Environmental Drivers of Amphibian Breeding Phenology across Multiple Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Field and Laboratory Methods
2.2. Statistical Analyses
2.2.1. Ordinal Day of Migration
2.2.2. Environmental Factors Affecting Timing of Migration
2.2.3. Annual Average Weather and Species Interactions
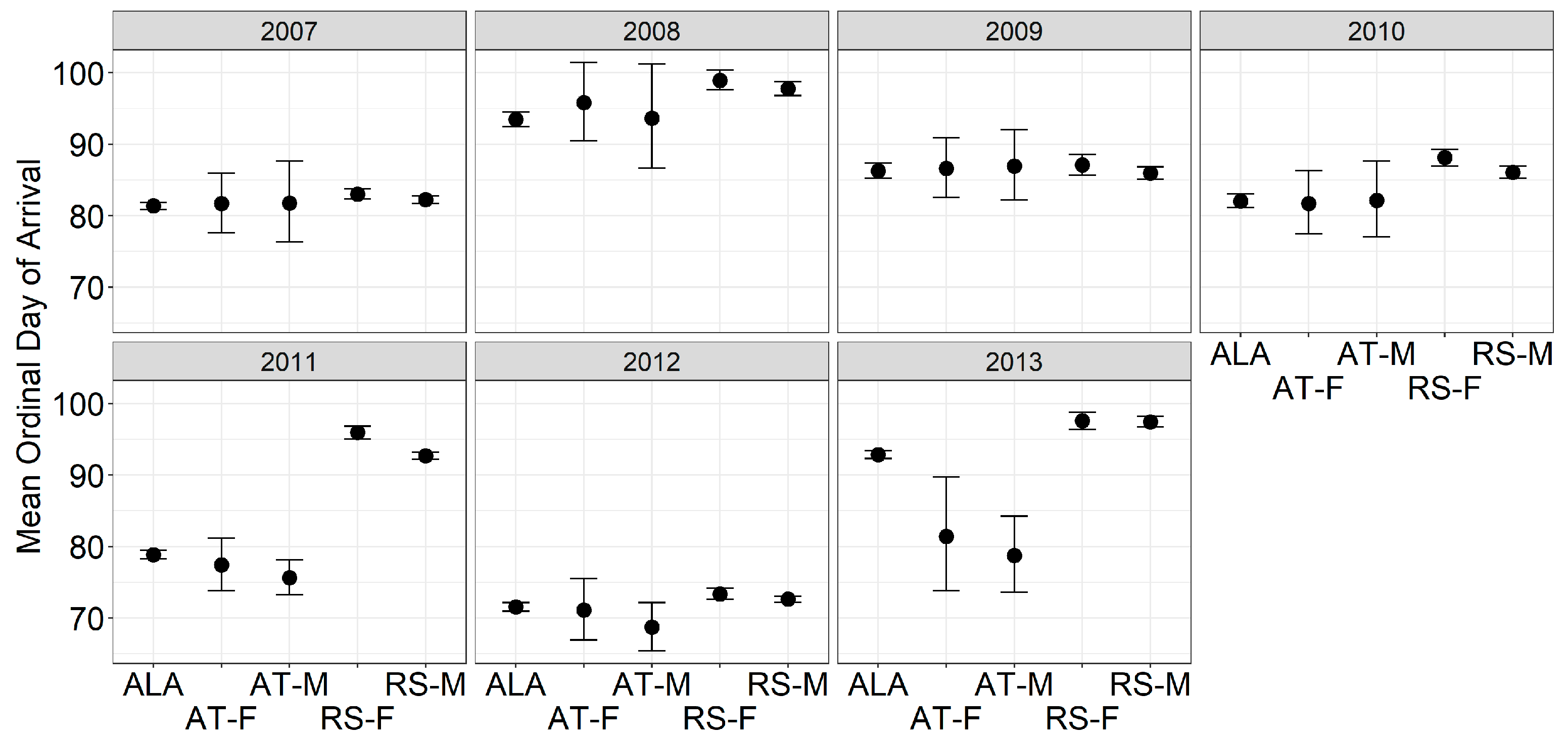
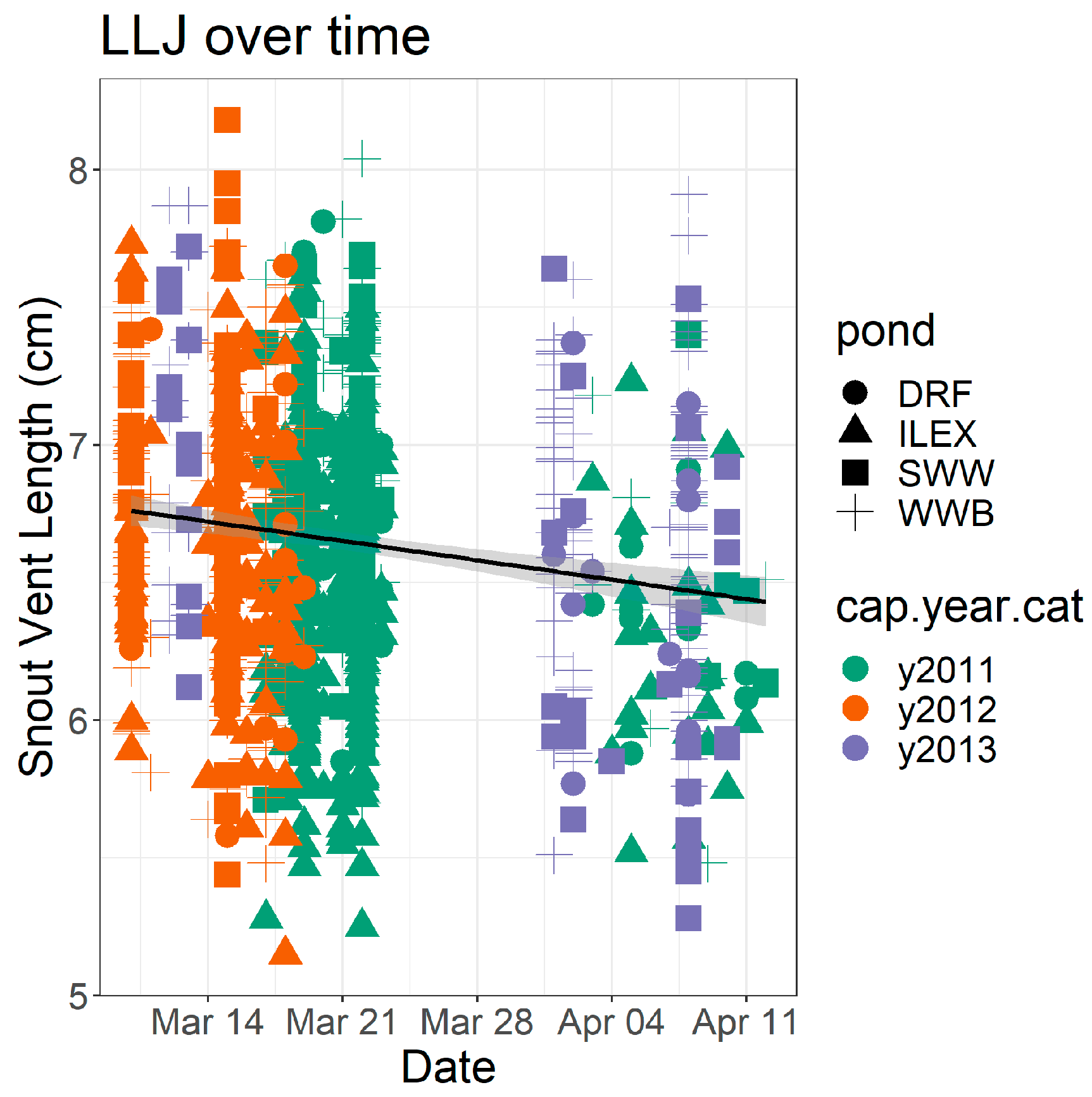
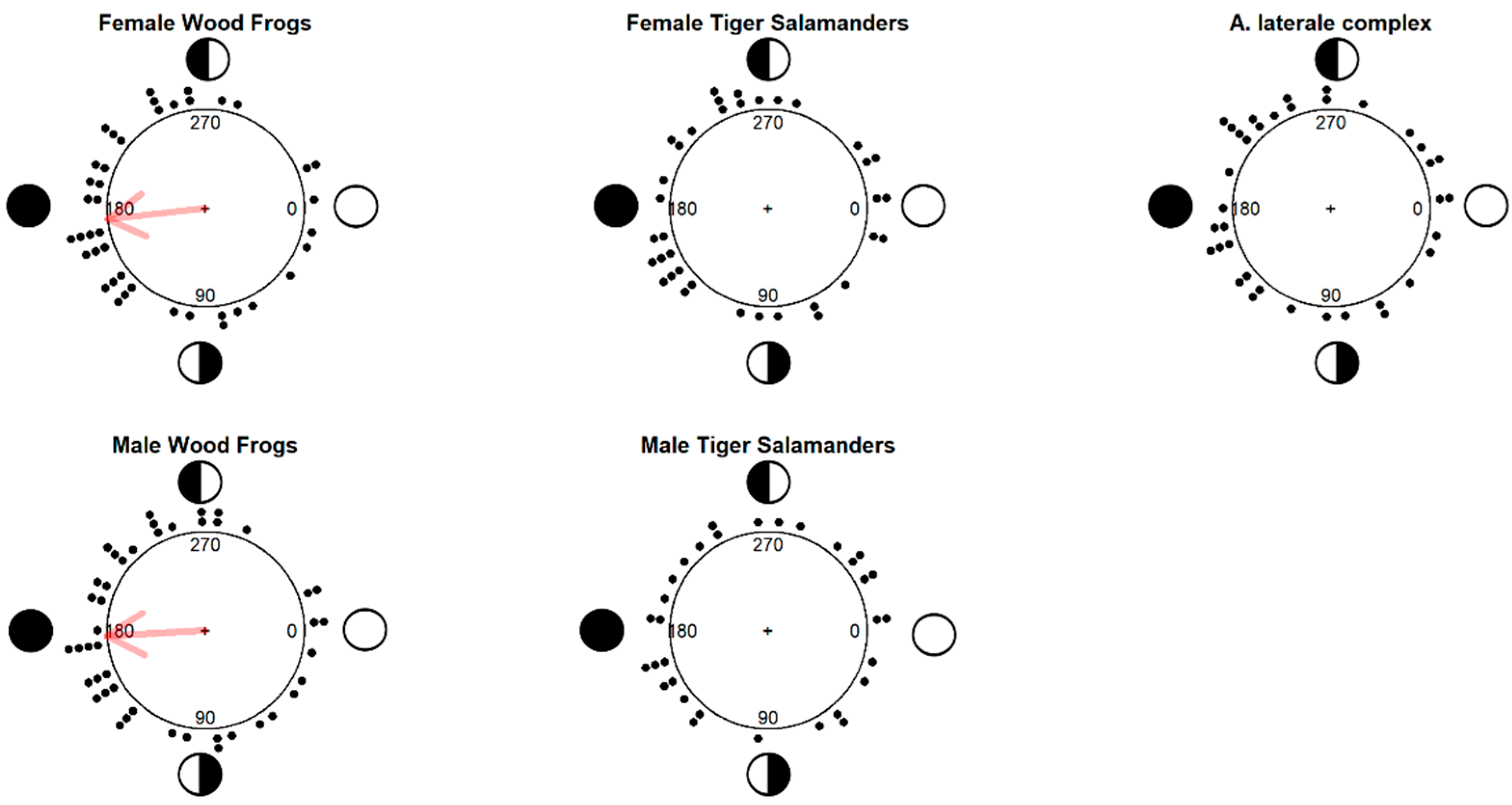
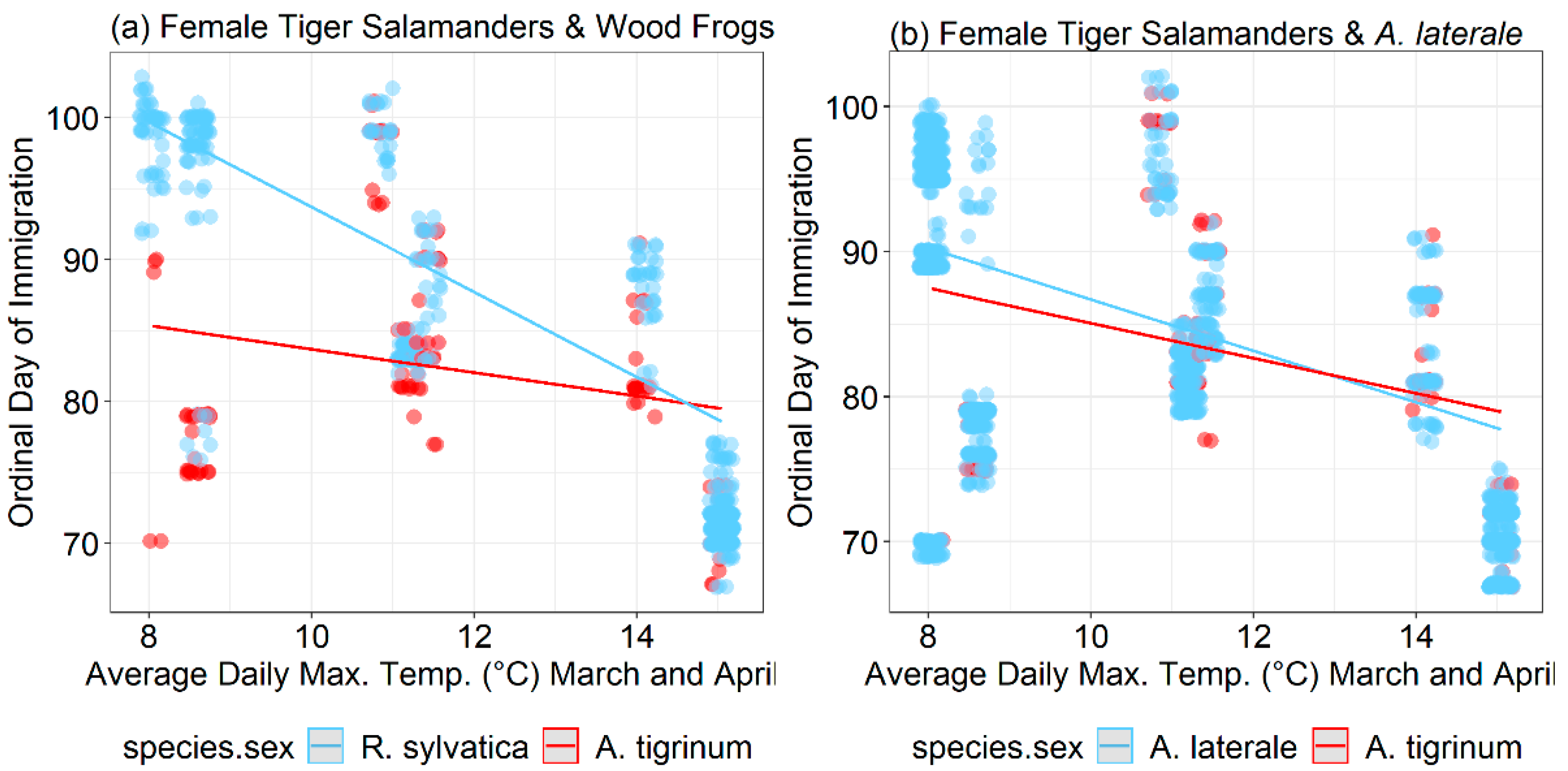
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramula, S.; Johansson, J.; Linden, A.; Jonzen, N. Linking phenological shifts to demographic change. Clim. Res. 2015, 63, 135–144. [Google Scholar] [CrossRef]
- Visser, M.E.; Gienapp, P. Evolutionary and demographic consequences of phenological mismatches. Nat. Ecol. Evol. 2019, 3, 879–885. [Google Scholar] [CrossRef]
- Yang, L.H.; Rudolf, V.H.W. Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol. Lett. 2010, 13, 1–10. [Google Scholar] [CrossRef]
- Inouye, D.W. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 2008, 89, 353–362. [Google Scholar] [CrossRef]
- Ozgul, A.; Childs, D.Z.; Oli, M.K.; Armitage, K.B.; Blumstein, D.T.; Olson, L.E.; Tuljapurkar, S.; Coulson, T. Coupled dynamics of body mass and population growth in response to environmental change. Nature 2010, 466, 482–485. [Google Scholar] [CrossRef]
- Rasmussen, N.L.; Rudolf, V.H.W. Individual and combined effects of two types of phenological shifts on predator-prey interactions. Ecology 2016, 97, 3414–3421. [Google Scholar] [CrossRef]
- Todd, B.D.; Scott, D.E.; Pechmann, J.H.K.; Gibbons, J.W. Climate change correlates with rapid delays and advancements in reproductive timing in an amphibian community. Proc. Royal Soc. B 2011, 278, 2191–2197. [Google Scholar] [CrossRef]
- Harnos, A.; Nora, A.; Kovacs, S.; Lang, Z.; Csorgo, T. Increasing protandry in the spring migration of the Pied Flycatcher (Ficedula hypoleuca) in Central Europe. J. Ornithol. 2015, 156, 543–546. [Google Scholar] [CrossRef]
- Ward, S.E.; Schulze, M.; Roy, B. A long-term perspective on microclimate and spring plant phenology in the Western Cascades. Ecosphere 2018, 9, e02451. [Google Scholar] [CrossRef]
