Climate Change May Pose Additional Threats to the Endangered Endemic Species Encalypta buxbaumioidea in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Occurrences Data
2.2. Environment Data
2.3. MaxEnt Model
2.4. Gap Analysis
3. Results
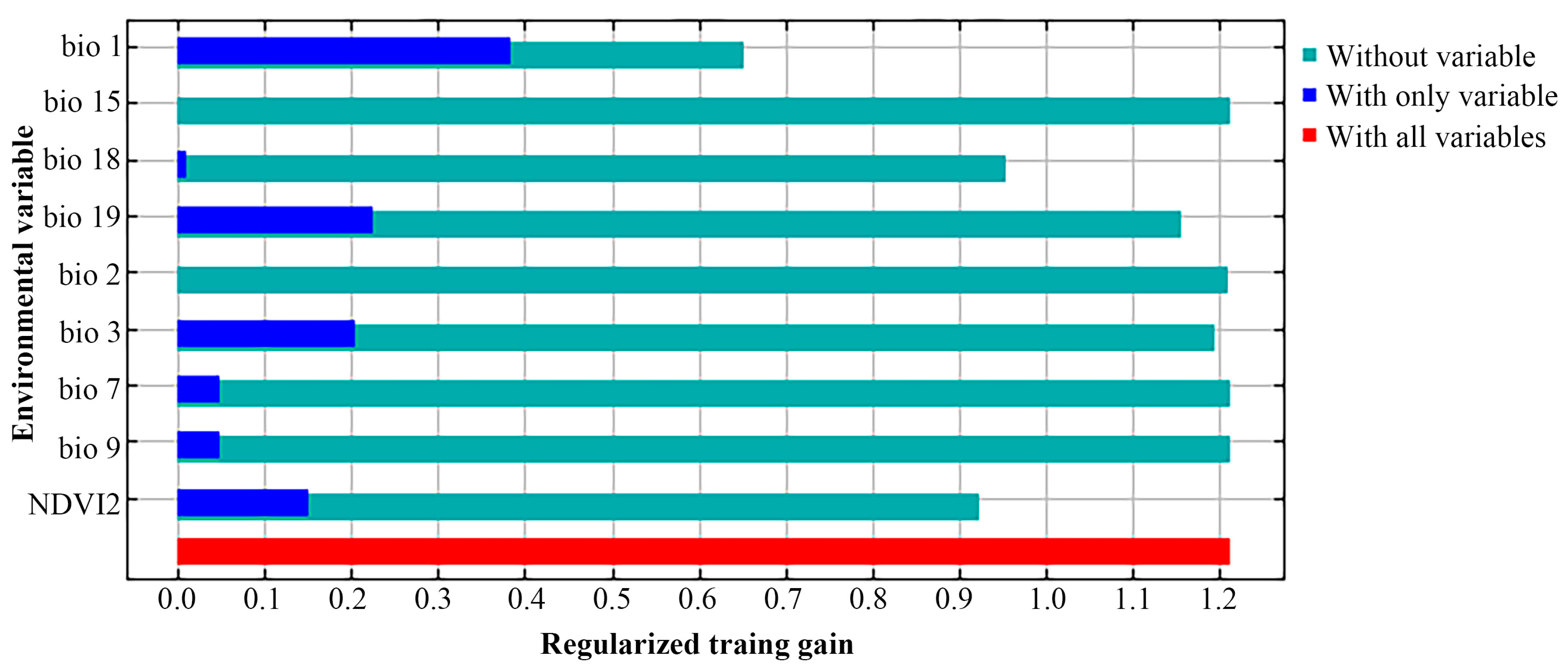
3.1. Model Performance and Important Environmental Variables
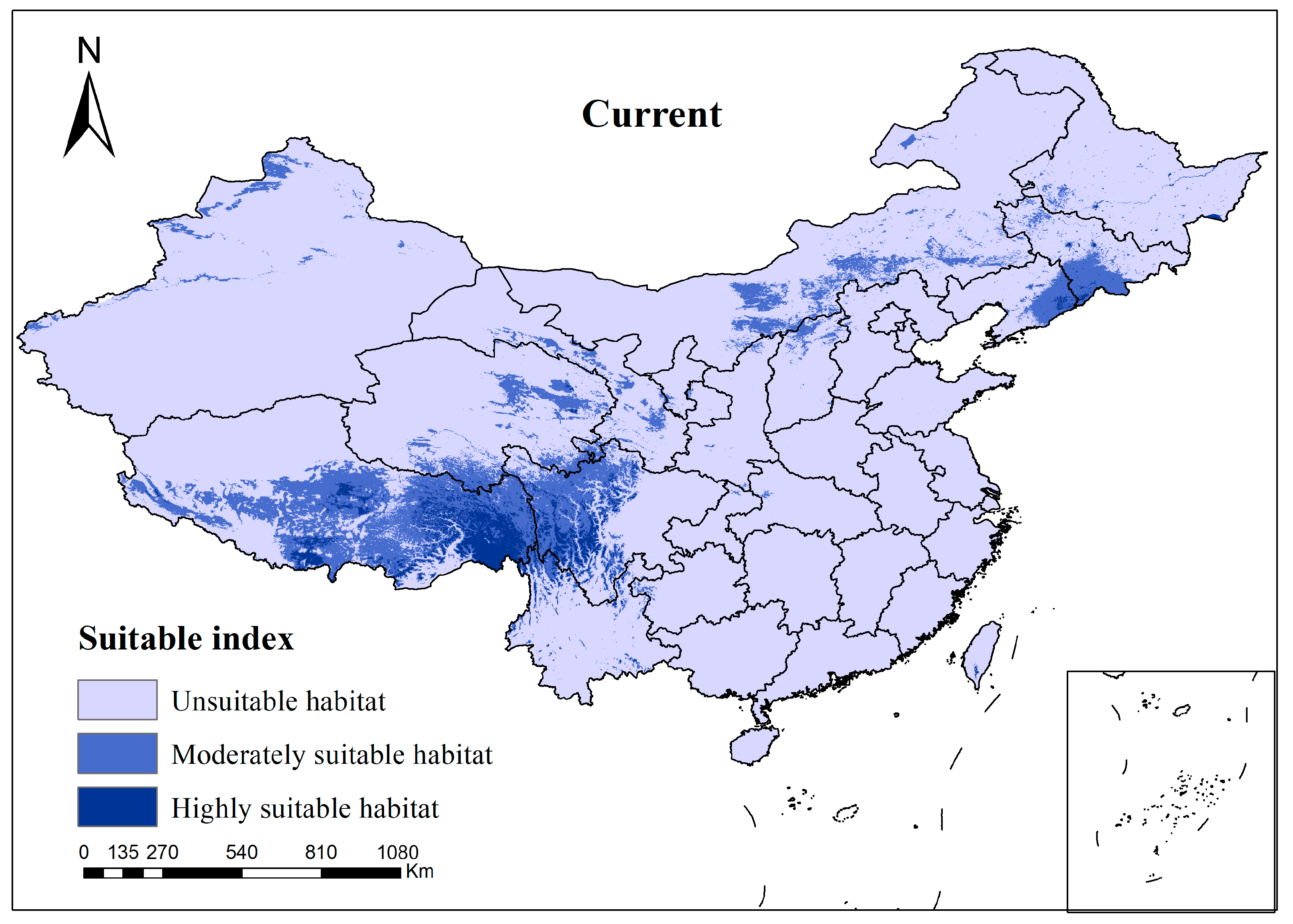
3.2. Species Distribution of Current Climate Scenario
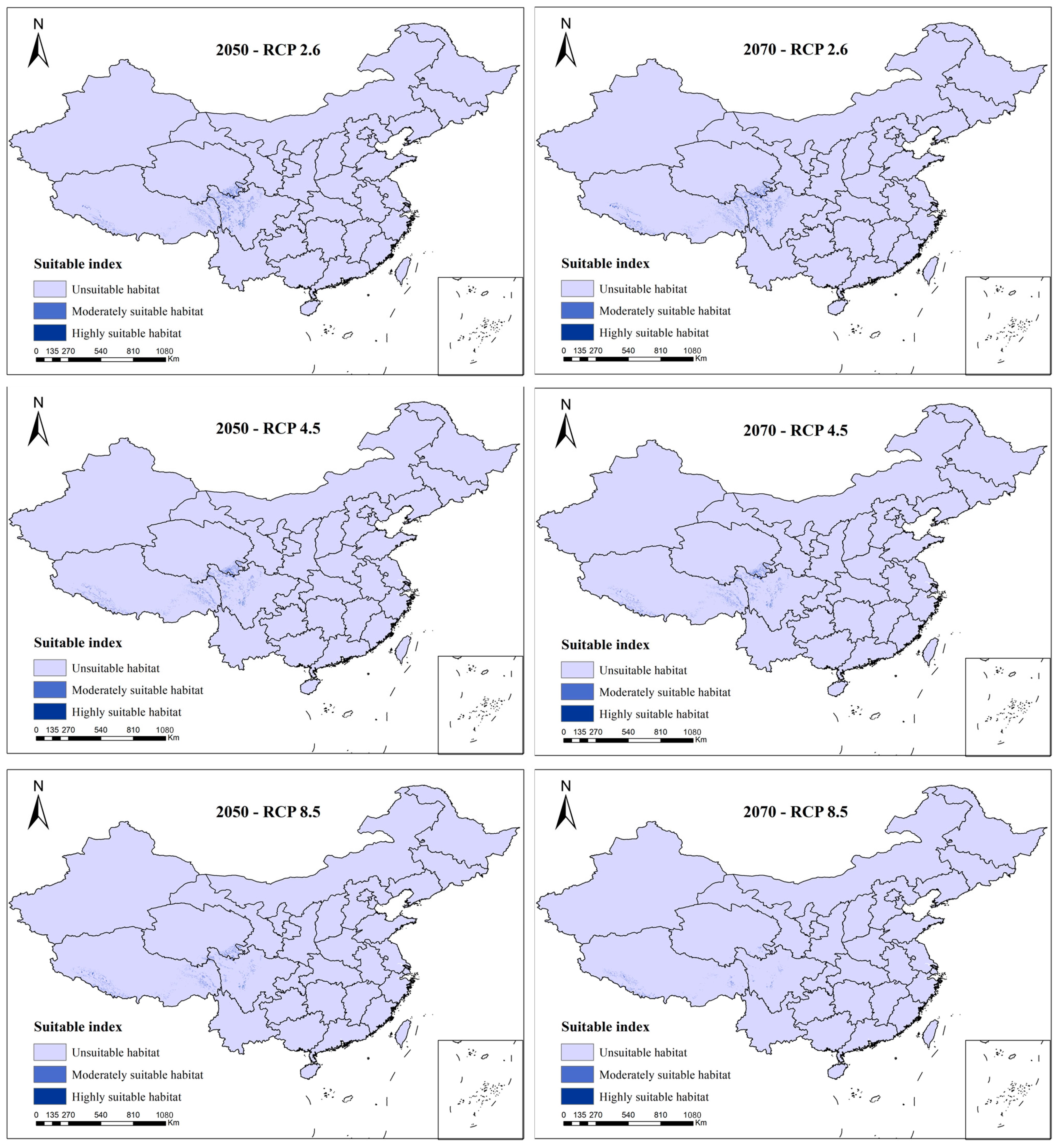
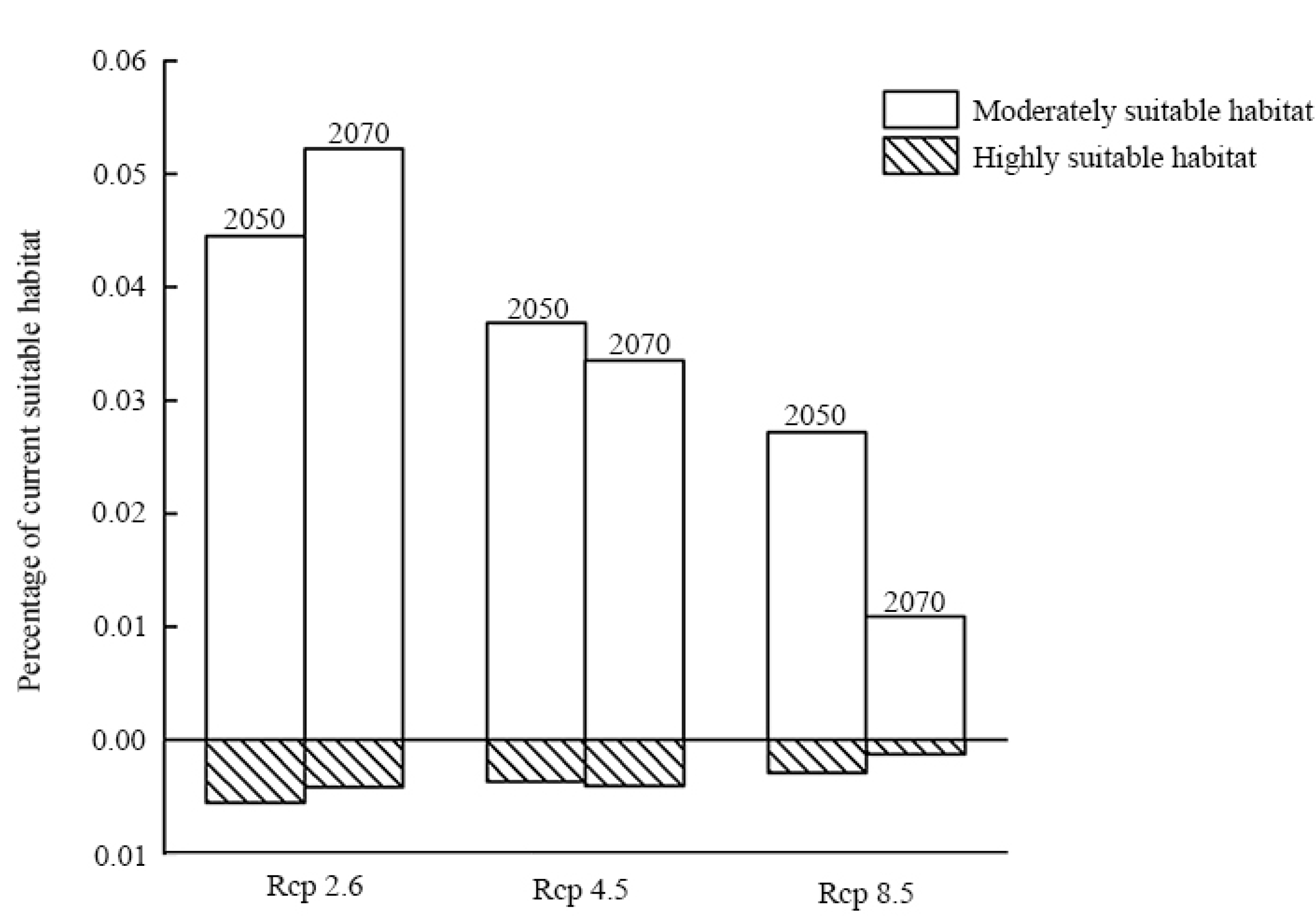
3.3. Species Distribution Change under Future Scenarios
3.4. Conservation Gaps
4. Discussion
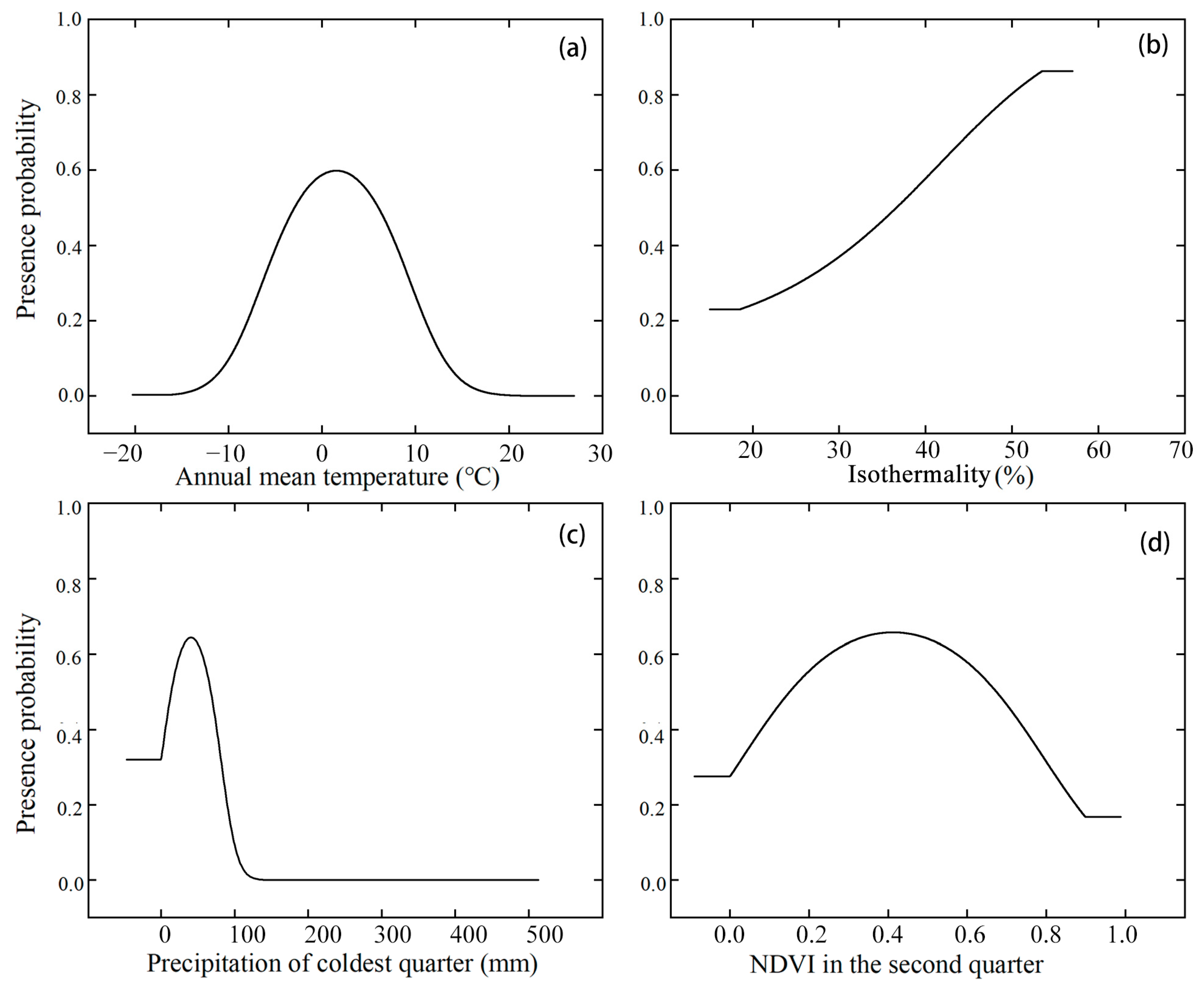
4.1. Environmental Variables Affecting the Distribution of E. buxbaumioidea
4.2. Analysis of Environmental Threats in Future Scenarios
4.3. Endangered Rank of E. buxbaumioidea
4.4. Protection Area
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markham, J. Rare species occupy uncommon niches. Sci. Rep. 2014, 4, 6012. [Google Scholar] [CrossRef]
- Rejmanek, M. Vascular plant extinctions in California: A critical assessment. Divers. Distrib. 2018, 24, 129–136. [Google Scholar] [CrossRef]
- Chape, S.; Harrison, J.; Spalding, M.; Lysenko, I. Measuring the extent and effectiveness of protected areas as an indicator for meeting global biodiversity targets. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Li, J.H. Scientific solutions for the functional zoning of nature reserves in China. Ecol. Model. 2008, 215, 237–246. [Google Scholar] [CrossRef]
