Effects of Floods on Zooplankton Community Structure in the Huayanghe Lake
Abstract
1. Introduction
2. Materials and Methods
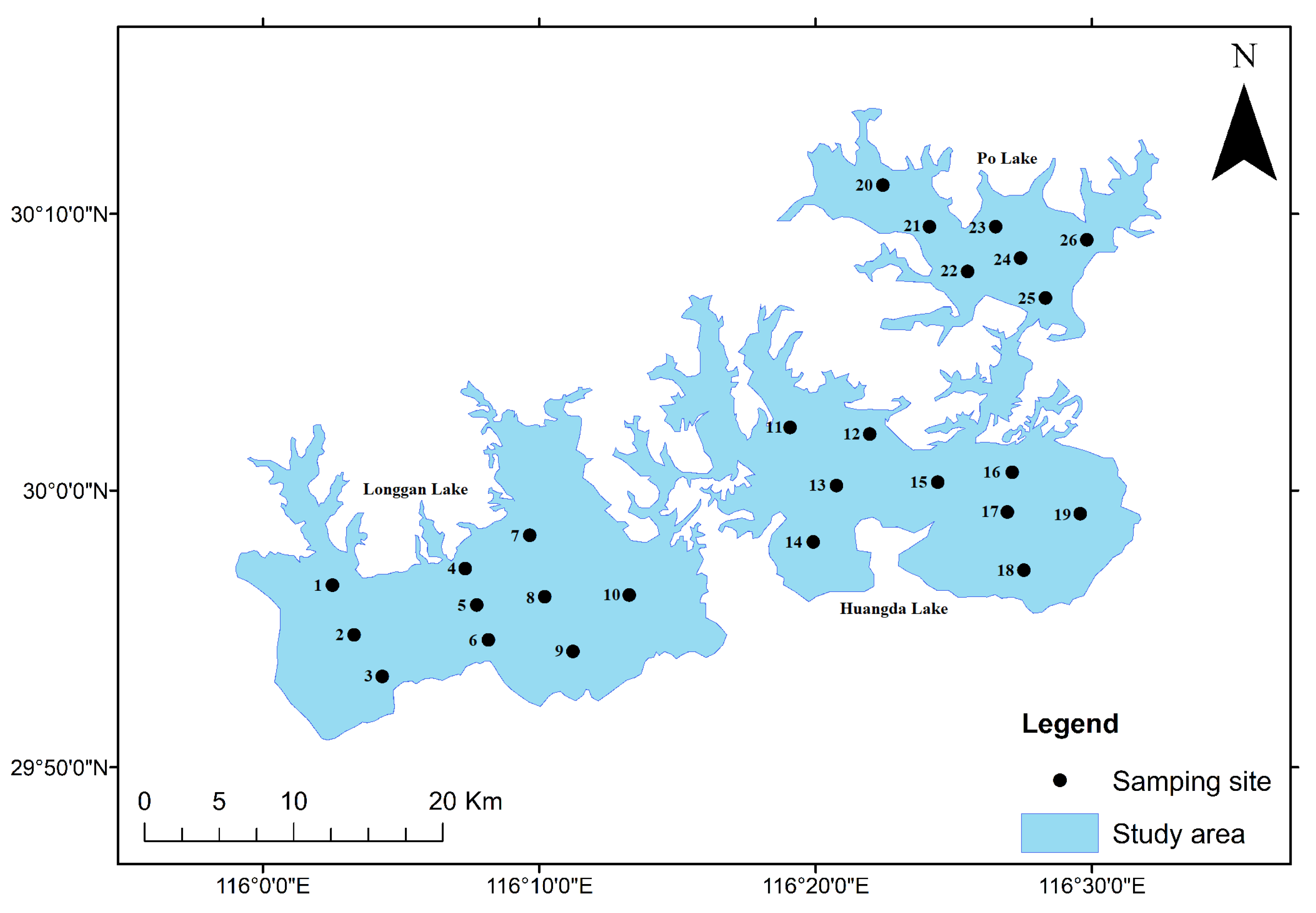
2.1. Study Area
2.2. Sampling Points and Sampling Time
2.3. Sample Collection and Processing
2.4. Data Processing
3. Results
3.1. Environmental Parameters of Huayanghe Lake
3.2. Zooplankton Species Composition and Dominant Species
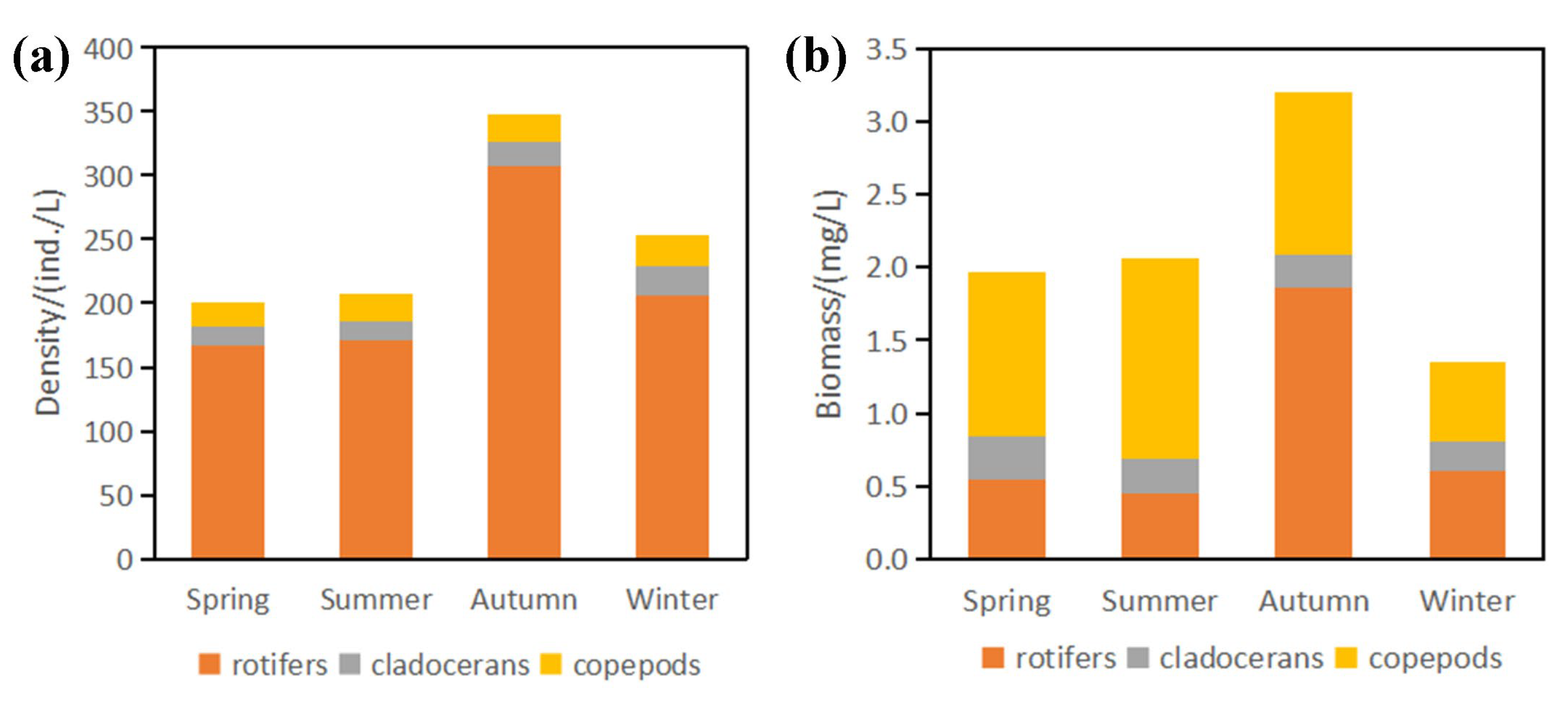
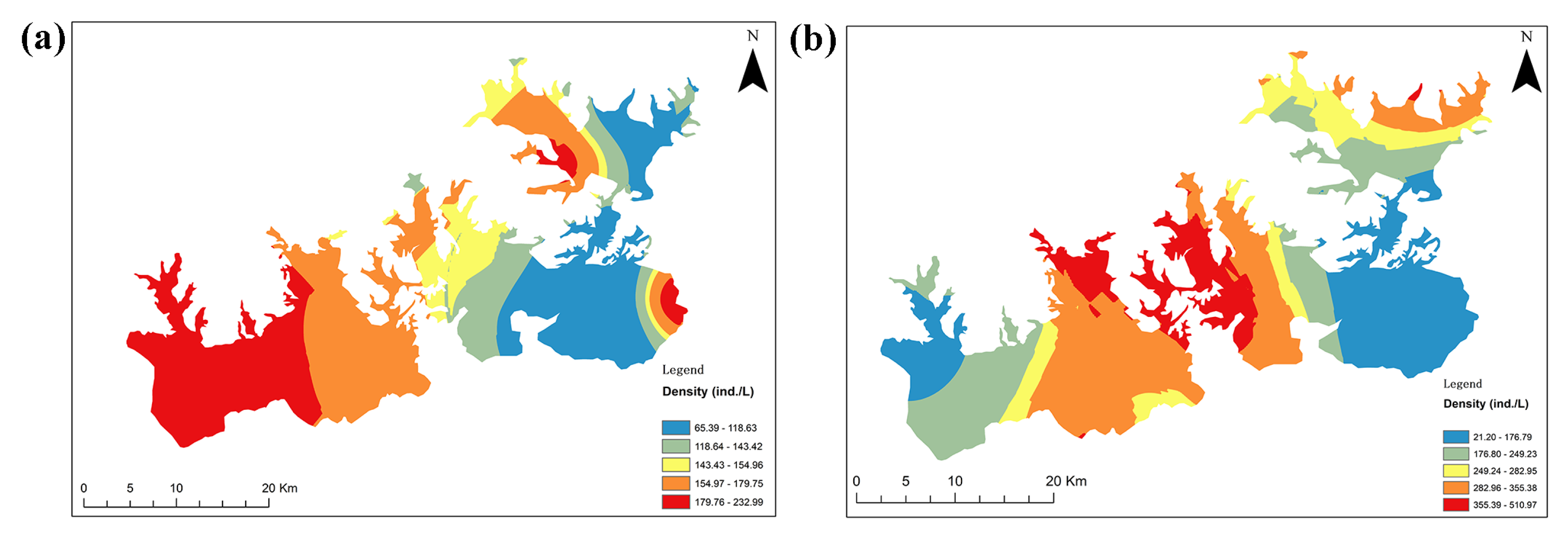
3.3. Zooplankton Density, Biomass and Species Diversity
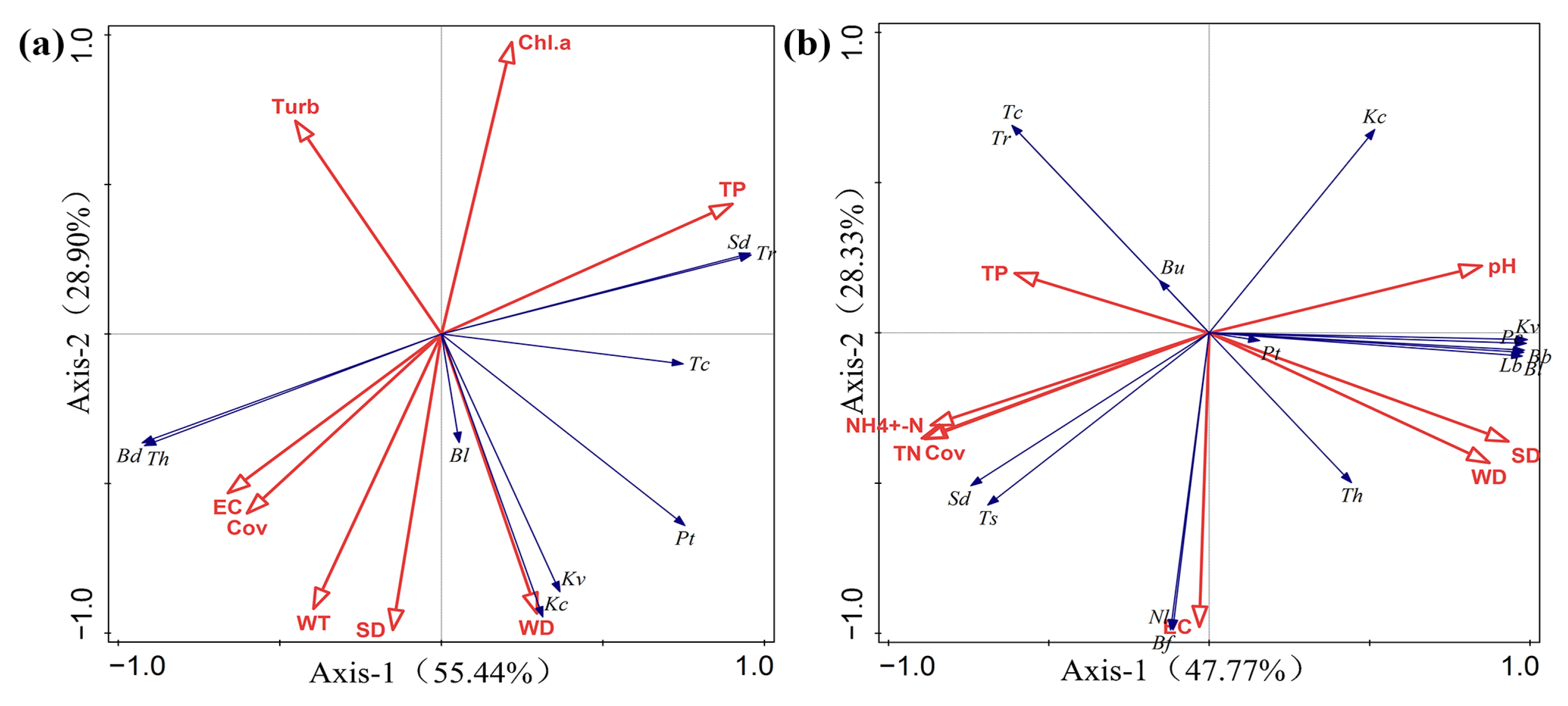
3.4. Relationship between the Zooplankton Community Structure and Environmental Parameters
4. Discussion
4.1. Effects of Floods on Environmental Parameters
4.2. Effects of Floods on the Zooplankton Community Structure
4.3. Relationship between Zooplankton and Environmental Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, B.; Yang, G.S.; Wan, R.R.; Lai, X.J.; Wagner, P.D. Impacts of hydrological alteration on ecosystem services changes of a large river-connected lake (Poyang Lake), China. J. Environ. Manag. 2022, 310, 114750. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, J.S.; Zhang, H.; Zhou, Q. Community structure and the water quality during different hydrological periods in Poyang Lake. Acta. Hydrobiol. Sin. 2021, 45, 1093–1103. [Google Scholar]
- Yang, S.; Hu, L.M.; Gao, R.; Liu, Q. Study of the problems and countermeasures in the ecosystem of Dongting Lake wetland. Environ. Sci. Manag. 2006, 31, 111–113. [Google Scholar]