- Cohen, J.M.; Lajeunesse, M.J.; Rohr, J.R. A global synthesis of animal phenological responses to climate change. Nat. Clim. Chang. 2018, 8, 224–228. [Google Scholar] [CrossRef]
- Parmesan, C. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Chang. Biol. 2007, 13, 1860–1872. [Google Scholar] [CrossRef]
- Chuine, I.; Regniere, J. Process-Based Models of Phenology for Plants and Animals. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 159–182. [Google Scholar] [CrossRef]
- Helm, B.; Ben-Shlomo, R.; Sheriff, M.J.; Hut, R.A.; Foster, R.; Barnes, B.M.; Dominoni, D. Annual rhythms that underlie phenology: Biological time-keeping meets environmental change. Proc. Royal Soc. B 2013, 280, 20130016. [Google Scholar] [CrossRef]
- Simmonds, E.G.; Cole, E.F.; Sheldon, B.C. Cue identification in phenology: A case study of the predictive performance of current statistical tools. J. Anim. Ecol. 2019, 88, 1428–1440. [Google Scholar] [CrossRef]
- Benard, M.F. Warmer winters reduce frog fecundity and shift breeding phenology, which consequently alters larval development and metamorphic timing. Glob. Chang. Biol. 2015, 21, 1058–1065. [Google Scholar] [CrossRef]
- Loman, J. Primary and secondary phenology. Does it pay a frog to spawn early? J. Zool. 2009, 279, 64–70. [Google Scholar] [CrossRef]
- Anderson, T.L.; Rowland, F.E.; Semlitsch, R.D. Variation in phenology and density differentially affects predator-prey interactions between salamanders. Oecologia 2017, 185, 475–486. [Google Scholar] [CrossRef]
- Lawler, S.P.; Morin, P.J. Temporal overlap, competition, and priority effects in larval anurans. Ecology 1993, 74, 174–182. [Google Scholar] [CrossRef]
- Gibbs, J.P.; Breisch, A.R. Climate warming and calling phenology of frogs near Ithaca, New York, 1900–1999. Conserv. Biol. 2001, 15, 1175–1178. [Google Scholar] [CrossRef]
- Klaus, S.P.; Lougheed, S.C. Changes in breeding phenology of eastern Ontario frogs over four decades. Ecol. Evol. 2013, 3, 835–845. [Google Scholar] [CrossRef]
