Effect of Enriched Substrate on the Growth of the Sea Cucumber Holothuria arguinensis Koehler and Vaney, 1906 Juveniles
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Data Analysis
3. Results
3.1. Survival Rate
3.2. Weight Analysis
3.3. Length Analysis
3.4. RSD Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polidoro, B.; Tognelli, M.; Harwell, H.; Elfes, C.; Cepeda, A.; Gonzalez-Maya, J.F.; Zarate-Charry, D.A.; Alvarado, J.J.; Benavides, M.; Conand, C.; et al. IUCN Red List workshop for sea cucumbers. SPC Beche-De-Mer Inf. Bull. 2011, 31, 65. [Google Scholar]
- Domínguez-Godino, J.A.; Slater, M.J.; Hannon, C.; González-Wangüermert, M. A new species for sea cucumber ranching and aquaculture: Breeding and rearing of Holothuria arguinensis. Aquaculture 2015, 438, 122–128. [Google Scholar] [CrossRef]
- Eriksson, H.; Robinson, G.; Slater, M.J.; Troell, M. Sea cucumber aquaculture in the Western Indian Ocean: Challenges for sustainable livelihood and stock improvement. Ambio 2012, 41, 109–121. [Google Scholar] [CrossRef]
- Purcell, S.W.; Williamson, D.H.; Ngaluafe, P. Chinese market prices of beche-de-mer: Implications for fisheries and aquaculture. Mar. Policy 2018, 91, 58–65. [Google Scholar] [CrossRef]
- Venâncio, E.; Félix, P.M.; Brito, A.C.; Sousa, J.; Azevedo e Silva, F.; Simões, T.; Narciso, L.; Amorim, A.; Dâmaso, L.; Pombo, A. Do broodstock diets influence viability and larval development of Holothuria mammata? Aquaculture 2021, 536, 736431. [Google Scholar] [CrossRef]