- Wan, R.R.; Yang, G.S.; Wang, X.L.; Qin, N.X.; Dai, X. Progress of research on the relationship between the Yangtze River and its connected lakes in the middle reaches. J. Lake Sci. 2014, 26, 1–8. [Google Scholar]
- Zhang, D.M.; Zhou, L.Z.; Song, Y.W. Effect of water level fluctuations on temporal-spatial patterns of foraging activities by the wintering hooded crane (Grus monacha). Avian. Res. 2015, 6, 169–177. [Google Scholar] [CrossRef]
- Cui, Y.H.; Wang, J. Influence of the sluice on water level and area of Yangtze river-connected lakes: A case study in Shengjin Lake. J. Water Resour Water Eng. 2018, 29, 47–52. [Google Scholar]
- Krylov, A.V.; Mendsaihan, B.; Ayushsuren, C.; Tsvetkov, A.I. Zooplankton of Pulsating Lakes Orog and Tatsyn-Tsagaan (West Mongolia) in the period of the beginning of the stabilization of water-level regime. Inland. Water. Biol. 2020, 13, 242–250. [Google Scholar] [CrossRef]
- Carpenter, S.R. Phosphorus control is critical to mitigating eutrophication. Proc. Natl. Acad. Sci. USA 2008, 105, 1039–1040. [Google Scholar] [CrossRef]
- Tong, Y.L.; Liang, T.; Wang, L.Q.; Li, K.X. Simulation on phosphorus release characteristics of Poyang Lake sediments under variable water levels and velocities. J. Geogr. Sci. 2017, 27, 697–710. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Mitchell, A.M. The effects of drying and re-flooding on the sediment and soil nutrient dynamics of lowland river-floodplain systems: A synthesis. Regul. Rivers: Res. Mgmt. 2000, 16, 457–467. [Google Scholar] [CrossRef]
