Abstract
Global climate change is causing unprecedented impacts on biodiversity. In India, there is little information available regarding how climate change affects biodiversity at the taxon/group level, and large-scale ecological analyses have been lacking. In this study, we demonstrated the applicability of eBird and GBIF (Global Biodiversity Information Facility), and produced national-scale forecasts to examine the possible impacts of climate change on terrestrial avifauna in India. Using data collected by citizen scientists, we developed fine-tuned Species Distribution Models (SDMs) and predicted 1091 terrestrial bird species that would be distributed in India by 2070 on two climatic surfaces (RCP 4.5 and 8.5), using Maximum Entropy-based species distribution algorithms. Of the 1091 species modelled, our findings indicate that 66–73% of bird species in India will shift to higher elevations or shift northward, and 58–59% of bird species (RCP 4.5 and 8.5) would lose a portion of their distribution ranges. Furthermore, distribution ranges of 41–40% of bird species would increase. Under both RCP scenarios (RCP 4.5 and 8.5), bird species diversity will significantly increase in regions above 2500 m in elevation. Both RCP scenarios predict extensive changes in the species richness of the western Himalayas, Sikkim, northeast India, and the western Ghats regions by 2070. This study has resulted in novel, high-resolution maps of terrestrial bird species richness across India, and we predict predominantly northward shifts in species ranges, similar to predictions made for avifauna in other regions, such as Europe and the USA.
1. Introduction
The continuing anthropogenic effects of greenhouse gas emissions and conversion of natural land areas have accelerated global climate change, posing an escalating threat to natural systems. This has resulted in interconnected, more severe, and often permanent effects on ecosystems and biodiversity [1,2]. According to the IPCC’s forecast, global mean temperatures are expected to rise by 0.3–4.8 °C by the end of the twenty-first century [3], and as per the recent forecast, they have increased by 0.8 °C during the last 150 years [2]. This rate of climate warming has the potential to significantly impact species distribution and community assemblages [4], with some studies predicting that the phenotype or range of as many as 80% of species may change as a result [5,6]. Furthermore, with 1.5 °C to 2 °C global warming, endemic species in biodiversity hotspots are expected to face double the risk of extinction, and more than tenfold if global warming rises to 3 °C [2].
The effects of climate warming on many organisms have been reported in several studies, with the most notable effects being species distribution [7,8], reproduction [9,10], and changes in demography [11,12,13]. Some species have been reported to adapt, to counteract the detrimental effects of climate change by extending or delaying their reproductive timing [14], or by leaving their existing breeding region and moving to more climatically suited breeding sites [15]. The distributions of avian species have shifted towards the poles and mountain tops in Eurasia and North America in recent decades, as temperatures have risen [16]. As a result, range shifts have become widely accepted as an indicator or proof of measurable climate change impact on avian species and communities [17,18,19]. Birds are fundamental to the majority of ecosystems; consequently, they are extremely sensitive to changes in ecosystems. Avian species are particularly more sensitive to the effects of anthropogenic and climate change, including range shifts, phenology, genetics, population level, and biogeographic alterations [8,20].
Predicting species distribution patterns under future climate change will greatly contribute to the academic fields of microevolution, ecology, biogeography, and global change biology [4], in addition to informing the planning of conservation measures for avian and other species [1,21,22,23]. In the context of conservation management, the expansion or contraction of each species’ range may influence prioritization for landscape-level or regional conservation. Predictions are critical to conservation management, and can help inform proactive initiatives to prevent climate change impacts on biodiversity [24,25,26]. Globally, several countries have published national-level assessments of how species are adapting to climate change [27,28,29,30,31,32,33,34]. For example, India, one of the world’s megadiverse countries, released its National Action Plan on Climate Change (NAPCC) in 2008. NAPCC had eight different national missions aimed at promoting the understanding of issues related to climate change, natural resource conservation, and energy efficiency. Experts have shared their views on limitations of the NAPCC, although back then it was still in its infancy [35]. However, more than ten years since the NACCP was launched, tracking the success and progress of its missions has proven to be a daunting task. So far, studies on the impacts of climate change in India have been largely conducted at the ecosystem-level, mostly focused on the Himalayas [36,37,38,39,40], as compared to taxa- or species-level assessments.
In general, studies on the effects of climate change on biodiversity in India are sparse. Only a few studies have evaluated the impacts of future climate change on the distribution of species or species-groups, including, for example, birds [41,42,43,44,45,46,47,48,49,50,51], plants [37,52,53,54,55,56,57], and mammals [58,59]. Although birds are considered essential indicators of climate change [60], the effects of climate change on the avifauna of India have not been adequately evaluated, and our understanding in this regard remains limited. This is partially because ground surveys are expensive and time-consuming [61,62], and in cases where population numbers are low, the rates of discovery success are low. In such situations, citizen science data, when properly validated and applied, may serve as a large and relatively accurate source of information on the ground, making it an essential and highly valuable tool for conservation planning [63,64,65,66,67,68,69,70]. In national-scale assessments, several studies have independently utilized citizen science datasets to examine climate change effects on the biogeography of individual species, as well as multiple groups on a regular basis, and to inform conservation strategies [71,72,73,74,75].
We conducted this study to answer the following questions: (i) how will climate change affect the range and distribution of Indian birds, and (ii) how will the bird species richness change from the present to the future (year 2070), under different climate change scenarios. We employed a Maximum Entropy (MaxEnt) modelling approach to predict the spatial distribution of 1091 terrestrial birds of India, and understand the direction and pattern of species range shifts, as well as the comparative change in species richness from the present to the future (year 2070) under different climatic projections (RCP 4.5 and RCP 8.5). The findings of the study will inform national-scale conservation planning and management of birds in response to climate change, and will determine the future conservation priorities for birds in India.
2. Materials and Methods
2.1. Species Occurrence Data
A database of presence records of bird species occurring in India was compiled through online, open-access citizen science databases (Global Biodiversity Information Facility [GBIF; https://www.gbif.org/] [76] accessed on 25 December 2021 and eBird [https://ebird.org/] [77], accessed on 25 December 2021), covering the Indian subcontinent. We compiled 28,437,430 (~28.4 million) occurrence locations of 1344 bird species in India from these databases. To match the temporal extent of climatic data and species recorded in India, species-wise occurrence records were reduced using data from years 1950 to 2021 only [78,79]. Following that, digitized maps of species distribution data, that are standard references compiled by Birdlife International and Handbook of the Birds of the World [80], were manually compared to identify vagrant or imprecise species occurrences, and all mismatched records were removed. As citizen science initiatives sometimes fall short of systematic sampling requirements [81,82], the following four criteria were used to reduce biases, and to avoid overfitting and high-variability predictions [83]:
- i.
- All duplicates were discarded, that is, records occurring within a 1 × 1 km2 cell already having a given species’ record to match the spatial resolution of climatic data for all the species.
- ii.
- All the occurrence records were further rarefied using the SpThin [83,84] package in R (R Core Team 2022).
- iii.
- All bird species with fewer than thirty independent localities were excluded [85,86].
- iv.
- All bird species with a limited sampling area (n < 10,000, Sq KM) were excluded. This includes species with small range areas, and endemic species occurring in pelagic, coastal, or island ecosystems.
As the choice of climate baseline and reduction of sampling bias affects model performance for each species, these steps were crucial to identifying problematic or inaccurate species occurrence data with incorrect climate values [87]. Species occurrences collected from the citizen science datasets are often subject to multiple sampling biases [88]. Geographic sampling biases in biodiversity research may have serious ramifications that must be taken into consideration [88,89,90,91]. Therefore, we used the sampbias package [92] in R (R Core Team 2022) to quantify the effects of geographic sampling biases and reduction through implemented data cleaning and thinning strategies. The results indicated that our final analysis-ready data had a lower level of sampling bias compared to the original data. Additional information on bias correction is provided in Supplementary S2 Figure S1. We were left with 1,912,725 (~1.9 million) independent occurrences of 1091 terrestrial bird species (out of 1344 species), and these were used to create species distribution models (SDMs) [78,79]. These 1091 bird species occur in India across 430 genera, 101 families, and 25 orders, and they include resident, as well as migratory, terrestrial birds. A complete list of the species included or excluded in our analyses is available in Supplementary S1.
2.2. Climate Data and Climate Scenarios
Generating SDMs depends upon various environmental factors related to the locations where specific bird species occur. Therefore, 29 environmental variables were compiled, including 19 bioclimatic variables summarizing aspects of precipitation and temperature for the Earth’s surface from WorldClim 1.4 layers [93], 5 topographic variables, and 5 variables from ENVIREM [http://envirem.github.io/] [94], accessed on 3 November 2020. Supplementary S2 Table S1 contains a list of the 29 environmental variables. Although topographic and ENVIREM variables are not commonly used in SDM, we included them here because numerous examples show that these variables can be used as proxies for other types of variables that are correlated with species’ physiological requirements (e.g., microclimate, edaphic conditions) [59,95,96,97,98,99]. Accordingly, all 29 variables were retained for SDM through MaxEnt built-in variable selection (via L1-regularization), because MaxEnt is reliable, relatively insensitive to correlation among variables, and model performance may be degraded by imposing additional variable selection procedures before running MaxEnt for all the bird species in consideration [100,101].