- Guo, Z.L.; Cui, G.F. Establishment of Nature Reserves in Administrative Regions of Mainland China. PLoS ONE 2015, 10, e0119650. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Graham, C.H.; Elith, J.; Hijmans, R.J.; Guisan, A.; Peterson, A.T.; Loiselle, B.A.; NCEAS Predicting Species Distributions Working Group. The influence of spatial errors in species occurrence data used in distribution models. J. Appl. Ecol. 2008, 45, 239–247. [Google Scholar] [CrossRef]
- Qin, A.L.; Liu, B.; Guo, Q.S.; Bussmann, R.W.; Ma, F.Q.; Jian, Z.J.; Xu, G.X.; Pei, S.X. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Yang, Z.B.; Bai, Y.; Alatalo, J.M.; Huang, Z.D.; Yang, F.; Pu, X.Y.; Wang, R.B.; Yang, W.; Guo, X.Y. Spatio-temporal variation in potential habitats for rare and endangered plants and habitat conservation based on the maximum entropy model. Sci. Total Environ. 2021, 784, e147080. [Google Scholar] [CrossRef]
- Condro, A.A.; Prasetyo, L.B.; Rushayati, S.B.; Santikayasa, I.P.; Iskandar, E. Predicting Hotspots and Prioritizing Protected Areas for Endangered Primate Species in Indonesia under Changing Climate. Biology 2021, 10, 154. [Google Scholar] [CrossRef]
- Wang, Q.H.; Zhang, J.; Liu, Y.; Jia, Y.; Jiao, Y.N.; Xu, B.; Chen, Z.D. Diversity, phylogeny, and adaptation of bryophytes: Insights from genomic and transcriptomic data. J. Exp. Bot. 2022, 73, 4306–4322. [Google Scholar] [CrossRef]
- Ogwu, M.C. Ecological and Economic Significance of Bryophytes. In Current State and Future Impacts of Climate Change on Biodiversity; IGI Global: Hershey, PA, USA, 2020; pp. 54–78. [Google Scholar] [CrossRef]