- While, G.M.; Uller, T. Quo vadis amphibia? Global warming and breeding phenology in frogs, toads and salamanders. Ecography 2014, 37, 921–929. [Google Scholar] [CrossRef]
- Green, D.M. Amphibian breeding phenology trends under climate change: Predicting the past to forecast the future. Glob. Chang. Biol. 2017, 23, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Semlitsch, R.D. Analysis of Climatic Factors Influencing Migrations of the Salamander Ambystoma talpoideum. Copeia 1985, 1985, 477. [Google Scholar] [CrossRef]
- Sexton, O.J.; Phillips, C.; Bramble, J.E. The effects of temperature and precipitation on the breeding migration of the spotted salamander (Ambystoma maculum). Copeia 1990, 1990, 781–787. [Google Scholar] [CrossRef]
- Timm, B.C.; McGarigal, K.; Compton, B.W. Timing of large movement events of pond-breeding amphibians in Western Massachusetts, USA. Biol. Conserv. 2007, 136, 442–454. [Google Scholar] [CrossRef]
- Brooks, G.C.; Smith, J.A.; Gorman, T.A.; Haas, C.A. Discerning the Environmental Drivers of Annual Migrations in an Endangered Amphibian. Copeia 2019, 107, 270–276. [Google Scholar] [CrossRef]
- Grant, R.A.; Chadwick, E.A.; Halliday, T. The lunar cycle: A cue for amphibian reproductive phenology? Anim. Behav. 2009, 78, 349–357. [Google Scholar] [CrossRef]
- Adamski, P.; Ćmiel, A.M.; Lipińska, A.M. Intraseasonal asynchrony as a factor boosting isolation within a metapopulation: The case of the clouded apollo. Insect. Sci. 2019, 26, 911–922. [Google Scholar] [CrossRef]
- Stanton, M.L.; Galen, C. Life on the edge: Adaptation versus environmentally mediated gene flow in the snow buttercup, Ranunculus adoneus. Am. Nat. 1997, 150, 143–178. [Google Scholar] [CrossRef]
- Marsh, D.M.; Fegraus, E.H.; Harrison, S. Effects of breeding pond isolation on the spatial and temporal dynamics of pond use by the tungara frog, Physalaemus pustulosus. J. Anim. Ecol. 1999, 68, 804–814. [Google Scholar] [CrossRef]
- Smith, M.A.; Green, D.M. Dispersal and the metapopulation paradigm in amphibian ecology and conservation: Are all amphibian populations metapopulations? Ecography 2005, 28, 110–128. [Google Scholar] [CrossRef]
