Effects of Different Types of Agricultural Land Use on the Occurrence of Common Aquatic Bugs (Nepomorpha, Heteroptera) in Habitats with Slow Flowing Water in Bulgaria, Southeast Europe
Abstract
1. Introduction
2. Materials and Methods
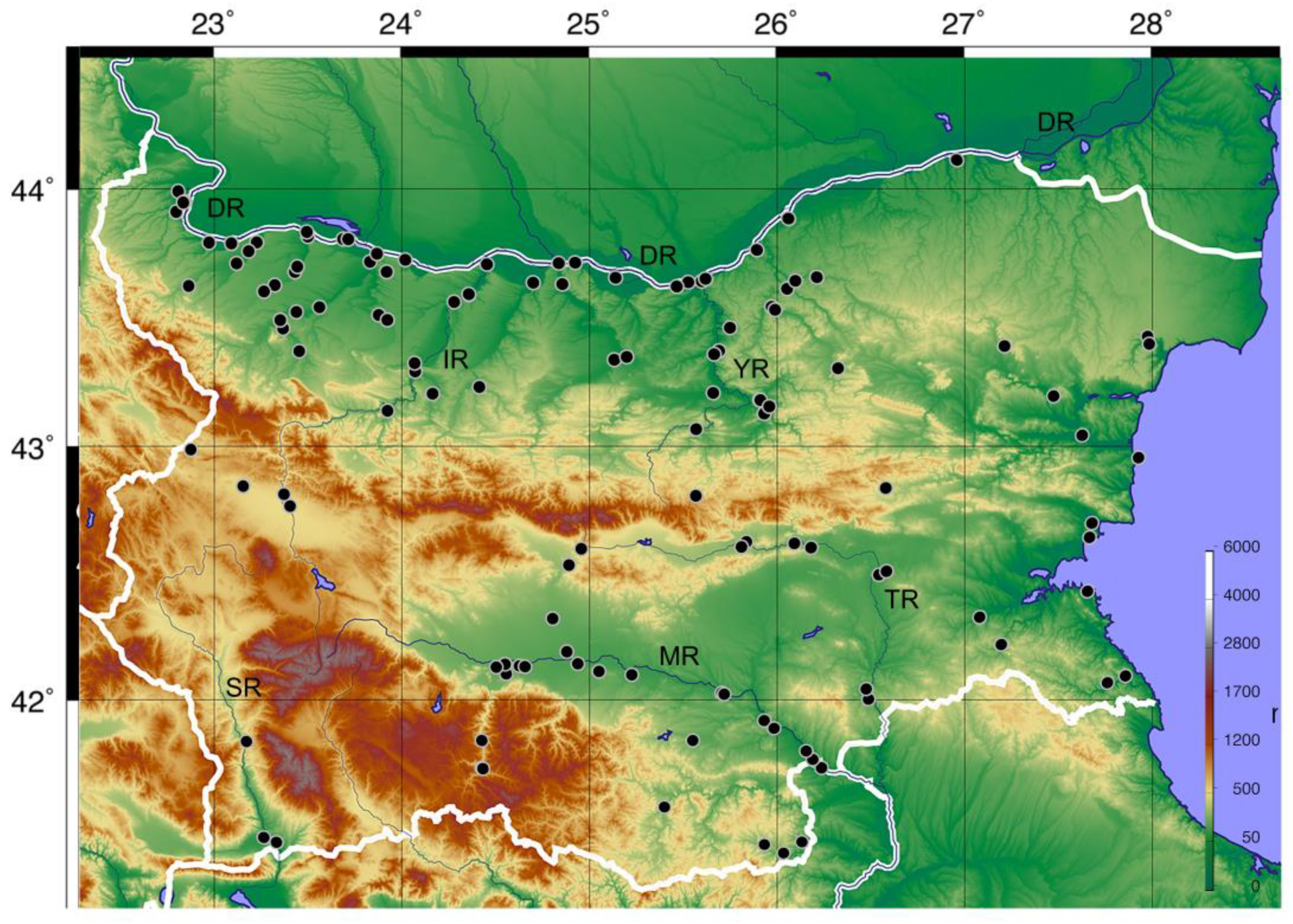
2.1. Study Area and Species Occurrence Data
2.2. Environmental Dataset
2.3. Modeling the Response of the Selected Species to the Agricultural Land Use Variables
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monroe, J.B.; Baxter, C.V.; Olden, J.D.; Angermeier, P.L. Freshwaters in the Public Eye: Understanding the Role of Images and Media in Aquatic Conservation. Fisheries 2009, 34, 581–585. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint Pollution of Surface Waters with Phosphorus and Nitrogen. Ecol. Appl. 1998, 8, 559. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human Alteration of the Global Nitrogen Cycle: Sources and Consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Bouraoui, F.; Grizzetti, B. Long Term Change of Nutrient Concentrations of Rivers Discharging in European Seas. Sci. Total Environ. 2011, 409, 4899–4916. [Google Scholar] [CrossRef]
- Windolf, J.; Thodsen, H.; Troldborg, L.; Larsen, S.E.; Bøgestrand, J.; Ovesen, N.B.; Kronvang, B. A Distributed Modelling System for Simulation of Monthly Runoff and Nitrogen Sources, Loads and Sinks for Ungauged Catchments in Denmark. J. Environ. Monit. 2011, 13, 2645–2658. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W. Recent Advances in the Understanding and Management of Eutrophication. Limnol. Oceanogr. 2006, 51, 356–363. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, F.; Shi, J.; Kung, H.-T. What Is the Relationship between Land Use and Surface Water Quality? A Review and Prospects from Remote Sensing Perspective. Environ. Sci. Pollut. Res. 2022, 29, 56887–56907. [Google Scholar] [CrossRef]
- Huber, A.; Bach, M.; Frede, H. Pollution of Surface Waters with Pesticides in Germany: Modeling Non-Point Source Inputs. Agric. Ecosyst. Environ. 2000, 80, 191–204. [Google Scholar] [CrossRef]
- Genito, D.; Gburek, W.J.; Sharpley, A.N. Response of Stream Macroinvertebrates to Agricultural Land Cover in a Small Watershed. J. Freshw. Ecol. 2002, 17, 109–119. [Google Scholar] [CrossRef]
- Lenat, D.R.; Crawford, J.K. Effects of Land Use on Water Quality and Aquatic Biota of Three North Carolina Piedmont Streams. Hydrobiologia 1994, 294, 185–199. [Google Scholar] [CrossRef]