- Qiu, S.; McComb, A.J. Effects of oxygen concentration on phosphorus release from reflooded air-dried wetland sediments. Mar. Freshwater. Res. 1994, 45, 1319–1328. [Google Scholar] [CrossRef]
- Dube, T.; DeNecker, L.; Van Vuren, J.H.; Wepener, V.; Smit, N.J.; Brendonck, L. Spatial and temporal variation of invertebrate community structure in flood-controlled tropical floodplain wetlands. J. Freshwater Ecol. 2017, 32, 1–15. [Google Scholar] [CrossRef]
- Meng, C.L.; Li, Q.H.; Li, H.M.; Hu, Y.; Li, Y. Comparison of community structure of zooplankton in Shuimodu Reservoir and Daotianhe Reservoir based on the path model. Ecol. Sci. 2021, 40, 1–12. [Google Scholar] [CrossRef]
- Mac Donagh, M.E.; Casco, M.A.; Claps, M.C. Plankton relationships under small water level fluctuations in a subtropical reservoir. Aquat Ecol. 2009, 43, 371–381. [Google Scholar] [CrossRef]
- Baranyi, C.; Hein, T.; Holarek, C.; Keckeis, S.; Schiemer, F. Zooplankton biomass and community structure in a Danube River floodplain system: Effects of hydrology. Freshw. Biol. 2002, 47, 473–482. [Google Scholar] [CrossRef]
- Cavan, E.L.; Henson, S.A.; Belcher, A.; Sanders, R. Role of zooplankton in determining the efficiency of the biological carbon pump. Biogeosciences 2017, 14, 177–186. [Google Scholar] [CrossRef]
- Ndah, A.B.; Meunier, C.L.; Kirstein, I.V.; Göbel, J.; Rönn, L.; Boersma, M. A systematic study of zooplankton-based indices of marine ecological change and water quality: Application to the European marine strategy framework directive (MSFD). Ecol. Indic. 2022, 135, 108587. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Zhou, L.Z. Biodiversity and Conservation in Anqing Floodplain Wetlands; Hefei University of Technology Press: Hefei, China, 2010; pp. 1–64. [Google Scholar]
