Gametogenic Cycle of the Oysters Pinctada capensis (Sowerby III, 1890) and Saccostrea cucullata (Born, 1778) (Class Bivalvia) in Inhaca Island, Southern Mozambique: A Subsidy for Bivalve Culture in the Region
Abstract
1. Introduction
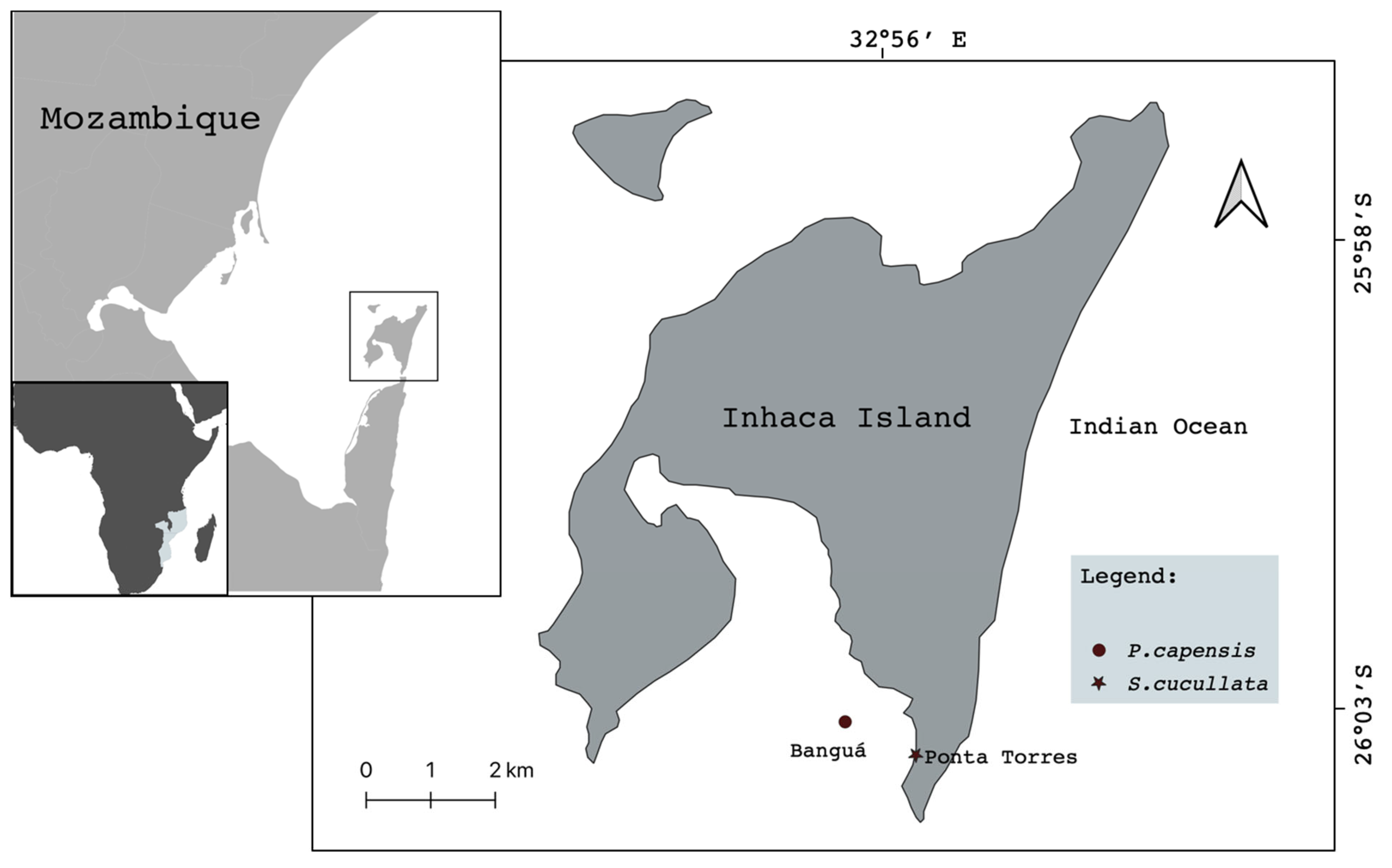
2. Materials and Methods
Data Analysis
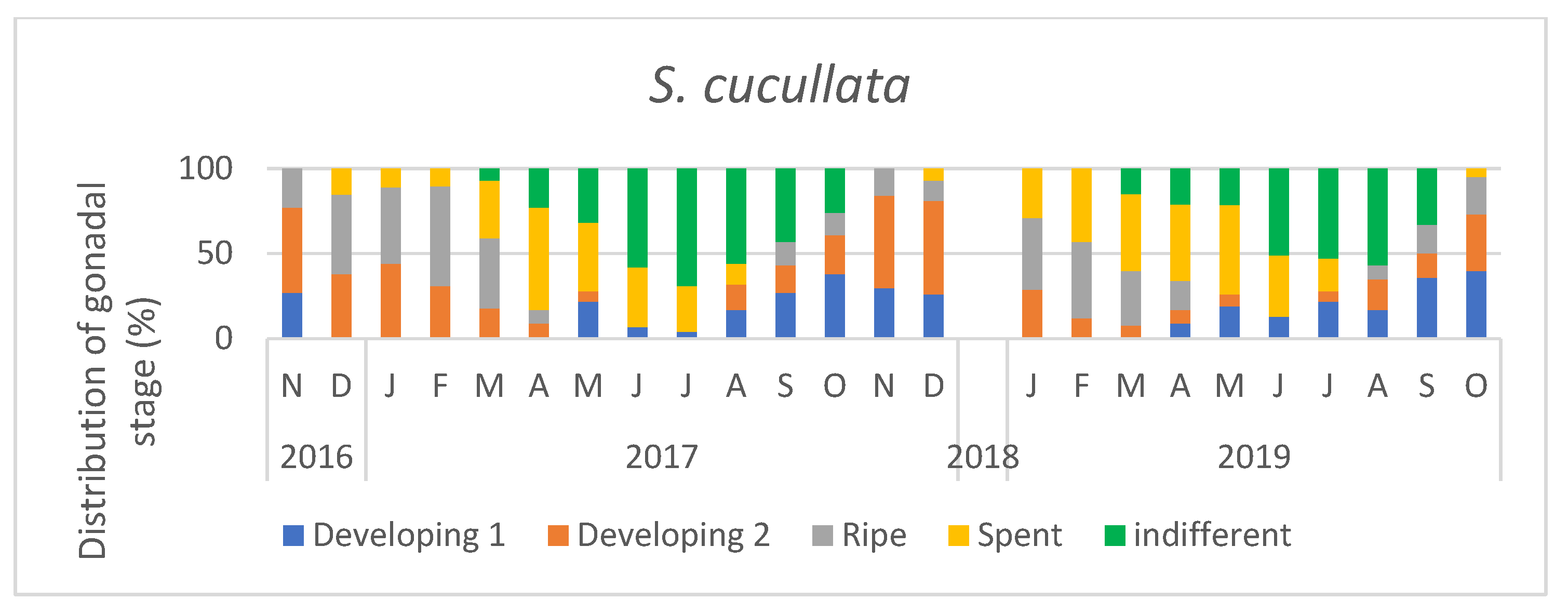
3. Results
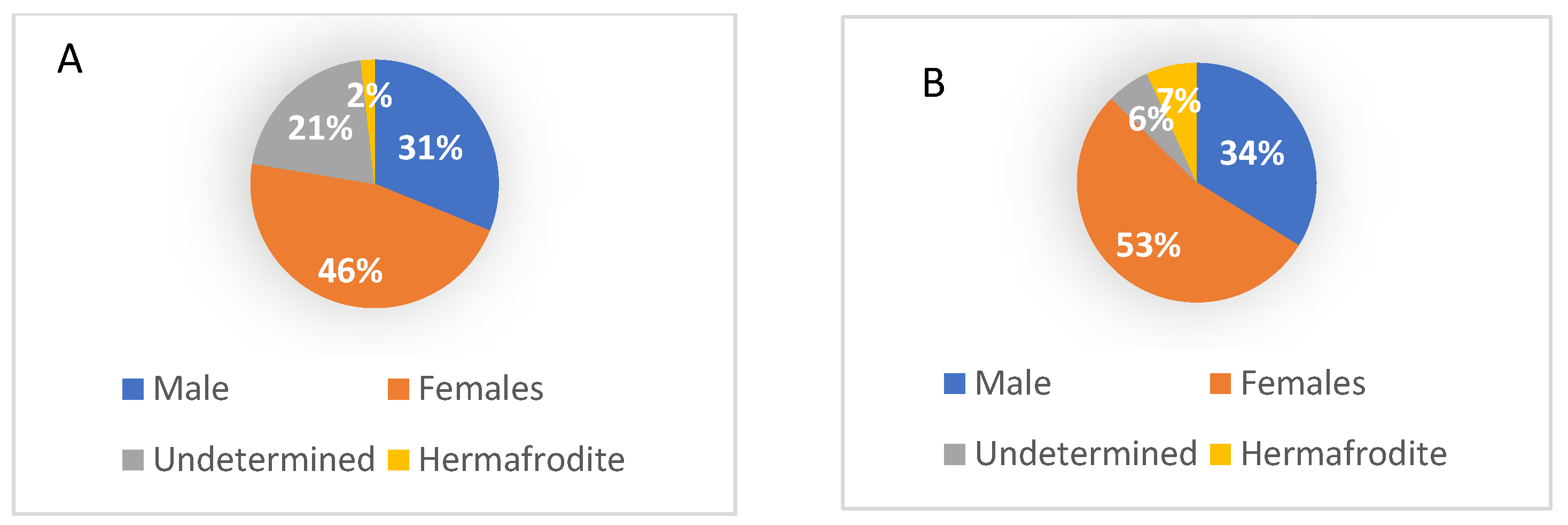
3.1. Sex Ratio
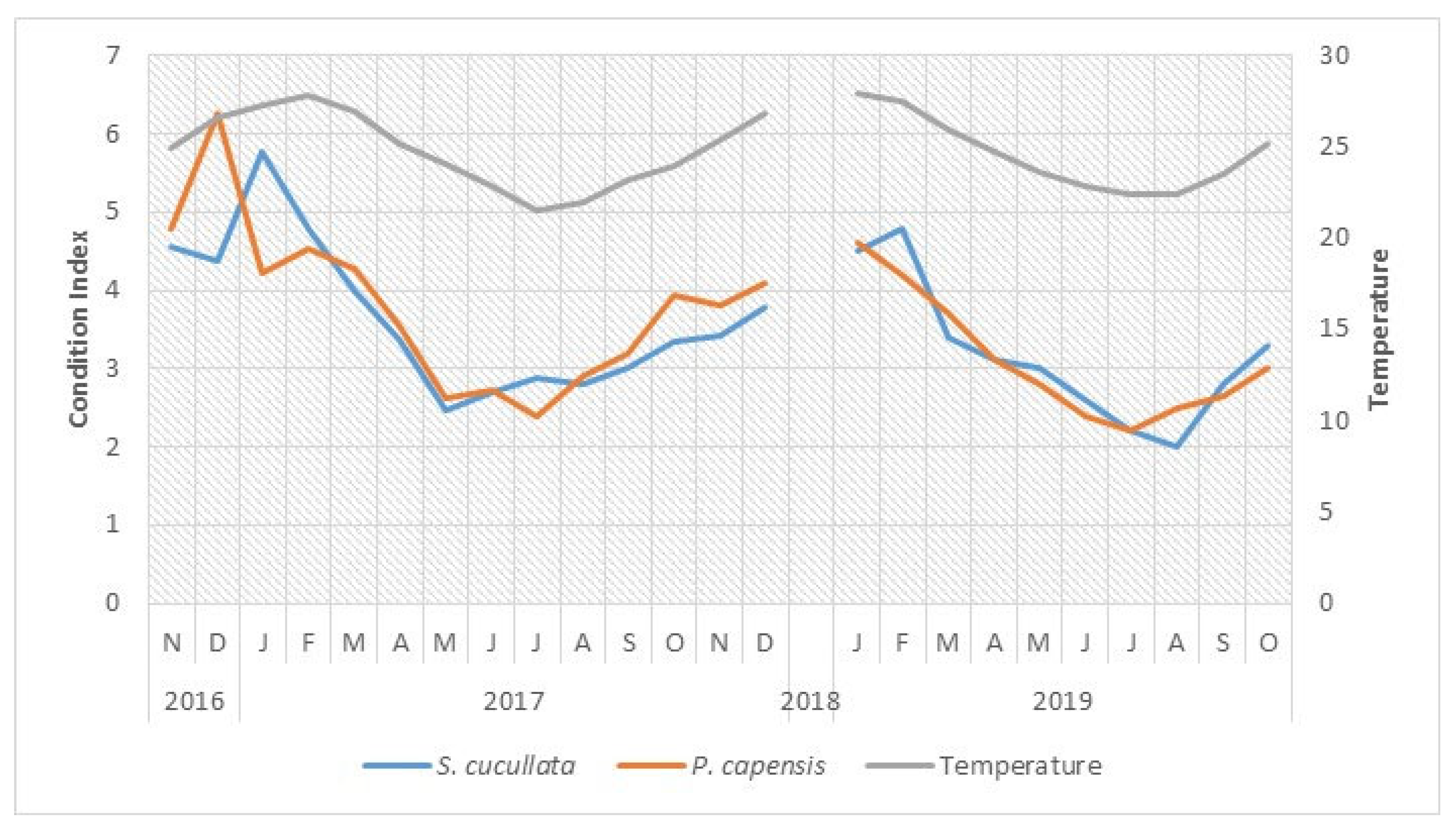
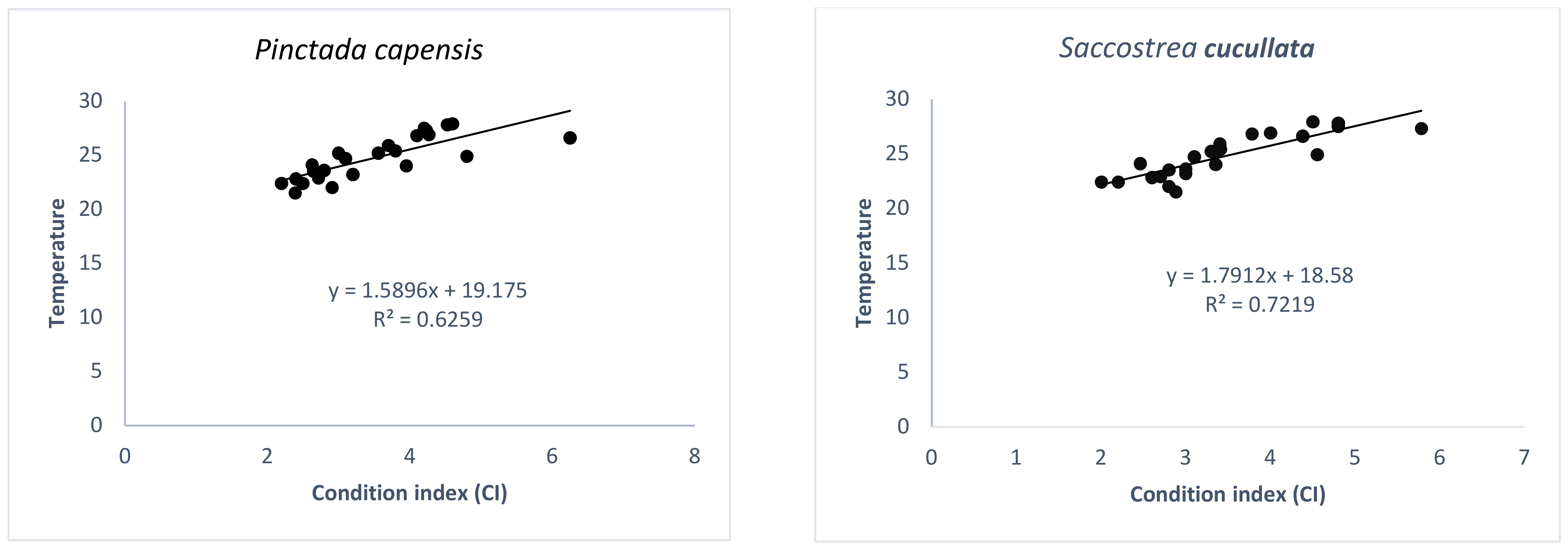
3.2. Temperature and the Condition Index
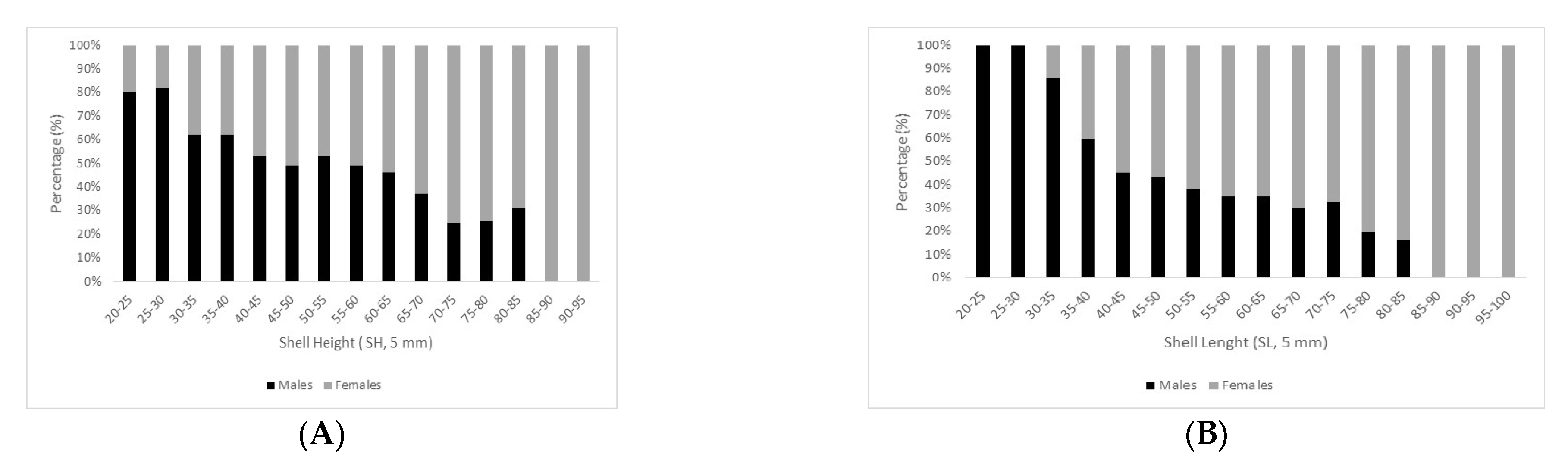
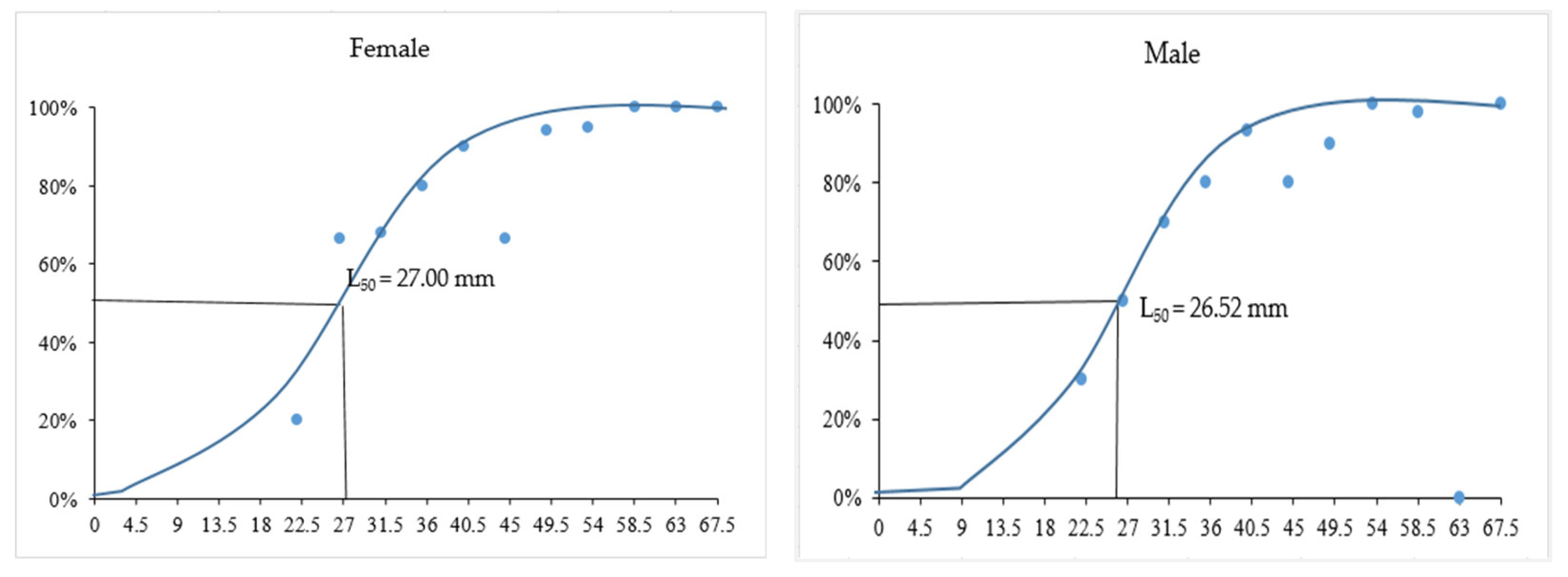
3.3. Minimum Size at First Maturity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quan, W.; Fan, R.; Wang, Y.; Humphries, A.T. Long-Term Oyster Recruitment and Growth are not influenced by Substrate Type in China: Implications for Sustainable Oyster Reef Restoration. J. Shellf. Res. 2017, 36, 79–86. [Google Scholar] [CrossRef]