- Riley, R.H.; Townsend, C.R.; Niyogi, D.K.; Arbuckle, C.A.; Peacock, K.A. Headwater Stream Response to Grassland Agricultural Development in New Zealand. New Zeal. J. Mar. Freshw. Res. 2003, 37, 389–403. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and Riverscapes: The Influence of Land Use on Stream Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Probst, M.; Berenzen, N.; Lentzen-Godding, A.; Schulz, R.; Liess, M. Linking land use variables and invertebrate taxon richness in small and medium-sized agricultural streams on a landscape level. Ecotoxicol. Environ. Saf. 2005, 60, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.K.; Wiederholm, T.; Rosenberg, D.M. Freshwater Biomonitoring Using Individual Organisms, Populations, and Species Assemblages of Benthic Macroinvertebrates. Freshw. Biomonitoring Benthic Macroinvertebrates 1993, 40, 40–158. [Google Scholar]
- Rosenberg, D.M.; Resh, V.H. Freshwater Biomonitoring and Benthic Macroinvertebrates; Springer: New York, NY, USA, 1993; ISBN 978-0-412-02251-7. [Google Scholar]
- Lock, K.; Adriaens, T.; Van De Meutter, F.; Goethals, P. Effect of Water Quality on Waterbugs (Hemiptera: Gerromorpha & Nepomorpha) in Flanders (Belgium): Results from a Large-Scale Field Survey. Ann. Limnol.-Int. J. Limnol. 2013, 49, 121–128. [Google Scholar] [CrossRef]
- Bakonyi, G.; Vásárhelyi, T.; Szabó, B. Pollution Impacts on Water Bugs (Nepomorpha, Gerromorpha): State of the Art and Their Biomonitoring Potential. Environ. Monit. Assess. 2022, 194, 1–25. [Google Scholar] [CrossRef]
- Jansson, A. Micronectae (Heteroptera, Corixidae) as Indicators of Water Quality in Two Lakes in Southern Finland. Proc. Ann. Zool. Fenn. 1977, 14, 118–124. [Google Scholar]
- Biesiadka, E.; Tabaka, K. Badania Nad Pluskwiakami (Heteroptera) Wodnymi Jezior Szczycieńskich (Woj. Olsztyńskie). Fragm. Faun. 1990, 33, 45–69. [Google Scholar] [CrossRef]
- Savage, A. The Distribution of Corixidae in Relation to the Water Quality of British Lakes: A Monitoring Model. Freshw. Forum 1994, 4, 32–61. [Google Scholar]
- Sládecek, V.; Sládecková, A. Corixidae as Indicators of Organic Pollution. Freshw. Forum 1994, 4, 211–213. [Google Scholar]
- Vásárhelyi, T.; Bakonyi, G. Seven Decades of Monitoring the Aquatic Bug Fauna of Lake Balaton (Heteroptera: Nepomorpha). Aquat. Insects 2012, 34, 33–43. [Google Scholar] [CrossRef]
- Karaouzas, I.; Gritzalis, K.C. Local and Regional Factors Determining Aquatic and Semi-Aquatic Bug (Heteroptera) Assemblages in Rivers and Streams of Greece. Hydrobiologia 2006, 573, 199–212. [Google Scholar] [CrossRef]