- Wang, J.Q.; Wu, J.P.; Yu, Y.B.; Wang, T.Y.; Cheng, G. The specific list, quantitative distribution and change of zooplankton in the season of spring and autumn in Poyang Lake. J. Lake Sci. 2003, 15, 345–352. [Google Scholar]
- Goswami, S.C. Zooplankton Methodology, Collection and Identification-A Field Manual; National Institute of Oceanography: Dona Paula, India, 2004; p. 26. [Google Scholar]
- Ruttner-Kolisko, A. Suggestions for biomass calculations of planktonic rotifers. Arch. Fur Hydrobiol. Beih. 1977, 21, 71–76. [Google Scholar]
- Downing, J.A. Assessment of secondary production: The first step. A manual on methods for the assessment of secondary productivity in fresh waters. IBP Handb. 1984, 17, 1–18. [Google Scholar]
- Zhang, W.C.; Zhang, C.X.; Xiao, T. Role of microzooplankton in marine planktonic ecosystem. Adv. Earth Sci. 2009, 24, 1195–1201. [Google Scholar]
- Wang, J.J. Chinese Freshwater Rotifers; Science Press: Beijing, China, 1961; pp. 24–289. [Google Scholar]
- Du, N.S. Index of Common Freshwater Cladocera in China; Science Press: Beijing, China, 1973; pp. 33–89. [Google Scholar]
- Shen, J.R. Fauna of China Arthropoda Crustacea Freshwater Copepoda; Science Press: Beijing, China, 1979; pp. 297–696. [Google Scholar]
- Jersabek, C.D.; Leitner, M.F. The Rotifer World Catalog; World Wide Web Electronic Publication. 2013. Available online: http://rotifera.hausdernatur.at/ (accessed on 5 December 2022).
