Consistent Nest Site Selection by Turtles across Habitats with Varying Levels of Human Disturbance
Abstract
1. Introduction
2. Methods
2.1. Field Data Collection
2.2. Nest Predation Experiment
2.3. Quantifying Urbanization
2.4. Statistical Analysis
3. Results
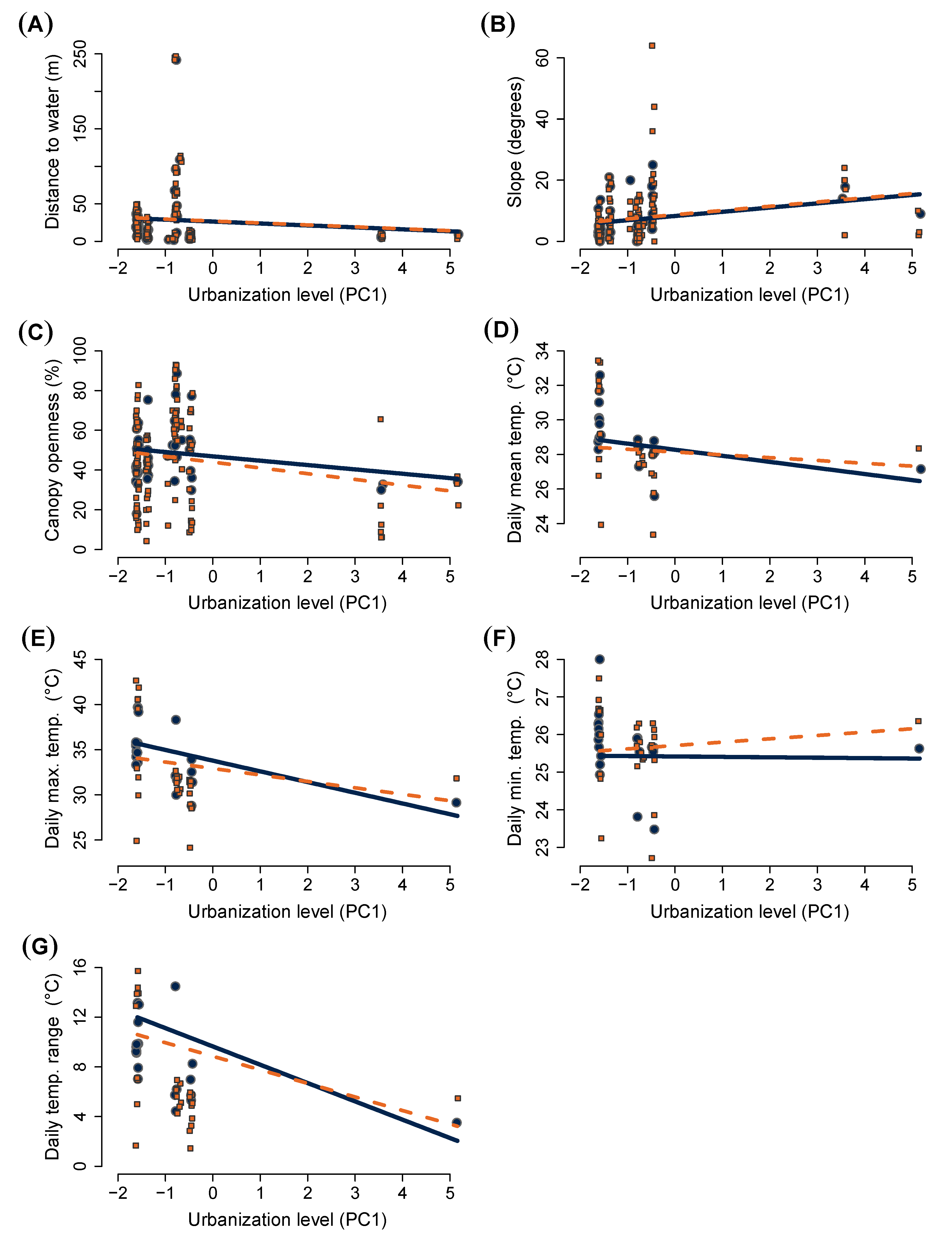
3.1. Nest Site Choice
3.2. Nest Predation Experiment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKinney, M.L. The Impacts of Urbanization on Native Species Are Poorly Studied, but Educating a Highly Urbanized Human Population about These Impacts Can Greatly Improve Species Conservation in All Ecosystems. BioScience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- Mcdonald, R.I.; Kareiva, P.; Forman, R.T.T. The Implications of Current and Future Urbanization for Global Protected Areas and Biodiversity Conservation. Biol. Conserv. 2008, 141, 1695–1703. [Google Scholar] [CrossRef]
- Pauchard, A.; Aguayo, M.; Peña, E.; Urrutia, R. Multiple Effects of Urbanization on the Biodiversity of Developing Countries: The Case of a Fast-Growing Metropolitan Area (Concepción, Chile). Biol. Conserv. 2006, 127, 272–281. [Google Scholar] [CrossRef]
- Czech, B.; Krausman, P.R.; Devers, P.K. Economic Associations among Causes of Species Endangerment in the United States. BioScience 2000, 50, 593. [Google Scholar] [CrossRef]
- Isaksson, C. Urbanization, Oxidative Stress and Inflammation: A Question of Evolving, Acclimatizing or Coping with Urban Environmental Stress. Funct. Ecol. 2015, 29, 913–923. [Google Scholar] [CrossRef]
- Alberti, M.; Correa, C.; Marzluff, J.M.; Hendry, A.P.; Palkovacs, E.P.; Gotanda, K.M.; Hunt, V.M.; Apgar, T.M.; Zhou, Y. Global Urban Signatures of Phenotypic Change in Animal and Plant Populations. Proc. Natl. Acad. Sci. USA 2017, 114, 8951–8956. [Google Scholar] [CrossRef]
- Szulkin, M.; Munshi-South, J.; Charmantier, A. Urban Evolutionary Biology; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Ritzel, K.; Gallo, T. Behavior Change in Urban Mammals: A Systematic Review. Front. Ecol. Evol. 2020, 8, 576665. [Google Scholar] [CrossRef]
- Kolbe, J.J.; Janzen, F.J. Impact of Nest-Site Selection on Nest Success and Nest Temperature in Natural and Disturbed Habitats. Ecology 2002, 83, 269–281. [Google Scholar] [CrossRef]
- Buxton, V.L.; Santymire, R.M.; Benson, T.J. Mixed Effects of Urbanization on Density, Nest Survival, and Nestling Corticosterone of a Generalist Passerine. Ecosphere 2018, 9, e02517. [Google Scholar] [CrossRef]
- Hope, S.F.; Hopkins, W.A.; Angelier, F. Parenting in the City: Effects of Urbanization on Incubation Behaviour and Egg Temperature in Great Tits, Parus major. Anim. Behav. 2022, 194, 1–11. [Google Scholar] [CrossRef]
