Abstract
The genus Abyssopathes Opresko, 2002, comprises deep-sea black corals known almost exclusively from lower bathyal and abyssal depths, mainly from seamounts covered by cobalt-rich crusts and areas of polymetallic nodules. The taxonomical position of the genus and its placement in the family Schizopathidae has been repeatedly questioned, but fruitlessly. Known only in extremely deep habitats, these corals have rarely been collected in a state suitable for morphological or molecular studies that could help to clarify their status. Recently, increasing attention has been paid to the study of fauna associated with deep-sea minerals. Using material of Abyssopathes lyra (Brook, 1889) sampled during these studies, we transfer the genus Abyssopathes from the family Schizopathidae to the family Cladopathidae based on morphological and molecular data. Morphological data includes six mesenteries in the polyps, a unique pinnulation pattern found only in genera within the Cladopathidae, and relatively short polyp tentacles, a feature typical of some cladopathids. Sequencing data, consisting of 626 bp from the mitochondrial cox1 gene, showed that Abyssopathes is 99% identical to Chrysopathes Opresko, 2003, Cladopathes Brook, 1889, Heteropathes Opresko, 2011, and Trissopathes Opresko, 2003 (all Cladopathidae), in this gene region.
1. Introduction
It is generally considered that species of the order Antipatharia occur primarily in the deep sea, with up to 75% of all hitherto known antipatharian species recorded below 50 m [1,2]. However, this approximation is possibly exaggerated, as many genera of the families Antipathidae, Myriopathidae, and Aphanipathidae are found predominantly at shallow (0–30 m) and mesophotic (30–150 m) depths [3,4] and only up to 31.6% of hitherto described species occur below 800 m [5]. Not all antipatharian families are equally represented in the deep sea. At depths of 800–3500 m, the dominant families are the Cladopathidae, Antipathidae, and Schizopathidae (13.68%, 14.74%, and 51.58%, respectively) [5]. Below 3500 m, the only families present are the Leiopathidae, Cladopathidae, and Schizopathidae, with the Schizopathidae comprising 85.71% of the black coral species known from the abyssal and hadal zones [5].
A close evolutionary relationship between Cladopathidae and Schizopathidae was revealed in the first molecular phylogenies of the order [6]. Both families share the absence of the cox1 intron in the mitochondrial genome [7]. The only reliable morphological feature that has been used to date to distinguish these two families is the number of polypar mesenteries: six in Cladopathidae and ten in Schizopathidae. Many features of gross morphology are shared between the two families: general patterns of pinnulation, presence of striatum in monopodial colonies, transversely elongated polyps, etc. The common morphological features of the Cladopathidae and Schizopathidae families make it difficult to accurately position a species that is devoid of polyps or has poorly preserved soft tissues when no histological or molecular methods can be used. It can be illustrated by the situation with Sibopathes macrospina Opresko, 1993, which originally was assigned to the family Cladopathidae based on the presence of only six mesenteries in the polyps [8], but molecular data showed that specimens having identical external morphology clustered with the morphologically similar genus Parantipathes Brook, 1889, in the family Schizopathidae [6,7,9]. However, until genetic-grade material of Sibopathes gephura van Pesch, 1914, the type species of the genus Sibopathes van Pesch, 1914, is collected and analyzed, the taxonomic position of the genus Sibopathes cannot be resolved [6,7].
The genus Abyssopathes Opresko, 2002 (Figure 1), is one of the deepest occurring genera of black corals and is found exclusively at lower bathyal and abyssal depths [3,5]. Although a species of the genus Schizopathes Brook, 1889, is known from hadal depths [3,5], in general, the genus Schizopathes more commonly occurs in the bathyal zone [5,10]. In contrast, all three hitherto known species of Abyssopathes were reported from a similar depth range (3000–5000 m) from oligotrophic deep basins, often in connection with seamounts covered by cobalt-rich crusts and areas of polymetallic nodules [3,11,12,13,14]. Bathypathes lyra Brook, 1889 (Figure 1 and Figure 2a), the type species of Abyssopathes, was originally included by Brook [11] in the genus Bathypathes Brook, 1889, subfamily Schizopathinae of the family Antipathidae. When the genus Abyssopathes was established [12], it was reclassified within the family Schizopathidae in the absence of any data on polyp structure [11,12]. The taxonomical position of the genus and its placement in the family Schizopathidae has been repeatedly questioned because of similarities in gross morphology to species of the family Cladopathidae (Figure 2 and Figure 3) [11,12]; however, these corals have rarely been collected in a state suitable for histological or molecular studies that could help to clarify their status.

Figure 1.
Abyssopathes lyra (Brook, 1889). In situ photo of specimen USNM 1453760 with a characteristic basket-like corallum. Photo courtesy of the NOAA OER (Note: color enhanced to show details).