- Madruga, A.S.; Félix, P.M.; Sousa, J.; e Silva, F.A.; Brito, A.C.; Mendes, S.; Pombo, A. Effect of rearing temperature in the growth of hatchery reared juveniles of the sea cucumber Holothuria arguinensis (Koehler & Vaney, 1906). Aquaculture 2022, 562, 738809. [Google Scholar]
- Domínguez-Godino, J.A.; González-Wangüemert, M. Breeding and larval development of Holothuria mammata, a new target species for aquaculture. Aquac. Res. 2018, 49, 1430–1440. [Google Scholar] [CrossRef]
- Domínguez-Godino, J.A.; González-Wangüemert, M. Holothuria arguinensis: A new sea cucumber species for aquaculture. SPC Beche-De-Mer Inf. Bull. 2019, 39, 60–64. [Google Scholar]
- González-Wangüemert, M.; Valente, S.; Henriques, F.; Domínguez-Godino, J.; Serrão, E. Setting preliminary biometric baselines for new target sea cucumbers species of the NE Atlantic and Mediterranean fisheries. Fish. Res. 2016, 179, 57–66. [Google Scholar] [CrossRef]
- Santos, R.; Dias, S.; Pinteus, S.; Silva, J.; Alves, C.; Tecelão, C.; Pedrosa, R.; Pombo, A. Sea cucumber Holothuria forskali, a new resource for aquaculture? Reproductive biology and nutraceutical approach. Aquac. Res. 2015, 47, 2307–2323. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Northern sea cucumber (Cucumaria frondosa): A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef]
- González-Wangüemert, M.; Domínguez-Godino, J.; Cánovas, F. The fast development of sea cucumber fisheries in the Mediterranean and NE Atlantic waters: From a new marine resource to its over-exploitation. Ocean Coast Manag. 2018, 151, 165–177. [Google Scholar] [CrossRef]
