol. 1985, 151, 67–71. [Google Scholar] [CrossRef]
- Rosen, B.H.; Berliner, M.D.; Petro, M.J. Protoplast induction in Chlorella pyrenoidosa. Plant Sci. 1985, 41, 23–30. [Google Scholar] [CrossRef]
- Takeda, H. Sugar composition of the cell wall and the taxonomy of Chlorella (Chlorophyceae). J. Phycol. 1991, 27, 224–232. [Google Scholar] [CrossRef]
- Takeda, H. Chemical composition of cell walls as a taxonomical marker. J. Plant Res. 1993, 106, 195–200. [Google Scholar] [CrossRef]
- Vorobyev, K.; Andronov, E.; Rautian, M.; Skoblo, I.; Migunova, A.; Kvitko, K. An atypical Chlorella symbiont from Paramecium bursaria. Protistology 2009, 6, 39–44. [Google Scholar]
- Krivina, E.S.; Temraleeva, A.D.; Bukin, Y.S. Species delimitation and cryptic diversity analysis of Parachlorella-clade microalgae (Chlorophyta). Microbiology 2021, 90, 455–469. [Google Scholar] [CrossRef]
- Müller, J.; Friedl, T.; Hepperle, D.; Lorenz, M.; Day, J.G. Distinction between multiple isolates of Chlorella vulgaris (Chlorophyta, Trebouxiophyceae) and testing for conspecificity using amplified fragment length polymorphism and its rDNA sequences. J. Phycol. 2005, 41, 1236–1247. [Google Scholar] [CrossRef]
- Barten, R.; Djohan, Y.; Evers, W.; Wijffels, R.; Barbosa, M. Towards industrial production of microalgae without temperature control: The effect of diel temperature fluctuations on microalgal physiology. J. Biotechnol. 2021, 336, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ras, M.; Steyer, J.-P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 153–164. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, S.; Ji, Y.; Schwaneberg, U.; Chi, Z. Progress toward a bicarbonate-based microalgae production system. Trends Biotechnol. 2022, 40, 180–193. [Google Scholar] [CrossRef]
- Bassi, A.; Saxena, P.; Aguirre, A.-M. Mixotrophic Algae Cultivation for Energy Production and Other Applications. In Algal Biorefineries: Volume 1: Cultivation of Cells and Products; Bajpai, R., Prokop, A., Zappi, M., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 177–202. [Google Scholar]
- Kessler, E. Comparative physiology, biochemistry, and the taxonomy of Chlorella (Chlorophyceae). Plant Syst. Evol. 1976, 125, 129–138. [Google Scholar] [CrossRef]


| # | Medium | Light/μmol Photons m−2 s−1 | Temperature | Aeration | Time of Sampling |
|---|---|---|---|---|---|
| 1 | solid BG-11 | 30, 12:12 h L/D | 22 °C | none | 20 days |
| 2 | liquid BG-11 +20 mM HEPES, pH 7.5 | 100, continuous light | 32 °C | 1.5–2% CO2 | 3 days |
| 3 | liquid TAP | 30, continuous light | 32° C 22 °C | none | 5 days 6 months |
| Amplified Sequence | Primer | Sequence (5′–3′) | PCR Conditions | Reference |
|---|---|---|---|---|
| SSU ~2200 bp | EukA F | AACCTGGTTGATCCTGCCAGT | 95 °C 10 min; 35 cycles (95 °C 30 s, 62 °C 30 s, and 72 °C 2 min); and 72 °C 6 min | [28] |
| 18L R | CACCTACGGAAACCTTGTTACGACTT | [29] | ||
| ITS1-5.8S- ITS2 785 bp | ITS5 F | GGAAGTAAAAGTCGTAACAAGG | 95 °C 10 min; 35 cycles (95 °C 30 s, 55 °C 30 s, and 72 °C 1 min); and 72 °C 6 min | [30] |
| ITS4 R | TCCTCCGCTTATTGATATGC |
| Characteristics | N. semenenkoi IPPAS C-1210 | N. thermophila ITBB HTA 1–65 | C. volutis CCAP 211/120 | C. sorokiniana SAG 211-8k | C. lewinii CCAP 211/90 | C. vulgaris SAG 211-11b |
|---|---|---|---|---|---|---|
| Cells | solitary | |||||
| Bristle | no | |||||
| Mucilage | no | |||||
| Adult cell shape | spherical or ellipsoidal | spherical or ellipsoidal | spherical | ellipsoidal or spherical | oval and egg shaped | always spherical |
