Relationship between Leaf Degradation and Pore Water Chemistry in Two Mangrove Forests of Southeastern Mexico
Abstract
1. Introduction
2. Materials and Methods
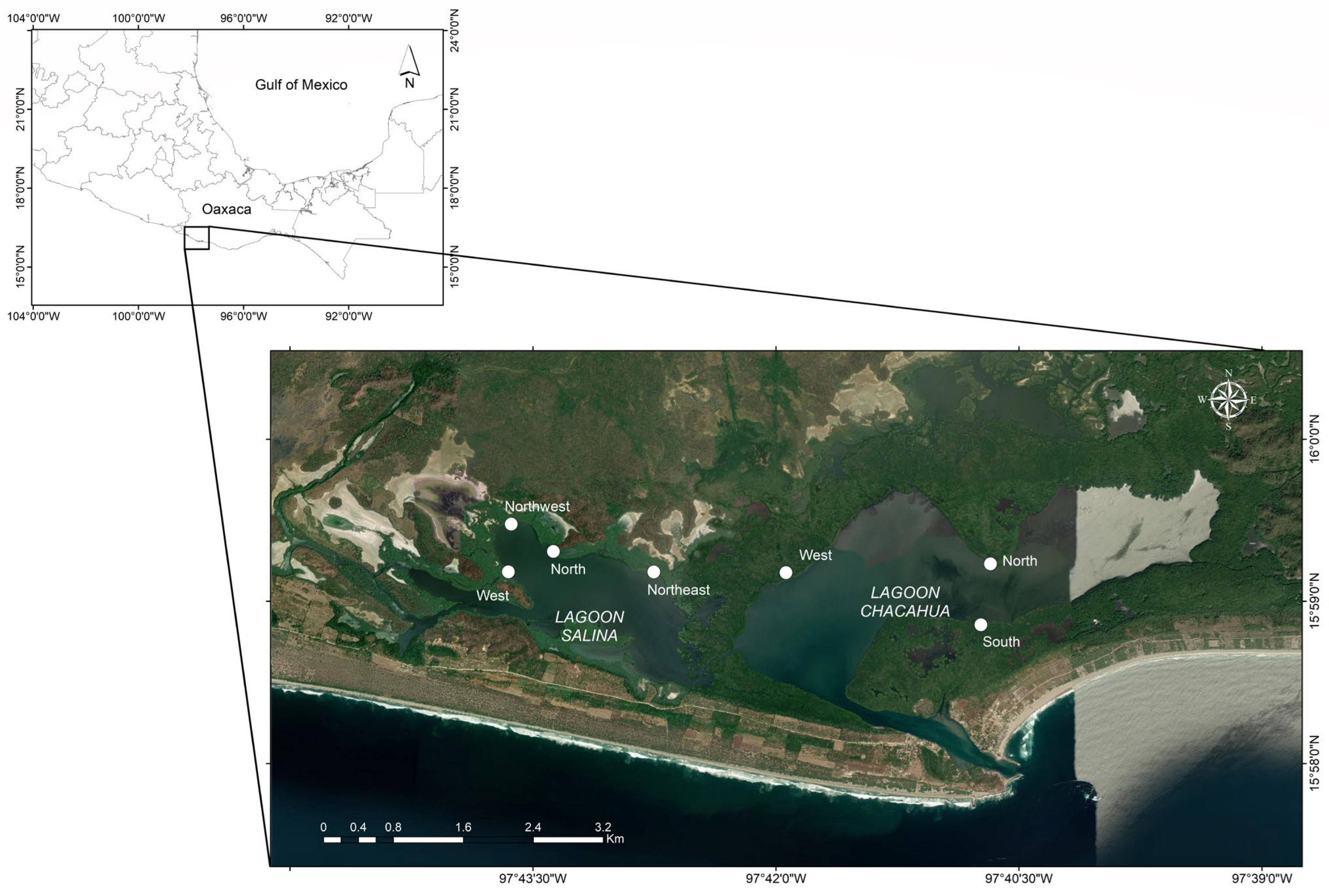
2.1. Study Area
2.2. Forest Attributes and Physiognomic Type
2.3. Interstitial Water Chemistry
2.4. Experimental Design for Determination of Leaf Degradation Rate
2.5. Statistical Analysis
3. Results
3.1. Forest Structure
3.2. Physicochemical Parameters
3.3. Leaf Degradation Rate
3.4. Predictive Models of Leaf Biomass Degradation in Relation to Interstitial Water Chemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lugo, A.E.; Snedaker, S.C. The Ecology of Mangroves. Annu. Rev. Ecol. Syst. 1974, 5, 39–64. [Google Scholar] [CrossRef]
- Khan, Z.R.; Midega, C.A.O.; Wadhams, L.J.; Pickett, J.A.; Mumuni, A. Evaluation of Napier Grass (Pennisetum purpureum) Varieties for Use as Trap Plants for the Management of African Stemborer (Busseola fusca) in a Push-Pull Strategy. Entomol. Exp. Appl. 2007, 124, 201–211. [Google Scholar] [CrossRef]
- Mata, T.B. Evolución de las temperaturas invernales en la segunda mitad del Siglo XVI en un sector del Sistema Central Español. Boletín De La Asoc. De Geógrafos Españoles 2008, 48, 311–325. [Google Scholar]
- Twilley, R.W.; Lugo, A.E.; Patterson-Zucca, C. Litter Production and Turnover in Basin Mangrove Forests in Southwest Florida. Ecology 1986, 67, 670–683. [Google Scholar] [CrossRef]
- Wafar, S.; Untawale, A.G.; Wafar, M. Litter Fall and Energy Flux in a Mangrove Ecosystem. Estuar. Coast. Shelf Sci. 1997, 44, 111–124. [Google Scholar] [CrossRef]
- Wolanski, E.; Chappell, J. The Response of Tropical Australian Estuaries to a Sea Level Rise. J. Mar. Syst. 1996, 7, 267–279. [Google Scholar] [CrossRef]
- Burbridge, P.R. Koesoebiono Management of Mangrove Exploitation in Indonesia. Appl. Geogr. 1982, 2, 39–54. [Google Scholar] [CrossRef]
- Turner, K. Economics and Wetland Management. Ambio 1991, 20, 59–63. [Google Scholar]
- Granek, E.F.; Compton, J.E.; Phillips, D.L. Mangrove-Exported Nutrient Incorporation by Sessile Coral Reef Invertebrates. Ecosystems 2009, 12, 462–472. [Google Scholar] [CrossRef]