To predict the ranges of Indian birds in the future, we used five different General Circulation Models (GCMs) for 2050 and 2070, based on Coupled Model Intercomparison Project (CMIP5) data, in both RCP 4.5 and RCP 8.5 scenarios: CCCma-CanESM2, CESM1-CAM5, CSIRO ACCESS13, IPSL-CM5AMR, and MIROC-MIROC5 [102,103]. In 2022, we are trending towards the high emissions RCP 8.5 scenario. RCP 2.6 represents an increasingly unlikely aggressive mitigation approach; thus, RCP 4.5 was selected as a minimal-impact scenario. The data has been analysed against two RCPs, and targets two time periods: year 2050 (average for years 2041–2060) and year 2070 (average for years 2061–2080), at approximately 1 × 1 km2 resolution. Each RCP (4.5 and 8.5) takes a different set of socioeconomic, technological, and political scenarios into account, representing optimistic to pessimistic greenhouse gas concentration trajectories. Here, we discuss the present climate versus the year 2070 climate scenarios with RCP 4.5 and RCP 8.5.
2.3. Distribution Range Modelling
MaxEnt 3.4.4 was used in this study for the distribution modelling exercise [104]. MaxEnt uses a machine learning approach for presence-only data, such as information from citizen science datasets, to produce reliable results [105]. The minimum convex polygon (MCP) was used at 100% of species occurrences, with a 2 degree buffer to derive the model calibration area [106,107]. This method generates models that represent suitable habitats within a known occurrence area (based on a buffered MCP), while excluding suitable habitats well outside of the observed range, and unsuitable habitats throughout the landscape [108].
A target-group background approach [109,110,111] was applied to reduce the influence of spatial sampling bias by using all bird species occurrence localities throughout the Indian sub-continent, for selecting background localities [112,113,114]. The objective of background data is not to speculate on absence locations, but rather to characterise the environment of the study region. In this sense, the background is identical regardless of where the species was discovered. Background data establish the environmental domain of the study, while presence data should establish the conditions under which a species is more likely than average to be present.
We generated species-specific fine-tuned MaxEnt models using ENMeval [115,116]. To choose the parameters that exhibit the greatest performance in MaxEnt based on relevance, predictive power, and complexity level, ENMeval assists in sorting through complex and numerous sets of parameters. The occurrence data was partitioned using the checkerboard2 method, resulting in 4-fold cross-validations. Models with Regularization Multiplier (RM) values ranging from 0.5 to 4.0 (increments of 0.5), and with six different Feature Classes (FC) combinations (L, LQ, H, LQH, LQHP, LQHPT; where L = linear, Q = quadratic, H = hinge, P = product and T = threshold), were built, resulting in 48 individual species model runs. To select the optimal model from all the models that were run, a sequential method was executed that relied on cross-validation results, selecting models with the lowest average test omission rate, and breaking ties with the highest average validation area under the receiver operating characteristic curve (AUC) [83,117]. To ascertain whether the environment associated with each partition is different from that of all the others, the Multivariate Environmental Similarity Surfaces (MESS) predictions were derived for each partition as the reference [101,107]. Details of optimal model tuning parameters are given in Supplementary S1. The Cloglog output was preferred from MaxEnt 3.4.4, which generates a Bernoulli Generalised Linear model based on the Poisson Distribution to estimate the probability of presence [118]. Cloglog transformation can improve model performance by reducing the effects of sample selection bias, while maintaining the same AUC [104].
The species distribution map was created using the average Cloglog output for each species and scenario with a mean ensemble of GCMs. The 10th percentile training presence values for each species were used as thresholds to convert Cloglog raster outputs to ‘presence/absence’ binary maps [83,86,119,120]. This threshold criterion was used to reduce over-predictions in final binary maps, and improve the recovery of species distributions [120]. Supplementary S1 contains all the required information for species distribution models.
Models were evaluated using multiple threshold-dependent and threshold-independent model evaluation criteria, as the best way to measure or evaluate the efficacy of ecological niche models (ENMs) is still being debated [121,122,123,124]. We derived the AUC plot based on the training and validation data (AUCTRAIN, AUCVAL). We also calculated the difference between training and validation AUC (AUCDIFF), which is expected to be high with overfitting models [124]. We came up with two omission rates (ORs) that, when compared to the corresponding theoretically anticipated omission rates, quantify model overfitting [83,121,122,125]: ORMTP (‘Minimum Training Presence’ omission rate) and OR10 (10% training omission rate). We also calculated the AUC ratio (pAUC Ratio) and associated p-value for the partial ROC performance metric, defined in [126] and implemented by the R package kuenm [127]. We estimated the continuous Boyce Index (CBIVAL, CBITRAIN) for training and validation data, as it only requires presences, and measures how much model predictions differ from the random distribution of the observed presences across the prediction gradients [128]. As a result, it is a suitable statistic for presence-only models. It fluctuates continuously between −1 and +1. Values close to zero indicate that the model is identical to a random model, while negative values indicate counter predictions, i.e., predicting poor quality areas where presences are more frequent. Positive values indicate a model whose present predictions are consistent with the distribution of presences in the evaluation dataset [129].
MaxEnt provides two metrics to determine the importance of input variables in the final model: percentage contribution, and permutation importance. While the MaxEnt model is being trained, it keeps track of which environmental variables are contributing to fitting the model [100]. The contribution for each variable is determined by randomly permuting the values of that variable among the training points (both presence and background), and measuring the resulting decrease in training AUC. A substantial decrease indicates that the model depends heavily on that variable [100]. We further analyse the resulting species distribution models through habitat preferences [130,131], ecoregions [132], and elevation gradients.
3. Results
3.1. Model Evaluation
After evaluating model performance, we retained 1091 species data occurring in India out of 1149 species, modelled for further analysis. For the 1091 species modelled, we obtained an AUCTRAIN value of 0.840 ± 0.096 (mean ± standard deviation) and AUCVAL equal to 0.828 ± 0.095, indicating better model abilities to discriminate between conditions of occurrence localities and those of background localities [121,133]. We found the mean AUCDIFF to be 0.012 ± 0.015, indicating a much better degree of model fitting. We also obtained omission rates of ORMTP = 0.108 ± 0.016 and ORMTP = 0.010 ± 0.018, quantifying a much lesser degree of overfitting in the models.
We estimated the pAUC Ratio = 1.958 ± 0.159, indicating that models performed better than random models. A pAUC ratio > 1 indicates that the model has performed better than random chance.
We obtained CBIVAL = 0.900 ± 0.097 and CBITRAIN = 0.968 ± 0.049, indicating excellent model performance. Details of species distribution model validation and evaluation metrics are given in Supplementary S1.
3.2. Variables Contribution and Importance
We found that environmental variables, such as Terrain Wetness Index, Elevation and Terrain Ruggedness Index, and Temperature and Precipitation Seasonality, provided maximum contributions and importance to the derived MaxEnt models (Table 1). Mean variable contribution and importance for all variables are in Table S2 of Supplementary S2.

Table 1.
Top five Mean Environmental Variables contribution and importance of derived MaxEnt models.
3.3. Species Range Change
By 2070, from the 1091 studied bird species, around 59.37% of species may lose some of their range, and around 40.62% may see an increase in their distribution range, as a result of climate change under RCP 4.5 or RCP 8.5 scenarios (Table 2).

Table 2.
Species range changes by the year 2070 for Indian terrestrial birds, along with threatened species under RCP 4.5 or RCP 8.5 scenarios. (n = no. of species, r = % range change, p = % species).
For the RCP 4.5 scenario, we found that 38 species with reduced ranges are threatened birds (critically endangered (n = 7), endangered (n = 11), vulnerable (n = 20)), with an average of 46.78% range loss. In the meantime, 26 threatened species (critically endangered (n = 3), endangered (n = 4), vulnerable (n = 19)) may experience an average range expansion of 61.89%. For the RCP 8.5 scenario, we found that 40 threatened species (critically endangered (n = 6), endangered (n = 11), vulnerable (n = 23)) with reduced ranges are threatened birds, with an average of 59.21% range loss, while 24 threatened species (critically endangered (n = 4), endangered (n = 4), vulnerable (n = 16)) may experience an average range expansion of about 68.41%. The statistics showed that the impact of climate change on the range reduction of partially migratory and migratory species is higher, respectively, 62.88% and 68.01% versus 54.08% for sedentary species for the RCP 8.5 2070 scenario; 63.40% and 68.01% versus 53.6% for the RCP 4.5 2070 scenario (Table 3).

Table 3.
Species range changes by the year 2070—comparison for resident and migratory birds in India under the RCP 4.5 or RCP 8.5 scenarios (n = no. of species, r = % range change, p = % species).
We were able to model 42 species from a total of 78 endemic species in India. By 2070, almost 75% of endemic bird species will a have reduced climatically suitable area, with −53.02% in the RCP 8.5 scenario and −37.87% in the RCP 4.5 scenario. By 2070, we may lose 100% of the climatically suitable environment for the following three bird species: the Trumpeter Finch (Bucanetes githagineus (Lichtenstein, 1823)), the Chinese Francolin (Francolinus pintadeanus (Scopoli, 1786)), and the Hypocolius (Hypocolius ampelinus Bonaparte, 1850), under both the RCP 4.5 and RCP 8.5 scenarios. Whereas, in the RCP 8.5 scenario, we may lose 100% of the climatically suitable habitat for the following five bird species: Collared Myna (Acridotheres albocinctus Godwin-Austen and Walden, 1875), Black-crowned Sparrow-lark (Eremopterix nigriceps (Gould, 1839)), Eurasian Oystercatcher (Haematopus ostralegus Linnaeus, 1758), Greater Adjutant (Leptoptilos dubius (Gmelin, 1789)), and Nilgiri Chilappan (Montecincla cachinnans (Jerdon, 1839)).