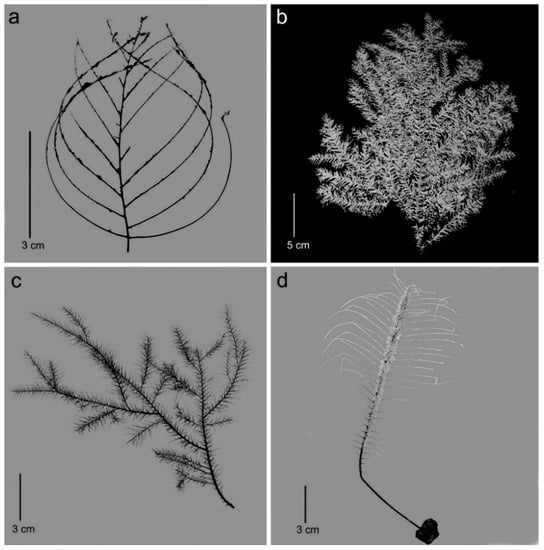
Figure 2.
Select genera of the family Cladopathidae: (a) Abyssopathes lyra (Brook, 1889) (syntype, NHMUK 1890-4-9-22); (b) Trissopathes pseudotristicha Opresko, 2003 (USNM 1070975); (c) Chrysopathes speciosa Opresko, 2003 (paratype, USNM 92520); (d) Heteropathes pacifica (Opresko, 2005) (holotype, USNM 1070758).
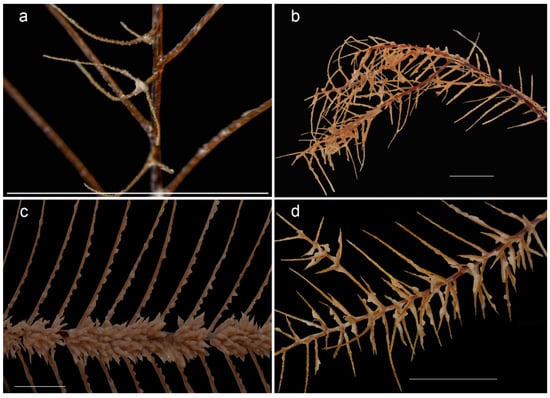
Figure 3.
Close-ups of select genera of the family Cladopathidae: (a) Abyssopathes lyriformis Opresko, 2002 (holotype, USNM 83567); (b) Sibopathes gephura van Pesch, 1914 (holotype ZMA Coel 3283), (c) Heteropathes pacifica (holotype, USNM 1070758), (d) Trissopathes pseudotristicha (holotype, RMNH Coel. 32045). Scale 10 mm.
Recently, the National Oceanic and Atmospheric Administration (NOAA) organized and implemented a Pacific-wide field campaign CAPSTONE (Campaign to Address Pacific monument Science, Technology, and Ocean NEeds). A number of previously poorly studied biotopes were investigated and sampled using the dual body, 6000 m-rated, ROV system Deep Discoverer (D2) [15]. On one of the dives to an abyssal habitat off the Phoenix Islands, a specimen of Abyssopathes lyra was collected at a depth of 5772.36 m and recovered on deck with the polyps in a reasonably good state of preservation, which allowed for both histological and molecular studies. Below we present our data to clarify the taxonomic position of this abyssal genus.
2. Materials and Methods
Morphological characters used to distinguish genera in the families Cladopathidae and Schizopathidae were analyzed based on existing literature [3,5,8,12,16,17,18,19,20,21] and original data (Table 1, Supplementary Table S1). For terminology, see [6,22].

Table 1.
Comparison of gross colony features of Abyssopathes Opresko, 2002, with other hitherto known genera of Cladopathidae and Schizopathidae [3,5,8,12,16,17,18,19,20,21].