- Chan-Keb, C.A.; Agraz-Hernández, C.M.; Pérez-Balan, R.A.; Gutiérrez-Alcántara, E.J.; Muñiz-Salazar, R.; Reyes-Castellano, J.E.; Osti-Sáenz, J. Phytotoxicity in Seedlings of Rhizophora mangle (L.) Exposed to 2,4-Dichlorophenoxyacetic Acid under Experimental Conditions. J. Mar. Sci. Eng. 2021, 9, 1417. [Google Scholar] [CrossRef]
- Agraz-Hernández, C.M.; Chan-Keb, C.A.; Muñiz-Salazar, R.; Pérez-Balan, R.A.; Vanegas, G.P.; Manzanilla, H.G.; Osti-Sáenz, J.; del Río Rodríguez, R. Pore Water Chemical Variability and Its Effect on Phenological Production in Three Mangrove Species under Drought Conditions in Southeastern Mexico. Diversity 2022, 14, 668. [Google Scholar] [CrossRef]
- Boonruang, P. The Degradation Rates of Mangrove Leaves of Rhizophora Apiculata (Bl.) and Avicennia marina (Forsk.) Vierh. At Phuket Island, Thailand. Phuket Mar. Biol. Center Res. Bull. 1978, 26, 1–7. [Google Scholar]
- Middleton, B.A.; Mckee, K.L. Degradation of Mangrove Tissues and Implications for Peat Formation in Belizean Island Forests. J. Ecol. 2001, 89, 818–828. [Google Scholar] [CrossRef]
- Dick, T.M.; Osunkoya, O.O. Influence of Tidal Restriction Floodgates on Decomposition of Mangrove Litter. Aquat. Bot. 2000, 68, 273–280. [Google Scholar] [CrossRef]
- Ashton, E.C.; Hogarth, P.J.; Ormond, R. Breakdown of Mangrove Leaf Litter in a Managed Mangrove Forest in Peninsular Malaysia BT—Diversity and Function in Mangrove Ecosystems; Dodd, R.S., Ed.; Springer: Dordrecht, The Netherlands, 1999; pp. 77–88. [Google Scholar]
- Newell, D.J. Health care research in the united kingdom. Community Health Stud. 1984, 8, 113–116. [Google Scholar] [CrossRef]
- Odum, W.E. The Ecology of the Mangroves of South Florida: A Community Profile (Classic Reprint); FB &C Limited: Washington, DC, USA, 2018; ISBN 9780267436002. [Google Scholar]
- Chan-Keb, C.A.; Agraz-Hernández, C.M.; Perez-Balan, R.A.; Gutierrez-Alcantara, E.J. Quimica Ambiental; Primera; ECOFARN: Mexico City, Mexico, 2022; ISBN 978-607-8695-82-9. [Google Scholar]
- Vargas Edgard Lagunas de Chacahua, ¿qué Hacer En Este Maravilla Natural? Available online: https://revistaquixe.com/2022/05/14/lagunas-de-chacahua-una-opcion-para-hacer-ecoturismo/ (accessed on 6 October 2022).