- Guo, X.; Wang, C.; Li, H.; Xu, Z. Diversity and Evolution of Living Oysters. J. Shellf. Res. 2018, 37, 755–771. [Google Scholar] [CrossRef]
- Angel, C.L. The Biology and Culture of Tropical Oysters; ICLARM Studies and Reviews: Manila, Philippines, 1986; p. 42. [Google Scholar]
- Kimani, E.N.; Mavuti, K.M.; Mukiama, T. The reproductive activity of the pearl oyster Pinctada imbricata Röding 1798 (Pteriidae) in Gazi Bay, Kenya. Tro. Zool. 2006, 19, 159–174. [Google Scholar]
- Kishore, P.; Vuibeqa, G.B.; Southgate, P.C. Developing a national spat collection program for pearl oysters in Fiji Islands supporting pearl industry development and livelihoods. Aquacul. Reports. 2018, 9, 46–52. [Google Scholar] [CrossRef]
- Saucedo, P.E.; Southgate, P. Reproduction, development and growth. In The Pearl Oyster; Southgate, P., Lucas, J., Eds.; Elsevier: Oxford, UK, 2008; Volume 1, pp. 131–186. [Google Scholar]
- Gomes, C.H.A.M.; Silva, F.C.; Lopes, G.R.; Melo, C.M.R. The reproductive cycle of the oyster Crassostrea gasar. Braz. J. Biol. 2014, 74, 967–976. [Google Scholar] [CrossRef]
- Pouvreau, S.; Gangnery, A.; Tiapari, J.; Lagarde, F. Gametogenic cycle and reproductive effort of the tropical blacklip pearl oyster, Pinctada margaritifera (Bivalvia: Pteriidae), cultivated in Takapoto atoll (French Polynesia). Aquat. Living Resour. 2000, 13, 37–48. [Google Scholar] [CrossRef]
- Aideed, M.S.; Ahmed, B.A.A.; Mukhaysin, A.A. Existence, growth, and reproduction of pearl oyster Pinctada margaritifera in Hadhramout coast/Gulf of Aden. Egypt. J. Aqua. Res. 2014, 40, 473–481. [Google Scholar] [CrossRef]