- Cheshmedjiev, S.D.; Karagiozova, T.L.; Michailov, M.A.; Valev, V.P. Revision of River & Lake Typology in Bulgaria within Ecoregion 12 (Pontic Province) and Ecoregion 7 (Eastern Balkans) According to the Water Framework Directive. Ecol. Balk. 2010, 2, 75–96. [Google Scholar]
- Stoyanova, T.; Traykov, I.; Bogoev, V.; Yaneva, I.; Vidinova, Y.; Tyufekchieva, V.; Kenderov, L. Composition of the Macrozoobenthos in Semi-Mountainous River in South-Western Bulgaria. Nat. Montenegrina 2013, 12, 803–811. [Google Scholar]
- Stoyanova, T.; Traykov, I. Assessment of the Ecological Status of Ogosta River, Northwestern Bulgaria, Based on the Macrozoobenthos and the General Physical and Chemical Quality Elements. Acta Zool. Bulg. 2014, (Suppl. 7), 173–178. [Google Scholar]
- Borisova, P.; Varadinova, E.; Yzunov, Y. Contemporary State of the Bottom Invertebrate Communities of the Tundzha River Basin (South-East Bulgaria). Acta Zool. Bulg. 2013, 65, 75–87. [Google Scholar]
- Kazakov, S. Structural and Functional Parameters of the Hydrozoocenosys in the Lower Danube Wetlands; Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences: Sofia, Bulgaria, 2017. [Google Scholar]
- Vidinova, Y.; Tyufekchieva, V.; Varadinova, E.; Stoichev, S.; Kenderov, L.; Dedov, I.; Uzunov, Y. Taxonomic List of Benthic Macroinvertebrate Communities of Inland Standing Water Bodies in Bulgaria. Acta Zool. Bulg. 2016, 68, 147–158. [Google Scholar]
- Stoianova, D.; Evtimova, V.; Kenderov, L.; Varadinova, E.D.; Kerakova, M.Y.; Ihtimanska, M.K.; Stefanov, T.; Soufi, R.A.; Tyufekchieva, V.; Vidinova, Y. New Localities and Habitat Suitability Modelling for the Riverine Water Bug Aphelocheirus Aestivalis (Fabricius, 1794) (Heteroptera: Aphelocheiridae) in Northern and Eastern Bulgaria. Acta Zool. Bulg. 2018, 70, 415–431. [Google Scholar]
- Park, J.; Sakelarieva, L.; Varadinova, E.; Evtimova, V.; Vidinova, Y.; Tyufekchieva, V.; Georgieva, G.; Ihtimanska, M.; Todorov, M. Taxonomic Composition and Dominant Structure of the Macrozoobenthos in the Maritsa River and Some Tributaries, South Bulgaria. Acta Zool. Bulg. 2022, in press. [Google Scholar]
- Cheshmedjiev, S.; Soufi, R.; Vidinova, Y.; Tyufekchieva, V.; Yaneva, I.; Uzunov, Y.; Varadinova, E. Multi-Habitat Sampling Method for Benthic Macroinvertebrate Communities in Different River Types in Bulgaria. Water Res. Manag. 2011, 1, 55–58. [Google Scholar]
- Joint Research Centre—European Commission CCM River and Catchment Database, Version 2.1 (CCM2). Available online: https://ossf.denny.one/tw/resourcecatalog/GIS/Map-Data/catchment-characterisation-and-modelling-ccm/visit.html (accessed on 11 February 2023).
