Drying Shapes Aquatic Fungal Community Assembly by Reducing Functional Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Study Area
2.2. Hydrological and Drying Stress Characterization
2.3. Aquatic Fungi Assemblages and Fungal Traits
2.4. Data Analysis
3. Results
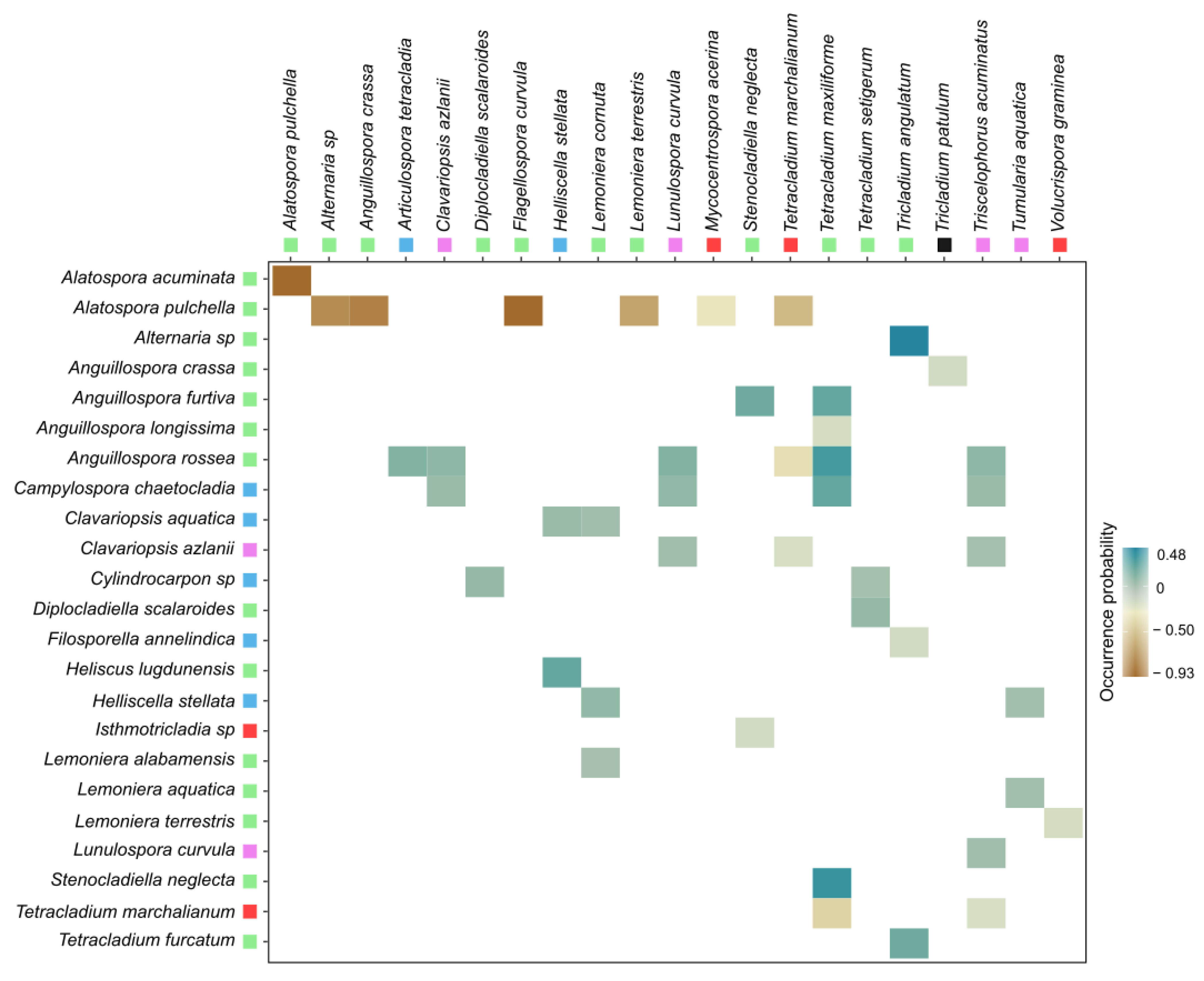
3.1. Co-occurrence Patterns of Aquatic Fungi
3.2. Functional Dissimilarity and Co-occurrence Patterns across Fungal Species
3.3. Functional Diversity and Community Assembly across the Drying Gradient
4. Discussion
4.1. Fungal Co-occurrence Patterns over the Drying Gradient
4.2. Assembly Processes of Fungal Communities over the Drying Gradient
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Grossart, H.-P.; Van den Wyngaert, S.; Kagami, M.; Wurzbacher, C.; Cunliffe, M.; Rojas-Jimenez, K. Fungi in aquatic ecosystems. Nat. Rev. Microbiol. 2019, 17, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Canhoto, C.; Gonçalves, A.L.; Bärlocher, F. Biology and ecological functions of aquatic hyphomycetes in a warming climate. Fungal Ecol. 2016, 19, 201–218. [Google Scholar] [CrossRef]
- Krauss, G.J.; Solé, M.; Krauss, G.; Schlosser, D.; Wesenberg, D.; Bärlocher, F. Fungi in freshwaters: Ecology, physiology and biochemical potential. FEMS Microbiol. Rev. 2011, 35, 620–651. [Google Scholar] [CrossRef] [PubMed]
- Arias-Real, R.; Gutiérrez-Cánovas, C.; Muñoz, I.; Pascoal, C.; Menéndez, M. Fungal biodiversity mediates the effects of drying on freshwater ecosystem functioning. Ecosystems 2022, 25, 780–794. [Google Scholar] [CrossRef]
- Manning, D.W.P.; Rosemond, A.D.; Gulis, V.; Benstead, J.P.; Kominoski, J.S. Nutrients and temperature additively increase stream microbial respiration. Glob. Change Biol. 2018, 24, e233–e247. [Google Scholar] [CrossRef]
- Kuehn, K.A. Lentic and lotic habitats as templets for fungal communities: Traits, adaptations, and their significance to litter decomposition within freshwater ecosystems. Fungal Ecol. 2016, 19, 135–154. [Google Scholar] [CrossRef]
- Arias-Real, R.; Menéndez, M.; Abril, M.; Oliva, F.; Muñoz, I. Quality and quantity of leaf litter: Both are important for feeding preferences and growth of an aquatic shredder. PLoS ONE 2018, 13, e0208272. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Chauvet, E.; Bärlocher, F.; Graça, M.A.S.; Canhoto, C. Top-down and bottom-up control of litter decomposers in streams. Freshw. Biol. 2014, 59, 2172–2182. [Google Scholar] [CrossRef]
- Grossart, H.-P.; Rojas-Jimenez, K. Aquatic fungi: Targeting the forgotten in microbial ecology. Curr. Opin. Microbiol. 2016, 31, 140–145. [Google Scholar] [CrossRef]
- Grossart, H.-P.; Hassan, E.A.; Masigol, H.; Arias-Andres, M.; Rojas-Jimenez, K. Inland water fungi in the anthropocene: Current and future perspectives. In Encyclopedia of Inland Waters, 2nd ed.; Mehner, T., Tockner, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 4, pp. 667–684. [Google Scholar] [CrossRef]