- Lankford, R.R. Coastal lagoons of Mexico their origin and classification. In Estuarine Processes; Academic Press: Cambridge, MA, USA, 1977; pp. 182–215. [Google Scholar] [CrossRef]
- Perez, N. Electronic Instrumentation for Environmental Control View Project National System for Identification of Agriculture Land Use with High Impact on Freshwater Quality (INIA Sa27) View Project. 2002. [Google Scholar]
- Garcia, E.A. Modificaciones a l Sistema de Clasificacion Climática de Köppen; Instituto de Geografía-UNAM: Mexico City, Mexico, 2004; ISBN 9703210104. [Google Scholar]
- Buenrostro-Silva, A.; Antonio-Gutiérrez, M.; García-Grajales, J. Mammals of the Parque Nacional Lagunas de Chacahua and La Tuza de Monroy. Acta Zoológica Mex. 2012, 28, 56–72. [Google Scholar] [CrossRef]
- Moreno-Casasola, P.; Rosas, H.L.; Mata, D.I.; Peralta, L.A.; Travieso-Bello, A.C.; Warner, B.G. Environmental and Anthropogenic Factors Associated with Coastal Wetland Differentiation in La Mancha, Veracruz, Mexico. Plant Ecol. 2009, 200, 37–52. [Google Scholar] [CrossRef]
- Agraz-Hernández, C.M.; Noriega-Trejo, R.; López-Portillo, J.; Flores-Verdugo, F.J.; José, J.; Claudia, J.-Z.; Agraz-Hernández, M.; José Jiménez-Zacarías, J. Guía de Campo Identificación de Los Manglares En México; Universidad Autonoma de Campeche, Instituto EPOMEX, CONAFOR: Campeche, Mexico, 2006. [Google Scholar]
- Flores-Verdugo, F.; Gonzalez-Farias, F.; Zamorano, D.S.; Ramirez-Garcia, P. Mangrove Ecosystems of the Pacific Coast of Mexico: Distribution, Structure, Litterfall, and Detritus Dynamics. In Coastal Plant Communities of Latin America; Academic Press: Cambridge, MA, USA, 1992; pp. 269–288. [Google Scholar] [CrossRef]
- Hermosilla, Z.; Jorge, C.; Romero, I.; Marti, E.; Cabañero, M. Diferencias Espaciales y Estacionales En El Contenido de Nutrientes, Demanda de Oxígeno y Potencial Redox En Sedimentos Bajo Una Instalación de Producción Acuícola En Jaulas. Bol. Inst. Esp. Ocean. 2005, 21, 29–35. [Google Scholar]
- Agraz Hernández, C.M.; García Zaragoza, C.; Iriarte-Vivar, S.; Flores-Verdugo, F.J.; Moreno Casasola, P.; García Zaragoza, Á.C.; Moreno Casasola, P.A. Forest Structure, Productivity and Species Phenology of Mangroves in the La Mancha Lagoon in the Atlantic Coast of Mexico. Wetl. Ecol Manag. 2011, 19, 273–293. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; American Public Health Association (Eds.) Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10, ISBN 9780875530130. [Google Scholar]