- Copernicus—The European Earth Observation Programme. Available online: https://land.copernicus.eu/copernicus-the-european-earth-observation-programme (accessed on 26 January 2023).
- QGIS Association QGIS Geographic Information System 2020. Available online: https://qgis.org/ (accessed on 11 February 2023).
- Kuemmerlen, M.; Schmalz, B.; Guse, B.; Cai, Q.; Fohrer, N.; Jähnig, S.C. Integrating Catchment Properties in Small Scale Species Distribution Models of Stream Macroinvertebrates. Ecol. Modell. 2014, 277, 77–86. [Google Scholar] [CrossRef]
- Kuemmerlen, M.; Stoll, S.; Sundermann, A.; Haase, P. Long-Term Monitoring Data Meet Freshwater Species Distribution Models: Lessons from an LTER-Site. Ecol. Indic. 2016, 65, 122–132. [Google Scholar] [CrossRef]
- R CoreTeam R: A Language and Environment for Statistical Computing 2022. Available online: https://www.r-project.org (accessed on 11 February 2023).
- Dudik, M.; Phillips, S.; Schapire, R. Maxent 2020. Available online: https://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 11 February 2023).
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudik, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography (Cop.) 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A Maximum Entropy Approach to Species Distribution Modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; p. 83. [Google Scholar]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography (Cop.) 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Smith, R.V.; Jordan, C.; Annett, J.A. A Phosphorus Budget for Northern Ireland: Inputs to Inland and Coastal Waters. J. Hydrol. 2005, 304, 193–202. [Google Scholar] [CrossRef]
- Łaszewski, M.; Fedorczyk, M.; Stępniewski, K. The Impact of Land Cover on Selected Water Quality Parameters in Polish Lowland Streams during the Non-Vegetative Period. Water 2022, 14, 3295. [Google Scholar] [CrossRef]
- Manjarres-López, D.P.; Andrades, M.S.; Sánchez-González, S.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Herrero-Hernández, E. Assessment of Pesticide Residues in Waters and Soils of a Vineyard Region and Its Temporal Evolution. Environ. Pollut. 2021, 284, 117463. [Google Scholar] [CrossRef]
- Dixon, J.; Garrity, D. Chapter 23. Perennial Crops and Trees Targeting the Opportunities within a Farming Systems Context. In Perennial Crops for Food Security, Proceedings of the FAO Expert Workshop, Rome, Italy, 28–30 August 2013; Batello, C., Wade, L., Cox, S., Pogna, N., Bozzini, A., Choptiany, J., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; pp. 307–324. [Google Scholar]
- Merow, C.; Smith, M.J.; Silander Jr, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography (Cop.) 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Ryszkowski, L.; Bartoszewicz, A.; Kędziora, A. Management of Matter Fluxes by Biogeochemical Barriers at the Agricultural Landscape Level. Landsc. Ecol. 1999, 14, 479–492. [Google Scholar] [CrossRef]
- Życzyńska-Bałoniak, I.; Szajdak, L.; Jaskulska, R. Impact of Biogeochemical Barriers on the Migration of Chemical Compounds with the Water of Agricultural Landscape. Polish J. Environ. Stud. 2005, 14, 671–676. [Google Scholar]
- Ryden, J.C.; Ball, P.R.; Garwood, E.A. Nitrate Leaching from Grassland. Nature 1984, 311, 50–53. [Google Scholar] [CrossRef]
- Jaguś, A. The Impact of Extensive Grazing on the Fertility of Mountain Streams on the Example of the Biała Woda Valley in the Pieniny Range (Polish Carpathians). J. Ecol. Eng. 2020, 21, 112–119. [Google Scholar] [CrossRef]
- Schmidt-Kloiber, A.; Hering, D. Www.Freshwaterecology.Info—An Online Tool That Unifies, Standardises and Codifies More than 20,000 European Freshwater Organisms and Their Ecological Preferences. Ecol. Indic. 2015, 53, 271–282. [Google Scholar] [CrossRef]
- Coulianos, C.-C.; Okland, J.; Okland, K.A. Norwegian Aquatic Bugs. Distribution and Ecology (Hemiptera-Heteroptera: Gerromorpha and Nepomorpha). Nor. J. Entomol. 2008, 55, 179–222. [Google Scholar]