- Duarte, S.; Baerlocher, F.; Cassio, F. Biogeography of aquatic hyphomycetes: Current knowledge and future perspectives. Fungal Ecol. 2015, 19, 169–181. [Google Scholar] [CrossRef]
- Bärlocher, F. Reproduction and dispersal in aquatic hyphomycetes. Mycoscience 2009, 50, 3–8. [Google Scholar] [CrossRef]
- Skoulikidis, N.T.; Sabater, S.; Datry, T.; Morais, M.M.; Buffagni, A.; Dörflinger, G.; Zogaris, S.; del Mar Sánchez-Montoya, M.; Bonada, N.; Kalogianni, E.; et al. Non-perennial Mediterranean rivers in Europe: Status, pressures, and challenges for research and management. Sci. Total Environ. 2017, 577, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cánovas, C.; Arias-Real, R.; Bruno, D.; Cabrerizo, M.J.; González-Olalla, J.M.; Picazo, F.; Romero, F.; Sánchez-Fernández, D.; Pallarés, S. Multiple-stressors effects on Iberian freshwaters: A review of current knowledge and future research priorities. Limnetica 2022, 41, 245–268. [Google Scholar] [CrossRef]
- Messager, M.L.; Lehner, B.; Cockburn, C.; Lamouroux, N.; Pella, H.; Snelder, T.; Tockner, K.; Trautmann, T.; Watt, C.; Datry, T. Global prevalence of non-perennial rivers and streams. Nature 2021, 594, 391–397. [Google Scholar] [CrossRef]
- Scheider, A.; Jost, A.; Coulon, C.; Silvestre, M.; Théry, S.; Ducharne, A. Global-scale river network extraction based on high-resolution topography and constrained by lithology, climate, slope, and observed drainage density. Geophys. Res. Lett. 2017, 44, 2773–2781. [Google Scholar] [CrossRef]
- Döll, P.; Schmied, H.M. How is the impact of climate change on river flow regimes related to the impact on mean annual runoff? A global-scale analysis. Environ. Res. Lett. 2012, 7, 014037. [Google Scholar] [CrossRef]
- Maynard, D.S.; Bradford, M.A.; Covey, K.R.; Lindner, D.; Glaeser, J.; Talbert, D.A.; Tinker, P.J.; Walker, D.M.; Crowther, T.W. Consistent trade-offs in fungal trait expression across broad spatial scales. Nat. Microbiol. 2019, 4, 846–853. [Google Scholar] [CrossRef]
- Crowther, T.W.; Maynard, D.S.; Crowther, T.R.; Peccia, J.; Smith, J.R.; Bradford, M.A. Untangling the fungal niche: The trait-based approach. Front. Microbiol. 2014, 5, 579. [Google Scholar] [CrossRef]
- Battin, T.J.; Besemer, K.; Bengtsson, M.M.; Romani, A.M.; Packmann, A.I. The ecology and biogeochemistry of stream biofilms. Nat. Rev. Microbiol. 2016, 14, 251–263. [Google Scholar] [CrossRef]
- Duarte, S.; Mora-Gómez, J.; Romanı, A.M. Responses of microbial decomposers to drought in streams may depend on the environmental context. Environ. Microbiol. Rep. 2017, 9, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Gionchetta, G.; Oliva, F.; Menéndez, M.; Lopez, P.; Anna, L. Key role of streambed moisture and flash storms for microbial resistance and resilience to long-term drought. Freshw. Biol. 2019, 64, 306–322. [Google Scholar] [CrossRef]