3.4. Species Range Centroid Shift
According to statistics on the distribution range centroid shifts of various species groups in 2070, more species would migrate northward, with longer average shift distances among various species groups in the RCP 8.5 scenario compared to RCP 4.5 (Table 4). This finding suggests that the northward shift of the range distributions may be a general trend. The fundamental range shift pattern is the same for the RCP 8.5 scenario, but migration distances are longer and more variable than they were in the RCP 4.5 scenario.

Table 4.
Species range centroid shift (n = no. of species, d = distance shift in Km, p = % species).
For the purpose of understanding the precise spatial patterns of range shifts under both the RCP 4.5 and RCP 8.5 scenarios in 2070, the displacement vectors of each bird species’ range are mapped over the geographical scope of India, and represented in polar form.
By 2070, most bird species would relocate to higher elevations in both scenarios (Figure 1). As a result, species diversity is expected to rise in plateaus and mountains, and fall in lower elevations.
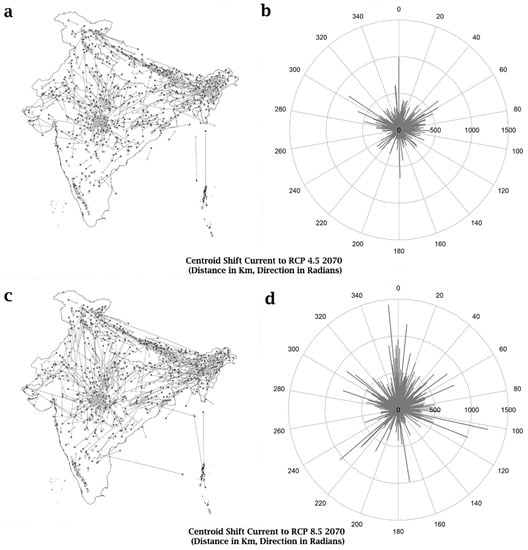
Figure 1.
Range centroid shifts of bird species in 2070 under RCP 4.5 and RCP 8.5. (a,c) depict changes in centroid and directions as a map in the respective RCP 4.5 and RCP 8.5 scenarios for 2070. (b,d) depict distance (in Km) and direction (in radians) changes as a polar graph in the respective RCP 4.5 and RCP 8.5 scenarios.
We further analyse the results via the habitats of bird species (Figure 2). We found five bird species (Alaemon alaudipes, Ammomanes deserti, Chlamydotis macqueenii, Columba eversmanni, Montifringilla adamsi) that are accustomed to desert habitats, showing an almost 212% range expansion as compared to their current distribution ranges in both RCP scenarios, likely because the Indian deserts are becoming wetter. According to the Indian Meteorological Department, over the past three decades, the rainfall pattern in the desert region of India has changed, and the number of rainy days per year has been on the rise [134].
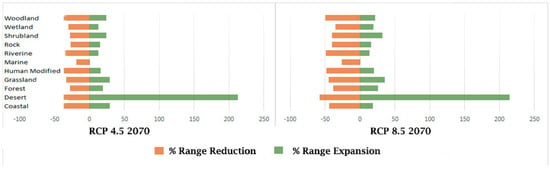
Figure 2.
Habitat–wise % changes in distribution ranges of bird species in 2070 under RCP 4.5 and RCP 8.5 scenarios in India.
3.5. Changes in Species Richness Pattern
To our knowledge, we have generated the first high-resolution full-taxon species richness map (Figure 3) of terrestrial bird species occurring in India, by superimposing the species distribution models. We also calculated changes in species richness for the RCP 4.5 and RCP 8.5 scenarios in 2070.
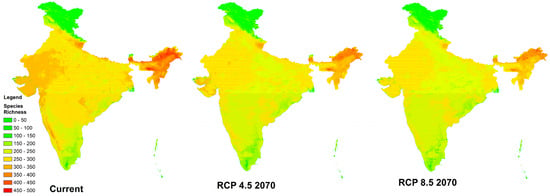
Figure 3.
High-resolution maps showcasing bird species richness for the current, RCP 4.5 2070, and RCP 8.5 2070 scenarios in India.
By 2070, areas showing drastic shifts in species richness for both RCP 4.5 and 8.5 scenarios include the western Himalayas, the state of Sikkim, northeast India, and the western Ghats, see Figure 4. Many lower elevation species are moving towards higher elevations due to the lack of suitable climates at existing lower elevational ranges. We also examined how species richness has changed across different ecoregions. The top five ecoregions with the greatest mean reductions in species richness by 2070, in both RCP 4.5 and 8.5 scenarios, are northeast India-Myanmar pine forests, Brahmaputra Valley semi-evergreen forests, Chin Hills-Arakan Yoma montane forests, Mizoram-Manipur-Kachin rainforests, and Meghalaya subtropical forests, while eastern Himalayan subalpine conifer forests, north-western Himalayan alpine shrub and meadows, northeast Himalayan subalpine conifer forests, eastern Himalayan alpine shrub and meadows, and western Himalayan alpine shrub and meadows will exhibit an increase in species richness (detailed ecoregion-wise comparison in Supplementary S2, Table S3). Additionally, states in northeast India exhibiting extensive reductions in bird species richness include Nagaland, Manipur, Assam, Meghalaya, and Mizoram (detailed ecoregion-wise comparison in Supplementary S2, Table S4). In contrast, Ladakh, Himachal Pradesh, parts of Uttarakhand, and Arunachal Pradesh exhibit an increase in species richness, in both RCP 4.5 and 8.5 scenarios by the year 2070.
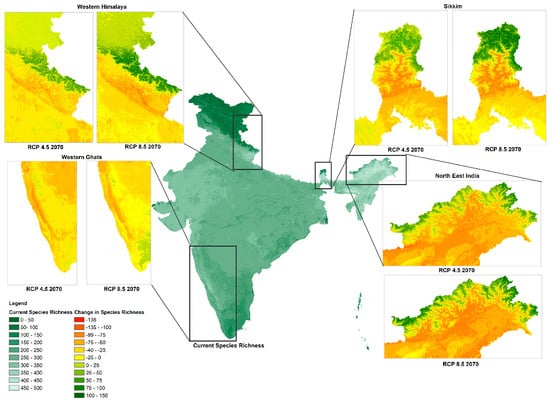
Figure 4.
Species richness change for RCP 4.5 and RCP 8.5 scenarios in 2070.
Both RCP 4.5 and 8.5 scenarios result in the majority of bird species moving to higher altitudes by 2070. We tracked how species diversity varied with elevation, and discovered that for all the species we had investigated together, the turning point occurred at 2000–2500 m, with diversity rising above it and falling below it. Detailed elevation gradient-wise comparison is provided in Supplementary S2, Table S5.
4. Discussion
Our study highlights the impacts of climate change on the distribution of bird species in India. Our findings indicate that with changing climatic conditions, more birds in India would face risks related to habitat loss in the next 50 years. Climate change has the potential to alter the distribution ranges of many birds in India, with ranges contracting or expanding, alongside shifts in elevation and latitude. Our study shows that long distance migratory birds’ habitat area (68%) is more vulnerable to climate change, because these birds’ migration processes are strongly tied to climate [31,135,136]. Future climate change is very likely to pose a greater threat to migratory birds than those of resident bird species, because range areas of migratory birds are more likely to shift northward and diminish in size. Past studies have predicted similar trends with migratory species under climate change projections [15,63,73,74,137,138,139,140]. The 30–42% of resident and 46–59% of threatened bird species that are vulnerable to reductions in their existing range sizes warrant special attention. The effects of range reduction may exhibit a higher rate of regional or local extinction of species in future climate scenarios, as found in other global and national level studies [8,73,141,142,143,144,145,146,147]. Around 22–27% of bird species that may see an increase in their range sizes might still be vulnerable in the future, if their extended/new suitable habitats lie outside of protected areas, or in an increasingly developed territory. Poaching and illegal trade, urbanization, and industrialization are major threats to bird populations as their distribution ranges move and expand into more climatically favourable, but densely populated environments [148,149].
Our study also found the majority of species shifting their ranges northward. Several studies in different parts of the world, including, for example, in China [73], Great Britain [150], Europe [151], and North America [152,153], as well as global meta-analyses [154,155], also predicted poleward shifts of bird ranges with increases in global temperature and climate change. We also found increasing species richness in higher elevations, especially in the Himalayas and Western Ghats. The Himalayan region is among the most vulnerable to climate change on a global scale. The rate of temperature increase across the Himalaya is three times the global average, and as a result, the Himalayan snowline has shifted upwards over time [156]. This shift has created new niches for birds in alpine regions, causing their altitude ranges to expand upward. Low- to mid-elevation species have also adapted to climate change by extending their altitudinal limits to higher elevations. In the Himalayan region, the effects of climate change manifest as glacial melt, changes in sowing and harvesting seasons, decreased crop productivity with the introduction of new invasive species and weeds, drying up of springs, shifts in the geographical range of species, alterations in the species composition of communities, and extinction of species [39,40,42,56,72,156,157,158,159,160,161,162,163,164,165,166,167]. The tropical mountain birds, including of the Himalayas and Western Ghats regions, are among the most susceptible to climate change [140]. More vulnerable to extinction risks are endemics and restricted range species, with little to no space for upward movement, or their inability to shift upwards due to intolerance to physiological limits imposed by geographical gradients. Similarly, species inhabiting higher elevations are at higher risk of extinction than the species inhabiting lower elevations, particularly in cases when there is no land or habitat available at higher elevations [168].