- Werner, E.E.; Yurewicz, K.L.; Skelly, D.K.; Relyea, R.A. Turnover in an amphibian metacommunity: The role of local and regional factors. Oikos 2007, 116, 1713–1725. [Google Scholar] [CrossRef]
- Takahashi, K.; Sato, T. Spatial variation in breeding phenology at small spatial scales: A stochastic effect of population size. Popul. Ecol. 2020, 62, 332–340. [Google Scholar] [CrossRef]
- Boes, M.W.; Benard, M.F. Carry-Over Effects in Nature: Effects of Canopy Cover and Individual Pond on Size, Shape and Locomotor Performance of metamorphosing Wood Frogs. Copeia 2013, 2013, 717–722. [Google Scholar] [CrossRef]
- Freidenburg, L.K.; Skelly, D.K. Microgeographical variation in thermal preference by an amphibian. Ecol. Lett. 2004, 7, 369–373. [Google Scholar] [CrossRef]
- Halverson, M.A.; Skelly, D.K.; Kiesecker, J.M.; Freidenburg, L.K. Forest mediated light regime linked to amphibian distribution and performance. Oecologia 2003, 134, 360–364. [Google Scholar] [CrossRef]
- Relyea, R.A. Local population differences in phenotypic plasticity: Predator-induced changes in wood frog tadpoles. Ecol. Monogr. 2002, 72, 77–93. [Google Scholar] [CrossRef]
- Wilbur, H.M. Competition, predation and structure of Ambystoma-Rana sylvatica community. Ecology 1972, 53, 3–21. [Google Scholar] [CrossRef]
- Sredl, M.J.; Collins, J.P. The effect of ontogeny on interspecific interactions in larval amphibians. Ecology 1991, 72, 2232–2239. [Google Scholar] [CrossRef]
- Berven, K.A. Factors affecting variation in reproductive traits within a population of wood frogs (Rana sylvatica). Copeia 1988, 1998, 605–615. [Google Scholar] [CrossRef]
- Rollins, H.B.; Benard, M.F. Challenges in predicting the outcome of competition based on climate change-induced phenological and body size shifts. Oecologia 2020, 193, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Bogart, J.P.; Bi, K.; Fu, J.Z.; Noble, D.W.A.; Niedzwiecki, J. Unisexual salamanders (genus Ambystoma) present a new reproductive mode for eukaryotes. Genome 2007, 50, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Bogart, J.P.; Bartoszek, J.; Noble, D.W.A.; Bi, K. Sex