- Angilletta, M.J., Jr.; Winters, R.S.; Dunham, A.E. Thermal Effects on the Energetics of Lizard Embryos: Implications for Hatchling Phenotypes. Ecology 2000, 81, 2957–2968. [Google Scholar] [CrossRef]
- Christian, K.A.; Tracy, C.R.; Porter, W.P. The Effect of Cold Exposure during Incubation of Sceloporus undulatus Eggs. Copeia 1986, 1986, 1012–1014. [Google Scholar] [CrossRef]
- Muth, A. Physiological Ecology of Desert Iguana (Dipsosaurus dorsalis) Eggs: Temperature and Water Relations. Ecology 1980, 61, 1335–1343. [Google Scholar] [CrossRef]
- Bodensteiner, B.L.; Mitchell, T.S.; Strickland, J.T.; Janzen, F.J. Hydric Conditions during Incubation Influence Phenotypes of Neonatal Reptiles in the Field. Funct. Ecol. 2015, 29, 710–717. [Google Scholar] [CrossRef]
- Bowne, D.R.; Cosentino, B.J.; Anderson, L.J.; Bloch, C.P.; Cooke, S.; Crumrine, P.W.; Dallas, J.; Doran, A.; Dosch, J.J.; Druckenbrod, D.L.; et al. Effects of Urbanization on the Population Structure of Freshwater Turtles across the United States. Conserv. Biol. 2018, 32, 1150–1161. [Google Scholar] [CrossRef]
- Jackson, N.; Cristescu, R.H.; Piza-Roca, C.; Littleford-Colquhoun, B.L.; Strickland, K.; Frère, C.H. Maternal Nesting Behaviour in City Dragons: A Species with Temperature-Dependent Sex Determination. J. Urban Ecol. 2019, 5, juz005. [Google Scholar] [CrossRef]
- Wilcove, D.S. Nest Predation in Forest Tracts and the Decline of Migratory Songbirds. Ecology 1985, 66, 1211–1214. [Google Scholar] [CrossRef]
- Jokimäki, J.; Huhta, E. Artificial Nest Predation and Abundance of Birds Along an Urban Gradient. Condor 2000, 102, 838–847. [Google Scholar] [CrossRef]
- Feinberg, J.A.; Burke, R.L. Nesting Ecology and Predation of Diamondback Terrapins, Malaclemys terrapin, at Gateway National Recreation Area, New York. J. Herpetol. 2003, 37, 517–526. [Google Scholar] [CrossRef]
- Marchand, M.N.; Litvaitis, J.A. Effects of Landscape Composition, Habitat Features, and Nest Distribution on Predation Rates of Simulated Turtle Nests. Biol. Conserv. 2004, 117, 243–251. [Google Scholar] [CrossRef]
- Foley, S.M.; Price, S.J.; Dorcas, M.E. Nest-Site Selection and Nest Depredation of Semi-Aquatic Turtles on Golf Courses. Urban Ecosyst. 2012, 15, 489–497. [Google Scholar] [CrossRef]
- Prange, S.; Gehrt, S.D.; Wiggers, E.P. Influences of Anthropogenic Resources on Raccoon (Procyon lotor) Movements and Spatial Distribution. J. Mammal. 2004, 85, 483–490. [Google Scholar] [CrossRef]
- Vincze, E.; Seress, G.; Lagisz, M.; Nakagawa, S.; Dingemanse, N.J.; Sprau, P. Does Urbanization Affect Predation of Bird Nests? A Meta-Analysis. Front. Ecol. Evol. 2017, 5, 29. [Google Scholar] [CrossRef]
- Eötvös, C.B.; Magura, T.; Lövei, G.L. A Meta-Analysis Indicates Reduced Predation Pressure with Increasing Urbanization. Landsc. Urban Plan. 2018, 180, 54–59. [Google Scholar] [CrossRef]