- Segers, H.; De Smet, W.H.; Fischer, C.; Fontaneto, D.; Michaloudi, E.; Wallace, R.L.; Jersabek, C.D. Towards a list of available names in zoology, partim Phylum Rotifera. Zootaxa 2012, 3179, 61–68. [Google Scholar] [CrossRef]
- Chatterjee, T.; Kotov, A.A.; Van Damme, K.; Chandrasekhar, S.V.A.; Padhye, S. An annotated checklist of the Cladocera (Crustacea: Branchiopoda) from India. Zootaxa 2013, 3667, 1–89. [Google Scholar] [CrossRef]
- Liu, X.Y.; Tang, P.; Liu, Y.H.; Xie, W.C.; Chen, C.; Li, T.; He, Q.P.; Bao, J.; Tiraferri, A.; Liu, B.C. Efficient removal of organic compounds from shale gas wastewater by coupled ozonation and moving-bed-biofilm submerged membrane bioreactor. Bioresour. Technol. 2022, 344, 126191. [Google Scholar] [CrossRef]
- He, C.Q.; Chen, Y.N.; Liu, C.; Jiang, Y.; Yin, R.Y.; Qiu, T.S. The role of reagent adding sequence in the NH4+-N recovery by MAP method. Sci. Rep. 2020, 10, 7672. [Google Scholar] [CrossRef]
- Chen, H.W.; Zhao, L.L.; Yu, F.B.; Du, Q.L. Detection of phosphorus species in water: Technology and strategies. Analyst 2019, 144, 7130–7148. [Google Scholar] [CrossRef]
- Rousso, B.Z.; Bertone, E.; Stewart, R.; Aguiar, A.; Chuang, A.; Hamilton, D.P.; Burford, M.A. Chlorophyll and phycocyanin in-situ fluorescence in mixed cyanobacterial species assemblages: Effects of morphology, cell size and growth phase. Water. Res. 2022, 212, 118127. [Google Scholar] [CrossRef]
- De Silva-Dávila, R.; Franco-Gordo, C.; Hochberg, F.G.; Godínez-Domínguez, E.; Avendaño-Ibarra, R.; Gómez-Gutiérrez, J.; Robinson, C.J. Cephalopod paralarval assemblages in the Gulf of California during 2004–2007. Mar. Ecol. Prog. Ser. 2015, 520, 123–141. [Google Scholar] [CrossRef]
- Margalef, D.R. Information theory in ecology. Gen. Syst. 1957, 32, 374–559. [Google Scholar]
- Pielou, E.C. An Introduction to Mathematical Ecology; Wiley-Interscience, Inc.: New York, NY, USA, 1969. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell. Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Zhu, W.J.; Hu, W.G.; Zhang, Y.D.; Jia, W.P.; Ma, D.C.; Gao, Y.; You, Y.; Zhang, X.R. Characteristics of zooplankton communities and their responses to water environmental factors during different hydrological periods of the Daquangou Reservoir. Asian. J. Ecotoxicol. 2020, 6, 243–251. [Google Scholar]
- Burns, N.M.; Rutherford, J.C.; Clayton, J.S. A monitoring and classification system for New Zealand lakes and reservoirs. Lake Reserv. Manage. 1999, 15, 255–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.X.; Dong, J.F.; Yang, H.; Van Zwieten, L.; Lu, H.; Alshameri, A.; Zhan, Z.H.; Chen, X.; Jiang, X.D.; et al. A critical review of methods for analyzing freshwater eutrophication. Water 2021, 13, 225. [Google Scholar] [CrossRef]
- Xiong, W.; Ni, P.; Chen, Y.Y.; Gao, Y.C.; Shan, B.Q.; Zhan, A.B. Zooplankton community structure along a pollution gradient at fine geographical scales in river ecosystems: The importance of species sorting over dispersal. Mol. Ecol. 2017, 26, 4351–4360. [Google Scholar] [CrossRef]
- Zhang, K. Characteristics of Zooplankton Communities in Tongjiang Lakes in the Anhui Section of Yangtze River and Their Relationship with Environmental Factors. Ph.D. Thesis, Anhui University, Hefei, China, 2019. [Google Scholar]
- Fang, Y.X.; He, C.Q.; Liang, X.; Jia, H.; Ma, Z.F.; Zhan, Y.W.; Zhang, Y.K.; Zhang, X.X. The Purifying Effect of Polluted Water by the Aquatic Plants. J. Hydroecol. 2010, 3, 36–40. [Google Scholar]