- Roggatz, C.C.; González-Wangüemert, M.; Pereira, H.; Vizetto-Duarte, C.; Rodrigues, M.J.; Barreira, L.; da Silva, M.M.; Varela, J.; Custódio, L. A first glance into the nutritional properties of the sea cucumber Parastichopus regalis from the Mediterranean Sea (SE Spain). Nat. Prod. Res. 2018, 32, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Gorospe, J.C.; Juinio-Meñez, M.A.; Southgate, P.C. Effects of shading on periphyton characteristics and performance of sandfish, Holothuria scabra Jaeger 1833, juveniles. Aquaculture 2019, 512, 734307. [Google Scholar] [CrossRef]
- Pei, S.; Dong, S.; Wang, F.; Tian, X.; Gao, Q. Effects of density on variation in individual growth and differentiation in endocrine response of Japanese sea cucumber (Apostichopus japonicus Selenka). Aquaculture 2012, 356, 398–403. [Google Scholar] [CrossRef]
- Félix, P.M.; Pombo, A.; Azevedo e Silva, F.; Simoes, T.; Marques, T.A.; Melo, R.; Rocha, C.; Sousa, J.; Venâncio, E.; Costa, J.L.; et al. Modelling the distribution of a commercial NE-Atlantic Sea Cucumber, Holothuria mammata: Demographic and abundance spatio-temporal patterns. Front. Mar. Sci. 2021, 8, 675330. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, W.L.; Xin, S.G.; Xing, W.Q. Analysis study of trace elements in abalone and sea cucumber. Spectrosc. Spectr. Anal. 2009, 29, 511–514. [Google Scholar]
- Azevedo e Silva, F.; Brito, A.C.; Simões, T.; Pombo, A.; Marques, T.A.; Rocha, C.; Sousa, J.; Venâncio, E.; Félix, P.M. Allometric relationships to assess ontogenetic adaptative changes in three NE Atlantic commercial sea cucumbers (Echinodermata, Holothuroidea). Aquat. Ecol. 2021, 55, 711–720. [Google Scholar] [CrossRef]