- Brown, G.P.; Shine, R. Maternal Nest-Site Choice and Offspring Fitness in a Tropical Snake (Tropidonophis mairii, Colubridae). Ecology 2004, 85, 1627–1634. [Google Scholar] [CrossRef]
- Wilson, D.S. Nest-Site Selection: Microhabitat Variation and Its Effects on the Survival of Turtle Embryos. Ecology 1998, 79, 1884–1892. [Google Scholar] [CrossRef]
- Warner, D.A.; Andrews, R.M. Nest-site Selection in Relation to Temperature And Moisture by the Lizard Sceloporus undulatus. Herpetologica 2002, 58, 399–407. [Google Scholar] [CrossRef]
- Pruett, J.E.; Addis, E.A.; Warner, D.A. The Influence of Maternal Nesting Behaviour on Offspring Survival: Evidence from Correlational and Cross-Fostering Studies. Anim. Behav. 2019, 153, 15–24. [Google Scholar] [CrossRef]
- Warner, D.A.; Shine, R. Maternal Nest-Site Choice in a Lizard with Temperature-Dependent Sex Determination. Anim. Behav. 2008, 75, 861–870. [Google Scholar] [CrossRef]
- Refsnider, J.M.; Janzen, F.J. Putting Eggs in One Basket: Ecological and Evolutionary Hypotheses for Variation in Oviposition-Site Choice. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 39–57. [Google Scholar] [CrossRef]
- Rand, A.S.; Dugan, B. Structure of Complex Iguana Nests. Copeia 1983, 1983, 705–711. [Google Scholar] [CrossRef]
- Madsen, T.; Shine, R. Life History Consequences of Nest-Site Variation in Tropical Pythons (Liasis fuscus). Ecology 1999, 80, 989–997. [Google Scholar] [CrossRef]
- Spencer, R.J. Experimentally Testing Nest Site Selection: Fitness Trade-Offs and Predation Risk in Turtles. Ecology 2002, 83, 2136–2144. [Google Scholar] [CrossRef]
- Ogden, J.C. Nesting by Wood Storks in Natural, Altered, and Artificial Wetlands in Central and Northern Florida. Colonial Waterbirds 1991, 14, 39–45. [Google Scholar] [CrossRef]
- Borges, F.J.A.; Marini, M.Â. Birds Nesting Survival in Disturbed and Protected Neotropical Savannas. Biodivers. Conserv. 2010, 19, 223–236. [Google Scholar] [CrossRef]
- James Reynolds, S.; Ibáñez-Álamo, J.D.; Sumasgutner, P.; Mainwaring, M.C. Urbanisation and Nest Building in Birds: A Review of Threats and Opportunities. J. Ornithol. 2019, 160, 841–860. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, Z.; Wang, B. Effect of Urbanization Intensity on Nest-Site Selection by Eurasian Magpies (Pica pica). Urban Ecosyst. 2020, 23, 1099–1105. [Google Scholar] [CrossRef]
- Turtle Conservation Coalition; Rhodin, A.G.J.; Walde, A.D.; Horne, B.D.; van Dijk, P.P.; Blanck, T.; Hudson, R. Turtles in Trouble: The World’s 25+ Most Endangered Tortoises and Freshwater Turtles—2011; IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, Turtle Conservation Fund, Turtle Survival Alliance, Turtle Conservancy, Chelonian Research Foundation, Conservation International, Wildlife Conservation Society, and San Diego Zoo Global: Lunenburg, MA, USA, 2011. [Google Scholar]