- Singh, R.; Joshi, H.; Singh, A. Bryophytes: Natural Biomonitors. In Natural Products Chemistry; Apple Academic Press: Palm Bay, FL, USA, 2020; pp. 139–153. [Google Scholar] [CrossRef]
- He, X.L.; He, K.S.; Hyvonen, J. Will bryophytes survive in a warming world? Perspect. Plant Ecol. Evol. Syst. 2016, 19, 49–60. [Google Scholar] [CrossRef]
- Alatalo, J.M.; Jagerbrand, A.K.; Erfanian, M.B.; Chen, S.B.; Sun, S.Q.; Molau, U. Bryophyte cover and richness decline after 18 years of experimental warming in alpine Sweden. AoB Plants 2020, 12, plaa061. [Google Scholar] [CrossRef] [PubMed]
- Camara, P.; Valente, D.V.; Sancho, L.G. Changes in the moss (Bryophyta) flora in the vicinity of the Spanish Juan Carlos I Station (Livingston island, Antarctica) over three decades. Polar Biol. 2020, 43, 1745–1752. [Google Scholar] [CrossRef]
- Kou, J.; Wang, T.J.; Yu, F.Y.; Sun, Y.W.; Feng, C.; Shao, X.M. The moss genus Didymodon as an indicator of climate change on the Tibetan Plateau. Ecol. Indic. 2020, 113, e106204. [Google Scholar] [CrossRef]
- Patiño, J.; Mateo, R.G.; Zanatta, F.; Marquet, A.; Aranda, S.C.; Borges, P.A.V.; Dirkse, G.; Gabriel, R.; Gonzalez-Mancebo, J.M.; Guisan, A.; et al. Climate threat on the Macaronesian endemic bryophyte flora. Sci. Rep. 2016, 6, 29156. [Google Scholar] [CrossRef]
- Wu, J.G. The danger and indeterminacy of forfeiting perching space of bryophytes from climate shift: A case study for 115 species in China. Environ. Monit. Assess. 2022, 194, 233. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Feng, C.T.; Liu, F.Z.; Li, J.S. Biodiversity conservation in China: A review of recent studies and practices. Env. Sci. Ecotechnol. 2020, 2, e100025. [Google Scholar] [CrossRef]