in unisexual salamanders: Discovery of a new sperm donor with ancient affinities. Heredity 2009, 103, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Brodman, R.; Krouse, H.D. How blue-spotted and small-mouthed salamander larvae coexist with their unisexual counterparts. Herpetologica 2007, 63, 135–143. [Google Scholar] [CrossRef]
- Greenwald, K.R.; Denton, R.D.; Gibbs, H.L. Niche partitioning among sexual and unisexual Ambystoma salamanders. Ecosphere 2016, 7, e01579. [Google Scholar] [CrossRef]
- Lowcock, L.A. Biotype, genomotype, and genotype: Variable effects of polyploidy and hybridity on ecological partitioning in a bisexual-unisexual community of salamanders. Can. J. Zool. 1994, 72, 104–117. [Google Scholar] [CrossRef]
- Van Drunen, S.G.; Linton, J.E.; Bogart, J.P.; McCarter, J.; Fotherby, H.; Sandilands, A.; Norris, D.R. Estimating critical habitat based on year-round movements of the endangered jefferson salamander (Ambystoma jeffersonianum) and their unisexual dependents. Can. J. Zool. 2020, 98, 117–126. [Google Scholar] [CrossRef]
- Bogart, J.P.; Klemens, M.W. Additional distributional records of Ambystoma laterale, A-jeffersonianum (Amphibia: Caudata) and their unisexual kleptogens in northeastern North America. Am. Mus. Novit. 2008, 2008, 1–58. [Google Scholar] [CrossRef]
- Bogart, J.P.; Klemens, M.W. Hybrids and genetic interactions of mole salamanders (Ambystoma jeffersonianum and A. laterale) (Amphibia, Caudata) in New York and New England. Am. Mus. Novit. 1997, 3218. [Google Scholar]
- Phillips, C.A.; Uzzell, T.; Spolsky, C.M.; Serb, J.M.; Szafoni, R.E.; Pollowy, T.R. Persistent high levels of tetraploidy in salamanders of the Ambystoma jeffersonianum complex. J. Herpetol. 1997, 31, 530. [Google Scholar] [CrossRef]
- Licht, L.E.; Bogart, J.P. Growth and sexual maturation in diploid and polyploid salamanders (Genus Ambstyoma). Can. J. Zool. 1989, 67, 812–818. [Google Scholar] [CrossRef]
- Lowcock, L.A.; Griffith, H.; Murphy, R.W. The Ambystoma laterale-jeffersonianum complex in central ontario: Ploidy structure, sex ratio, and breeding dynamics in a bisexual-unisexual community. Copeia 1991, 1991, 87. [Google Scholar] [CrossRef]