- Congdon, J.D.; Dunham, A.E.; Sels, R.C.V.L. Delayed Sexual Maturity and Demographics of Blanding’s Turtles (Emydoidea blandingii): Implications for Conservation and Management of Long-Lived Organisms. Conserv. Biol. 1993, 7, 826–833. [Google Scholar] [CrossRef]
- Congdon, J.D.; Tinkle, D.W.; Breitenbach, G.L.; van Loben Sels, R.C. Nesting Ecology and Hatching Success in the Turtle Emydoidea blandingi. Herpetologica 1983, 39, 417–429. [Google Scholar]
- Schwanz, L.E.; Spencer, R.J.; Bowden, R.M.; Janzen, F.J. Climate and Predation Dominate Juvenile and Adult Recruitment in a Turtle with Temperature-Dependent Sex Determination. Ecology 2010, 91, 3016–3026. [Google Scholar] [CrossRef]
- Refsnider, J.M.; Reedy, A.M.; Warner, D.A.; Janzen, F.J. Do Trade-Offs between Predation Pressures on Females versus Nests Drive Nest-Site Choice in Painted Turtles? Biol. J. Linn. Soc. 2015, 116, 847–855. [Google Scholar] [CrossRef]
- Vanek, J.P.; Glowacki, G.A. Assessing the Impacts of Urbanization on Sex Ratios of Painted Turtles (Chrysemys picta). Diversity 2019, 11, 72. [Google Scholar] [CrossRef]
- Anđelković, M.; Bogdanović, N. Amphibian and Reptile Road Mortality in Special Nature Reserve Obedska Bara, Serbia. Animals 2022, 12, 561. [Google Scholar] [CrossRef]
- Patrick, D.A.; Gibbs, J.P. Population Structure and Movements of Freshwater Turtles across a Road-Density Gradient. Landsc. Ecol. 2010, 25, 791–801. [Google Scholar] [CrossRef]
- Steen, D.A.; Gibbs, J.P. Effects of Roads on the Structure of Freshwater Turtle Populations. Conserv. Biol. 2004, 18, 1143–1148. [Google Scholar] [CrossRef]
- Witherington, B.E. Behavioral Responses of Nesting Sea Turtles to Artificial Lighting. Herpetologica 1992, 48, 31–39. [Google Scholar]
- Steen, D.A.; Aresco, M.J.; Beilke, S.G.; Compton, B.W.; Condon, E.P.; Kenneth Dodd Jr., C.; Forrester, H.; Gibbons, J.W.; Greene, J.L.; Johnson, G.; et al. Relative Vulnerability of Female Turtles to Road Mortality. Anim. Conserv. 2006, 9, 269–273. [Google Scholar] [CrossRef]
- Tiatragul, S.; Hall, J.M.; Warner, D.A. Nestled in the City Heat: Urban Nesting Behavior Enhances Embryo Development of an Invasive Lizard. J. Urban Ecol. 2020, 6, juaa001. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; van Dijk, P.P.; Rhodin, A.G.J.; Nash, S.D. Turtle Hotspots: An Analysis of the Occurrence of Tortoises and Freshwater Turtles in Biodiversity Hotspots, High-Biodiversity Wilderness Areas, and Turtle Priority Areas. Chelonian Conserv. Biol. 2015, 14, 2–10. [Google Scholar] [CrossRef]
- Najbar, B.; Szuszkiewicz, E. Nest-Site Fidelity of the European Pond Turtle Emys orbicularis (LINNAEUS, 1758) (Testudines: Emydidae) in Western Poland. Acta Zool. Crac.-Ser. A Vertebr. 2007, 50, 1–8. [Google Scholar] [CrossRef]