- Xing, R.; Ma, W.; Shao, Y.; Cao, X.; Chen, L.; Jiang, A. Factors that affect the growth and photosynthesis of the filamentous green algae, Chaetomorpha valida, in static sea cucumber aquaculture ponds with high salinity and high pH. PeerJ 2019, 7, e6468. [Google Scholar] [CrossRef] [PubMed]
- Sabilu, K.; Supriyono, E.; Nirmala, K.; Ketjulan, R.; Hamzah, M.; Rahman, A.; Patadjai, R.S.; Alwi, L.O.; Sabilu, M. Application of compost in the different of pen culture substrates type for the intensification of sea cucumber, Holothuria scabra (Jaeger 1883) culture. IOP Conf. Ser. Earth Environ. Sci. 2022, 1033, 012018. [Google Scholar] [CrossRef]
- Sinsona, M.J.; Juinio-Meñez, M.A. Effects of sediment enrichment with macroalgae, Sargassum spp., on the behavior, growth, and survival of juvenile sandfish, Holothuria scabra. Aquac. Rep. 2018, 12, 56–63. [Google Scholar] [CrossRef]
- Magcanta, M.L.M.; Sornito, M.B.; Espadero, A.D.A.; Bacosa, H.P.; Uy, W.H. Growth, survival, and behavior of early juvenile sandfish Holothuria scabra (Jaeger, 1883) in response to feed types and salinity levels under laboratory conditions. Philipp. J. Sci. 2021, 150, 871–884. [Google Scholar] [CrossRef]
- Olaya-Restrepo, J.; Erzini, K.; González-Wangüemert, M. Estimation of growth parameters for the exploited sea cucumber Holothuria arguinensis from south Portugal. Fish. Bull. 2018, 116, 1–8. [Google Scholar]
- Altamirano, J.P.; Recente, C.P.; Rodriguez, J.C., Jr. Substrate preference for burying and feeding of sandfish Holothuria scabra juveniles. Fish. Res. 2017, 186, 514–523. [Google Scholar] [CrossRef]