The availability of citizen science datasets, in which citizens document species observations in a non-standardized manner, is growing [169,170,171,172]. This information could fill in the gaps where scientific observations are lacking, for a variety of studies. Over the past two decades, an increasing percentage of the Indian population has taken up birding as an educational or recreational activity, and has shared their observations in open-source databases. This study, aided by such citizen science data, could potentially be used as a starting point for further investigations regarding site-based conservation planning, community ecology, ecosystem service accounting, and ecological processes studies. To reduce the possibility of mistakes in species identification and locale, eBird is one of the sources where each record is checked by an expert reviewer.
Despite their limitations, citizen science datasets have the advantage of providing extensive occurrence data in a short period of time and, with proper bias removal methods, may allow for fast and reliable assessments. Recently, conservation and management decisions based on species distribution models informed by citizen science data have gained increasing acceptance as reliable methods for protecting biodiversity [73,173,174]. In this study, we used the presence thinning and background selection methods for sampling bias correction [84,90,110,175]. Nonetheless, we discovered that some island species (Andaman and Nicobar and Lakshadweep Islands), pelagic, data-deficient, or small range restricted species did not lend themselves to modelling, due to their limited sampling background area.
These changes have significant ecological ramifications that go far beyond just the numerical loss or addition of species in India. A change in climate will not result in an overall, unidirectional change in the relative habitat suitability of protected areas. However, along with species range shifts, the conservation priority areas will also change because of future climatic influences. Therefore, setting biodiversity conservation goals, as well as formulating effective policies and implementing actions, must consider the ever-changing climate and its multi sectoral impacts.
5. Potential Limitations and Future Directions
Our methodology is based on common presumptions found in all multi-species studies that use species distribution models. In order to determine climatic tolerance from the observed distribution of the species, these models first make the assumption that species are in equilibrium with the environment, and that all pertinent climatic factors that may have an impact on species presence are taken into account. The main drawbacks of this strategy include the potential omission of important climatic variables from models, and the possibility that a variety of variables, other than climatic tolerance, may affect the current and future distribution of bird species, such as habitat loss, hunting, and exploitation. As a result, the assumptions of species distribution models are frequently broken [176].
Another caution is that species distribution models, which ignore interspecies interactions, are predicated on the idea that species react to climate change independently. Species interactions within and between trophic levels may have a significant impact on whether a specific taxon can persist in its current range or colonise new areas [177,178].
6. Conclusions
Species Distribution Models are effective at predicting the occurrence of numerous species. However, we emphasise that models must be validated properly prior to use in decision-making and other applications. MaxEnt is a useful tool for assessing the potential impacts of climate change on species distributions, in order to gain insight into how species may respond in the event of future climate change. By simulating the future distribution ranges of 1091 extant terrestrial Indian birds, we demonstrate how their climatically appropriate distribution ranges are predicted to experience significant shifts under climate change. These shifts are projected to be consistent across a wide range of climate scenarios, and vary geographically. This study can be considered one of the first attempts from India at conducting a thorough analysis of bird distributions based on point data. The growth of bird watching through citizen science initiatives, and the expansion of data size and quality, offer researchers and government agencies unprecedented access to distribution data. We anticipate that periodic assessments, such as the analysis in this study, will be continuously updated to address various issues faced in biodiversity conservation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15030404/s1, Supplementary S1: All species details with records and model evaluations and validation parameters. Supplementary S2 Table S1: List of environmental variables. Supplementary S2 Table S2: MaxEnt mean variable contribution and importance. Supplementary S2 Table S3: Changes in species richness across different ecoregions. Supplementary S2 Table S4: Changes in species richness across the different states. Supplementary S2 Table S5: Changes in species richness across the different elevation gradients. Supplementary S2 Figure S1: Bias correction results for presence data using sampbias packages.
Author Contributions
A.D. conceived the idea and collected data, developed an analytical framework, performed analyses and wrote original draft. R.S., A.S. and D.G. improved and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All original data are available from the respective portals, Global Biodiversity Information Facility [GBIF; https://www.gbif.org/], accessed on 25 December 2021, eBird [https://ebird.org/home], accessed on 25 December 2021; ENVIREM [http://envirem.github.io/], accessed on 3 November 2020 and Worldclim [https://www.worldclim.org/], accessed on 3 November 2020. An open-access data repository for this study’s results, and the necessary code to replicate them, can be found at https://github.com/arpitdeomurari/IndianBirds_ClimateChange, accessed on 25 December 2021 (will be archived in Zenodo once accepted).
Acknowledgments
We thank Christi Sylvia and Harpalsinh Chudasama for their valuable suggestions on manuscript aspects. We appreciate the three anonymous reviewers’ thorough comments, which helped the manuscript become much better.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of Climate Change on the Future of Biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability. Working Group II Contribution to the IPCC Sixth Assessment Report; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Hu, J.; Jiang, Z.; Chen, J.; Qiao, H. Niche Divergence Accelerates Evolution in Asian Endemic Procapra Gazelles. Sci. Rep. 2015, 5, 10069. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2007: The Physical Science Basis—Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Trisos, C.H.; Merow, C.; Pigot, A.L. The Projected Timing of Abrupt Ecological Disruption from Climate Change. Nature 2020, 580, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Anthelme, F.; Cavieres, L.A.; Dangles, O. Facilitation among Plants in Alpine Environments in the Face of Climate Change. Front. Plant Sci. 2014, 5, 387. [Google Scholar] [CrossRef]
- Trautmann, S. Climate Change Impacts on Bird Species. In Bird Species: How They Arise, Modify and Vanish; Tietze, D.T., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 217–234. ISBN 978-3-319-91689-7. [Google Scholar]
- Lameris, T.K.; Scholten, I.; Bauer, S.; Cobben, M.M.P.; Ens, B.J.; Nolet, B.A. Potential for an Arctic-Breeding Migratory Bird to Adjust Spring Migration Phenology to Arctic Amplification. Glob. Change Biol. 2017, 23, 4058–4067. [Google Scholar] [CrossRef]
- Saalfeld, S.T.; Hill, B.L.; Hunter, C.M.; Frost, C.J.; Lanctot, R.B. Warming Arctic Summers Unlikely to Increase Productivity of Shorebirds through Renesting. Sci. Rep. 2021, 11, 15277. [Google Scholar] [CrossRef]
- Bagaria, P.; Thapa, A.; Sharma, L.K.; Joshi, B.D.; Singh, H.; Sharma, C.M.; Sarma, J.; Thakur, M.; Chandra, K. Distribution Modelling and Climate Change Risk Assessment Strategy for Rare Himalayan Galliformes Species Using Archetypal Data Abundant Cohorts for Adaptation Planning. Clim. Risk Manag. 2021, 31, 100264. [Google Scholar] [CrossRef]
- Rakhimberdiev, E.; Duijns, S.; Karagicheva, J.; Camphuysen, C.J.; Dekinga, A.; Dekker, R.; Gavrilov, A.; ten Horn, J.; Jukema, J.; Saveliev, A.; et al. Fuelling Conditions at Staging Sites Can Mitigate Arctic Warming Effects in a Migratory Bird. Nat. Commun. 2018, 9, 4263. [Google Scholar] [CrossRef]