- Roosenburg, W.M. Maternal Condition and Nest Site Choice: An Alternative for the Maintenance of Environmental Sex Determination? Am. Zool. 1996, 36, 157–168. [Google Scholar] [CrossRef]
- Doody, J.S.; Guarino, E.N.Z.O.; Harlow, P.; Corey, B. Quantifying Nest Site Choice in Reptiles Using Hemispherical Photography and Gap Light Analysis. Herpetol. Rev. 2006, 37, 49–51. [Google Scholar]
- Wilhoft, D.C.; Del Baglivo, M.G.; Del Baglivo, M.D. Observations on Mammalian Prediation of Snapping Turtle Nests (Reptilia, Testudines, Chelydridae). J. Herpetol. 1979, 13, 435–438. [Google Scholar] [CrossRef]
- Marchand, M.N.; Litvaitis, J.A.; Maier, T.J.; DeGraaf, R.M. Use of Artificial Nests to Investigate Predation on Freshwater Turtle Nests. Wildl. Soc. Bull. 2002, 30, 1092–1098. [Google Scholar]
- Ratnaswamy, M.J.; Warren, R.J.; Kramer, M.T.; Adam, M.D. Comparisons of Lethal and Nonlethal Techniques to Reduce Raccoon Depredation of Sea Turtle Nests. J. Wildl. Manag. 1997, 61, 368–376. [Google Scholar] [CrossRef]
- Dawson, S.J.; Adams, P.J.; Huston, R.M.; Fleming, P.A. Environmental Factors Influence Nest Excavation by Foxes. J. Zool. 2014, 294, 104–113. [Google Scholar] [CrossRef]
- Strickland, J.; Colbert, P.; Janzen, F.J. Experimental Analysis of Effects of Markers and Habitat Structure on Predation of Turtle Nests. J. Herpetol. 2010, 44, 467–470. [Google Scholar] [CrossRef]
- Buzuleciu, S.A.; Crane, D.P.; Parker, S.L. Scent of Disinterred Soil as an Olfactory Cue Used by Raccoons to Locate Nests of Diamond-Backed Terrapins. Herpetol. Conserv. Biol. 2016, 11, 539–551. [Google Scholar]
- Congdon, J.D.; Breitenbach, G.L.; van Loben Sels, R.C.; Tinkle, D.W. Reproduction and Nesting Ecology of Snapping Turtles (Chelydra serpentina) in Southeastern Michigan. Herpetologica 1987, 43, 39–54. [Google Scholar]
- Holcomb, S.R.; Carr, J.L. Mammalian Depredation of Artificial Alligator Snapping Turtle (Macrochelys temminckii) Nests in North Louisiana. Southeast. Nat. 2013, 12, 478–491. [Google Scholar] [CrossRef]
- Riley, J.L.; Litzgus, J.D. Cues Used by Predators to Detect Freshwater Turtle Nests May Persist Late into Incubation. Can. Field-Nat. 2014, 128, 179–188. [Google Scholar] [CrossRef]
- Hill, R.A.; Weber, M.H.; Leibowitz, S.G.; Olsen, A.R.; Thornbrugh, D.J. The Stream-Catchment (StreamCat) Dataset: A Database of Watershed Metrics for the Conterminous United States. J. Am. Water Resour. Assoc. 2016, 52, 120–128. [Google Scholar] [CrossRef]
- Butler, D.G.; Cullis, B.R.; Gilmour, A.R.; Gogel, B.J. Asreml: Asreml() Fits the Linear Mixed Model. R Package Version 3.0. 2009. Available online: www.vsni.co.uk (accessed on 22 November 2022).