- Dawley, E.M.; Dawley, R.M. Species discrimination by chemical cues in a unisexual-bisexual complex of salamanders. J. Herpetol. 1986, 20, 114. [Google Scholar] [CrossRef]
- Uzzell, T.M., Jr. Relations of the diploid and triploid species of the Ambystoma jeffersonianum Complex (Amphibia, Caudata). Copeia 1964, 1964, 257–300. [Google Scholar] [CrossRef]
- Werner, E.E.; Skelly, D.K.; Relyea, R.A.; Yurewicz, K.L. Amphibian species richness across environmental gradients. Oikos 2007, 116, 1697–1712. [Google Scholar] [CrossRef]
- Ramsden, C.; Beriault, K.; Bogart, J.P. A nonlethal method of identification of Ambystoma laterale, Ambystoma jeffersonianum and sympatric unisexuals. Mol. Ecol. Notes. 2006, 6, 261–264. [Google Scholar] [CrossRef]
- Teltser, C.; Greenwald, K.R. Survivorship of ploidy-variable unisexual ambystoma salamanders across developmental stages. Herpetologica 2015, 71, 81–87. [Google Scholar] [CrossRef]
- Julian, S.E.; King, T.L. Novel tetranucleotide microsatellite DNA markers for the wood frog, Rana sylvatica. Mol Ecol. Notes. 2003, 3, 256–258. [Google Scholar] [CrossRef]
- Lowcock, L.A.; Licht, L.E.; Bogart, J.P. Nomenclature in hybrid complexes of Ambystoma (Urodela, Ambystomatidae)-No case for the erection of hybrid species. Syst. Zool. 1987, 36, 328–336. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package 1.7.2. In R Foundation for Statistical Computing; 2022; Volume 34. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2021. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 4 October 2022).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. 2022. Available online: https://cran.r-project.org/package=DHARMa (accessed on 4 October 2022).
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). 2020. Available online: https://cran.r-project.org/package=AICcmodavg (accessed on 4 October 2022).
- Agostinelli, C.; Lund, U. R Package “Circular”: Circular Statistics (Version 0.4-93). 2017. Available online: https://r-forge.r-project.org/projects/circular/ (accessed on 4 October 2022).