- Rapacciuolo, G.; Maher, S.P.; Schneider, A.C.; Hammond, T.T.; Jabis, M.D.; Walsh, R.E.; Iknayan, K.J.; Walden, G.K.; Oldfather, M.F.; Ackerly, D.D.; et al. Beyond a Warming Fingerprint: Individualistic Biogeographic Responses to Heterogeneous Climate Change in California. Glob. Change Biol. 2014, 20, 2841–2855. [Google Scholar] [CrossRef]
- Walther, G.R. Community and Ecosystem Responses to Recent Climate Change. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2019–2024. [Google Scholar] [CrossRef]
- Zhu, B.R.; Verhoeven, M.A.; Velasco, N.; Sanchez-Aguilar, L.; Zhang, Z.; Piersma, T. Current Breeding Distributions and Predicted Range Shifts under Climate Change in Two Subspecies of Black-Tailed Godwits in Asia. Glob. Change Biol. 2022, 28, 5416–5426. [Google Scholar] [CrossRef]
- Lehikoinen, A.; Virkkala, R. North by North-West: Climate Change and Directions of Density Shifts in Birds. Glob. Change Biol. 2016, 22, 1121–1129. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jetz, W. The Effect of Range Changes on the Functional Turnover, Structure and Diversity of Bird Assemblages under Future Climate Scenarios. Glob. Change Biol. 2015, 21, 2917–2928. [Google Scholar] [CrossRef]
- Ramírez-Albores, J.E.; Prieto-Torres, D.A.; Gordillo-Martínez, A.; Sánchez-Ramos, L.E.; Navarro-Sigüenza, A.G. Insights for Protection of High Species Richness Areas for the Conservation of Mesoamerican Endemic Birds. Divers. Distrib. 2021, 27, 18–33. [Google Scholar] [CrossRef]
- Wayman, J.P.; Sadler, J.P.; Pugh, T.A.M.; Martin, T.E.; Tobias, J.A.; Matthews, T.J. Assessing Taxonomic and Functional Change in British Breeding Bird Assemblages over Time. Glob. Ecol. Biogeogr. 2022, 31, 925–939. [Google Scholar] [CrossRef]
- Pautasso, M.; Döring, T.F.; Garbelotto, M.; Pellis, L.; Jeger, M.J. Impacts of Climate Change on Plant Diseases—Opinions and Trends. Eur. J. Plant Pathol. 2012, 133, 295–313. [Google Scholar] [CrossRef]
- Morecroft, M.D.; Crick, H.Q.P.; Duffield, S.J.; Macgregor, N.A. Resilience to Climate Change: Translating Principles into Practice. J. Appl. Ecol. 2012, 49, 547–551. [Google Scholar] [CrossRef]
- Sekercioglu, C.H.; Schneider, S.H.; Fay, J.P.; Loarie, S.R. Climate Change, Elevational Range Shifts, and Bird Extinctions. Conserv. Biol. 2008, 22, 140–150. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological Responses to Recent Climate Change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Dawson, T.P.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond Predictions: Biodiversity Conservation in a Changing Climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef]
- McMahon, S.M.; Harrison, S.P.; Armbruster, W.S.; Bartlein, P.J.; Beale, C.M.; Edwards, M.E.; Kattge, J.; Midgley, G.; Morin, X.; Prentice, I.C. Improving Assessment and Modelling of Climate Change Impacts on Global Terrestrial Biodiversity. Trends Ecol. Evol. 2011, 26, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.M.; Leadley, P.W.; Proença, V.; Alkemade, R.; Scharlemann, J.P.W.; Fernandez-Manjarrés, J.F.; Araújo, M.B.; Balvanera, P.; Biggs, R.; Cheung, W.W.L.; et al. Scenarios for Global Biodiversity in the 21st Century. Science 2010, 330, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate Warming and the Decline of Amphibians and Reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Beresford, A.E.; Buchanan, G.M.; Donald, P.F.; Butchart, S.H.M.; Fishpool, L.D.C.; Rondinini, C. Poor Overlap between the Distribution of Protected Areas and Globally Threatened Birds in Africa. Anim. Conserv. 2011, 14, 99–107. [Google Scholar] [CrossRef]
- Coetzee, B.W.T.; Robertson, M.P.; Erasmus, B.F.N.; van Rensburg, B.J.; Thuiller, W. Ensemble Models Predict Important Bird Areas in Southern Africa Will Become Less Effective for Conserving Endemic Birds under Climate Change. Glob. Ecol. Biogeogr. 2009, 18, 701–710. [Google Scholar] [CrossRef]
- Garnett, S.; Franklin, D.C. (Eds.) Climate Change Adaptation Plan for Australian Birds; CSIRO Publishing: Collingwood, VIC, Australia, 2014; ISBN 978-0-643-10802-8. [Google Scholar]
- Gill, J.A.; Alves, J.A.; Gunnarsson, T.G. Mechanisms Driving Phenological and Range Change in Migratory Species. Philos. Trans. R. Soc. B 2019, 374, 20180047. [Google Scholar] [CrossRef]
- Huntley, B.; Collingham, Y.C.; Willis, S.G.; Green, R.E. Potential Impacts of Climatic Change on European Breeding Birds. PLoS ONE 2008, 3, e1439. [Google Scholar] [CrossRef]
- Scott, D.; Lemieux, C. Climate Change and Protected Area Policy and Planning in Canada. For. Chron. 2005, 81, 696–703. [Google Scholar] [CrossRef]
- Sintayehu, D.W. Impact of Climate Change on Biodiversity and Associated Key Ecosystem Services in Africa: A Systematic Review. Ecosyst. Health Sustain. 2018, 4, 225–239. [Google Scholar] [CrossRef]
- Pandve, H. India’s National Action Plan on Climate Change. Indian J. Occup. Environ. Med. 2009, 13, 17. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Gopalakrishnan, R.; Jayaraman, M.; Bala, G.; Joshi, N.V.; Sukumar, R.; Ravindranath, N.H. Impact of Climate Change on Indian Forests: A Dynamic Vegetation Modeling Approach. Mitig. Adapt. Strateg. Glob. Change 2011, 16, 119–142. [Google Scholar] [CrossRef]
- Maikhuri, R.K.; Phondani, P.C.; Dhyani, D.; Rawat, L.S.; Jha, N.K.; Kandari, L.S. Assessment of Climate Change Impacts and Its Implications on Medicinal Plants-Based Traditional Healthcare System in Central Himalaya, India. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 1827–1835. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, P.; Archie, K.M.; Gupta, A.K.; Joshi, P.K.; Valente, D.; Petrosillo, I. Climate Change Adaptation in the Western-Himalayas: Household Level Perspectives on Impacts and Barriers. Ecol. Indic. 2018, 84, 27–37. [Google Scholar] [CrossRef]
- Tewari, V.P.; Verma, R.K.; von Gadow, K. Climate Change Effects in the Western Himalayan Ecosystems of India: Evidence and Strategies. For. Ecosyst. 2017, 4, 13. [Google Scholar] [CrossRef]
- Upgupta, S.; Sharma, J.; Jayaraman, M.; Kumar, V.; Ravindranath, N.H. Climate Change Impact and Vulnerability Assessment of Forests in the Indian Western Himalayan Region: A Case Study of Himachal Pradesh, India. Clim. Risk Manag. 2015, 10, 63–76. [Google Scholar] [CrossRef]
- Chhetri, B.; Badola, H.K.; Barat, S. Predicting Climate-Driven Habitat Shifting of the near Threatened Satyr Tragopan (Tragopan satyra; Galliformes) in the Himalayas. Avian Biol. Res. 2018, 11, 221–230. [Google Scholar] [CrossRef]
- Chhetri, B.; Badola, H.K.; Barat, S. Modelling Climate Change Impacts on Distribution of Himalayan Pheasants. Ecol. Indic. 2021, 123, 107368. [Google Scholar] [CrossRef]
- Jha, K.K.; Jha, R. Study of Vulture Habitat Suitability and Impact of Climate Change in Central India Using MaxEnt. J. Resour. Ecol. 2021, 12, 30–42. [Google Scholar] [CrossRef]
- Jose, V.S.; Nameer, P.O. The Expanding Distribution of the Indian Peafowl (Pavo cristatus) as an Indicator of Changing Climate in Kerala, Southern India: A Modelling Study Using MaxEnt. Ecol. Indic. 2020, 110, 105930. [Google Scholar] [CrossRef]
- Menon, S.; Peterson, A.T. Projected Climate Change Effects on Nuthatch Distribution. Raffles Bull. Zool. 2009, 57, 569–575. [Google Scholar]
- Ramesh, V.; Gopalakrishna, T.; Barve, S.; Melnick, D.J. Citizen Science Driven Species Distribution Models Estimate Drastically Smaller Range Sizes and Higher Threat Levels for Western Ghats Endemic Birds. Biol. Conserv. 2017, 210, 205–221. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, N.; Kumar, M.; Singh, R. Modelling Habitat Suitability of Western Tragopan (Tragopan melanocephalus) a Range-Restricted Vulnerable Bird Species of the Himalayan Region, in Response to Climate Change. Clim. Risk Manag. 2020, 29, 100241. [Google Scholar] [CrossRef]
- Sreekumar, E.R.; Nameer, P.O. Impact of Climate Change on Two High-Altitude Restricted and Endemic Flycatchers of The Western Ghats, India. Curr. Sci. 2021, 121, 1335. [Google Scholar] [CrossRef]
- Sreekumar, E.R.; Nameer, P.O. A MaxEnt Modelling Approach to Understand the Climate Change Effects on the Distributional Range of White-Bellied Sholakili Sholicola Albiventris (Blanford, 1868) in the Western Ghats, India. Ecol. Inform. 2022, 70, 101702. [Google Scholar] [CrossRef]
- Sutton, L.J.; McClure, C.J.W.; Kini, S.; Leonardi, G. Climatic Constraints on Laggar Falcon (Falco jugger) Distribution Predicts Multidirectional Range Movements under Future Climate Change Scenarios. J. Raptor Res. 2020, 54, 1–17. [Google Scholar] [CrossRef]