A colony of Abyssopathes (USNM 1453760; subsample BPBM D2466) (Figure 1) was collected by the NOAA Ship Okeanos Explorer (NOAA OER) as a part of the CAPSTONE project during cruise EX1703, Dive 16 to the Howland and Baker unit of the Pacific Remote Islands Marine National Monument (PRIMNM) and the Phoenix Islands Protected Area (PIPA). The specimen was preserved in 95% ethanol on board the ship. Dive 16 was to an unnamed hadal trough off the Phoenix Islands (4.38° S, 173° W) at a depth of 5772.36 m. The sampled colony was identified as Abyssopathes lyra, the type species of the genus. Whole genomic DNA was extracted from a subsample of this specimen at the Smithsonian Institution, and the 5′-end of the mitochondrial cytochrome c oxidase subunit I (cox1) gene was sequenced via traditional Sanger sequencing in both the forward and reverse direction. After trimming low-quality base calls from the ends of each read, 626 base pairs of overlapping sequence remained (GenBank Accession Number MT350286). This 626 bp fragment of cox1 was added to the igrC multiple sequence alignment from [6]. Additionally, schizopathid and cladopathid mitochondrial cox1 sequences were extracted from the complete mitogenome-based phylogeny presented in [6], as well as a recent paper describing three species within the newly established genus Diplopathes Opresko, 2022 [21], and added to the igrC alignment, resulting in a total of 88 taxa. The igrC alignment was truncated on the 5′ and 3′ end to exactly match the length [626 bp] of the Abyssopathes lyra sequence. XSEDE on the CIPRES Science Gateway v3.3 [23] was used to construct a maximum likelihood-based phylogeny using IQ-Tree v2.1.2 with 1000 ultrafast bootstrap replicates and the GTR+I+G model of sequence evolution [24]. The resulting phylogenetic reconstruction was used to infer the evolutionary relationship of Abyssopathes lyra to representatives of four genera within the family Cladopathidae (Chrysopathes Opresko, 2003, Cladopathes Brook, 1889, Heteropathes Opresko, 2011, and Trissopathes Opresko, 2003) and eleven genera within the family Schizopathidae (Alternatipathes Molodtsova and Opresko, 2017, Bathypathes Brook, 1889, Dendropathes Opresko, 2005, Dendrobathypathes Opresko, 2002, Diplopathes, Lillipathes Opresko, 2002, Parantipathes, Saropathes Opresko, 2002, cf. Sibopathes van Pesch, 1914, Telopathes MacIsaac and Best, 2013, and Stauropathes Opresko, 2002).
The sample identified as cf. Sibopathes macrospina in the phylogenetic reconstructions [6,7,9] is morphologically very similar to Sibopathes, but was not evaluated histologically or by any other means to determine the number of mesenteries in the polyps.
Genetic distance estimates were obtained using the Kimura 2-Parameter within MEGA X [25]. K2P parameters included uniform rates among sites and pairwise deletion of gaps/missing data.
Polyps of the same specimen (subsample BPBM D2466) were subjected to histological analysis. Two fragments of a pinnule with a polyp were re-hydrated through a graded ethanol series (95–30%), then placed in Carlisle’s solution [26,27] and soaked for 24 h. After the Carlisle’s solution treatment, the fragments were rinsed and dehydrated through a graded ethanol series (30–95%), followed by: (1) four changes of isopropanol; (2) mixtures isopropanol:mineral oil 5:1 and 2:1; and (3) mineral oil alone. The samples were then infiltrated in two changes of Histomix® paraffin medium (BioVitrum, Bolshoy Prospekt Vasilyevsky Island, 68, lit. A, St. Petersburg, 199106, Russia) at 56 °C. The resulting Histomix® soaked samples were embedded in Histomix ®, blocked, and sectioned at 5 µm. Sections of polyps were mounted on microscopic slides, deparaffinized, re-hydrated, stained with Mayer’s hemalum [27], dehydrated, dealcoholized in toluol, and mounted with synthetic mounting media “Bio Mount” (BioVitrum, Russia).
3. Results
We have considered morphological, histological, and molecular data in re-evaluating the taxonomic position and relationships of Abyssopathes.
3.1. Morphological Analysis
Two of the three species in the genus Abyssopathes are similar in general appearance to species of Hexapathes Kinoshita, 1910, and Heteropathes (Figure 3a,c, Table 1, Supplementary Table S1) in that they have two rows of simple, elongate, lateral primary pinnules, as well as one or more rows of anterior primary pinnules which can be simple or subpinnulate. Furthermore, the pinnulation pattern in which the two lowermost lateral pinnules are subopposite and all the others are arranged alternately is found exclusively in Hexapathes, Heteropathes, and Abyssopathes (Table 1, Supplementary Table S1). In contrast, there are no genera in the family Schizopathidae that have a similar pinnulation pattern. It should be noted that Abyssopathes anomala Molodtsova and Opresko, 2017, is uniquely different from the other two species of Abyssopathes in that it lacks anterior primary pinnules. The presence of anterior pinnules is a characteristic of all other taxa within the family Cladopathidae, and its absence in A. anomala suggests that it is an autapomorphic character state.
In situ photographs of Abyssopathes lyra (Figure 1 and Figure 4a) indicate that the tentacles, when fully expanded, are relatively short compared to the transverse diameter of the polyp. A similar condition has also been observed in some in situ photos of the polyps of species of Heteropathes (Figure 4b), suggesting a possible shared characteristic; however, actual measurements of the tentacle length of living polyps are not available. In contrast, the tentacles of the polyps in genera in the family Schizopathidae are usually much longer in proportion to the transverse diameter (Figure 4c,d). Polyps of Abyssopathes, however, differ from those of other members of the Cladopathidae (Trissopathes, Cladopathes, Hexapathes, and Heteropathes) in having a very short oral cone.