- Wang, Y.T.; Wang, W.C.; Zhou, Z.Z.; Wan, X.; Zhang, Y.X. Effect of fast restoration of aquatic vegetation on phytoplankton community after removal of purse seine culture in Huayanghe Lakes. Sci. Total Environ. 2021, 768, 144024. [Google Scholar] [CrossRef]
- Usenko, O.M.; Sakevich, A.I. Allelopathic influence of higher aquatic plants on the functional activity of plankton algae. Hydrobiology 2005, 41, 54–66. [Google Scholar]
- Guo, W.L.; Zhou, Z.Z.; Chen, J.W.; Zheng, X.D.; Ye, X.X. Effects of extreme flooding on aquatic vegetation cover in Shengjin Lake, China. Hydrol. Process. 2022, 36, e14459. [Google Scholar]
- Peng, X.; Ning, X.R.; Sun, J.; Le, F.F. Responses of phytoplankton growth on nutrient enrichments in the northern South China Sea. Acta Ecol. Sin. 2006, 26, 3959–3968. [Google Scholar]
- Golubkov, M.; Golubkov, S. Impact of the construction of new port facilities on primary production of plankton in the Neva Estuary (Baltic Sea). Front. Mar. Sci. 2022, 9, 851043. [Google Scholar] [CrossRef]
- Yan, D.H.; Deng, W.; He, Y. The evolution of pH-value of natural water and its course of formation Tumen water systems. Agro Environ. Prot. 2001, 20, 4–6. [Google Scholar]
- Huang, C.; Liu, M.D.; Liu, S.P.; Chen, Z.X.; Liang, X.Y. Prediction and analysis of the influence of suspension on aquatic life in waterway regulation in the middle reaches of the Yangtze River: Taking the section from Xinzhou to Jiujiang river as an example. Environ. Impact Assess. 2019, 41, 50–55+60. [Google Scholar]
- Boon, P.I.; Bunn, S.E.; Green, J.D.; Shiel, R.J. Consumption of cyanobacteria by freshwater zooplankton: Implications for the success of “top-down” control of cyanobacterial blooms in Australia. Mar. Freshw. Res. 1994, 45, 875–887. [Google Scholar] [CrossRef]
- AL-Keriawy, H.A.H. Impact of fish farming in floating cages on zooplankton community in Euphrates River, Iraq. IOP. Conf. Ser. Earth. Environ Sci. 2021, 722, 012042. [Google Scholar] [CrossRef]
- Gazonato Neto, A.J.; Silva, L.C.D.; Saggio, A.A.; Rocha, O. Zooplankton communities as eutrophication bioindicators in tropical reservoirs. Biota Neotrop. 2014, 14, 1–12. [Google Scholar] [CrossRef]
- Ortega-Cisneros, K.; Scharler, U.M.; Whitfield, A.K. Inlet mouth phase influences density, variability and standing stocks of plankton assemblages in temporarily open/closed estuaries. Estuar. Coast. Shelf. Sci. 2014, 136, 139–148. [Google Scholar] [CrossRef]
- Viroux, L. Zooplankton development in two large lowland rivers, the Moselle (France) and the Meuse (Belgium). J. Plankton Res. 1997, 19, 1743–1762. [Google Scholar] [CrossRef]
- Ge, Y.W.; Zhang, K.; Yang, X.D. Long-term succession of aquatic plants reconstructed from palynological records in a shallow freshwater lake. Sci. Total Environ. 2018, 643, 312–323. [Google Scholar] [CrossRef]
- Sun, X.Y.; Arnott, S.E. Interactive effects of increased salinity and heatwaves on freshwater zooplankton communities in simultaneous and sequential treatments. Freshw. Biol. 2022, 67, 1604–1617. [Google Scholar] [CrossRef]
- Xu, M. The Research on the Community Structure of Metazoan Zooplanktons in Lake Jiaoganghu and Lake Huayanghe. Master’s Thesis, Anhui University, Hefei, China, 2016. [Google Scholar]
- Zhang, Y.X. The Effect of Removing the Fisher Purse Seine on Zooplankton Communities Structure in the Huayanghe Lakes. Master’s Thesis, Anhui University, Hefei, China, 2021. [Google Scholar]