- Cao, T.; Gao, C. A new species of Encalypta (Musci) from China. Acta Bryolichenolog. Asiat. 1990, 2, 1–4. [Google Scholar]
- Cao, T.; Gao, C.; Horton, D.G. Fissidentaceae-Ptychomitriaceae; English Version; Science Press: Beijing, China; Missouri Botanical Garden: New York, NY, USA, 2001; Volume 2, pp. 103–113. [Google Scholar]
- Zhao, J.C.; Li, X.Q.; Tang, W.B. Taxonomy and distribution of Encalyptaceae, Musci. Acta Bot. Boreali Occident. Sin. 2002, 22, 453–466. [Google Scholar]
- He, Q.; Jia, Y. Assessing the threat status of China’s bryophytes. Biodivers. Sci. 2017, 25, 774–780. [Google Scholar] [CrossRef]
- Cao, T.; Zhu, R.; Guo, S.; Zuo, B.; Yu, J. A brief report of the first red list of endangered bryophytes in China. Bull. Bot. Res. 2006, 26, 756–762. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Gent, P.R.; Danabasoglu, G.; Donner, L.J.; Holland, M.M.; Hunke, E.C.; Jayne, S.R.; Lawrence, D.M.; Neale, R.B.; Rasch, P.J.; Vertenstein, M.; et al. The Community Climate System Model Version 4. J. Clim. 2011, 24, 4973–4991. [Google Scholar] [CrossRef]
- Xu, X.L. Spatial Distribution Dataset of Quarterly Vegetation Indices (NDVI) in China; Data Registration and Publication System for Resource and Environment Science and Data Center, Chinese Academy of Sciences: Beijing, China, 2018; Available online: http://www.resdc.cn/DOI (accessed on 24 March 2021). [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Manel, S.; Williams, H.C.; Ormerod, S.J. Evaluating presence-absence models in ecology: The need to account for prevalence. J. Appl. Ecol. 2001, 38, 921–931. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Freeman, E.A.; Moisen, G. PresenceAbsence: An R package for presence absence analysis. J. Stat. Softw. 2008, 23, 1–31. [Google Scholar] [CrossRef]