- Gosling, E. Bivalve Mollusks: Biology, Ecology and Culture. Reproduction, Settlement and Recruitment; Fishing News Books, Ed.; Fishing News Books: Oxford, UK; London, UK, 2003; p. 455. [Google Scholar]
- Avendaño, M.; Le Pennec, M. Intraspecific variation in gametogenesis in two populations of the Chilean molluscan bivalve, Argopecten purpuratus (Lamarck). Aqua. Res. 1997, 28, 175–182. [Google Scholar] [CrossRef]
- Silva, P.P.; Peso-Aguiar, M.C.; Ribeiro, G. Ciclo Gametogênico e Comportamento Reprodutivo de Iphigenia brasiliana (Mollusca, Bivalvia, Donacidae) no Estuário do Rio Subaé; Baía de Todos os Santos: Bahia, Brasil, 2012; p. 11. [Google Scholar]
- Saucedo, P.; Rodruguez-Jaramillo, C.; Aldana-Aviles, C.; Monsalvo-Spencer, P.; Reynoson-Granados, T. Gonadic conditioning of the calafia mother-of-pear oyster, Pinctada mazatlanica (Hanley, 1956) under two temperature regime. Aquacultura 2001, 195, 103–119. [Google Scholar] [CrossRef]
- Dye, A.H. Episodic recruitment of the rock oyster Saccostrea cucullata (Born, 1778) on the Transkei coast. S. Afr. J. Mar. Sci. 1990, 25, 185–187. [Google Scholar]
- Torigoe, K. Oysters in Japan. J. Sci. -Hiroshima Univ. Ser. B Div. 1 1981, 29, 291–481. [Google Scholar]
- Roughley, T.C. The Cult of the Goldfish; Angus, Robertson ltd.: Sydney, Australia, 1933; pp. Xiii + 146. [Google Scholar]
- Dinamani, P. The morphology of the larval shell of Saccostrea glomerata (Gould, 1850) and a comparative study of the larval shell in the genus Crassostrea Sacco, 1897 (Ostreidae). J. Molluscan Stud. 1976, 42, 95–107. [Google Scholar]
- Van Someren, W.R.; Whitehead, B.A. An investigation of the biology and culture of an East African oyster Crassostrea cucullata. Fish. Publ. Lond. 1961, 14, 1–41. [Google Scholar]
- Everett, B.I.; van der Elst, R.; Schleyer, M.H. A natural history of the Bazaruto Archipelago, Mozambique. Ocen. Res. Inst. Spec. Ed. 2008, 8, SAAMBR/WWF. [Google Scholar]
- De Boer, W.F.; Prins, H.H.T. The community structure of a tropical intertidal mudflat under human exploitation. ICES J. Mar. Sci. 2001, 59, 1237–1247. [Google Scholar] [CrossRef]
- De Boer, W.F.; Pereira, T.; Guissamulo, A. Comparing recent and abandoned shell middens to detect the impact of human exploitation on the intertidal ecosystem. Aquat. Ecol. 2000, 34, 287–297. [Google Scholar] [CrossRef]
- Kalk, M. A Natural History of Inhaca Island, Mozambique, 3rd ed.; Witwatersrand University Press: Johannesburg, South Africa, 1995. [Google Scholar]
- Emanuelsson, A.; Isaksson, D. Inhaca Marine Biology Research Station. Sweden, 2016. Available online: www.globalreporting.net (accessed on 10 January 2023).