- Nosek, J.N.; Vásárhelyi, T.; Bakoyi, G.; Oertel, N. Spatial pattern of water bugs (Nepomorpha, Gerromorpha) at different scales in the Szigetköz (Hungary). Biol. Bratislava 2007, 62, 345–350. [Google Scholar] [CrossRef]
- Ilie, D.M.; Olosutean, H. Structure and Seasonal Dynamics of Water Bugs Communities (Heteroptera: Nepomorpha) in Anthropic and Natural Ponds from South-Eastern Transylvania: The Role of Vegetation and Water Supply. In Advances in Environment, Ecosystems and Sustainable Tourism; Marascu-Klein, V., Panaitescu, F.V., Panaitescu, M., Eds.; WSEAS Press: Brasov, Romania, 2013; p. 213À218. [Google Scholar]
- Gligorovic, B.; Savic, A.; Protic, L.; Pešic, V. Oceanological and Hydrobiological Studies Ecological Patterns of Water Bug (Hemiptera: Heteroptera) Assemblages in Karst Springs: A Case Study from Central Montenegro. Oceanol. Hydrobiol. Stud. 2016, 45, 554–563. [Google Scholar] [CrossRef]
- EU-STAR EU-STAR. Standardization of River Classifications. Protocols. Energy, Environment and Sustainable Development Programme. 2005. Available online: http://www.eu-star.at/pdf/LatvianMacroinvertebrateSamplingProtocol.pdf (accessed on 26 January 2023).
- Cheshmedjiev, S.; Varadinova, E. Chapter 5. Demersal Macroinvertabrates. In Biological Analysis and Ecological Status Assessment of Bulgarian Surface Water Ecosystems; Belkinova, D., Gecheva, G., Cheshmedzhiev, S., Dimitrova-Dyulgerova, I., Mladenov, R., Marinov, M., Teneva, I., Stoyanov, P., Ivanov, P., Mihov, S., et al., Eds.; Plovdiv University Press: Plovdiv, Bulgaria, 2013; pp. 147–162. [Google Scholar]
- Papáček, M. On the benthic water bug Aphelocheirus aestivalis (Fabricius, 1794) (Heteroptera, Aphelocheiridae): Minireview. Entomol. Austriaca 2012, 19, 9–19. [Google Scholar]
- Manko, P. Interesujące stwierdzenia trzech rzadkich i zagrozonych merolimnicznych gatunków owadów na Słowacji. Forum Faunistyczne 2011, 1, 56–62. [Google Scholar]
- Popham, E.J. On the respiration of aquatic Hemiptera Heteroptera with special reference to the Corixidae. Proc. Zool. Soc. Lond. 1960, 135, 209–242. [Google Scholar] [CrossRef]
- Kaczmarczyk-Ziemba, A.; Krepski, T. First report on Wolbachia endosymbiosis in freshwater Aphelocheirus aestivalis (Heteroptera: Aphelocheiridae) and its potential impact on genetic diversity of host. Entomol. Sci. 2020, 23, 44–56. [Google Scholar] [CrossRef]
- Kurzatkowska, A. Preference of Micronectidae (Heteroptera: Corixidae) for Low Trophism Lakes: Data from Mazurian Lake District(Northeastern Poland). J. Entomol. Res. Soc. 2003, 5, 1–12. [Google Scholar]
- Günther, H.; Hoffmann, H.-J.; Melber, A.; Remane, R.; Simon, H.; Winkelmann, H. Rote Liste Der Wanzen (Heteroptera) (Bearbeitungsstand: 1997). In Rote Liste gefährdeter Tiere Deutschlands. Bundesamt für Naturschutz; Remane, R., Simon, H., Winkelmann, H., Eds.; Bundesamt für Naturschutz: Bonn-Bad Godesberg, Germany, 1998; pp. 235–242. [Google Scholar]
- Płaska, W. Water Bugs (Heteroptera Aquatica) as an Indicator of Ecological State of Running Watres (Preliminary Studies). Acta Agrophysica 2003, 1, 493–499. [Google Scholar]
- Whitney, R.J. The Thermal Resistance of Mayfly Nymphs from Ponds and Streams. J. Exp. Biol. 1939, 16, 374–385. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Calosi, P. Oxygen Limits Heat Tolerance and Drives Heat Hardening in the Aquatic Nymphs of the Gill Breathing Damselfly Calopteryx Virgo (Linnaeus, 1758). J. Therm. Biol. 2012, 37, 224–229. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Bilton, D.T. Respiratory Control in Aquatic Insects Dictates Their Vulnerability to Global Warming. Biol. Lett. 2013, 9, 20130473. [Google Scholar] [CrossRef]
- Verberk, W.; Sommer, U.; Davidson, R.L.; Viant, M.R. Anaerobic Metabolism at Thermal Extremes: A Metabolomic Test of the Oxygen Limitation Hypothesis in an Aquatic Insect. Integr. Comp. Biol. 2013, 53, 609–619. [Google Scholar] [CrossRef]
- Artfakta. Available online: https://artfakta.se/artbestamning (accessed on 26 January 2023).