- Paidere, J. Influence of flooding frequency on zooplankton in the floodplains of the Daugava River (Latvia). Acta Zool Litu. 2009, 19, 306–313. [Google Scholar] [CrossRef]
- Tockner, K.; Malard, F.; Ward, J.V. An extension of the flood pulse concept. Hydrol. Process. 2000, 14, 2861–2883. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Bini, L.M.; Bozelli, R.L. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 2007, 579, 1–13. [Google Scholar] [CrossRef]
- Higuti, J.; Velho, L.F.M.; Lansac-Toha, F.A.; Martens, K. Pleuston communities are buffered from regional flood pulses: The example of ostracods in the Parana River floodplain, Brazil. Freshwat. Biol. 2007, 52, 1930–1943. [Google Scholar] [CrossRef]
- da Conceição, E.d.O.; Higuti, J.; de Campos, R.; Martens, K. Effects of flood pulses on persistence and variability of pleuston communities in a tropical floodplain lake. Hydrobiologia 2018, 807, 175–188. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ralph, T.J.; Ryder, D.S.; Hunter, S.J.; Shiel, R.J.; Segers, H. Spatial dissimilarities in plankton structure and function during flood pulses in a semi-arid floodplain wetland system. Hydrobiologia 2015, 747, 19–31. [Google Scholar] [CrossRef]
- Bozelli, R.L.; Thomaz, S.M.; Padial, A.A.; Lopes, P.M.; Bini, L.M. Floods decrease zooplankton beta diversity and environmental heterogeneity in an Amazonian floodplain system. Hydrobiologia 2015, 753, 233–241. [Google Scholar] [CrossRef]
- Braghin, L.S.M.; Figueiredo, B.R.S.; Meurer, T.; Michelan, T.S.; Simoes, N.R.; Bonecker, C.C. Zooplankton diversity in a dammed river basin is maintained by preserved tributaries in a tropical floodplain. Aquat. Ecol. 2015, 49, 175–187. [Google Scholar] [CrossRef]
- O’Hare, M.T.; Baattrup-Pedersen, A.; Baumgarte, I.; Freeman, A.; Gunn, I.D.M.; Lázár, A.N.; Sinclair, R.; Wade, A.J.; Bowes, M.J. Responses of aquatic plants to eutrophication in rivers: A revised conceptual model. Front. Plant. Sci. 2018, 9, 451. [Google Scholar] [CrossRef]
- Pretty, T.J. Boreal Subarctic Lake Water Quality, Zooplankton Communities, and Benthic Macroinvertebrate Communities in areas Impacted by Wildfire. Master’s Thesis, Wilfrid Laurier University, Waterloo, ON, Canada, 2020. [Google Scholar]
- Chiba, S.; Batten, S.; Martin, C.S.; Ivory, S.; Miloslavich, P.; Weatherdon, L.V. Zooplankton monitoring to contribute towards addressing global biodiversity conservation challenges. J. Plankton Res. 2018, 40, 509–518. [Google Scholar] [CrossRef]
- Fullgrabe, L.; Grosjean, P.; Gobert, S.; Lejeune, P.; Leduc, M.; Engels, G.; Dauby, P.; Boissery, P.; Richir, J. Zooplankton dynamics in a changing environment: A 13-year survey in the northwestern Mediterranean Sea. Mar. Environ Res. 2020, 159, 104962. [Google Scholar] [CrossRef]
- Dorche, E.E.; Shahraki, M.Z.; Farhadian, O.; Keivany, Y. Seasonal variations of plankton structure as bioindicators in Zayandehrud Dam Lake, Iran. Limnol. Rev. 2018, 18, 157–165. [Google Scholar] [CrossRef]
- Song, J.; Hou, C.Y.; Liu, Q.; Wu, X.F.; Wang, Y.J.; Yi, Y.J. Spatial and temporal variations in the plankton community because of water and sediment regulation in the lower reaches of Yellow River. J. Clean. Prod. 2020, 261, 120972. [Google Scholar] [CrossRef]