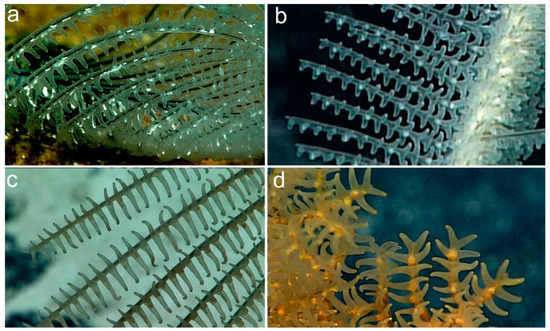
Figure 4.
Polyps of Cladopathidae and Schizopathidae: (a) Abyssopathes lyra (USNM 1453760); (b) Heteropathes pacifica; (c) Bathypathes cf patula Brook, 1889; (d) Stauropathes staurocrada Opresko, 2002. In situ photos taken by the ROV Deep Discoverer (NOAA Ship Okeanos Explorer, Cruises EX 1703, EX1708, and EX1504L2). Photos courtesy of the NOAA OER.
3.2. Histological Analysis
Carlisle’s solution treatment [26,27] of the samples of Abyssopathes lyra (BPBM D2466) gave adequate results in softening the scleroproteinaceous axial material of the pinnule, and successful sectioning was carried out without seriously affecting the soft tissue. However, a study of the resulting microscopic slides indicated that the initial preservation of the delicate polyp tissue in 95% ethanol caused the dissolution of the gland cells and probably also some damage to the polyp epidermis (Figure 5a–c). Sections of two polyps of Abyssopathes lyra (BPBM D2466) were obtained. Polyps of A. lyra demonstrated a few unique morphological features not reported in previous studies of schizopathids or cladopathids [3,8,11,28,29]. Thus, both sectioned polyps were found to have a very thick mesogleal layer in the polyp wall (30–65 µm) and tentacles (20–35 µm). In addition, a cross-sectional view of the upper part of the polyps revealed that the stomodeum has a very distinct Maltese cross or four-leaf outline (Figure 5a,b and Figure 6a). The cross becomes more elongated in a transverse direction lower down in the polyp at a level corresponding to the base of the tentacles (Figure 5a), but it still retains a recognizable four-leaf shape. It should be noted that a stomodeum of a similar but more elongated shape was reported in Cladopathes plumosa Brook, 1889 (Figure 6c) [11], although it has not been found in any non-cladopathid taxa.
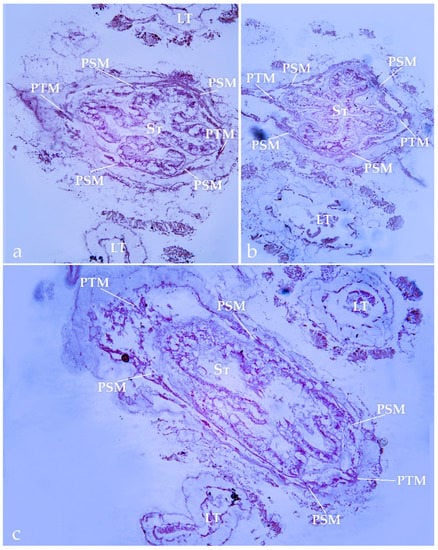
Figure 5.
Histological section of polyps of Abyssopathes lyra (BPBM D2466). (a) cross-section of the oral cone near the base of the tentacles; (b) cross-section of the same polyp at the middle of the oral cone; (c) cross-section of another polyp at the middle of the oral cone. LT—lateral tentacle; St—stomodeum; PSM—primary sagittal mesentery; PTM—primary transversal mesentery. Scale 100 mkm.
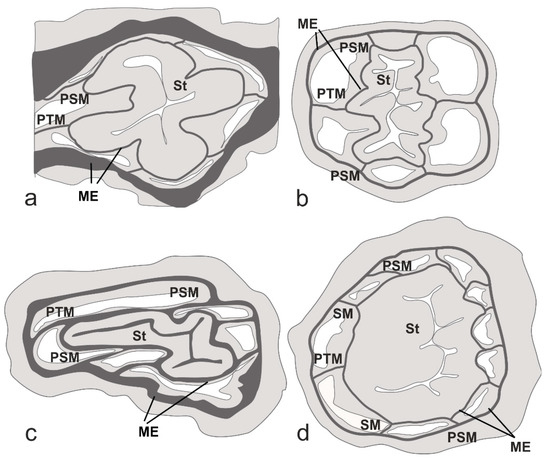
Figure 6.