| Young cell shape | spherical to ellipsoidal | spherical to ellipsoidal | spherical to slightly oval | ellipsoidal | oval | spherical |
| Cell size (μm) | 3.5−6.3 | 1.5−2.5 | 5.0−6.5 | 4.5–5.5 × 3.5–5.4 | 4.0–6.0 | 2–6 |
| Chloroplast | single, parietal, and cup-shaped | single, parietal, and cup-shaped | single, parietal, and cup- or saucer-shaped | single, shallow, and cup-shaped | single, parietal, and cup-, girdle- or saucer-shaped | single and deep cup-shaped |
| Pyrenoid | single, spherical, and 0.4−0.8 μm in diameter | single, spherical, and 0.4−0.6 μm in diameter | single, ellipsoid to spherical | single | single andbroadly ellipsoidal to spherical | single |
| Starch envelope | two starch halves | |||||
| Cell wall | 20–40 nm single-layer in young cells, and 100–200 nm non-homogenous in mature cells | ~60–80 nm and double-layered | ND | 22 nm and single-layered in young cells; 60 nm and multilayered in older cells | ND | 180–200 nm |
| Main storage products | Lipids and starch | Starch | ND | Starch and lipids | ND | Lipids and starch |
| Reproduction | by 2–8 autospores | by 2–4 (sometimes more, needs clarification) autospores | ND | by 2–8 (16) autospores | ND | by 2–16 autospores |
| Intron in the SSU | 440 bp | no | no | no | no | no |
| Type location | freshwater reservoir | rooftops | freshwater reservoir | freshwater reservoir | soil in pond | freshwater reservoir |
| NaCl optimum/limits | 0 M/2M | ND | ND | ND/1–3% (0.2–0.6 M) | ND | ND/3–4% (=0.6–0.7 M) |
| Temperature optimum/limits | 30 °C/36 °C (41 °C–43 h) | 33 °C/42 °C (45–3h) | ND | 38–39 °C/42 °C | ND | 20–25 °C/28–30 °C |
| pH optimum/lower, upper limits | 8–9/4, 11 | ND | ND | ND/3.5–5 | ND | 5–8/4, 9.5 |
| Organic C sources acetate glucose galactose mannose glycerol | + + + − − | +? N/D N/D N/D N/D | ND | − + + − N/D | ND | + + N/D N/D + (under light) |
| N sources nitrate ammonium urea tryptone/peptone yeast extract casamino acids | + + + + + + | N/D + N/D N/D N/D N/D | + N/D N/D N/D N/D N/D | + + N/D N/D − + | + N/D N/D N/D N/D N/D | + N/D N/D + N/DN/D |
| References | Present study | [7] | [6] | [3,51,57,58,59,60] | [6] | [1,59,60,61,62,63,64,65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krivina, E.S.; Bobrovnikova, L.A.; Temraleeva, A.D.; Markelova, A.G.; Gabrielyan, D.A.; Sinetova, M.A. Description of Neochlorella semenenkoi gen. et. sp. nov. (Chlorophyta, Trebouxiophyceae), a Novel Chlorella-like Alga with High Biotechnological Potential. Diversity 2023, 15, 513. https://doi.org/10.3390/d15040513
Krivina ES, Bobrovnikova LA, Temraleeva AD, Markelova AG, Gabrielyan DA, Sinetova MA. Description of Neochlorella semenenkoi gen. et. sp. nov. (Chlorophyta, Trebouxiophyceae), a Novel Chlorella-like Alga with High Biotechnological Potential. Diversity. 2023; 15(4):513. https://doi.org/10.3390/d15040513
Chicago/Turabian StyleKrivina, Elena S., Lidia A. Bobrovnikova, Anna D. Temraleeva, Alexandra G. Markelova, David A. Gabrielyan, and Maria A. Sinetova. 2023. "Description of Neochlorella semenenkoi gen. et. sp. nov. (Chlorophyta, Trebouxiophyceae), a Novel Chlorella-like Alga with High Biotechnological Potential" Diversity 15, no. 4: 513. https://doi.org/10.3390/d15040513
APA StyleKrivina, E. S., Bobrovnikova, L. A., Temraleeva, A. D., Markelova, A. G., Gabrielyan, D. A., & Sinetova, M. A. (2023). Description of Neochlorella semenenkoi gen. et. sp. nov. (Chlorophyta, Trebouxiophyceae), a Novel Chlorella-like Alga with High Biotechnological Potential. Diversity, 15(4), 513. https://doi.org/10.3390/d15040513