- Yousefi, M.; Ahmadi, M.; Nourani, E.; Rezaei, A.; Kafash, A.; Khani, A.; Sehhatisabet, M.E.; Adibi, M.A.; Goudarzi, F.; Kaboli, M. Habitat Suitability and Impacts of Climate Change on the Distribution of Wintering Population of Asian Houbara Bustard Chlamydotis Macqueenii in Iran. Bird Conserv. Int. 2017, 27, 294–304. [Google Scholar] [CrossRef]
- Chitale, V.S.; Behera, M.D.; Roy, P.S. Future of Endemic Flora of Biodiversity Hotspots in India. PLoS ONE 2014, 9, e115264. [Google Scholar] [CrossRef]
- Ghosh, B.G.; Garai, S.; Rahaman, S.M.; Khatun, M.; Mohammad, N.; Mishra, Y.; Ranjan, A.; Tiwari, S. Assessing Potential Habitat Distribution Range of the Endangered Tree Species Pterocarpus Marsupium Roxb. Under the Climate Change Scenario in India. Trees For. People 2021, 6, 100124. [Google Scholar] [CrossRef]
- Hebbar, K.B.; Abhin, P.S.; Sanjo Jose, V.; Neethu, P.; Santhosh, A.; Shil, S.; Prasad, P.V.V. Predicting the Potential Suitable Climate for Coconut (Cocos nucifera L.) Cultivation in India under Climate Change Scenarios Using the MaxEnt Model. Plants 2022, 11, 731. [Google Scholar] [CrossRef]
- Kailash, B.R.; Charles, B.; Ravikanth, G.; Setty, S.; Kadirvelu, K. Identifying the Potential Global Distribution and Conservation Areas for Terminalia Chebula, an Important Medicinal Tree Species under Changing Climate Scenario. Trop. Ecol. 2022, 63, 584–595. [Google Scholar] [CrossRef]
- Manish, K.; Telwala, Y.; Nautiyal, D.C.; Pandit, M.K. Modelling the Impacts of Future Climate Change on Plant Communities in the Himalaya: A Case Study from Eastern Himalaya, India. Model. Earth Syst. Environ. 2016, 2, 92. [Google Scholar] [CrossRef]
- Priti, H.; Aravind, N.A.; Uma Shaanker, R.; Ravikanth, G. Modeling Impacts of Future Climate on the Distribution of Myristicaceae Species in the Western Ghats, India. Ecol. Eng. 2016, 89, 14–23. [Google Scholar] [CrossRef]
- Chatterjee, P.; Tripathy, B.; Chandra, K.; Saha, G.K.; Mondal, K. Climate Change Alarms the Survival of Near Threatened Species Malayan Giant Squirrel (Ratufa bicolor Sparrman, 1778) in India. JMAM 2020, 45, 289–302. [Google Scholar] [CrossRef]
- Kanagaraj, R.; Araujo, M.B.; Barman, R.; Davidar, P.; De, R.; Digal, D.K.; Gopi, G.V.; Johnsingh, A.J.T.; Kakati, K.; Kramer-Schadt, S.; et al. Predicting Range Shifts of Asian Elephants under Global Change. Divers. Distrib. 2019, 25, 822–838. [Google Scholar] [CrossRef]
- Fraixedas, S.; Lindén, A.; Piha, M.; Cabeza, M.; Gregory, R.; Lehikoinen, A. A State-of-the-Art Review on Birds as Indicators of Biodiversity: Advances, Challenges, and Future Directions. Ecol. Indic. 2020, 118, 106728. [Google Scholar] [CrossRef]
- Rushton, S.P.; Ormerod, S.J.; Kerby, G. New Paradigms for Modelling Species Distributions? J. Appl. Ecol. 2004, 41, 193–200. [Google Scholar] [CrossRef]
- Vogiatzakis, I.N.; Griffiths, G.H.; Mannion, A.M. Environmental Factors and Vegetation Composition, Lefka Ori Massif, Crete, S. Aegean. Glob. Ecol. Biogeogr. 2003, 12, 131–146. [Google Scholar] [CrossRef]
- Abolafya, M.; Onmuş, O.; Şekercioǧlu, Ç.H.; Bilgin, R.; Şekercioğlu, Ç.H.; Bilgin, R. Using Citizen Science Data to Model the Distributions of Common Songbirds of Turkey Under Different Global Climatic Change Scenarios. PLoS ONE 2013, 8, e68037. [Google Scholar] [CrossRef]
- Devictor, V.; Whittaker, R.J.; Beltrame, C. Beyond Scarcity: Citizen Science Programmes as Useful Tools for Conservation Biogeography. Divers. Distrib. 2010, 16, 354–362. [Google Scholar] [CrossRef]
- Fink, D.; Auer, T.; Johnston, A.; Ruiz-Gutierrez, V.; Hochachka, W.M.; Kelling, S. Modeling Avian Full Annual Cycle Distribution and Population Trends with Citizen Science Data. Ecol. Appl. 2020, 30, e02056. [Google Scholar] [CrossRef]
- Kujala, H.; Moilanen, A.; Gordon, A. Spatial Characteristics of Species Distributions as Drivers in Conservation Prioritization. Methods Ecol. Evol. 2018, 9, 1121–1132. [Google Scholar] [CrossRef]
- Moilanen, A.; Lehtinen, P.; Kohonen, I.; Jalkanen, J.; Virtanen, E.A.; Kujala, H. Novel Methods for Spatial Prioritization with Applications in Conservation, Land Use Planning and Ecological Impact Avoidance. Methods Ecol. Evol. 2022, 13, 1062–1072. [Google Scholar] [CrossRef]
- Robinson, O.J.; Ruiz-Gutierrez, V.; Fink, D. Correcting for Bias in Distribution Modelling for Rare Species Using Citizen Science Data. Divers. Distrib. 2018, 24, 460–472. [Google Scholar] [CrossRef]
- Snäll, T.; Kindvall, O.; Nilsson, J.; Pärt, T. Evaluating Citizen-Based Presence Data for Bird Monitoring. Biol. Conserv. 2011, 144, 804–810. [Google Scholar] [CrossRef]
- Wijewardhana, U.A.; Meyer, D.; Jayawardana, M. Statistical Models for the Persistence of Threatened Birds Using Citizen Science Data: A Systematic Review. Glob. Ecol. Conserv. 2020, 21, e00821. [Google Scholar] [CrossRef]
- Coxen, C.L.; Frey, J.K.; Carleton, S.A.; Collins, D.P. Species Distribution Models for a Migratory Bird Based on Citizen Science and Satellite Tracking Data. Glob. Ecol. Conserv. 2017, 11, 298–311. [Google Scholar] [CrossRef]
- Girish, K.S.; Srinivasan, U. Community Science Data Provide Evidence for Upward Elevational Range Shifts by Eastern Himalayan Birds. Biotropica 2022, 54, 1457–1465. [Google Scholar] [CrossRef]
- Hu, R.; Gu, Y.; Luo, M.; Lu, Z.; Wei, M.; Zhong, J. Shifts in Bird Ranges and Conservation Priorities in China under Climate Change. PLoS ONE 2020, 15, e0240225. [Google Scholar] [CrossRef]
- Liang, J.; Xing, W.; Zeng, G.; Li, X.; Peng, Y.; Li, X.; Gao, X.; He, X. Where Will Threatened Migratory Birds Go under Climate Change? Implications for China’s National Nature Reserves. Sci. Total Environ. 2018, 645, 1040–1047. [Google Scholar] [CrossRef]
- Peterson, A.T.; Navarro-Siguenza, A.G.; Martinez-Meyer, E.; Cuervo-Robayo, A.P.; Berlanga, H.; Soberon, J.; Navarro-Sigüenza, A.G.; Martínez-Meyer, E.; Cuervo-Robayo, A.P.; Berlanga, H.; et al. Twentieth Century Turnover of Mexican Endemic Avifaunas: Landscape Change versus Climate Drivers. Sci. Adv. 2015, 1, e1400071. [Google Scholar] [CrossRef]
- GBIF. GBIF Occurrence Download. Available online: http://www.gbif.org/ (accessed on 25 December 2021).
- eBird EBird: An Online Database of Bird Distribution and Abundance [Web Application]. Available online: http://www.ebird.org (accessed on 25 December 2021).
- Jayadevan, P.; Jayapal, R.; Pittie, A. A Checklist of the Birds of India. Indian BIRDS 2016, 11, 113–172. [Google Scholar]
- Sullivan, B.L.; Wood, C.L.; Iliff, M.J.; Bonney, R.E.; Fink, D. EBird: A Citizen-Based Bird Observation Network in the Biological Sciences. Biol. Conserv. 2009, 142, 2282–2292. [Google Scholar] [CrossRef]
- BirdLife International. BirdLife International and Handbook of the Birds of the World Bird Species Distribution Maps of the World Version 2020.1; BirdLife International: Cambridge, UK, 2020. [Google Scholar]
- Isaac, N.J.B.; van Strien, A.J.; August, T.A.; de Zeeuw, M.P.; Roy, D.B. Statistics for Citizen Science: Extracting Signals of Change from Noisy Ecological Data. Methods Ecol. Evol. 2014, 5, 1052–1060. [Google Scholar] [CrossRef]
- Lagoze, C. EBird: Curating Citizen Science Data for Use by Diverse Communities. Int. J. Digit. Curation 2014, 9, 71–82. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making Better Maxent Models of Species Distributions: Complexity, Overfitting and Evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. SpThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Van Eupen, C.; Maes, D.; Herremans, M.; Swinnen, K.R.R.; Somers, B.; Luca, S. The Impact of Data Quality Filtering of Opportunistic Citizen Science Data on Species Distribution Model Performance. Ecol. Model. 2021, 444, 109453. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; Elith, J.; Dudík, M.; Ferrier, S.; Huettmann, F.; et al. Effects of Sample Size on the Performance of Species Distribution Models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial Filtering to Reduce Sampling Bias Can Improve the Performance of Ecological Niche Models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Meyer, C.; Weigelt, P.; Kreft, H. Multidimensional Biases, Gaps and Uncertainties in Global Plant Occurrence Information. Ecol. Lett. 2016, 19, 992–1006. [Google Scholar] [CrossRef]
- Cayuela, L.; Golicher, D.J.; Newton, A.C.; Kolb, M.; de Alburquerque, F.S.; Arets, E.J.M.M.; Alkemade, J.R.M.; Pérez, A.M. Species Distribution Modeling in the Tropics: Problems, Potentialities, and the Role of Biological Data for Effective Species Conservation. Trop. Conserv. Sci. 2009, 2, 319–352. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The Importance of Correcting for Sampling Bias in MaxEnt Species Distribution Models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Rocchini, D.; Hortal, J.; Lengyel, S.; Lobo, J.M.; Jiménez-Valverde, A.; Ricotta, C.; Bacaro, G.; Chiarucci, A. Accounting for Uncertainty When Mapping Species Distributions: The Need for Maps of Ignorance. Prog. Phys. Geogr. Earth Environ. 2011, 35, 211–226. [Google Scholar] [CrossRef]