- Meyer, K. Likelihood Calculations to Evaluate Experimental Designs to Estimate Genetic Variances. Heredity 2008, 101, 212–221. [Google Scholar] [CrossRef]
- Wolak, M.E. Nadiv: An R Package to Create Relatedness Matrices for Estimating Non-Additive Genetic Variances in Animal Models. Methods Ecol. Evol. 2012, 3, 792–796. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Reale, J.A.; Blair, R.B. Nesting Success and Life-History Attributes of Bird Communities Along an Urbanization Gradient. Urban Habitats 2005, 3, 1–14. [Google Scholar]
- Sumasgutner, P.; Nemeth, E.; Tebb, G.; Krenn, H.W.; Gamauf, A. Hard Times in the City–Attractive Nest Sites but Insufficient Food Supply Lead to Low Reproduction Rates in a Bird of Prey. Front. Zool. 2014, 11, 48. [Google Scholar] [CrossRef]
- Arnfield, A.J. Two Decades of Urban Climate Research: A Review of Turbulence, Exchanges of Energy and Water, and the Urban Heat Island. Int. J. Climatol. 2003, 23, 1–26. [Google Scholar] [CrossRef]
- Peng, S.; Piao, S.; Ciais, P.; Friedlingstein, P.; Ottle, C.; Bréon, F.-M.; Nan, H.; Zhou, L.; Myneni, R.B. Surface Urban Heat Island Across 419 Global Big Cities. Environ. Sci. Technol. 2012, 46, 696–703. [Google Scholar] [CrossRef]
- Breitenbach, G.L.; Congdon, J.D.; van Loben Sels, R.C. Winter Temperatures of Chrysemys picta Nests in Michigan: Effects on Hatchling Survival. Herpetologica 1984, 40, 76–81. [Google Scholar]
- Noble, D.W.A.; Stenhouse, V.; Schwanz, L.E. Developmental Temperatures and Phenotypic Plasticity in Reptiles: A Systematic Review and Meta-Analysis. Biol. Rev. 2018, 93, 72–97. [Google Scholar] [CrossRef]
- Packard, G.C.; Packard, M.J.; Miller, K.; Boardman, T.J. Influence of Moisture, Temperature, and Substrate on Snapping Turtle Eggs and Embryos. Ecology 1987, 68, 983–993. [Google Scholar] [CrossRef]
- Cagle, K.D.; Packard, G.C.; Miller, K.; Packard, M.J. Effects of the Microclimate in Natural Nests on Development of Embryonic Painted Turtles, Chrysemys picta. Funct. Ecol. 1993, 7, 653–660. [Google Scholar] [CrossRef]
- Chovanec, A. Man-Made Wetlands in Urban Recreational Areas—A Habitat for Endangered Species? Landsc. Urban Plan. 1994, 29, 43–54. [Google Scholar] [CrossRef]
- Orams, M.B. Feeding Wildlife as a Tourism Attraction: A Review of Issues and Impacts. Tour. Manag. 2002, 23, 281–293. [Google Scholar] [CrossRef]
- Spencer, R.J.; Thompson, M.B. The Significance of Predation in Nest Site Selection of Turtles: An Experimental Consideration of Macro- and Microhabitat Preferences. Oikos 2003, 102, 592–600. [Google Scholar] [CrossRef]
- Janzen, F.J.; Morjan, C.L. Repeatability of Microenvironment-Specific Nesting Behaviour in a Turtle with Environmental Sex Determination. Anim. Behav. 2001, 62, 73–82. [Google Scholar] [CrossRef]
- Murray, M.H.; Becker, D.J.; Hall, R.J.; Hernandez, S.M. Wildlife Health and Supplemental Feeding: A Review and Management Recommendations. Biol. Conserv. 2016, 204, 163–174. [Google Scholar] [CrossRef]
- Leighton, P.A.; Horrocks, J.A.; Kramer, D.L. Conservation and the Scarecrow Effect: Can Human Activity Benefit Threatened Species by Displacing Predators? Biol. Conserv. 2010, 143, 2156–2163. [Google Scholar] [CrossRef]
- Thorington, K.K.; Bowman, R. Predation Rate on Artificial Nests Increases with Human Housing Density in Suburban Habitats. Ecography 2003, 26, 188–196. [Google Scholar] [CrossRef]