- Lu, Y.P.; Liu, H.C.; Chen, W.; Yao, J.; Huang, Y.Q.; Zhang, Y.; He, X.Y. Conservation planning of the genus Rhododendron in Northeast China based on current and future suitable habitat distributions. Biodivers. Conserv. 2021, 30, 673–697. [Google Scholar] [CrossRef]
- Coe, K.K.; Sparks, J.P.; Belnap, J. Physiological Ecology of Dryland Biocrust Mosses. Photosynth. Bryophyt. Early Land Plants 2014, 37, 291–308. [Google Scholar] [CrossRef]
- Frahm, J.-P. Bryophytes. In Inselbergs; Springer: Berlin/Heidelberg, Germany, 2000; pp. 91–102. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhao, L.; Song, X.T.; Wang, Q.G.; Kou, J.; Jiang, Y.B.; Shao, X.M. Morphological traits of Bryum argenteum and its response to environmental variation in arid and semi-arid areas of Tibet. Ecol. Eng. 2019, 136, 101–107. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.B.; Song, X.T.; Tang, J.W.; Kou, J.; Fan, Y.J.; Shao, X.M. Temperature, not precipitation, drives the morphological traits of Didymodon rigidulus in Tibet. Ecol. Indic. 2021, 133, e108401. [Google Scholar] [CrossRef]
- Zhang, H. Morphological Characteristics and Geographical Distribution of Mielichhoferia mielichhoferiana(Bryaceae, Bryophyta). Acta Bot. Boreali Occident. Sin. 2014, 34, 851–854. [Google Scholar] [CrossRef]