- Zizka, A.; Antonelli, A.; Silvestro, D. Sampbias, a Method for Quantifying Geographic Sampling Biases in Species Distribution Data. Ecography 2021, 44, 25–32. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Title, P.O.; Bemmels, J.B. ENVIREM: An expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography 2018, 41, 291–307. [Google Scholar] [CrossRef]
- Feeley, K.J.; Bravo-Avila, C.; Fadrique, B.; Perez, T.M.; Zuleta, D. Climate-Driven Changes in the Composition of New World Plant Communities. Nat. Clim. Change 2020, 10, 965–970. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Kallimanis, A.; Strid, A.; Dimopoulos, P. Plant Endemism Centres and Biodiversity Hotspots in Greece. Biology 2021, 10, 72. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Nijs, I.; Lenoir, J. Incorporating Microclimate into Species Distribution Models. Ecography 2019, 42, 1267–1279. [Google Scholar] [CrossRef]
- Pecchi, M.; Marchi, M.; Burton, V.; Giannetti, F.; Moriondo, M.; Bernetti, I.; Bindi, M.; Chirici, G. Species Distribution Modelling to Support Forest Management. A Literature Review. Ecol. Model. 2019, 411, 108817. [Google Scholar] [CrossRef]
- Urbina-Cardona, N.; Blair, M.E.; Londoño, M.C.; Loyola, R.; Velásquez-Tibatá, J.; Morales-Devia, H. Species Distribution Modeling in Latin America: A 25-Year Retrospective Review. Trop. Conserv. Sci. 2019, 12, 194008291985405. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in Ecological Niche Modeling: Confusions and Challenges. Ecol. Evol. 2019, 9, 10365–10376. [Google Scholar] [CrossRef]
- Harris, R.M.B.; Grose, M.R.; Lee, G.; Bindoff, N.L.; Porfirio, L.L.; Fox-Hughes, P. Climate Projections for Ecologists. Wiley Interdiscip. Rev. Clim. Change 2014, 5, 621–637. [Google Scholar] [CrossRef]
- Sanderson, B.M.; Knutti, R.; Caldwell, P. A Representative Democracy to Reduce Interdependency in a Multimodel Ensemble. J. Clim. 2015, 28, 5171–5194. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-Source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The Crucial Role of the Accessible Area in Ecological Niche Modeling and Species Distribution Modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The Art of Modelling Range-Shifting Species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Kremen, C.; Cameron, A.; Moilanen, A.; Phillips, S.J.; Thomas, C.D.; Beentje, H.; Dransfield, J.; Fisher, B.L.; Glaw, F.; Good, T.C.; et al. Aligning Conservation Priorities across Taxa in Madagascar with High-Resolution Planning Tools. Science 2008, 320, 222–226. [Google Scholar] [CrossRef]
- Anderson, R.P.; Raza, A. The Effect of the Extent of the Study Region on GIS Models of Species Geographic Distributions and Estimates of Niche Evolution: Preliminary Tests with Montane Rodents (Genus Nephelomys) in Venezuela. J. Biogeogr. 2010, 37, 1378–1393. [Google Scholar] [CrossRef]
- Barber, R.A.; Ball, S.G.; Morris, R.K.A.; Gilbert, F. Target-Group Backgrounds Prove Effective at Correcting Sampling Bias in Maxent Models. Divers. Distrib. 2022, 28, 128–141. [Google Scholar] [CrossRef]
- Botella, C.; Joly, A.; Monestiez, P.; Bonnet, P.; Munoz, F. Bias in Presence-Only Niche Models Related to Sampling Effort and Species Niches: Lessons for Background Point Selection. PLoS ONE 2020, 15, e0232078. [Google Scholar] [CrossRef] [PubMed]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting Pseudo-Absences for Species Distribution Models: How, Where and How Many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Inman, R.; Franklin, J.; Esque, T.; Nussear, K. Comparing Sample Bias Correction Methods for Species Distribution Modeling Using Virtual Species. Ecosphere 2021, 12, e03422. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample Selection Bias and Presence-Only Distribution Models: Implications for Background and Pseudo-Absence Data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for Customizable and Reproducible Modeling of Species’ Niches and Distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R Package for Conducting Spatially Independent Evaluations and Estimating Optimal Model Complexity for Maxent Ecological Niche Models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Kass, J.M.; Anderson, R.P.; Espinosa-Lucas, A.; Juárez-Jaimes, V.; Martínez-Salas, E.; Botello, F.; Tavera, G.; Flores-Martínez, J.J.; Sánchez-Cordero, V. Biotic Predictors with Phenological Information Improve Range Estimates for Migrating Monarch Butterflies in Mexico. Ecography 2020, 43, 341–352. [Google Scholar] [CrossRef]
- Fithian, W.; Elith, J.; Hastie, T.; Keith, D.A. Bias Correction in Species Distribution Models: Pooling Survey and Collection Data for Multiple Species. Methods Ecol. Evol. 2015, 6, 424–438. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Liu, R.G.P.; Berry, C.; Dawson, P.M.; Pearson, T.P.; Liu, C.; Berry, P.M.; Dawson, T.P.; et al. Selecting Thresholds of Occurrence in the Prediction of Species Distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting Thresholds for the Prediction of Species Occurrence with Presence-Only Data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B.; Peterson, A.T.; Soberón, J.; Pearson, R.G. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011; ISBN 978-1-4008-4067-0. [Google Scholar]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A Misleading Measure of the Performance of Predictive Distribution Models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological Niche Modeling in Maxent: The Importance of Model Complexity and the Performance of Model Selection Criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting Species Distributions from Small Numbers of Occurrence Records: A Test Case Using Cryptic Geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking Receiver Operating Characteristic Analysis Applications in Ecological Niche Modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. Kuenm: An R Package for Detailed Development of Ecological Niche Models Using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Boyce, M.S.; Vernier, P.R.; Nielsen, S.E.; Schmiegelow, F.K.A. Evaluating Resource Selection Functions. Ecol. Model. 2002, 157, 281–300. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the Ability of Habitat Suitability Models to Predict Species Presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Tobias, J.A.; Sheard, C.; Pigot, A.L.; Devenish, A.J.M.; Yang, J.; Sayol, F.; Neate-Clegg, M.H.C.; Alioravainen, N.; Weeks, T.L.; Barber, R.A.; et al. AVONET: Morphological, Ecological and Geographical Data for All Birds. Ecol. Lett. 2022, 25, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.A.; Pigot, A.L. Integrating Behaviour and Ecology into Global Biodiversity Conservation Strategies. Philos. Trans. R. Soc. B 2019, 374, 20190012. [Google Scholar] [CrossRef] [PubMed]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An Ecoregion-Based Approach to Protecting Half the Terrestrial Realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- India Meteorological Department Climate Research and Services (CRS), Ministry of Earth Sciences (MoES). Statement on Climate of India during 2021; India Meteorological Department Climate Research and Services (CRS), Ministry of Earth Sciences (MoES): New Delhi, India, 2022. [Google Scholar]
- Dunn, R.R.; Harris, N.C.; Colwell, R.K.; Koh, L.P.; Sodhi, N.S. The Sixth Mass Coextinction: Are Most Endangered Species Parasites and Mutualists? Proc. R. Soc. B Biol. Sci. 2009, 276, 3037–3045. [Google Scholar] [CrossRef]
- Van Doren, B.M.; Conway, G.J.; Phillips, R.J.; Evans, G.C.; Roberts, G.C.M.; Liedvogel, M.; Sheldon, B.C. Human Activity Shapes the Wintering Ecology of a Migratory Bird. Glob. Change Biol. 2021, 27, 2715–2727. [Google Scholar] [CrossRef]
- Cleasby, I.R.; Bodey, T.W.; Vigfusdottir, F.; McDonald, J.L.; McElwaine, G.; Mackie, K.; Colhoun, K.; Bearhop, S. Climatic Conditions Produce Contrasting Influences on Demographic Traits in a Long-Distance Arctic Migrant. J. Anim. Ecol. 2017, 86, 285–295. [Google Scholar] [CrossRef]
- Kassara, C.; Gangoso, L.; Mellone, U.; Piasevoli, G.; Hadjikyriakou, T.G.; Tsiopelas, N.; Giokas, S.; López-López, P.; Urios, V.; Figuerola, J.; et al. Current and Future Suitability of Wintering Grounds for a Long-Distance Migratory Raptor. Sci. Rep. 2017, 7, 8798. [Google Scholar] [CrossRef]
- Liang, J.; Peng, Y.; Zhu, Z.; Li, X.; Xing, W.; Li, X.; Yan, M.; Yuan, Y. Impacts of Changing Climate on the Distribution of Migratory Birds in China: Habitat Change and Population Centroid Shift. Ecol. Indic. 2021, 127, 107729. [Google Scholar] [CrossRef]