- Havemann, N.; Gossner, M.M.; Hendrich, L.; Morinière, J.; Niedringhaus, R.; Schäfer, P.; Raupach, M.J. From water striders to water bugs: The molecular diversity of aquatic Heteroptera (Gerromorpha, Nepomorpha) of Germany based on DNA barcodes. PeerJ 2018, 6, e4577. [Google Scholar] [CrossRef]
- Linke, S.; Gifford, T.; Desjonquères, C.; Tonolla, D.; Aubin, T.; Barclay, L.; Karaconstantis, C.; Kennard, M.J.; Rybak, F.; Sueur, J. Freshwater ecoacoustics as a tool for continuous ecosystem monitoring. Front. Ecol. Environ. 2018, 16, 231–238. [Google Scholar] [CrossRef]
- Aukema, B.; Cuppen, J.G.M.; Nieser, N.; Tempelman, D. Verspreidingsatlas Nederlandse Wantsen (Hemiptera: Heteroptera). Deel I: Dipsocoromorpha, Nepomorpha, Gerromorpha & Leptopodomorpha. [Distributional atlas of Dutch true bugs (Hemiptera: Heteroptera). Vol. I: Dipsocoromorpha, Nepomorpha, Gerromorpha & Leptopodomorpha]; European Invertebrate Survey: Leiden, The Netherlands, 2002. [Google Scholar]
- Teyrovsky, V. Piispevek k Faunistice a Eko1ogii Klest’anek (Rod Sigara F.) Tekoucich Vo. Acta Univ. Palacki. Oomucensis 1960, 5, 89–165. [Google Scholar]
- Huxley, T. Provisional Atlas of the British Aquatic Bugs (Hemiptera, Heteroptera); Centre for Ecology and Hydrology Biological records Centre: Huntingdon, UK, 2003; ISBN 1870393678. [Google Scholar]
- Kovac, D. Zur Uberwinterung Der Wasserwanze Plea Minutissima Leach (Heteroptera: Pleidae): Diapause Mit Hilfe Der Plastronatmung. Nachr. Entomol. Ver. Apollo NF 1982, 3, 59–76. [Google Scholar]
- Papáček, M. Small aquatic bugs (Nepomorpha) with slight or underestimated economic importance. In Heteroptera of Economic Importance; Schaefer, C.W., Panizzi, A.R., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 591–600. [Google Scholar]