- Dalgish, J.; Anderson, S. A Field Experiment on Learning by Raccoons. J. Mammal. 1979, 60, 620–622. [Google Scholar] [CrossRef]
- Pelech, S.A.; Smith, J.N.M.; Boutin, S. A Predator’s Perspective of Nest Predation: Predation by Red Squirrels Is Learned, Not Incidental. Oikos 2010, 119, 841–851. [Google Scholar] [CrossRef]
- Packard, G.C.; Packard, M.J.; Birchard, G.F. Sexual Differentiation and Hatching Success by Painted Turtles Incubating in Different Thermal and Hydric Environments. Herpetologica 1989, 45, 385–392. [Google Scholar]
- Tucker, J.K.; Paukstis, G.L. Hatching Success of Turtle Eggs Exposed to Dry Incubation Environment. J. Herpetol. 2000, 34, 529–534. [Google Scholar] [CrossRef]
- Temple, S.A. Predation on Turtle Nests Increases near Ecological Edges. Copeia 1987, 1987, 250–252. [Google Scholar] [CrossRef]
- Buhlmann, K.A.; Coffman, G. Fire Ant Predation of Turtle Nests and Implications for the Strategy of Delayed Emergence. J. Elisha Mitchell Sci. Soc. 2001, 117, 94–100. [Google Scholar]
- Lloyd, R.B.; Warner, D.A. Maternal Nest-Site Choice Does Not Affect Egg Hatching Success in an Invasive Turtle Population. Behaviour 2019, 156, 265–285. [Google Scholar] [CrossRef]

| Disturbance Level | Study Area | Pond Size (m2) | Coordinates | Nesting Study vs. Nest Predation Experiment |
|---|---|---|---|---|
| High | Town Creek Park | 4561 | 32.582539, −85.476735 | Both |
| High | Kiesel Park | 742 | 32.587040, −85.542433 | Nesting study |
| High | Longleaf Villas | 3131 | 32.570633, −85.506619 | Nesting study |
| High | Agricultural Heritage Park | 8907 | 32.594622, −85.675574 | Predation experiment |
| Intermediate | Fisheries pond S10 | 11,558 | 32.669121, −85.508862 | Both |
| Intermediate | Fisheries pond S11 | 11,485 | 32.671127, −85.507211 | Both |
| Intermediate | Fisheries pond S2 | 7224 | 32.683346, −85.516154 | Nesting study |
| Intermediate | Fisheries pond S23 | 5600 | 32.678296, −85.517820 | Nesting study |
| Intermediate | Fisheries pond S24 | 7085 | 32.680441, −85.518099 | Nesting study |
| Intermediate | Fisheries pond S29 | 11,716 | 32.669498, −85.501004 | Nesting study |
| Intermediate | Fisheries pond S30 | 38,263 | 32.674933, −85.495792 | Nesting study |
| Intermediate | Fisheries pond S8 east | 5598 | 32.672734, −85.507651 | Nesting study |
| Intermediate | Fisheries pond S8 west | 37,512 | 32.672084, −85.509432 | Nesting study |
| Low | Tuskegee National Forest oxbow pond | 7342 | 32.439472, −85.635536 | Both |
| Low | Notasulga pond | 11,899 | - | Predation experiment |
| PC1 | PC2 | PC3 | PC4 | PC5 | |
|---|---|---|---|---|---|
| Impervious surface | 0.4624 | 0.0363 | −0.6312 | −0.6137 | −0.0988 |
| Developed area within catchment | 0.4732 | 0.0675 | −0.0300 | −0.2563 | 0.8396 |
| Density of roads | 0.4045 | 0.7692 | 0.4165 | 0.0355 | −0.2641 |
| Housing units | 0.4270 | −0.5903 | 0.6012 | 0.3196 | −0.0742 |
| Human population | 0.4649 | −0.2319 | −0.2562 | −0.6740 | −0.4583 |
| Proportion of variance explained | 0.8862 | 0.08643 | 0.0215 | 0.0046 | 0.0011 |
| Dependent Variables | Study Area Type | iButton Depth |
|---|---|---|
| Slope Intermediate disturbance High disturbance | F2,124 = 5.792, p = 0.004 β = 5.106 (1.602), p = 0.002 β = 6.233 (2.707), p = 0.023 | - |
| Canopy openness (%) Intermediate disturbance High disturbance | F2,126 = 4.573, p = 0.012 β = 7.852 (4.238), p = 0.066 β = −10.898 (7.197), p = 0.133 | - |