- Coleine, C.; Stajich, J.E.; Selbmann, L. Fungi are key players in extreme ecosystems. Trends Ecol. Evol. 2022, 37, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Gionchetta, G.; Artigas, J.; Arias-Real, R.; Oliva, F.; Romaní, A.M. Multi-model assessment of hydrological and environmental impacts on streambed microbes in Mediterranean catchments. Environ. Microbiol. 2020, 22, 2213–2229. [Google Scholar] [CrossRef] [PubMed]
- Chauvet, E.; Cornut, J.; Sridhar, K.R.; Selosse, M.A.; Bärlocher, F. Beyond the water column: Aquatic hyphomycetes outside their preferred habitat. Fungal Ecol. 2016, 19, 112–127. [Google Scholar] [CrossRef]
- Selosse, M.-A.; Vohník, M.; Chauvet, E. Out of the rivers: Are some aquatic hyphomycetes plant endophytes? New Phytol. 2008, 178, 3–7. [Google Scholar] [CrossRef]
- Sridhar, K.R. Fungi in the tree canopy—An appraisal. In Applies Mycology; Rai, M., Bridge, P., Eds.; CAB International: London, UK, 2009; pp. 73–91. [Google Scholar] [CrossRef]
- Ghate, S.D.; Sridhar, K.R. Diversity of aquatic hyphomycetes in streambed sediments of temporary streamlets of Southwest India. Fungal Ecol. 2015, 14, 53–61. [Google Scholar] [CrossRef]
- Cornut, J.; Chauvet, E.; Mermillod-Blondin, F.; Assemat, F.; Elger, A. Aquatic Hyphomycete Species Are Screened by the Hyporheic Zone of Woodland Streams. Appl. Environ. Microbiol. 2014, 80, 1949–1960. [Google Scholar] [CrossRef]
- Concostrina-Zubiri, L.; Prieto, M.; Hurtado, P.; Escudero, A.; Martínez, I. Functional diversity regulates the effects of habitat degradation on biocrust phylogenetic and taxonomic diversities. Ecol. Appl. 2022, 32, e2599. [Google Scholar] [CrossRef]
- Horner-Devine, M.C.; Silver, J.M.; Leibold, M.A.; Bohannan, B.J.M.; Colwell, R.K.; Fuhrman, J.A.; Green, J.L.; Kuske, C.R.; Martiny, J.B.; Muyzer, G.; et al. A Comparison of taxon co-occurrence patterns from macro- and microorganisms. Ecology 2007, 88, 1345–1353. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Li, Y.; Shipley, B. Community divergence and convergence along experimental gradients of stress and disturbance. Ecology 2018, 99, 775–781. [Google Scholar] [CrossRef]
- Gutiérrez-Cánovas, C.; Sánchez-Fernández, D.; Cañedo-Argüelles, M.; Millán, A.; Velasco, J.; Acosta, R.; Fortuño, P.; Otero, N.; Soler, A.; Bonada, N. Do all roads lead to Rome? Exploring community trajectories in response to anthropogenic salinization and dilution of rivers. Phil. Trans. R. Soc. B. 2019, 374, 20180009. [Google Scholar] [CrossRef]
- Maestre, F.T.; Callaway, R.M.; Valladares, F.; Lortie, C.J. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 2009, 97, 199–205. [Google Scholar] [CrossRef]
- Hammarlund, S.P.; Harcombe, W.R. Refining the stress gradient hypothesis in a microbial community. Proc. Natl. Acad. Sci. USA 2019, 116, 15760–15762. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Nemergut, D.R.; Pu, Z.; Jiang, L. Phylogenetic limiting similarity and competitive exclusion. Ecol. Lett. 2011, 14, 782–787. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of Maintenance of Species Diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Schwilk, D.W.; Ackerly, D.D. A trait-based test for habitat filtering: Convex hull volume. Ecology 2006, 87, 1465–1471. [Google Scholar] [CrossRef]