Schematic sections of the oral cone of polyps of Cladopathidae (a–c) and Schizopathidae (d). (a) Abyssopathes lyra (same section as shown in Figure 5a); (b) Hexapathes heterosticha Kinoshita, 1910 [30]; (c) Cladopathes plumosa [11]; (d) Parantipathes sp. (North Atlantic); St—stomodeum; PSM—primary sagittal mesentery; PTM—primary transversal mesentery; SM—secondary mesentery.
In all the studied histological sections, only six primary mesenteries and no secondary mesenteries were found (Figure 5). Two mesenteries were connected to each lateral fold of the stomodeum, and one was connected to each transversal fold (Figure 5). The presence of only six mesenteries is the morphological feature that is characteristic of the family Cladopathidae (Figure 6a–c) [16].
3.3. Molecular Analysis
A BLAST search in GenBank revealed that the cox1 nucleotide sequence from Abyssopathes lyra (USNM 1453760) is a 99% match (Percent identity: 620/626) to the following taxa: Heteropathes pacifica (USNM 1234550, MBARI T886-A8), Trissopathes pseudotristicha Opresko, 2003 (USNM 1482134, ROV Jason II, Dive J2095-2-1-3, and USNM 98848, HAS-31), and Chrysopathes formosa Opresko, 2003 (USNM 1484087, ABL ID 41-99-6), all taxa in the family Cladopathidae.
Based on 626 bp of the mitochondrial cox1 gene, genetic distances within the family Cladopathidae ranged from 0 to 1.95%. When comparing cladopathids to their nearest neighbor within the Schizopathidae (Telopathes magna MacIsaac and Best, 2013 [BAL103-1]), genetic distances ranged from 3.59 to 5.43%. However, the lower values were based on a comparison to Trissopathes grasshoffi Molodtsova, Altuna, and Hall-Spencer, 2019 (MT318862) and Cladopathes cf. plumosa (MT318861), which only had 167 bp and 258 bp, respectively. Not considering MT318862 and MT318861, the smallest genetic distance is 4.30%.
Results of the phylogenetic reconstruction for the families Cladopathidae and Schizopathidae are shown in Figure 7. Abyssopathes lyra groups in a clade containing other members of the family Cladopathidae. Within the Cladopathidae clade, which was recovered with 100% bootstrap support, Heteropathes pacifica (USNM 1234550), Trissopathes pseudotristicha (USNM 98848), and Cladopathes cf. plumosa (MT318861) are genetically identical. Grouping with this tri-generic complex is Trissopathes cf tetracrada Opresko, 2003 (MT318840), but it is genetically distinct with a K2P genetic distance of 0.16%. Three representative species of Chrysopathes (C. formosa [USNM 1015355, USNM 1484087 and NC_008411], C. speciosa [USNM 1484095] and C. cf. micracantha Opresko and Loiola, 2008 [MT318860]) are genetically distinct from one another and form a monophyletic clade. Abyssopathes lyra groups sister to the Chrysopathes clade, although with little support (52). Based on genetic distance, Trissopathes grasshoffi (MT318862) appears to be closest to Abyssopathes; however, the cox1 sequence for T. grasshoffi is only 167 bp in length, and thus this putative close relationship is likely an artifact of a lack of comparable sequence data.
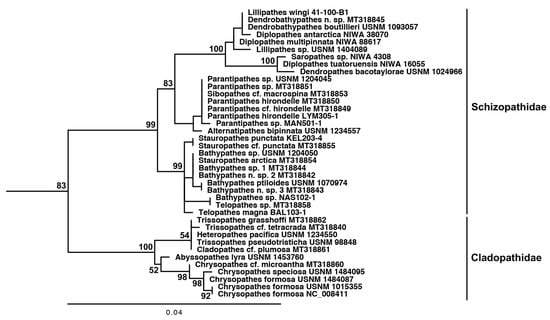
Figure 7.
Partial results of a maximum likelihood-based phylogenetic reconstruction using mitochondrial cox1 sequence data focusing specifically on the families Cladopathidae and Schizopathidae. The MAFFT LINS-i v7-based alignment consisted of 88 sequences and 626 sites. Akaike Information Criterion within jModelTest v2.1.1 selected the GTR+I+G model of sequence evolution (p-inv: 0.5690; gamma shape: 1.6810). IQ-Tree v2.1.2 utilized a BioNJ starting tree. Node support is based on 1000 ultrafast bootstrap replicates. The tree was rooted to sea anemones (Metridium senile (Linnaeus, 1761) and Nematostella sp.) and zoanthids (Palythoa sp. and Savalia savaglia (Bertoloni, 1819)). Following morphology-based taxonomic IDs are internal specimen codes followed by museum accession numbers (where applicable).