- Wormworth, J.; Sekercioğlu, C. Winged Sentinels: Birds and Climate Change; Cambridge University Press: Port Melbourne, VIC, Australia, 2011; ISBN 978-0-521-12682-3. [Google Scholar]
- Cahill, A.E.; Aiello-Lammens, M.E.; Fisher-Reid, M.C.; Hua, X.; Karanewsky, C.J.; Yeong Ryu, H.; Sbeglia, G.C.; Spagnolo, F.; Waldron, J.B.; Warsi, O.; et al. How Does Climate Change Cause Extinction? Proc. R. Soc. B Biol. Sci. 2013, 280, 20121890. [Google Scholar] [CrossRef]
- Hannah, L.; Roehrdanz, P.R.; Marquet, P.A.; Enquist, B.J.; Midgley, G.; Foden, W.; Lovett, J.C.; Corlett, R.T.; Corcoran, D.; Butchart, S.H.M.; et al. 30% Land Conservation and Climate Action Reduces Tropical Extinction Risk by More than 50%. Ecography 2020, 43, 943–953. [Google Scholar] [CrossRef]
- Harris, G.; Pimm, S.L. Range Size and Extinction Risk in Forest Birds. Conserv. Biol. 2008, 22, 163–171. [Google Scholar] [CrossRef]
- Loiseau, N.; Mouquet, N.; Casajus, N.; Grenié, M.; Guéguen, M.; Maitner, B.; Mouillot, D.; Ostling, A.; Renaud, J.; Tucker, C.; et al. Global Distribution and Conservation Status of Ecologically Rare Mammal and Bird Species. Nat. Commun. 2020, 11, 5071. [Google Scholar] [CrossRef]
- Administrator, S. Global Patterns of Geographic Range Size in Birds. PLoS Biol. 2007, 132, e208. [Google Scholar] [CrossRef]
- Peterson, A.T.; Sánchez-Cordero, V.; Soberón, J.; Stockwell, D.R.B.; Bartley, J.; Ortega-Huerta, M.A.; Buddemeier, R.H. Future Projections for Mexican Faunas under Global Climate Change Scenarios. Nature 2002, 416, 626–629. [Google Scholar] [CrossRef]
- Wormworth, J.; Mallon, K. Bird Species and Climate Change: The Global Status Report: A Synthesis of Current Scientific Understanding of Anthropogenic Climate Change Impacts on Global Bird Species Now, and Projected Future Effects; Climate Risk Pty Ltd.: Fairlight, NSW, Australia, 2006; ISBN 978-0-646-46827-3. [Google Scholar]
- BirdLife International. State of the World’s Birds 2022: Insights and Solutions for the Biodiversity Crisis; BirdLife International: Cambridge, UK, 2022; p. 87. [Google Scholar]
- The SoIB Partnership. SoIB State of India’s Birds, 2020: Range, Trends and Conservation Status; The SoIB Partnership: Gandhinagar, India, 2020; p. 50. [Google Scholar]
- Gillings, S.; Balmer, D.E.; Fuller, R.J. Directionality of Recent Bird Distribution Shifts and Climate Change in Great Britain. Glob. Change Biol. 2015, 21, 2155–2168. [Google Scholar] [CrossRef]
- Devictor, V.; van Swaay, C.; Brereton, T.; Brotons, L.; Chamberlain, D.; Heliölä, J.; Herrando, S.; Julliard, R.; Kuussaari, M.; Lindström, Å.; et al. Differences in the Climatic Debts of Birds and Butterflies at a Continental Scale. Nat. Clim. Change 2012, 2, 121–124. [Google Scholar] [CrossRef]
- Auer, S.K.; King, D.I. Ecological and Life-History Traits Explain Recent Boundary Shifts in Elevation and Latitude of Western North American Songbirds. Glob. Ecol. Biogeogr. 2014, 23, 867–875. [Google Scholar] [CrossRef]
- Hitch, A.T.; Leberg, P.L. Breeding Distributions of North American Bird Species Moving North as a Result of Climate Change. Conserv. Biol. 2007, 21, 534–539. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Thomas, C.D.; Lennon, J.J. Birds Extend Their Ranges Northwards. Nature 1999, 399, 213. [Google Scholar] [CrossRef]
- Xu, J.; Grumbine, R.E.; Shrestha, A.; Eriksson, M.; Yang, X.; Wang, Y.; Wilkes, A. The Melting Himalayas: Cascading Effects of Climate Change on Water, Biodiversity, and Livelihoods. Conserv. Biol. 2009, 23, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Aryal, A.; Shrestha, U.B.; Ji, W.; Ale, S.B.; Shrestha, S.; Ingty, T.; Maraseni, T.; Cockfield, G.; Raubenheimer, D. Predicting the Distributions of Predator (Snow Leopard) and Prey (Blue Sheep) under Climate Change in the Himalaya. Ecol. Evol. 2016, 6, 4065–4075. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.L.; Wikramanayake, E.; Shrestha, R.; Areendran, G.; Gyeltshen, K.; Maheshwari, A.; Mazumdar, S.; Naidoo, R.; Thapa, G.J.; Thapa, K. Conservation and Climate Change: Assessing the Vulnerability of Snow Leopard Habitat to Treeline Shift in the Himalaya. Biol. Conserv. 2012, 150, 129–135. [Google Scholar] [CrossRef]
- Kaul, R.; Kalsi, R.S.; Singh, R.; Basnet, H.; Awan, M.N. Cheer Pheasant (Catreus wallichii) and the Conservation Paradox: Importance of Unprotected Areas. Diversity 2022, 14, 785. [Google Scholar] [CrossRef]
- Rana, S.K.; Rawal, R.S.; Dangwal, B.; Bhatt, I.D.; Price, T.D. 200 Years of Research on Himalayan Biodiversity: Trends, Gaps, and Policy Implications. Front. Ecol. Evol. 2021, 8, 516. [Google Scholar] [CrossRef]
- Schild, A. ICIMOD’s Position on Climate Change and Mountain Systems; The Case of the Hindu Kush-Himalayas. Mt. Res. Dev. 2008, 28, 328–331. [Google Scholar] [CrossRef]
- Sharma, E.; Tse-Ring, K.; Chettri, N.; Shrestha, A. IMBC-Plenary Session 1: Climate Change and Its Implications for Mountain Biodiversity in the Himalayas-Trends, Perception and Impacts of Climate Change. In Proceedings of the International Mountain Biodiversity Conference, Kathmandu, Nepal, 16–18 November 2008. [Google Scholar]
- Shekhar, M.S.; Chand, H.; Kumar, S.; Srinivasan, K.; Ganju, A. Climate-Change Studies in the Western Himalaya. Ann. Glaciol. 2010, 51, 105–112. [Google Scholar] [CrossRef]
- Shrestha, U.B.; Shrestha, B.B. Climate Change Amplifies Plant Invasion Hotspots in Nepal. Divers. Distrib. 2019, 25, 1599–1612. [Google Scholar] [CrossRef]
- Singh, P.B.; Mainali, K.; Jiang, Z.; Thapa, A.; Subedi, N.; Awan, M.N.; Ilyas, O.; Luitel, H.; Zhou, Z.; Hu, H. Projected Distribution and Climate Refugia of Endangered Kashmir Musk Deer Moschus Cupreus in Greater Himalaya, South Asia. Sci. Rep. 2020, 10, 1511. [Google Scholar] [CrossRef]
- Telwala, Y.; Brook, B.W.; Manish, K.; Pandit, M.K. Climate-Induced Elevational Range Shifts and Increase in Plant Species Richness in a Himalayan Biodiversity Epicentre. PLoS ONE 2013, 8, e57103. [Google Scholar] [CrossRef]
- You, Q.L.; Ren, G.Y.; Zhang, Y.Q.; Ren, Y.Y.; Sun, X.B.; Zhan, Y.J.; Shrestha, A.B.; Krishnan, R. An Overview of Studies of Observed Climate Change in the Hindu Kush Himalayan (HKH) Region. Adv. Clim. Change Res. 2017, 8, 141–147. [Google Scholar] [CrossRef]
- Lawler, J.J.; Shafer, S.L.; White, D.; Kareiva, P.; Maurer, E.P.; Blaustein, A.R.; Bartlein, P.J. Projected Climate-Induced Faunal Change in the Western Hemisphere. Ecology 2009, 90, 588–597. [Google Scholar] [CrossRef]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The Global Diversity of Birds in Space and Time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef]
- Miller, D.A.W.; Gardner, B.; Reich, B.J.; Singh, S.; Pacifici, K.; Stauffer, G.; Mckerrow, A.; Collazo, J.A. Integrating Multiple Data Sources in Species Distribution Modeling: A Framework for Data Fusion. Ecology 2016, 98, 840–850. [Google Scholar]
- Pearce-Higgins, J.W.; Green, R.E.; Pearce-Higgins, J.W.; Green, R.E. Using Models to Predict the Effects of Climate Change on Birds. In Birds and Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 201–249. [Google Scholar]
- Sohl, T.L. The Relative Impacts of Climate and Land-Use Change on Conterminous United States Bird Species from 2001 to 2075. PLoS ONE 2014, 9, e112251. [Google Scholar] [CrossRef]
- Blair, M.E.; Le, M.D.; Xu, M. Species Distribution Modeling to Inform Transboundary Species Conservation and Management under Climate Change: Promise and Pitfalls. Front. Biogeogr. 2022, 14, 1–11. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Jarnevich, C.S.; Pearse, I.S.; Smyth, R.L.; Auer, S.; Cook, G.L.; Edwards, T.C.; Guala, G.F.; Howard, T.G.; Morisette, J.T.; et al. Development and Delivery of Species Distribution Models to Inform Decision-Making. BioScience 2019, 69, 544–557. [Google Scholar] [CrossRef]
- Hertzog, L.R.; Besnard, A.; Jay-Robert, P. Field Validation Shows Bias-Corrected Pseudo-Absence Selection Is the Best Method for Predictive Species-Distribution Modelling. Divers. Distrib. 2014, 20, 1403–1413. [Google Scholar] [CrossRef]
- Santini, L.; Benítez-López, A.; Maiorano, L.; Čengić, M.; Huijbregts, M.A.J. Assessing the Reliability of Species Distribution Projections in Climate Change Research. Divers. Distrib. 2021, 27, 1035–1050. [Google Scholar] [CrossRef]
- Early, R.; Keith, S.A. Geographically Variable Biotic Interactions and Implications for Species Ranges. Glob. Ecol. Biogeogr. 2019, 28, 42–53. [Google Scholar] [CrossRef]
- Pigot, A.L.; Tobias, J.A. Species Interactions Constrain Geographic Range Expansion over Evolutionary Time. Ecol. Lett. 2013, 16, 330–338. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).