- Graça, D.; Fernandes, I.; Cássio, F.; Pascoal, C. Eco-physiological responses of aquatic fungi to three global change stressors highlight the importance of intraspecific trait variability. Microb. Ecol. 2022. [Google Scholar] [CrossRef]
- Duarte, S.; Pascoal, C.; Cássio, F.; Bärlocher, F. Aquatic hyphomycete diversity and identity affect leaf litter decomposition in microcosms. Oecologia 2006, 147, 658–666. [Google Scholar] [CrossRef]
- Arias-Real, R.; Gutiérrez-Cánovas, C.; Menéndez, M.; Granados, V.; Muñoz, I. Diversity mediates the responses of invertebrate density to duration and frequency of rivers’ annual drying regime. Oikos 2021, 130, 2148–2160. [Google Scholar] [CrossRef]
- Graça, M.A.S.; Bärlocher, F.; Gessner, M.O. Methods to Study Litter Decomposition: A Practical Guide; Springer: Berlin, Germany, 2005. [Google Scholar] [CrossRef]
- Sati, S.C.; Pathak, R. New root endophytic water borne conidial fungi from Kumaun Himalaya. Curr. Bot. 2017, 8, 12–16. [Google Scholar] [CrossRef]
- Thakur, S.B. Survival of Some Aquatic Hyphomycetes under Dry Conditions. Mycologia 1977, 69, 843–845. [Google Scholar] [CrossRef]
- Sati, S.C.; Belwal, M. Aquatic hyphomycetes as endophytes of riparian plant roots. Mycologia 2005, 97, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Gulis, V.; Marvanová, L.; Descals, E. An Illustrated Key to the Common Temperate Species of Aquatic Hyphomycetes BT. In Methods to Study Litter Decomposition: A Practical Guide; Graça, M.A.S., Bärlocher, F., Gessner, M.O., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 153–167. [Google Scholar] [CrossRef]
- Sanpera-Calbet, I.; Ylla, I.; Romaní, A.M.; Sabater, S.; Muñoz, I. Drought effects on resource quality in a Mediterranean stream: Fatty acids and sterols as indicators. Limnetica 2017, 36, 29–43. [Google Scholar] [CrossRef]
- Bärlocher, F. Effects of drying and freezing autumn leaves on leaching and colonization by aquatic hyphomycetes. Freshw. Biol. 1992, 28, 1–7. [Google Scholar] [CrossRef]
- Põlme, S.; Abarenkov, K.; Henrik Nilsson, R.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Boonmee, S.; Wanasinghe, D.N.; Calabon, M.S.; Huanraluek, N.; Chandrasiri, S.K.U.; Jones, G.E.B.; Rossi, W.; Leonardi, M.; Singh, S.K.; Rana, S.; et al. Fungal diversity notes 1387–1511: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2021, 111, 1–335. [Google Scholar] [CrossRef]
- Kitching, R.L. An ecological study of water-filled tree-holes and their position in the woodland ecosystem. J. Anim. Ecol. 1971, 40, 281–302. [Google Scholar] [CrossRef]
- Gönczöl, J.; Révay, Á. Treehole fungal communities: Aquatic, aero-aquatic and dematiaceous hyphomycetes. Fungal Divers. 2003, 12, 19–34. Available online: https://www.fungaldiversity.org/fdp/sfdp/FD12-19-34.pdf (accessed on 10 December 2022).