4. Discussion
Brook [11] had established the Schizopathinae for taxa whose polyps were said to be divided along the transverse axis into three sections, each bearing one pair of tentacles, which Brook regarded as “dimorphic zooids” (i.e., two gonozooids and one gastrozooid for each polyp). In Schizopathes, these divisions of a polyp were associated with external “peristomal involutions” (constrictions of the coenenchyme between the sagittal and each lateral pair of tentacles) and with internal “mesogloeal septa” hanging down from the interior surface of the coelenteron. All the genera placed in the subfamily Schizopathinae were considered by Brook to have “dimorphic” polyps with external “peristomal involutions” and internal “mesogloeal septa”; however, Brook [11] did not specifically describe these features for the polyps of Bathypathes, Taxipathes, or Cladopathes, and his illustrations suggest that, although the polyps of these genera were transversely elongated as in Schizopathes, they did not possess these features. Brook did note that the polyps of Schizopathes had ten mesenteries, six primary and four secondary mesenteries; however, the secondary mesenteries were found only in the upper part of the oral cone. Brook [11] implied that the same condition occurred in species of Bathypathes, although he did not specifically describe this condition in any species of Bathypathes, including B. lyra. Since then, ten distinctive mesenteries were reported in Bathypathes arctica (Lütken, 1871) [31], a species subsequently transferred to Stauropathes [12]. Later, Pasternak [32] reported six primary and four secondary mesenteries in Bathypathes patula from Kuril-Kamchatka Trench, but it is questionable if the material studied actually belonged to the genus Bathypathes [33]. On the other hand, only six primary mesenteries and no secondary mesenteries were found in the polyps of the type specimens of Cladopathes plumosa [11] (Figure 6c) and Hexapathes heterosticha [30] (Figure 6b), and in addition, C. plumosa has a stomodeum similar in shape to that in A. lyra, a character not reported in any non-cladopathid genus. Therefore, the number of mesenteries and the shape of the stomodeum tie Abyssopathes more closely to the Cladopathidae than to the Schizopathidae.
Although the presence of only six mesenteries in the polyps has been used as a diagnostic feature of the family Cladopathidae, it has yet to be determined whether this feature is exclusive to the family. Despite the fact that only six mesenteries have been found in the polyps of the type material of both nominal species of Sibopathes [8,28], the position of this genus has not yet been confirmed with molecular data (see above). Specimens morphologically identified as S. macrospina have been shown to be genetically closely related to Parantipathes in the family Schizopathidae [6,7,9] (also see Figure 7), but these have not been evaluated histologically to determine the number of mesenteries in the polyps. If these specimens have only six mesenteries, as reported for the type species, it would indicate that this character state occurs in the Schizopathidae as well as the Cladopathidae. More histological studies of taxa of both the Schizopathidae and Cladopathidae are needed to determine if the character state of having only six mesenteries appeared multiple times and scattered between the two families.
The available genetic data clearly place Abyssopathes lyra in a clade containing only members of the family Cladopathidae. Abyssopathes lyra was found to group sister to the Chrysopathes clade, although with little support. Morphologically, the two genera are quite different. Species of Chrysopathes have primary pinnules arranged in six rows, whereas, in the other genera, including Abyssopathes (but excluding Sibopathes for reasons explained above), the primary pinnules occur in only three or four rows. As noted above, morphologically, Abyssopathes most closely resembles Heteropathes and Hexapathes. Additional genetic studies are needed to clarify the phylogenetic relationship of these taxa.
In conclusion, we consider the molecular and morphological evidence sufficient to transfer the genus Abyssopathes to the family Cladopathidae. After this taxonomic amendment, Cladopathidae becomes the second dominant family in the deep sea below 3500 m, with four species (29% of all known black corals) recorded from the abyssal and hadal zones.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15030436/s1, Table S1: Comparison of Abyssopathes Opresko, 2002 with other hitherto known genera of Cladopathidae and Schizopathidae.
Author Contributions
Conceptualization, T.N.M., D.M.O. and M.R.B.; methodology, D.M.O., M.R.B. and T.N.M.; morphological analysis D.M.O. and T.N.M., resources, D.M.O., T.N.M. and M.R.B.; molecular analysis M.R.B., M.O., Y.M.B., T.W.N. and R.F.R.; histological analysis T.N.M., U.V.S. and G.A.K.; writing—original draft preparation, D.M.O., T.N.M. and M.R.B.; writing—review and editing, D.M.O., T.N.M. and M.R.B.; supervision, M.R.B.; funding acquisition, D.M.O. and T.N.M. All authors have read and agreed to the published version of the manuscript.
Funding
Histological part of research was funded by RSF, grant number 22-24-00873 to TNM.
Institutional Review Board Statement
The study did not require ethical approval. Not applicable.
Data Availability Statement
cox1 DNA sequence (GenBank Accession Number MT350286) obtained in the present study is submitted to the NCBI database and publicly available there (https://www.ncbi.nlm.nih.gov/nuccore/MT350286.1/ accessed on 30 December 2022).