- Juinio-Meñez, M.A.; Evangelio, J.C.; Miralao, S.J.A. Trial grow-out culture of sea cucumber Holothuria scabra in sea cages and pens. Aquac. Res. 2014, 45, 1332–1340. [Google Scholar] [CrossRef]
- Juinio-Meñez, M.A.; Tech, E.D.; Ticao, I.P.; Gorospe, J.R.C.; Edullantes, C.M.A.; Rioja, R.A.V. Adaptive and integrated culture production systems for the tropical sea cucumber Holothuria scabra. Fish. Res. 2017, 186, 502–513. [Google Scholar] [CrossRef]
- Hair, C. Sandfish (Holothuria scabra) production and sea-ranching trial in Fiji. In Asia-Pacific Tropical Sea Cucumber Aquaculture, ACIAR Proceedings; Hair, C.A., Pickering, T.D., Mills, D.J., Eds.; Australian Centre for International Agricultural Research: Canberra, Austrilia, 2012; Volume 136, pp. 129–141. [Google Scholar]
- Brito, A.; Newton, A.; Tett, P.; Fernandes, T. F Temporal and spatial variability of microphytobenthos in a shallow lagoon: Ria Formosa (Portugal). Estuar. Coast. Shelf Sci. 2009, 83, 67–76. [Google Scholar] [CrossRef]
- Feng, J.; Anisuzzaman, M.; U-Cheol, J.; Jong-Kuk, C.; Hak-Sun, Y.; Seung-Wan, K.; Seok-Joong, K. Comparison of fatty acid composition of wild and cultured sea cucumber Apostichopus japonicus. Korean J. Fish Aqua. Sci. 2016, 49, 474–485. [Google Scholar]
- Feng, J.; Jong-Kuk, C.; U-Cheol, J.; Anisuzzaman, M.; Chung-Ho, R.; Byeong-dae, C.; Seok-Joong, K. Effects of fermented fecal solid diets on growth of the sea cucumber Apostichopus japonicus. Korean J. Fish Aqua. Sci. 2016, 49, 161–167. [Google Scholar]