- Buss, N.; Swierk, L.; Hua, J. Amphibian breeding phenology influences offspring size and response to a common wetland contaminant. Front. Zool. 2021, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.K.; Saenz, D.; Rudolf, V.H.W. Shifts in phenological distributions reshape interaction potential in natural communities. Ecol. Lett. 2018, 21, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.K.; Rudolf, V.H.W. Shifts in phenological mean and synchrony interact to shape competitive outcomes. Ecology 2019, 100, e02826. [Google Scholar] [CrossRef] [PubMed]
- John-Alder, H.B.; Morin, P.J.; Lawler, S. Thermal physiology, phenology and distribution of tree frogs. Am. Nat. 1988, 132, 506–520. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Holzapfel, C.M. Genetic shift in photoperiodic response correlated with global warming. Proc. Natl. Acad. Sci. USA 2001, 98, 14509–14511. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C.; Richardson, J.L.; Freidenfelds, N.A. Plasticity and genetic adaptation mediate amphibian and reptile responses to climate change. Evol. Appl. 2014, 7, 88–103. [Google Scholar] [CrossRef]
- Morbey, Y.E.; Ydenberg, R.C. Protandrous arrival timing to breeding areas: A review. Ecol. Lett. 2001, 4, 663–673. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Structure and Dynamics of Two Breeding Populations of the Eastern Tiger Salamander, Ambystoma tigrinum. Copeia 1983, 1983, 608. [Google Scholar] [CrossRef]
- Williams, R.N.; Gopurenko, D.; Kemp, K.R.; Williams, B.; DeWoody, J.A. Breeding chronology, sexual dimorphism, and genetic diversity of congeneric ambystomatid salamanders. J. Herpetol. 2009, 43, 438–449. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Drake, D.L. Structure and dynamics of Lithobates sylvaticus (Wood Frog) at the periphery of its range in Missouri. Southeast. Nat. 2015, 14, 329–341. [Google Scholar] [CrossRef]
- Hocking, D.J.; Rittenhouse, T.A.G.; Rothermel, B.B.; Johnson, J.R.; Conner, C.A.; Harper, E.B.; Semlitsch, R.D. Breeding and recruitment phenology of amphibians in Missouri oak-hickory forests. Am. Midl. Nat. 2008, 160, 41–60. [Google Scholar] [CrossRef]
- Paton, P.; Stevens, S.; Longo, L. Seasonal phenology of amphibian breeding and recruitment at a pond in Rhode Island. Northeast. Nat. 2000, 7, 255. [Google Scholar] [CrossRef]
- Buchinger, T.J.; Hondorp, D.W.; Krueger, C.C. Local diversity in phenological responses of migratory lake sturgeon to warm winters. Oikos 2022, 2022, e08977. [Google Scholar] [CrossRef]
- Schiesari, L. Pond canopy cover: A resource gradient for anuran larvae. Freshw. Biol. 2006, 51, 412–423. [Google Scholar] [CrossRef]
- Werner, E.E.; Glennemeier, K.S. Influence of forest canopy cover on the breeding pond distributions of several amphibian species. Copeia 1999, 1999, 1–12. [Google Scholar] [CrossRef]
- Skelly, D.K. Microgeographic countergradient variation in the wood frog, Rana sylvatica. Evolution 2004, 58, 160–165. [Google Scholar]
- Urban, M.C. Microgeographic adaptations of spotted salamander morphological defenses in response to a predaceous salamander and beetle. Oikos 2010, 119, 646–658. [Google Scholar] [CrossRef]
- Hardy, L.M.; Raymond, L.R. The breeding migration of the mole salamander, Ambystoma talpoideum,in Louisiana. J. Herpetol. 1980, 14, 327. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Scott, D.E.; Pechmann, J.H.K.; Gibbons, J.W. Phenotypic variation in the arrival time of breeding salamanders-individual repeatability and environmental influences. J. Anim. Ecol. 1993, 62, 334–340. [Google Scholar] [CrossRef]
- Briggler, J.T.; Johnson, J.E.; Rambo, D.D. Demographics of a ringed salamander (Ambystoma annulatum) breeding migration. Southwest. Nat. 2004, 49, 209–217. [Google Scholar] [CrossRef]
- Uzzell, T.M.; Goldblatt, S.M. Serum Proteins of Salamanders of the Ambystoma jeffersonianum Complex, and the Origin of the Triploid Species of this Group. Evolution 1967, 21, 345. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.J.; Zydlewski, J.D.; Calhoun, A.J.K. Using Passive Integrated Transponder (PIT) systems for terrestrial detection of blue-spotted salamanders (Ambystoma laterale) in situ. Herpetol. Conserv. Biol. 2014, 9, 97–105. [Google Scholar]