- Skoog, D.A.; West, D.M.; Holler, F.J.; Crouch, S.R. Fundamentals of Analytical Chemistry; Cengage Learning: Belmont, CA, USA, 2013; ISBN 9781285607191. [Google Scholar]
- Bosire, J.O.; Dahdouh-Guebas, F.; Kairo, J.G.; Kazungu, J.; Dehairs, F.; Koedam, N. Litter Degradation and CN Dynamics in Reforested Mangrove Plantations at Gazi Bay, Kenya. Biol. Conserv. 2005, 126, 287–295. [Google Scholar] [CrossRef]
- Agraz-Hernández, C.M.; Expósito-Díaz, G.; Osti-Sáenz, J.; Chan-Keb, C.; Lerandi Juárez, R.; Lecanda Terán, C.; Ledezma, S.; García Zaragoza, C. Criterios Para La Rehabilitación Hidrológica Del Mangle En El Parque Nacional Laguna de Chacahua, Oaxaca. Informe Técnico. Universidad Autónoma de Campeche. Comisión Federal de Electricidad; Convenio Agraz-Hernandez/2008/Rehabilitacion/Oaxaca/Pi/Uac/Cfe; Universidad Autónoma de Campeche: Campeche, Mexico, 2011. [Google Scholar]
- Tomlinson, P.B. The Botany of Mangroves, 2nd ed.; Cambridge University Press: Cambridge, UK, 2016; ISBN 9781107080676. [Google Scholar]
- Sobrado, M.A. Effect of High External NaCl Concentration on the Osmolality of Xylem Sap, Leaf Tissue and Leaf Glands Secretion of the Mangrove Avicennia germinans (L.) L. Flora 2001, 196, 63–70. [Google Scholar] [CrossRef]
- Chale, F.M.M. Degradation of Mangrove Leaf Litter under Aerobic Conditions. Hydrobiologia 1993, 257, 177–183. [Google Scholar] [CrossRef]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Ake-Castillo, J.A.; Vazquez, G.; Lopez-Portillo, J. Litterfall and Decomposition of Rhizophora mangle L. in a Coastal Lagoon in the Southern Gulf of Mexico. Hydrobiologia 2006, 559, 101–111. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 2010; ISBN 9780131008465. [Google Scholar]
- Contreras, J.; Scholz, C.H.; King, G.C.P. A Model of Rift Basin Evolution Constrained by First-Order Stratigraphic Observations. J. Geophys. Res. Solid Earth 1997, 102, 7673–7690. [Google Scholar] [CrossRef]
- Cruz Lazo, J. Estudio Del Espectro Trofico de Cuatro Especies Icticas En La Laguna de Chacahua. Bachelor’s Thesis, Universidad Nacional Autónoma de México, Oaxaca, México, 1987. [Google Scholar]
- Dagar, J.C.; Singh, N.T.; Mongia, A.D. Characteristics of Mangrove Soils and Vegetation of Bay Islands in India BT—Towards the Rational Use of High Salinity Tolerant Plants: Vol. 1 Deliberations about High Salinity Tolerant Plants and Ecosystems; Lieth, H., Al Masoom, A.A., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 59–80. ISBN 978-94-011-1858-3. [Google Scholar]
- Saifullah, S.M.; Khan, S.H.; Ismail, S. Distribution of Nickel in a Polluted Mangrove Habitat of the Indus Delta. Mar. Pollut. Bull. 2002, 44, 570–576. [Google Scholar] [CrossRef]