| Average daily mean temperature Intermediate disturbance High disturbance | F2,19 = 1.946, p = 0.170 β = −1.005 (1.168), p = 0.400 β = 1.179 (2.272), p = 0.610 | β = −1.778 (1.246) p = 0.170 |
| Average daily maximum temperature Intermediate disturbance High disturbance | F2,19 = 3.565, p = 0.048 β = −2.627 (2.076), p = 0.221 β = 2.263 (4.039), p = 0.582 | β = −3.877 (2.214) p = 0.096 |
| Average daily minimum temperature Intermediate disturbance High disturbance | F2,19 = 0.131, p = 0.878 β = −0.036 (0.645), p = 0.956 β = 0.588 (1.256), p = 0.645 | β = −0.417 (0.688) p = 0.552 |
| Average daily temperature range Intermediate disturbance High disturbance | F2,19 = 5.587, p = 0.012 β = −2.591 (1.573), p = 0.116 β = 1.675 (3.061), p = 0.590 | β = −3.460 (1.678) p = 0.053 |
| Urbanization Level (PC1) | Nest Type | Urbanization Level x Nest Type Interaction | iButton Depth | Residual Variances | Equal Variance Likelihood Ratio Test | |
|---|---|---|---|---|---|---|
| Distance from water | β = −2.589 (4.504) p = 0.567 | β = 0.625 (0.523) p = 0.186 | β = 0.020 (0.328) p = 0.951 | - | N = 4.875 (2.331 to 8.453) A = 12.889 (10.507 to 16.048) | λ1 = 5.025 p = 0.012 |
| Slope | β = 1.377 (0.831) p = 0.058 | β = 0.428 (0.898) p = 0.594 | β = 0.006 (0.562) p = 0.991 | - | N = 19.166 (12.147 to 29.477) A = 23.457 (18.740 to 29.871) | λ1 = 0.263 p = 0.304 |
| % canopy openness | β = −2.189 (1.826) p = 0.124 | β = −2.931 (2.259) p = 0.228 | β = −0.704 (1.415) p = 0.619 | - | N = 90.671 (49.070 to 153.127) A = 241.750 (191.827 to 306.462) | λ1 = 4.712 p = 0.015 |
| Average daily mean temp. | β = −0.339 (0.372) p = 0.095 | β = −0.134 (0.409) p = 0.522 | β = 0.189 (0.262) p = 0.470 | β = −0.021 (0.707) p = 0.973 | N = 0.029 (0 to 1.228) A = 2.659 (1.530 to 4.302) | λ1 = 2.781 p = 0.048 |
| Average daily maximum temp. | β = −0.333 (0.819) p = 0.077 | β = −0.871 (0.962) p = 0.150 | β = 0.472 (0.571) p = 0.408 | β = −1.435 (1.1494) p = 0.335 | N = 3.777 (0 to 20.031) A = 8.985 (1.899 to 17.140) | λ1 = 0.321 p = 0.286 |
| Average daily minimum temp. | β = 0.032 (0.232) p = 0.994 | β = 0.291 (0.183) p = 0.174 | β = 0.100 (0.115) p = 0.385 | β = −0.072 (0.437) p = 0.865 | N = 0.008 (0 to 0.279) A = 0.512 (0.317 to 0.835) | λ1 = 3.064 p = 0.040 |
| Average daily temp. range | β = 0.482 (1.002) p = 0.222 | β = −0.793 (1.051) p = 0.241 | β = 0.382 (0.578) p = 0.508 | β = −3.296 (1.605) p = 0.040 | N = 11.348 (3.224 to 23.909) A = 1.751 (0.828 to 7.223) | λ1 = 1.205 p = 0.136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folkerts Caldwell, M.; López-Pérez, J.E.; Warner, D.A.; Wolak, M.E. Consistent Nest Site Selection by Turtles across Habitats with Varying Levels of Human Disturbance. Diversity 2023, 15, 275. https://doi.org/10.3390/d15020275
Folkerts Caldwell M, López-Pérez JE, Warner DA, Wolak ME. Consistent Nest Site Selection by Turtles across Habitats with Varying Levels of Human Disturbance. Diversity. 2023; 15(2):275. https://doi.org/10.3390/d15020275
Chicago/Turabian StyleFolkerts Caldwell, Molly, Jorge E. López-Pérez, Daniel A. Warner, and Matthew E. Wolak. 2023. "Consistent Nest Site Selection by Turtles across Habitats with Varying Levels of Human Disturbance" Diversity 15, no. 2: 275. https://doi.org/10.3390/d15020275
APA StyleFolkerts Caldwell, M., López-Pérez, J. E., Warner, D. A., & Wolak, M. E. (2023). Consistent Nest Site Selection by Turtles across Habitats with Varying Levels of Human Disturbance. Diversity, 15(2), 275. https://doi.org/10.3390/d15020275