Acknowledgments
The specimen on which this paper is based was collected by ROV Deep Discoverer during NOAA Cruise EX1703, “Discovering the Deep”, to the Howland/Baker Unit of the Pacific Remote Islands Marine National Monument and the Phoenix Islands Protected Area on the R/V Okeanos Explorer under Permit Number PBRNo. 1/17 issued by the Republic of Kiribati. Thanks to the NOAA Office of Ocean Exploration and Research and the crew of the R/V Okeanos Explorer. Additional samples used in the molecular analysis were provided by R. Stone and J.F. Karinen of NOAA’s Auk Bay Marine Laboratory, L. Londsten of the Monterey Bay Aquarium Research Institute, and S. France of the University of Louisiana, Lafayette. We would like to thank the Academic Editor and Reviewers for their constructive criticism and technical remarks that helped to improve this paper. Funding for DMO was provided, in part, by a grant from the U.S. Department of Justice to the Smithsonian Institution. DMO and MRB are Research Associates at the Smithsonian Institution and gratefully acknowledge that affiliation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cairns, S.D. Deep-water corals: An overview with special reference to diversity and distribution of deep-water scleractinian corals. Bull. Mar. Sci. 2007, 81, 311–322. [Google Scholar]
- Wagner, D.; Luck, D.G.; Toonen, R.J. The biology and ecology of black corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia). Adv. Mar. Biol. 2012, 63, 67–132. [Google Scholar] [CrossRef] [PubMed]
- Molodtsova, T.N.; Opresko, D.M. Black corals (Anthozoa: Antipatharia) of the Clarion-Clipperton Fracture Zone. Mar. Biodivers. 2017, 47, 349–365. [Google Scholar] [CrossRef]
- Bo, M.; Montgomery, A.D.; Opresko, D.M.; Wagner, D.; Bavestrello, G. Antipatharians of the mesophotic zone: Four case studies; Coral Reefs of the World. In Mesophotic Coral Ecosystems; Loya, Y.P., Bridge, T.K., Eds.; Springer: Cham, Switzerland, 2019; pp. 683–708. [Google Scholar]
- Molodtsova, T.N.; Opresko, D.M.; Wagner, D. Description of a new and widely distributed species of Bathypathes (Cnidaria: Anthozoa: Antipatharia: Schizopathidae) previously misidentified as Bathypathes alternata Brook, 1889. PeerJ 2022, 10, e12638. [Google Scholar] [CrossRef] [PubMed]
- Brugler, M.R.; Opresko, D.M.; France, S.C. The evolutionary history of the order Antipatharia (Cnidaria: Anthozoa: Hexacorallia) as inferred from mitochondrial and nuclear DNA: Implications for black coral taxonomy and systematics. Zool. J. Linn. Soc. 2013, 169, 312–361. [Google Scholar] [CrossRef]
- Barrett, N.J.; Hogan, R.I.; Allcock, A.L.; Molodtsova, T.; Hopkins, K.; Wheeler, A.J.; Yesson, C. Phylogenetics and mitogenome organisation in black corals (Anthozoa: Hexacorallia: Antipatharia): An order-wide survey inferred from complete mitochondrial genomes. Front. Mar. Sci. 2020, 7, 440. [Google Scholar] [CrossRef]
- Opresko, D.M. A new species of Sibopathes (Cnidaria: Anthozoa: Antipatharia: Antipathidae) from the Gulf of Mexico. Proc. Biol. Soc. Wash. 1993, 106, 195–203. [Google Scholar]
- Chimienti, G.; Terraneo, T.I.; Vicario, S.; Marchese, F.; Purkis, S.J.; Abdulla Eweida, A.; Rodrigue, M.; Benzoni, F. A new species of Bathypathes (Cnidaria, Anthozoa, Antipatharia, Schizopathidae) from the Red Sea and its phylogenetic position. ZooKeys 2022, 1116, 1–22. [Google Scholar] [CrossRef]
- Opresko, D.M. Review of the genus Schizopathes (Cnidaria: Antipatharia: Schizopathidae) with a description of a new species from the Indian Ocean. Proc. Biol. Soc. Wash. 1997, 110, 157–166. [Google Scholar]
- Brook, G. Report on the Antipatharia collected by HMS Challenger during the years 1873–1876. In Report on the Scientific Results of the Voyage of HMS “Challenger” during the Years 1873–76 under the Command of Captain George S. Nares, R.N., F.R.S. and the Late Captain Frank Tourle Thomson, R.N., Zoology. XXII; Thomson, S.C.W., Murray, J., Eds.; HM Stationery Office: London, UK; Edinburgh, UK; Dublin, UK, 1889. [Google Scholar]