- Sanay González, R. Simulacion de la circulacion en el Sistema Lagunar Chacahua-Pastoria, Oaxaca. Masters’s Thesis, Universidad Nacional Autónoma de México, Oaxaca, Mexico, 1997. [Google Scholar]
- Boto, K.G.; Wellington, J.T. Soil Characteristics and Nutrient Status in a Northern Australian Mangrove Forest. Estuaries 1984, 7, 61–69. [Google Scholar] [CrossRef]
- Cintrón-Molero, G.; Schaeffer-Novelli, Y. Ecology and Management of New World Mangroves. In Coastal Plant Communities of Latin America; Academic Press: Cambridge, MA, USA, 1992; pp. 233–258. [Google Scholar] [CrossRef]
- Yáñez-Arancibia, A.; Day, J.W.; Twilley, R.R.; Day, R.H. Mangrove Swamps: Sentinel Ecosystem in Front of the Climatic Change, Gulf of Mexico. Madera Y Bosques 2014, 20, 39–75. [Google Scholar]
- Pool, D.J.; Snedaker, S.C.; Lugo, A.E. Structure of Mangrove Forests in Florida, Puerto Rico, Mexico, and Costa Rica. Biotropica 1977, 9, 195–212. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Marins, R.V. River Damming and Changes in Mangrove Distribution. ISME/GLOMIS Electron. J. 2002, 2, 1–4. [Google Scholar]
- Tam, N.F.Y.; Wong, Y.S.; Lan, C.Y.; Wang, L.N. Litter Production and Decomposition in a Subtropical Mangrove Swamp Receiving Wastewater. J. Exp. Mar. Bio. Ecol. 1998, 226, 1–18. [Google Scholar] [CrossRef]
- Hogarth, P.J. The Biology of Mangroves and Seagrasses Biology of Habitats; Oxford University Press: New York, NY, USA, 2015; ISBN 0198716540/9780198716549. [Google Scholar]
- Steinke, T.D.; Barnabas, A.D.; Somaru, R. Structural Changes and Associated Microbial Activity Accompanying Decomposition of Mangrove Leaves in Mgeni Estuary. S. Afr. J. Bot. 1990, 56, 39–48. [Google Scholar] [CrossRef]
- Dutta, R.K.; Agrawal, M. Litterfall, Litter Decomposition and Nutrient Release in Five Exotic Plant Species Planted on Coal Mine Spoils. Pedobiologia 2001, 45, 298–312. [Google Scholar] [CrossRef]
- Gessner, M.O.; Chauvet, E.; Dobson, M. A Perspective on Leaf Litter Breakdown in Streams. Oikos 1999, 85, 377–384. [Google Scholar] [CrossRef]
- Saenger, P. Mangrove Ecology, Silviculture and Conservation, 1st ed.; Springer: Dordrecht, The Netherlands, 2010; ISBN 978-94-015-9962-7. [Google Scholar]
- Bouillon, S.; Koedam, N.; Raman, A.; Dehairs, F. Primary Producers Sustaining Macro-Invertebrate Communities in Intertidal Mangrove Forests. Oecologia 2002, 130, 441–448. [Google Scholar] [CrossRef]
- Torres, A.; Andrade, E.; Garcia-Caceres, R. Syntonic divergence of plants and animals. Her. Tver State Univ. Ser. Biol. Ecol. 2018, 3, 336–377. [Google Scholar] [CrossRef]