- Pereira, I.J.F.; do Nascimento, F.R. Avaliação dos Recursos Naturais na Ilha Da Inhaca (Oceano Índico, Moçambique). Prim. Aproximação 2016, 36, 307–325. [Google Scholar]
- Gaspar, M.B.; Santos, M.N.; Vasconcelos, P.; Monteiro, C.C. Shell Morphometric relationships of the most common bivalve species (Mollusca: Bivalvia) of Algarve coast (Southern Portugal). Hydrobiology 2002, 477, 73–80. [Google Scholar] [CrossRef]
- Lenz, T.; Boehs, G. Ciclo reproductivo del ostin de manglar Crassostrea rhizophorae (Bivalvia: Ostreidae) en la Bah a de camamu Bahia, Brasil. Rev. Biol. Trop. 2011, 59, 137–149. [Google Scholar]
- Walne, P.R. Experiments on the culture of the Butterfish Venerupis deccussata L. Aquaculture 1976, 8, 371–381. [Google Scholar] [CrossRef]
- Guillou, J.; Bachelet, G.; Desprez, M.; Ducrotoy, J.P. Les modalités de la reproduction de la coque Cerastoderma eduleser le littoral français de la Manche et de l’Atlantique. Aquat. Living Resour. 1990, 3, 29–41. [Google Scholar] [CrossRef]
- King, M. Fisheries Biology. Assessement and Management; Fishing News Books: Oxford, UK, 1995; p. 341. [Google Scholar]
- Arbuckle, J.L. IBM SPSS Amos 20 User’s Guide; Amos Development Corporation Inc: Meadville, PE, USA, 2001; pp. 226–229. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2010; p. 947. [Google Scholar]
- Lee, J.S.; Lee, Y.G.; Kang, S.W.; Park, J.S.; Lee, D.G.; Jeon, M.A.; Ju, S.M. Intersexuality of Crassostrea gigas and Ruditapes philippinarum in Southern Coastal Waters of Korea. Environ. Health Toxicol. 2010, 25, 287–294. [Google Scholar]
- Christo, S.W. Biologia Reprodutiva e Ecologia de Ostras do Gênero Crassostrea (Sacco, 1897) na Baía de Guaratuba (Paraná–Brasil): Um Subsídio ao Cultivo. Ph.D. Thesis, Tese Doutorado Em Ciências Biológicas-Zoologia, Universidade Federal do Paraná, Curitiba, Brazil, 2006; p. 146. [Google Scholar]
- Castilho-Westphal, G.G.; Magnani, F.P.; Ostrensky, A. Gonad morphology and reproductive cycle of the mangrove oyster Crassostrea brasiliana (Lamarck, 1819) in the Baía de Guaratuba, Paraná, Brazil. Acta Zool. 2015, 96, 99–107. [Google Scholar] [CrossRef]
- Derbali, A.; Jarboui, O.; Ghorbel, M.; Dhieb, K. Reproductive biology of the pearl oyster, Pinctada radiata (Mollusca: Pteriidae), in northern Kerkennah Island (Gulf of Gabès). Cah. Biol. Mar. 2009, 50, 215–222. [Google Scholar]
- Hwang, J.J. Reproduction cycles of the pearl oysters, Pinctada fucata (Gould) and Pinctada margaritifera(Linnaeus) (Bivalvia: Pteriidae) in southwestern Taiwan waters. J. Mar. Sci. Tech. 2007, 15, 67–75. [Google Scholar] [CrossRef]
- Pakhmode, A.; Mohitea, S.A.; Takarb, S.; Gurjarc, U.R. Reproductive biology of rock oyster, Saccostrea cucullata (Born, 1778) along Aare-Ware rocky shore of Ratnagiri, Maharashtra, India. Indian J. Geo Mar. Sci. 2021, 50, 802–809. [Google Scholar]
- O’Connor, W.A.; Lawler, N.F. Reproductive condition of the pearl oyster, Pinctada imbricata (Roding), in Port Stephens, New South Wales (Australia). Aquacult. Res. 2004, 35, 385–396. [Google Scholar] [CrossRef]
- Aswani, K.; Volety, S.; Tolley, G.; Savarese, M.; Winstead, J.T. Role of anthropogenic and environmental variability on the physiological and ecological responses of oysters in southwest Florida estuaries. J. Shellfish Res. 2004, 23, 315–316. [Google Scholar]