- Johansson, V.; Lonnell, N.; Sundberg, S.; Hylander, K. Release thresholds for moss spores: The importance of turbulence and sporophyte length. J. Ecol. 2014, 102, 721–729. [Google Scholar] [CrossRef]
- Zanatta, F.; Engler, R.; Collart, F.; Broennimann, O.; Mateo, R.G.; Papp, B.; Munoz, J.; Baurain, D.; Guisan, A.; Vanderpoorten, A. Bryophytes are predicted to lag behind future climate change despite their high dispersal capacities. Nat. Commun. 2020, 11, 5601. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, Q.A.; Peng, C.H.; Wu, N.; Wang, Y.F.; Fang, X.Q.; Gao, Y.H.; Zhu, D.; Yang, G.; Tian, J.Q.; et al. The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan Plateau. Global Change Biol. 2013, 19, 2940–2955. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.D.; Liu, S.L.; Dong, S.K.; Su, X.K.; Wang, X.X.; Wu, X.Y.; Wu, L.; Zhang, X. Analysis of vegetation change associated with human disturbance using MODIS data on the rangelands of the Qinghai-Tibet Plateau. Rangeland J. 2015, 37, 77–87. [Google Scholar] [CrossRef]
- Chen, D.; Xu, B.; Yao, T.; Guo, Z.; Cui, P.; Chen, F.; Zhang, R.; Zhang, X.; Zhang, Y.; Fan, J.; et al. Assessment of past, present and future environmental changes on the Tibetan Plateau. Sci. Bull. 2015, 60, 3025–3035. [Google Scholar]
- Chen, B.X.; Zhang, X.Z.; Tao, J.; Wu, J.S.; Wang, J.S.; Shi, P.L.; Zhang, Y.J.; Yu, C.Q. The impact of climate change and anthropogenic activities on alpine grassland over the Qinghai-Tibet Plateau. Sci. Bull. 2014, 189, 11–18. [Google Scholar] [CrossRef]
- Tingstad, L.; Grytnes, J.A.; Saetersdal, M.; Gjerde, I. Using Red List species in designating protection status to forest areas: A case study on the problem of spatio-temporal dynamics. Biodivers. Conserv. 2020, 29, 3429–3443. [Google Scholar] [CrossRef]