- Koivusaari, P.; Tejesvi, M.V.; Tolkkinen, M.; Markkola, A.; Mykrä, H.; Pirttilä, A.M. Fungi originating from tree leaves contribute to fungal diversity of litter in streams. Front. Microbiol. 2019, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Leroy, C.J.; Fischer, D.G.; Halstead, K.; Pryor, M.; Bailey, J.K.; Schweitzer, J.A. A fungal endophyte slows litter decomposition in streams. Freshw. Biol. 2011, 56, 1426–1433. [Google Scholar] [CrossRef]
- Veech, J.A. A probabilistic model for analysing species co-occurrence. Glob. Ecol. Biogeogr. 2013, 22, 252–260. [Google Scholar] [CrossRef]
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. cooccur: Probabilistic Species Co-Occurrence Analysis in R. J. Stat. Softw. Code Snippets 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Arias-Real, R.; Menéndez, M.; Muñoz, I.; Pascoal, C. Drying shapes the ecological niche of aquatic fungi with implications on ecosystem functioning. Sci. Total Environ. 2022, 859, 160374. [Google Scholar] [CrossRef]
- Dolédec, S.; Chessel, D.; Gimaret-Carpentier, C. Niche separation in community analysis: A new method. Ecology 2000, 81, 2914–2927. [Google Scholar] [CrossRef]
- Pavoine, S.; Vallet, J.; Dufour, A.-B.; Gachet, S.; Daniel, H. On the challenge of treating various types of variables: Application for improving the measurement of functional diversity. Oikos 2009, 118, 391–402. [Google Scholar] [CrossRef]
- Maire, E.; Grenouillet, G.; Brosse, S.; Villéger, S. How many dimensions are needed to accurately assess functional diversity? A pragmatic approach for assessing the quality of functional spaces. Glob. Ecol. Biogeogr. 2015, 24, 728–740. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Arias-Real, R.; Gutiérrez-Cánovas, C.; Menéndez, M.; Muñoz, I. Drying niches of aquatic macroinvertebrates identify potential biomonitoring indicators in intermittent and ephemeral streams. Ecol. Indic. 2022, 142, 109263. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Fox, J.W. Interpreting the ‘selection effect’ of biodiversity on ecosystem function. Ecol. Lett. 2005, 8, 846–856. [Google Scholar] [CrossRef]
- Melbinger, A.; Vergassola, M. The Impact of Environmental Fluctuations on Evolutionary Fitness Functions. Sci. Rep. 2015, 5, 15211. [Google Scholar] [CrossRef]
- Gulis, V. Are there any substrate preferences in aquatic hyphomycetes? Mycol. Res. 2001, 105, 1088–1093. [Google Scholar] [CrossRef]
- Thomas, K.; Chilvers, G.A.; Norris, R.H. Aquatic hyphomycetes from different substrates: Substrate preference and seasonal occurrence. Mar. Freshw. Res. 1992, 43, 491–509. [Google Scholar] [CrossRef]
- Denelle, P.; Violle, C.; Consortium, D.; Munoz, F. Generalist plants are more competitive and more functionally similar to each other than specialist plants: Insights from network analyses. J. Biogeogr. 2020, 47, 1922–1933. [Google Scholar] [CrossRef]
- Morris, D.W. Coexistence of specialist and generalist rodents via habitat selection. Ecology 1996, 77, 2352–2364. [Google Scholar] [CrossRef]
- Badyaev, A.V. Stress-induced variation in evolution: From behavioural plasticity to genetic assimilation. Proc. R. Soc. B Biol. Sci. 2005, 272, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Arribas, P.; Gutiérrez-Cánovas, C.; Botella-Cruz, M.; Cañedo-Argüelles, M.; Carbonell, J.A.; Millán, A.; Pallarés, S.; Velasco, J.; Sánchez-Fernández, D. Insect communities in saline waters consist of realised but not fundamental niche specialists. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180008. [Google Scholar] [CrossRef]