- Opresko, D.M. Revision of the Antipatharia (Cnidaria: Anthozoa). Part II. Schizopathidae. Zool. Meded. 2002, 76, 411–422. [Google Scholar]
- Horowitz, J.; Opresko, D.M.; Bridge, T.C.L. Black corals (Anthozoa: Antipatharia) from the deep (916 m-2542 m) Coral Sea, north-eastern Australia. Zootaxa 2018, 4472, 307–326. [Google Scholar] [CrossRef]
- Pasternak, F.A. Antipatharia; Elsevier: Copenhagen, Denmark, 1977; pp. 164–167. [Google Scholar]
- Kennedy, B.R.C.; Cantwell, K.; Malik, M.; Kelley, C.; Potter, J.; Elliott, K.; Lobecker, E.; Gray, L.M.; Sowers, D.; White, M.P.; et al. The unknown and the unexplored: Insights into the Pacific Deep-Sea following NOAA CAPSTONE expeditions. Front. Mar. Sci. 2019, 6, 480. [Google Scholar] [CrossRef]
- Opresko, D.M. Revision of the Antipatharia. (Cnidaria: Anthozoa). Part III. Cladopathidae. Zool. Meded. 2003, 77, 495–536. [Google Scholar]
- Opresko, D.M. New genera and species of antipatharian corals (Cnidaria: Anthozoa) from the North Pacific. Zool. Meded. 2005, 79, 129–165. [Google Scholar]
- Molodtsova, T.N. New species of Hexapathes Kinoshita, 1910 (Anthozoa, Antipatharia, Cladopathidae) from the South-West Pacific. Zoosystema 2006, 28, 597–606. [Google Scholar]
- Opresko, D.M.; Loyola, L.L. Two new species of Chrysopathes (Cnidaria: Anthozoa: Antipatharia) from the Western Atlantic. Zootaxa 2008, 1707, 49–59. [Google Scholar]
- Opresko, D.M.; Molodtsova, T.N. New species of deep-sea Antipatharians from the North Pacific (Cnidaria: Anthozoa: Antipatharia), Part 2. Zootaxa 2021, 4999, 401–422. [Google Scholar] [CrossRef]
- Opresko, D.M.; Stewart, R.; Voza, T.; Tracey, D.; Brugler, M.R. New genus and species of black coral from the SW Pacific and Antarctica (Cnidaria: Anthozoa: Antipatharia: Schizopathidae). Zootaxa 2022, 5169, 31–48. [Google Scholar] [CrossRef]
- Opresko, D.; Tracey, D.M.; Mackay, E. Antipatharia (Black Corals) for the New Zealand Region: A Field Guide of Commonly Sampled New Zealand Black corals INCLUDING Illustrations Highlighting Technical Terms and Black Coral Morphology; Ministry for Primary Industries: Wellington, New Zeeland, 2014; Volume 131. [Google Scholar]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: A community resource for phylogenetic analyses. In Proceedings of the 2011 TeraGrid Conference: Extreme Digital Discovery, Salt Lake, UT, USA, 18–21 July 2011; pp. 1–8. [Google Scholar]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Carlisle, D.B. Softening chitin for histology. Nature 1960, 187, 1132–1133. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.; Berry, D.; Kary, C.K. Insect Histology: Practical Laboratory Techniques, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; p. 368. [Google Scholar]
- van Pesch, A.J. The Antipatharia of the Siboga Expedition. In Siboga-Expaditie XVII; Weber, M., Ed.; E.J. Brill: Leyden, The Netherlands, 1914; pp. 1–258. [Google Scholar]
- Wagner, D.; Waller, R.G.; Toonen, R.J. Sexual reproduction of Hawaiian black corals, with a review of the reproduction of antipatharians (Cnidaria: Anthozoa: Hexacorallia). Invertebr. Biol. 2011, 130, 211–225. [Google Scholar] [CrossRef]
- Kinoshita, K. On a new antipatharian Hexapathes heterosticha, n.g. et n.sp. Annot. Zool. Jpn. 1910, 7, 231–234. [Google Scholar]
- Pax, F. Die Antipatharien und Madreporarien des arktischen Gebietes. Fauna Arctica 1932, 6, 267–280. [Google Scholar]
- Pasternak, F. Deep-sea antipatharians of Kuril-Kamchatka Trench. Tr. Inst. Oceanol. AN SSSR. 1958, 27, 180–191. [Google Scholar]
- Molodtsova, T.N. Black corals (Antipatharia: Anthozoa: Cnidaria) of North-East Atlantic. In Biogeography of the North Atlantic seamounts; Mironov, A.N., Gebruk, A.V., Southward, A.J., Eds.; KMK Press: Moscow, Russia, 2006; pp. 141–151. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).