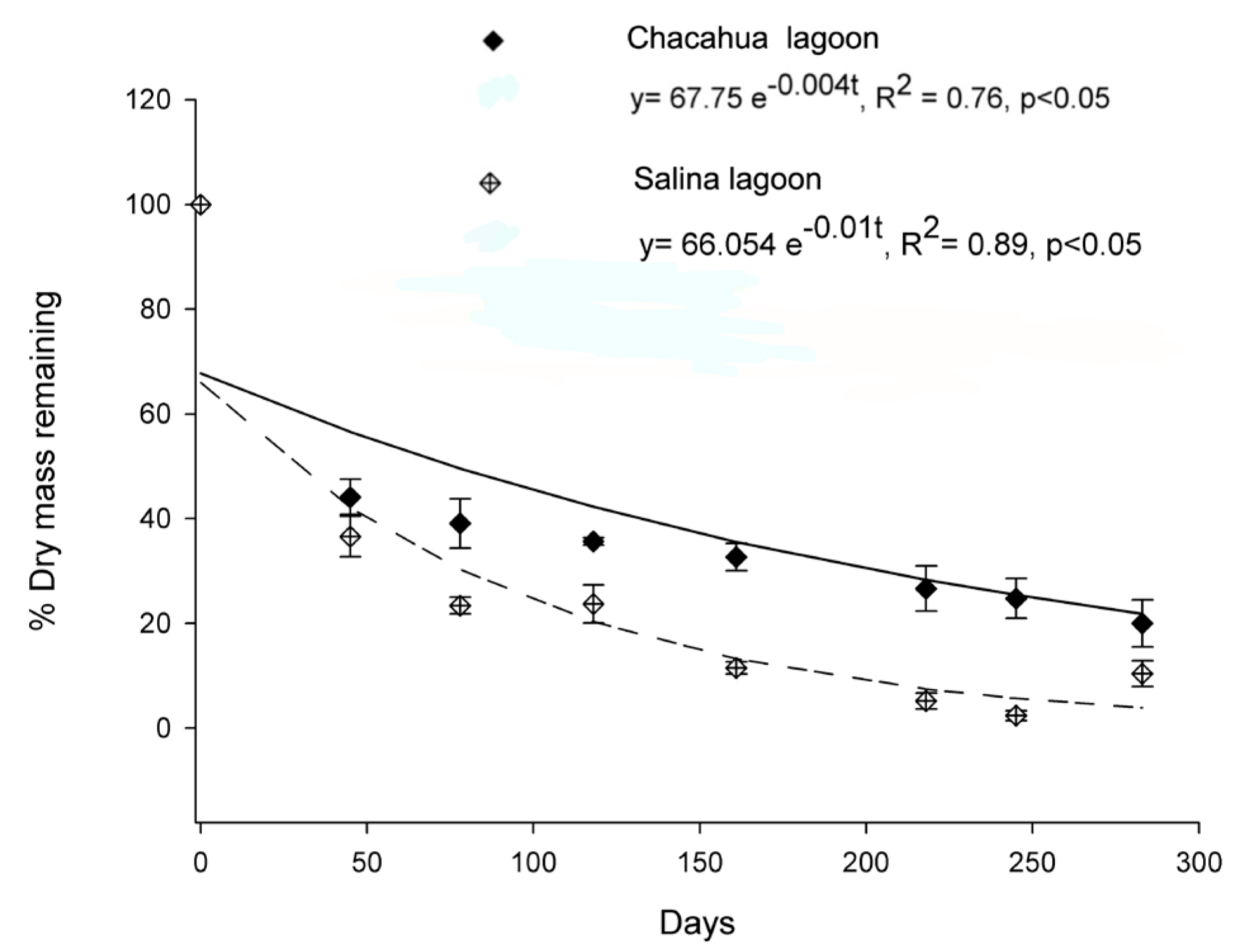
| Factor | gl | Leaf Degradation Rate (%) | Salinity (UPS) | Redox Potential (mV) | SO42− (mg/L) | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| A: Lagoon | 1 | 98.2 | 0.0001 ** | 95.91 | 0.0001 ** | 134.36 | 0.0001 ** | 17.31 | 0.0001 ** |
| B: Season | 1 | 103.3 | 0.0001 ** | 1.21 | 0.2725 NS | 45.88 | 0.0001 ** | 26.69 | 0.0001 ** |
| AXB | 1 | 4.8 | 0.030 * | 6.94 | 0.0095 * | 5.26 | 0.0235 * | 4.73 | 0.0315 * |
| Error | 206 | ||||||||
| Lagoon/Dependent Dependent Variable (Y) | Equation | R2 | p |
|---|---|---|---|
| Salina lagoon | |||
| Degraded biomass (%) | Y = 157.2 − 1.1 X1 | 0.82 | 0.013 |
| Degraded biomass (%) | Y = 86.18 − 0.17 X2 | 0.89 | 0.015 |
| Degraded biomass (%) | Y = 91.77 − 0.0033 X3 | 0.23 | 0.523 |
| Chacahua lagoon | |||
| Degraded biomass (%) | Y = −54.65 + 3.34 X1 | 0.62 | 0.072 |
| Degraded biomass (%) | Y = 48.43 − 0.099 X2 | 0.94 | 0.005 |
| Degraded biomass (%) | Y = 84.83 − 0.012 X3 | 0.88 | 0.017 |
| Redox potential (mV) | Y = −352.9 + 0.109 X3 | 0.75 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan-Keb, C.A.; Agraz-Hernández, C.M.; Pérez-Balan, R.A.; Expósito-Díaz, G.; Gutiérrez-Alcántara, E.J.; Muñiz-Salazar, R.; Osti-Sáenz, J.; Reyes-Castellano, J.E. Relationship between Leaf Degradation and Pore Water Chemistry in Two Mangrove Forests of Southeastern Mexico. Diversity 2023, 15, 432. https://doi.org/10.3390/d15030432
Chan-Keb CA, Agraz-Hernández CM, Pérez-Balan RA, Expósito-Díaz G, Gutiérrez-Alcántara EJ, Muñiz-Salazar R, Osti-Sáenz J, Reyes-Castellano JE. Relationship between Leaf Degradation and Pore Water Chemistry in Two Mangrove Forests of Southeastern Mexico. Diversity. 2023; 15(3):432. https://doi.org/10.3390/d15030432
Chicago/Turabian StyleChan-Keb, Carlos A., Claudia M. Agraz-Hernández, Román A. Pérez-Balan, Gilberto Expósito-Díaz, Eduardo J. Gutiérrez-Alcántara, Raquel Muñiz-Salazar, Juan Osti-Sáenz, and Jordán E. Reyes-Castellano. 2023. "Relationship between Leaf Degradation and Pore Water Chemistry in Two Mangrove Forests of Southeastern Mexico" Diversity 15, no. 3: 432. https://doi.org/10.3390/d15030432
APA StyleChan-Keb, C. A., Agraz-Hernández, C. M., Pérez-Balan, R. A., Expósito-Díaz, G., Gutiérrez-Alcántara, E. J., Muñiz-Salazar, R., Osti-Sáenz, J., & Reyes-Castellano, J. E. (2023). Relationship between Leaf Degradation and Pore Water Chemistry in Two Mangrove Forests of Southeastern Mexico. Diversity, 15(3), 432. https://doi.org/10.3390/d15030432