- Purvis, A.; Gittleman, J.L.; Cowlishaw, G.; Mace, G.M. Predicting extinction risk in declining species. Proc. R. Soc. B-Biol. Sci. 2000, 267, 1947–1952. [Google Scholar] [CrossRef]
- Rodrigues, A.S.L.; Gaston, K.J. How large do reserve networks need to be? Ecol. Lett. 2001, 4, 602–609. [Google Scholar] [CrossRef]


| Specimen Number | Longitude (°) | Latitude (°) | Altitude (m) | Matrix | Source |
|---|---|---|---|---|---|
| 2019P110Y001 | 87.44 | 28.95 | 5209 | Soil | BAU |
| 20200808SSL097 | 97.60 | 29.48 | 4442 | Soil | BAU |
| 20200808SWZ065 | 96.85 | 29.39 | 4147 | Soil | BAU |
| 20200811SGL025 | 96.76 | 29.57 | 4213 | Soil | BAU |
| 20200811SSL031 | 96.04 | 29.37 | 4128 | Soil | BAU |
| 20200812SGL018 | 97.29 | 30.15 | 4505 | Soil | BAU |
| 20200812SWZ002 | 97.46 | 28.66 | 4357 | Soil | BAU |
| 5291 | 98.00 | 29.04 | 5080 | Soil on the rock surface | PE |
| 2015037 | 116.24 | 42.60 | 1598 | Soil | IMNU |
| 3881 | 116.07 | 43.91 | 993 | Soil | IMU |
| 70 | 117.53 | 43.59 | 1444 | Soil | IMU |
| 98775-a | 114.60 | 41.80 | - | Soil | HBNU |
| Environmental Conditions | Localities | |
|---|---|---|
| Qinghai–Tibet Plateau | Mongolian Plateau | |
| Annual mean temperature (°C) | −3–10 | 0–6 |
| Mean diurnal range (°C) | 10–15 | 11–14 |
| Microhabitats | Soil surface in a shaded habitat | Soil surface in a shaded habitat |
| Altitude (m) | 4128–5209 | About 1600 |
| Temperature annual range (°C) | 25–32 | 46–52 |
| Vegetation | Thicket; grassland; alpine vegetation; coniferous forest | Grassland; cultivated vegetation |