- Laguerre, H.; Raymond, G.; Plan, P.; Améziane, N.; Bailly, X.; Le Chevalier, P. First description of embryonic and larval development, juvenile growth of the black sea-cucumber Holothuria forskali (Echinodermata: Holothuroidea), a new species for aquaculture in the north-eastern Atlantic. Aquaculture 2020, 521, 734961. [Google Scholar] [CrossRef]
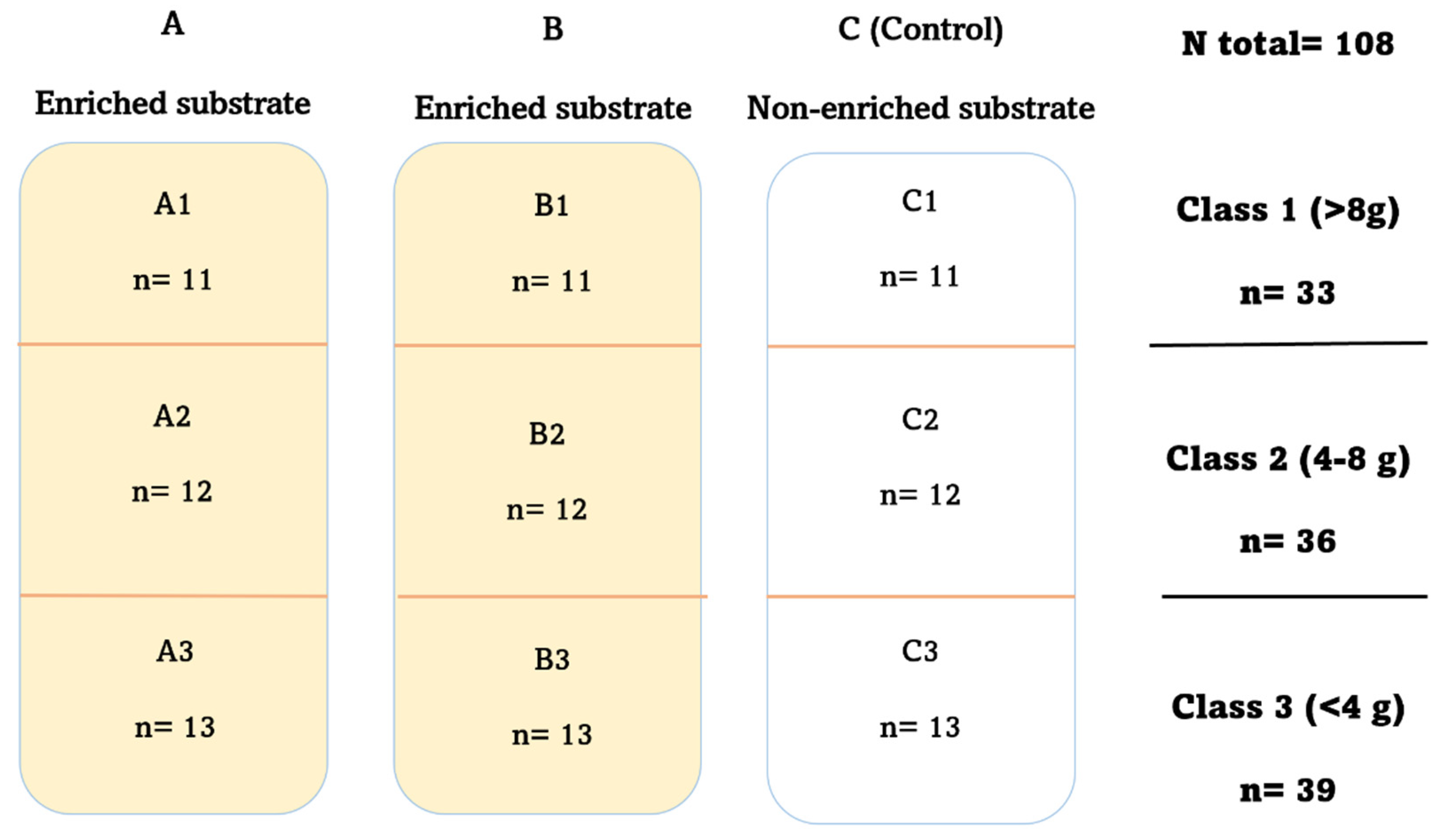

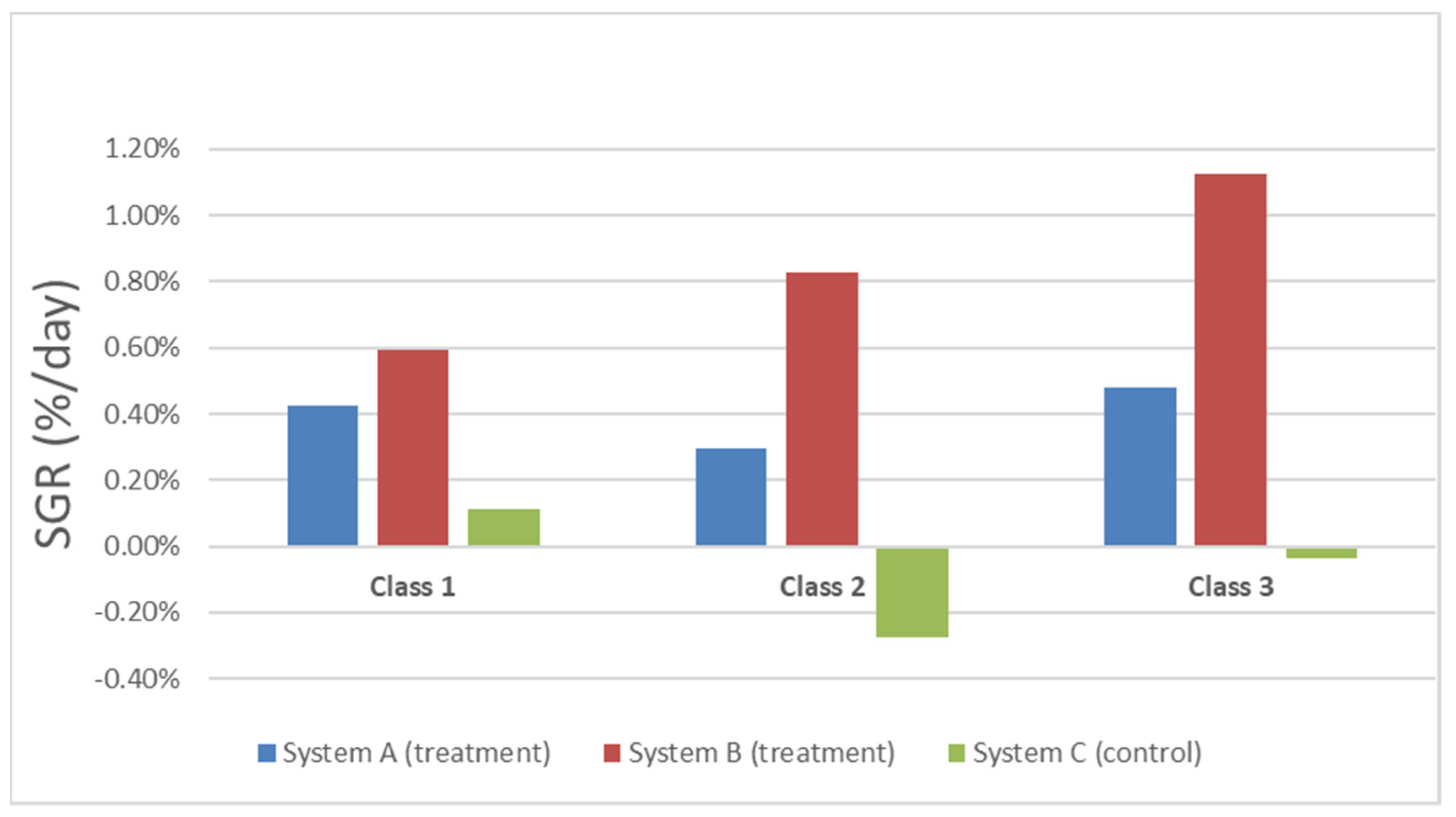

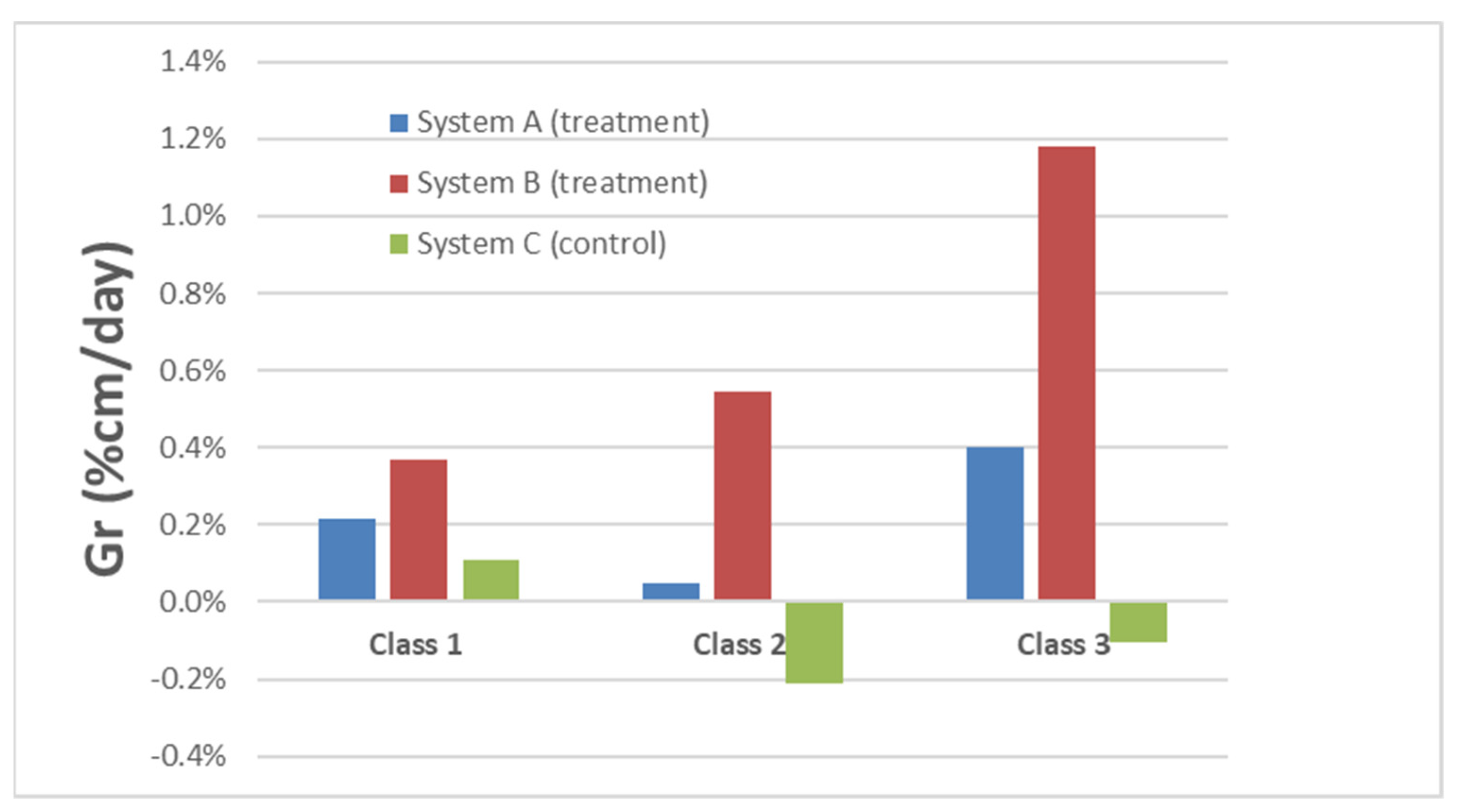

| Class 1 (>8 g) | Class 2 (4–8 g) | Class 3 (<4 g) | Total % | |
|---|---|---|---|---|
| System A | 3.03 | 2.70 | 0 | 5.73 |
| System B | 0 | 0 | 0 | 0 |
| System C | 0 | 5.55 | 7.69 | 13.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, T.; Azevedo e Silva, F.; Sousa, J.; Félix, P.M.; Pombo, A. Effect of Enriched Substrate on the Growth of the Sea Cucumber Holothuria arguinensis Koehler and Vaney, 1906 Juveniles. Diversity 2023, 15, 458. https://doi.org/10.3390/d15030458
Rodrigues T, Azevedo e Silva F, Sousa J, Félix PM, Pombo A. Effect of Enriched Substrate on the Growth of the Sea Cucumber Holothuria arguinensis Koehler and Vaney, 1906 Juveniles. Diversity. 2023; 15(3):458. https://doi.org/10.3390/d15030458
Chicago/Turabian StyleRodrigues, Tiago, Francisco Azevedo e Silva, João Sousa, Pedro M. Félix, and Ana Pombo. 2023. "Effect of Enriched Substrate on the Growth of the Sea Cucumber Holothuria arguinensis Koehler and Vaney, 1906 Juveniles" Diversity 15, no. 3: 458. https://doi.org/10.3390/d15030458
APA StyleRodrigues, T., Azevedo e Silva, F., Sousa, J., Félix, P. M., & Pombo, A. (2023). Effect of Enriched Substrate on the Growth of the Sea Cucumber Holothuria arguinensis Koehler and Vaney, 1906 Juveniles. Diversity, 15(3), 458. https://doi.org/10.3390/d15030458