- Gutiérrez-Cánovas, C.; Millán, A.; Velasco, J.; Vaughan, I.P.; Ormerod, S.J. Contrasting effects of natural and anthropogenic stressors on beta diversity in river organisms. Glob. Ecol. Biogeogr. 2013, 22, 796–805. [Google Scholar] [CrossRef]
- Herbst, D.B. Gradients of salinity stress, environmental stability and water chemistry as a templet for defining habitat types and physiological strategies in inland salt waters. Hydrobiologia 2001, 466, 209–219. [Google Scholar] [CrossRef]
- Liancourt, P.; Callaway, R.M.; Michalet, R. Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology 2005, 86, 1611–1618. [Google Scholar] [CrossRef]
- Noel, L.; Bärlocher, F.; Culp, J.M.; Seena, S. Nutrient enrichment and flow regulation impair structure and function of a large river as revealed by aquatic hyphomycete species richness, biomass, and decomposition rates. Freshw. Sci. 2016, 35, 1148–1163. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Lírio, A.V.; Graça, M.A.S.; Canhoto, C. Fungal species diversity affects leaf decomposition after drought. Int. Rev. Hydrobiol. 2016, 101, 78–86. [Google Scholar] [CrossRef]
- Sommer, B.; Harrison, P.L.; Beger, M.; Pandolfi, J.M. Trait-mediated environmental filtering drives assembly at biogeographic transition zones. Ecology 2014, 95, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Soliveres, S.; Maestre, F.T.; Bowker, M.A.; Torices, R.; Quero, J.L.; Garcia-Gomez, M.; Cabrera, O.; Cea, A.P.; Coaguila, D.; Eldridge, D.J.; et al. Functional traits determine plant co-occurrence more than environment or evolutionary relatedness in global drylands. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 164–173. [Google Scholar] [CrossRef]
- Medeiros, A.O.; Pascoal, C.; Graça, M.A.S. Diversity and activity of aquatic fungi under low oxygen conditions. Freshw. Biol. 2009, 54, 142–149. [Google Scholar] [CrossRef]
- Duarte, S.; Cássio, F.; Pascoal, C. Environmental drivers are more important for structuring fungal decomposer communities than the geographic distance between streams. Limnetica 2017, 36, 491–506. [Google Scholar] [CrossRef]
- Gulis, V.; Su, R.; Kuehn, K.A. Fungal decomposers in freshwater environments. In The Structure and Function of Aquatic Microbial Communities; Springer: Berlin, Germany, 2019; pp. 121–155. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Diversity, taxonomy and phylogeny of the Fungi. Biol. Rev. 2019, 94, 2101–2137. [Google Scholar] [CrossRef]
- Kakumanu, M.L.; Cantrell, C.L.; Williams, M.A. Microbial community response to varying magnitudes of desiccation in soil: A test of the osmolyte accumulation hypothesis. Soil Biol. Biochem. 2013, 57, 644–653. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Menezes, B.S.; Martins, F.R.; Dantas Carvalho, E.C.; Souza, B.C.; Silveira, A.P.; Loiola, M.I.B.; Araújo, F.S. Assembly rules in a resource gradient: Competition and abiotic filtering determine the structuring of plant communities in stressful environments. PLoS ONE. 2020, 15, e0230097. [Google Scholar] [CrossRef] [PubMed]
- Le Bagousse-Pinguet, Y.; Gross, N.; Maestre, F.T.; Maire, V.; de Bello, F.; Fonseca, C.R.; Kattge, J.; Valencia, E.; Leps, J.; Liancourt, P. Testing the environmental filtering concept in global drylands. J. Ecol. 2017, 105, 1058–1069. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; De Bello, F.; Quétier, F.; Grigulis, K.; Robson, T.M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef]