| Variable | Description | Unit |
|---|---|---|
| Bio1 | Annual mean temperature | °C |
| Bio2 | Mean diurnal range | °C |
| Bio3 | Isothermality | % |
| Bio7 | Temperature annual range | °C |
| Bio9 | Mean temp. of driest quarter | °C |
| Bio15 | Precipitation seasonality (coefficient of variation) | % |
| Bio18 | Precipitation of warmest quarter | mm |
| Bio19 | Precipitation of coldest quarter | mm |
| NDVI2 | NDVI in the second quarter | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Song, X.; Ye, Y.; Gu, J.; Wang, R.; Zhuogabayong; Zhao, D.; Shao, X. Climate Change May Pose Additional Threats to the Endangered Endemic Species Encalypta buxbaumioidea in China. Diversity 2023, 15, 269. https://doi.org/10.3390/d15020269
Liao Y, Song X, Ye Y, Gu J, Wang R, Zhuogabayong, Zhao D, Shao X. Climate Change May Pose Additional Threats to the Endangered Endemic Species Encalypta buxbaumioidea in China. Diversity. 2023; 15(2):269. https://doi.org/10.3390/d15020269
Chicago/Turabian StyleLiao, Yujia, Xiaotong Song, Yanhui Ye, Jiqi Gu, Ruihong Wang, Zhuogabayong, Dongping Zhao, and Xiaoming Shao. 2023. "Climate Change May Pose Additional Threats to the Endangered Endemic Species Encalypta buxbaumioidea in China" Diversity 15, no. 2: 269. https://doi.org/10.3390/d15020269
APA StyleLiao, Y., Song, X., Ye, Y., Gu, J., Wang, R., Zhuogabayong, Zhao, D., & Shao, X. (2023). Climate Change May Pose Additional Threats to the Endangered Endemic Species Encalypta buxbaumioidea in China. Diversity, 15(2), 269. https://doi.org/10.3390/d15020269