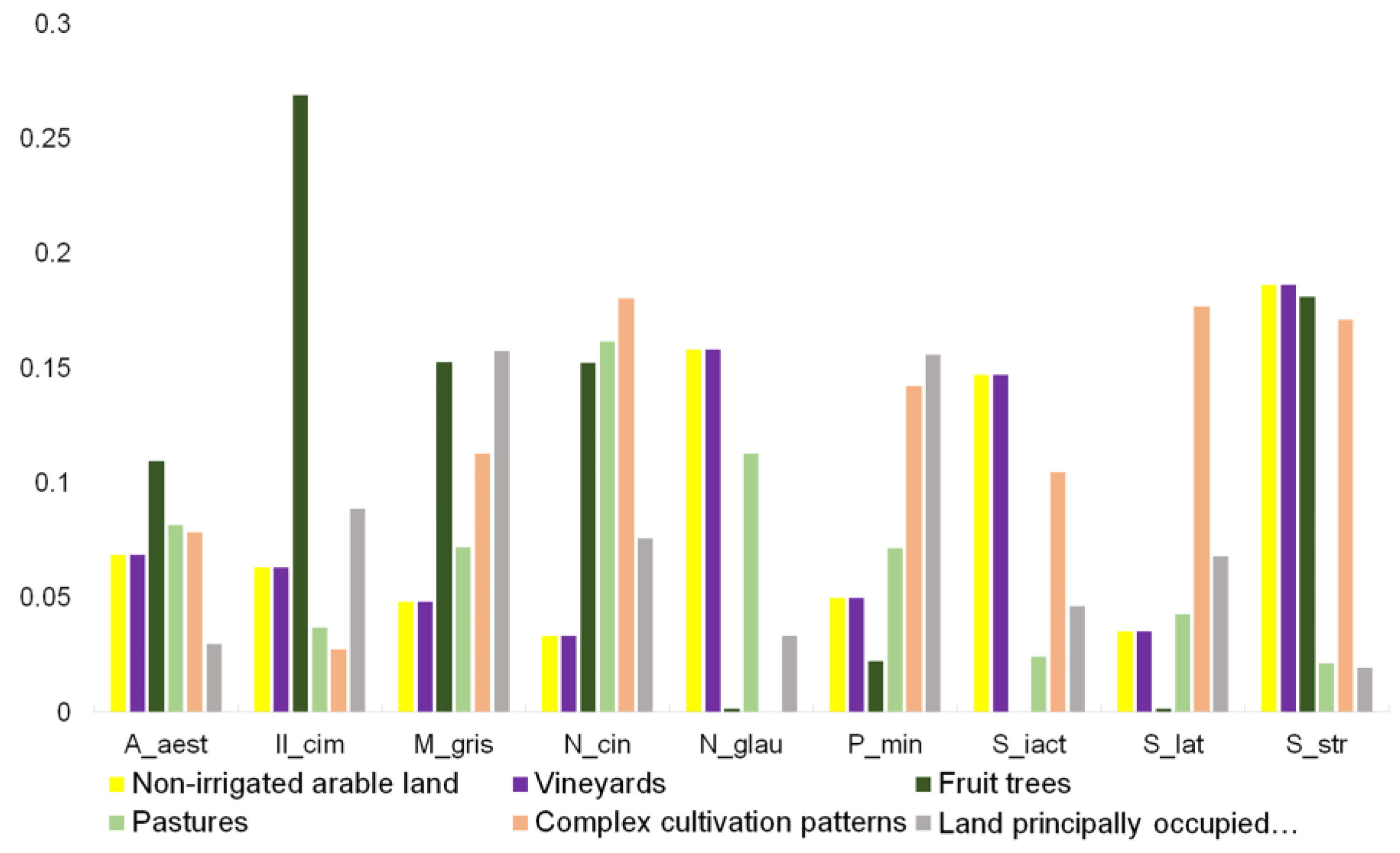

| LEVEL 1 | LEVEL 2 | LEVEL 3 |
|---|---|---|
| 2. Agricultural areas | 2.1. Arable land | 2.1.1. Non-irrigated arable land |
| 2.2. Permanent crops | 2.2.1. Vineyards | |
| 2.2.2. Fruit trees and berry plantations | ||
| 2.3. Pastures | 2.3.1. Pastures | |
| 2.4. Heterogeneous agricultural areas | 2.4.2. Complex cultivation patterns | |
| 2.4.3. Land principally occupied by agriculture, with significant areas of natural vegetation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoianova, D. Effects of Different Types of Agricultural Land Use on the Occurrence of Common Aquatic Bugs (Nepomorpha, Heteroptera) in Habitats with Slow Flowing Water in Bulgaria, Southeast Europe. Diversity 2023, 15, 292. https://doi.org/10.3390/d15020292
Stoianova D. Effects of Different Types of Agricultural Land Use on the Occurrence of Common Aquatic Bugs (Nepomorpha, Heteroptera) in Habitats with Slow Flowing Water in Bulgaria, Southeast Europe. Diversity. 2023; 15(2):292. https://doi.org/10.3390/d15020292
Chicago/Turabian StyleStoianova, Desislava. 2023. "Effects of Different Types of Agricultural Land Use on the Occurrence of Common Aquatic Bugs (Nepomorpha, Heteroptera) in Habitats with Slow Flowing Water in Bulgaria, Southeast Europe" Diversity 15, no. 2: 292. https://doi.org/10.3390/d15020292
APA StyleStoianova, D. (2023). Effects of Different Types of Agricultural Land Use on the Occurrence of Common Aquatic Bugs (Nepomorpha, Heteroptera) in Habitats with Slow Flowing Water in Bulgaria, Southeast Europe. Diversity, 15(2), 292. https://doi.org/10.3390/d15020292