- Van Gelder, J.J.; Olders, J.H.J.; Bosch, J.W.G.; Starmans, P.W. Behavior and body temperature of the hibernating common toads Bufo bufo. Holarct. Ecol. 1986, 9, 225–228. [Google Scholar]
- Marquez, R.; Beltran, J.F.; Llusia, D.; Penna, M.; Narins, P.M. Synthetic rainfall vibrations evoke toad emergence. Curr. Biol. 2016, 26, R1270–R1271. [Google Scholar] [CrossRef]
- Grant, R.; Halliday, T.; Chadwick, E. Amphibians’ response to the lunar synodic cycle-a review of current knowledge, recommendations, and implications for conservation. Behav. Ecol. 2013, 24, 53–62. [Google Scholar] [CrossRef]
- Onorati, M.; Vignoli, L. The darker the night, the brighter the stars: Consequences of nocturnal brightness on amphibian reproduction. Biol. J. Linn. 2017, 120, 961–976. [Google Scholar] [CrossRef]
- Beebee, T.J.C. Amphibian breeding and climate. Nature 1995, 374, 219–220. [Google Scholar] [CrossRef]
- Arietta, A.Z.A.; Freidenburg, L.K.; Urban, M.C.; Rodrigues, S.B.; Rubinstein, A.; Skelly, D.K. Phenological delay despite warming in wood frog Rana sylvatica reproductive timing: A 20-year study. Ecography 2020, 43, 1791–1800. [Google Scholar] [CrossRef]
- Vasconcelos, D.; Calhoun, A.J.K. Movement patterns of adult and juvenile Rana sylvatica (LeConte) and Ambystoma maculatum (Shaw) in three restored seasonal pools in Maine. J. Herpetol. 2004, 38, 551–561. [Google Scholar] [CrossRef]
- Todd, B.D.; Winne, C.T. Ontogenetic and interspecific variation in timing of movement and responses to climatic factors during migrations by pond-breeding amphibians. Can. J. Zool. 2006, 84, 715–722. [Google Scholar] [CrossRef]
- Boone, M.D.; Scott, D.E.; Niewiarowski, P.H. Effects of hatching time for larval ambystomatid salamanders. Copeia 2002, 2002, 511–517. [Google Scholar] [CrossRef]
- Alford, R.A. Variation in predator phenology affects predator performance and prey community composition. Ecology 1989, 70, 206–219. [Google Scholar] [CrossRef]


| Species-Sex Model | Temp. Lag | Precip. Lag | Ord. Day | Moon | K | AICc | Delta AICc | AICc Wt |
|---|---|---|---|---|---|---|---|---|
| Alat 1 | 2-day mean | day of mig. | yes | no | 5 | 732.99 | 0.00 | 0.60 |
| Alat 2 | 2-day mean | day of mig. | yes | NS | 7 | 734.79 | 1.80 | 0.24 |
| Atig F 1 | 4-day mean | day of mig. | yes | no | 5 | 204.71 | 0.00 | 0.28 |
| Atig F 2 | 5-day mean | day of mig. | yes | no | 5 | 205.04 | 0.33 | 0.24 |
| Atig F 3 | 4-day mean | day of mig. | yes | NS | 7 | 206.13 | 1.42 | 0.14 |
| Atig F 4 | 5-day mean | day of mig. | yes | NS | 7 | 206.31 | 1.60 | 0.13 |
| Atig M 1 | 2-day mean | day of mig. | yes | no | 5 | 203.77 | 0.00 | 0.29 |
| Atig M 2 | 2-day mean | day of mig. | yes | NS | 7 | 204.15 | 0.38 | 0.24 |
| Rsyl F 1 | day of migration | 3-day sum | yes | NS | 8 | 517.63 | 0.00 | 0.28 |
| Rsyl F 2 | day of migration | 3-day sum | yes | no | 6 | 518.24 | 0.60 | 0.21 |
| Rsyl F 3 | day of migration | 2-day sum | yes | NS | 8 | 518.24 | 0.60 | 0.21 |
| Rsyl F 4 | day of migration | 2-day sum | yes | no | 6 | 519.29 | 1.66 | 0.12 |
| Rsyl M 1 | day of migration | 2-day sum | yes | no | 5 | 609.75 | 0.00 | 0.28 |
| Rsyl M 2 | day of migration | 3-day sum | yes | no | 5 | 610.19 | 0.44 | 0.22 |
| Rsyl M 3 | day of migration | 2-day sum | yes | NS | 7 | 610.79 | 1.05 | 0.16 |
| Rsyl M 4 | day of migration | 3-day sum | yes | NS | 7 | 611.47 | 1.72 | 0.12 |
| Rsyl M 5 | day of migration | 4-day sum | yes | no | 5 | 611.53 | 1.78 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benard, M.F.; Greenwald, K.R. Environmental Drivers of Amphibian Breeding Phenology across Multiple Sites. Diversity 2023, 15, 253. https://doi.org/10.3390/d15020253
Benard MF, Greenwald KR. Environmental Drivers of Amphibian Breeding Phenology across Multiple Sites. Diversity. 2023; 15(2):253. https://doi.org/10.3390/d15020253
Chicago/Turabian StyleBenard, Michael F., and Katherine R. Greenwald. 2023. "Environmental Drivers of Amphibian Breeding Phenology across Multiple Sites" Diversity 15, no. 2: 253. https://doi.org/10.3390/d15020253
APA StyleBenard, M. F., & Greenwald, K. R. (2023). Environmental Drivers of Amphibian Breeding Phenology across Multiple Sites. Diversity, 15(2), 253. https://doi.org/10.3390/d15020253