- Flynn, D.F.B.; Gogol-Prokurat, M.; Nogeire, T.; Molinari, N.; Richers, B.T.; Lin, B.B.; Simpson, N.; Mayfield, M.M.; DeClerck, F. Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 2009, 12, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Giaramida, L.; Reich, P.B.; Khachane, A.N.; Hamonts, K.; Edwards, C.; Lawton, L.A.; Singh, B.K. Lack of functional redundancy in the relationship between microbial diversity and ecosystem functioning. J. Ecol. 2016, 104, 936–946. [Google Scholar] [CrossRef]
- Mori, A.S.; Isbell, F.; Fujii, S.; Makoto, K.; Matsuoka, S.; Osono, T. Low multifunctional redundancy of soil fungal diversity at multiple scales. Ecol. Lett. 2016, 19, 249–259. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- Granados, V.; Gutiérrez-Cánovas, C.; Arias-Real, R.; Obrador, B.; Harjung, A.; Butturini, A. The interruption of longitudinal hydrological connectivity causes delayed responses in dissolved organic matter. Sci. Total Environ. 2020, 713, 136619. [Google Scholar] [CrossRef]
- Li, Y.; Ge, Y.; Wang, J.; Shen, C.; Wang, J.; Liu, Y.-J. Functional redundancy and specific taxa modulate the contribution of prokaryotic diversity and composition to multifunctionality. Mol. Ecol. 2021, 30, 2915–2930. [Google Scholar] [CrossRef]


| Trait | Category | Relationship | Drying Effect | References |
|---|---|---|---|---|
| Primary lifestyle | Litter saprotrophic | − | Drying causes early leaf abscission affecting their basal resource | [50,51,52] |
| Wood saprotrophic | + | |||
| Plant pathogenic | + | |||
| Mycoparasite | + | |||
| Decay substrate | Litter | − | ||
| Root | + | |||
| Wood | + | |||
| Habitat | Aquatic | − | Drying favors non-aquatic fungi | [52,53] |
| Non-aquatic | + | |||
| Tree-holes | Yes | + | Tree-holes offer an alternative aquatic habitat during drying | [28,54,55] |
| No | − | |||
| Endophyte capacity | Yes | + | Capacity to inhabit plant roots or leaves provides independence from drying | [26,27,56,57] |
| No | − | |||
| Conidia shape | Branched | − | Conidia/spore shape determines fungal dispersal ability within streambed sediments, which allows them to survive during the dry phase and become physiologically active in the wet phase | [13,26,29,30] |
| Tetraradiate | − | |||
| Filiform | + | |||
| Sigmoid | − | |||
| Compact | − | |||
| Clove-shaped | − | |||
| Spore shape | Ascospores | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Real, R.; Hurtado, P.; Gionchetta, G.; Gutiérrez-Cánovas, C. Drying Shapes Aquatic Fungal Community Assembly by Reducing Functional Diversity. Diversity 2023, 15, 289. https://doi.org/10.3390/d15020289
Arias-Real R, Hurtado P, Gionchetta G, Gutiérrez-Cánovas C. Drying Shapes Aquatic Fungal Community Assembly by Reducing Functional Diversity. Diversity. 2023; 15(2):289. https://doi.org/10.3390/d15020289
Chicago/Turabian StyleArias-Real, Rebeca, Pilar Hurtado, Giulia Gionchetta, and Cayetano Gutiérrez-Cánovas. 2023. "Drying Shapes Aquatic Fungal Community Assembly by Reducing Functional Diversity" Diversity 15, no. 2: 289. https://doi.org/10.3390/d15020289
APA StyleArias-Real, R., Hurtado, P., Gionchetta, G., & Gutiérrez-Cánovas, C. (2023). Drying Shapes Aquatic Fungal Community Assembly by Reducing Functional Diversity. Diversity, 15(2), 289. https://doi.org/10.3390/d15020289