- Alves, R. Biologia de Pteria hirundo, Ostra Perlífera Nativa do Brasil. Ph.D. Thesis, Universidade de Santa Catarina, Florianópolis, Brazil, 2010; p. 164. [Google Scholar]
- Paula, J.; Pinto, I.; Guambe, I.; Monteiro, S.; Gove, D.; Guerreiro, J. Seasonal cycle of planktonic communities at Inhaca Island, southern Mozambique. J. Plankton Res. 1998, 20, 2165–2178. [Google Scholar] [CrossRef]
- Kang, C.K.; Park, M.S.; Lee, P.Y.; Choi, W.J.; Lee, W.C. Seasonal variation in condition, reproductive activity and biochemical composition of the acific oyster Crassostrea gigas, in suspended culture in two coastal bays of Korea. J. Shellfish Res. 2000, 19, 771–778. [Google Scholar]
- Beer, A.C.; Southgate, P.C. Collection of pearl oyster (family Pteriidae) spat at Orpheus Island Great Barrier Reef (Australia). J. Shellfish Res. 2000, 19, 821–826. [Google Scholar]
- Lannan, J.E.; Robinson, A.K.; Breese, W.P. Broodstock management of Crassostrea gigas: II. Broodstock conditioning to maximize larval survival. Aqua 1980, 21, 337–345. [Google Scholar] [CrossRef]
- Tenjing, S.Y. Population dynamics of the edible rock oyster Saccostrea cucullata (Born, 1778) along the south west coast of India. Indian J. Fish 2020, 67, 16–22. [Google Scholar]


| Stage | Characteristics |
|---|---|
| Indifferent | No gonad visible. This has two possible explanations—adults with recovering gonads after spawning or immature juveniles |
| Developing I | Gonad tissue visible, but it is very difficult to distinguish sex |
| Developing II | Gonad tissue are evident and sexes can be distinguished. Gametes are abundant, but the majority of the spermatozoids are hardly moving and pedunculated oocytes are present |
| Ripe | Gonad with rapid moving spermatozoids or spherical oocytes |
| Spent | Gonads are empty and thin. Coexistence of cells being reabsorbed and mature cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafambissa, M.; Rodrigues, M.; Taimo, T.; Andrade, C.; Lindegart, M.; Macia, A. Gametogenic Cycle of the Oysters Pinctada capensis (Sowerby III, 1890) and Saccostrea cucullata (Born, 1778) (Class Bivalvia) in Inhaca Island, Southern Mozambique: A Subsidy for Bivalve Culture in the Region. Diversity 2023, 15, 361. https://doi.org/10.3390/d15030361
Mafambissa M, Rodrigues M, Taimo T, Andrade C, Lindegart M, Macia A. Gametogenic Cycle of the Oysters Pinctada capensis (Sowerby III, 1890) and Saccostrea cucullata (Born, 1778) (Class Bivalvia) in Inhaca Island, Southern Mozambique: A Subsidy for Bivalve Culture in the Region. Diversity. 2023; 15(3):361. https://doi.org/10.3390/d15030361
Chicago/Turabian StyleMafambissa, Mizeque, Mery Rodrigues, Torres Taimo, Carlos Andrade, Mats Lindegart, and Adriano Macia. 2023. "Gametogenic Cycle of the Oysters Pinctada capensis (Sowerby III, 1890) and Saccostrea cucullata (Born, 1778) (Class Bivalvia) in Inhaca Island, Southern Mozambique: A Subsidy for Bivalve Culture in the Region" Diversity 15, no. 3: 361. https://doi.org/10.3390/d15030361
APA StyleMafambissa, M., Rodrigues, M., Taimo, T., Andrade, C., Lindegart, M., & Macia, A. (2023). Gametogenic Cycle of the Oysters Pinctada capensis (Sowerby III, 1890) and Saccostrea cucullata (Born, 1778) (Class Bivalvia) in Inhaca Island, Southern Mozambique: A Subsidy for Bivalve Culture in the Region. Diversity, 15(3), 361. https://doi.org/10.3390/d15030361