- Taipale, S.J.; Ventelä, A.M.; Litmanen, J.; Anttila, L. Poor nutritional quality of primary producers and zooplankton driven by eutrophication is mitigated at upper trophic levels. Ecol. Evol. 2022, 12, e8687. [Google Scholar] [CrossRef]
- Cai, Q.H. Multivariate analysis for the relationship between zooplankton and phytoplankton of Lake Donghu, Wuhan. J. Grad. Sch. Acad. Sinica. 1995, 12, 97–102. [Google Scholar]


| Environmental Factors/Times | EWF | RWF | ||||||
|---|---|---|---|---|---|---|---|---|
| Spring May 2020 | Summer Aug. 2020 | Autumn Nov. 2020 | Winter Feb. 2021 | Spring May 2021 | Summer Aug. 2021 | Autumn Nov. 2021 | Winter Feb. 2022 | |
| WT/(°C) | 24.57 ± 0.39 | 32.47 ± 0.65 | 23.82 ± 1.21 | 9.33 ± 0.19 | 18.26 ± 0.43 | 33.26 ± 1.89 | 19.90 ± 0.58 | 11.79 ± 0.98 |
| pH | 7.09 ± 0.16 | 9.06 ± 0.52 | 8.64 ± 0.51 | 8.30 ± 0.25 | 8.94 ± 0.22 | 8.65 ± 0.41 | 8.72 ± 0.40 | 7.56 ± 0.13 |
| DO/(mg/L) | 8.94 ± 0.72 | 10.38 ± 2.33 | 9.16 ± 0.87 | 11.51 ± 0.05 | 9.34 ± 0.20 | 9.21 ± 2.12 | 10.05 ± 0.31 | 11.27 ± 0.42 |
| EC/(µs/cm) | 240.20 ± 53.70 | 188.07 ± 30.39 | 181.08 ± 18.86 | 141.6 ± 15.19 | 229.55 ± 26.72 | 237.50 ± 36.78 | 141.33 ± 29.03 | 148.68 ± 30.14 |
| SD/m | 0.39 ± 0.19 | 0.93 ± 0.43 | 0.73 ± 0.15 | 0.06 ± 0.03 | 0.37 ± 0.07 | 0.63 ± 0.37 | 0.57 ± 0.10 | 0.21 ± 0.10 |
| WD/m | 1.98 ± 0.41 | 5.96 ± 0.87 | 6.77 ± 0.93 | 1.30 ± 0.14 | 2.32 ± 0.19 | 3.05 ± 0.49 | 3.26 ± 0.49 | 3.21 ± 0.58 |
| Turb/(NTU) | 54.81 ± 44.32 | 11.20 ± 7.99 | 12.64 ± 5.29 | 35.49 ± 10.32 | 25.94 ± 6.32 | 10.23 ± 2.31 | 46.52 ± 19.52 | 23.39 ± 13.31 |
| TN/(mg/L) | 1.81 ± 0.51 | 2.39 ± 1.45 | 1.41 ± 0.30 | 1.24 ± 0.38 | 2.36 ± 0.54 | 0.47 ± 0.05 | 0.33 ± 0.18 | 0.31 ± 0.04 |
| NH4+-N/(mg/L) | 0.07 ± 0.05 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.06 ± 0.01 | 0.10 ± 0.05 | 0.04 ± 0 | 0.05 ± 0.02 | 0.01 ± 0 |
| TP/(mg/L) | 0.03 ± 0.07 | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.13 ± 0.05 | 0.05 ± 0.10 | 0.02 ± 0.09 | 0.03 ± 0.03 | 0.06 ± 0.06 |
| Chl.a/(µg/L) | 4.32 ± 4.82 | 3.25 ± 2.65 | 3.74 ± 3.82 | 7.25 ± 5.83 | 2.64 ± 2.03 | 2.77 ± 3.02 | 3.01 ± 0.97 | 5.35 ± 3.95 |
| Cov/(%) | 5 | 35 | 2 | 1 | 10 | 40 | 2 | 1 |
| Sunshine duration/(h) | 6.93 | 7.83 | 5.72 | 1.43 | 6.93 | 7.83 | 5.72 | 1.43 |
| Species | Species Dominance (Y) | |||||||
|---|---|---|---|---|---|---|---|---|
| May 2020 | Aug. 2020 | Nov. 2020 | Feb. 2021 | May 2021 | Aug. 2021 | Nov. 2021 | Feb. 2022 | |
| Trichocerca capucina | 0.045 | 0.031 | - | - | - | 0.020 | - | - |
| Trichocerca stylata | 0.030 | - | - | - | 0.022 | 0.026 | 0.020 | - |
| Trichocerca rousseleti | - | - | 0.091 | 0.068 | - | 0.047 | - | - |
| Brachionus budapestinensis | - | - | - | - | 0.034 | 0.077 | - | - |
| Brachionus urceolaris | - | - | - | - | 0.022 | 0.050 | 0.033 | - |
| Keratella cochlearis | 0.121 | 0.100 | 0.041 | 0.094 | 0.215 | - | 0.058 | 0.026 |
| Keratella valga | - | 0.055 | - | - | 0.028 | 0.056 | 0.061 | 0.035 |
| Notholca labis | - | 0.020 | - | - | - | 0.185 | 0.023 | 0.180 |
| Polyarthra euryptera | - | - | - | - | - | 0.032 | - | - |
| Polyarthra trigla | 0.081 | - | 0.241 | - | 0.090 | - | 0.112 | 0.155 |
| Lecane bulla | - | - | 0.024 | - | 0.024 | - | - | 0.061 |
| Bosmina longirostris | 0.059 | - | - | 0.020 | 0.022 | - | - | |
| Bosmina fatalis | 0.025 | - | - | 0.024 | - | 0.027 | - | - |
| Bosminopsis deitersi | - | - | 0.037 | 0.031 | - | - | 0.095 | |
| Sinocalanus doerrii | 0.025 | - | 0.028 | - | - | 0.023 | - | - |
| Thermocyclops hyalinus | - | 0.022 | - | - | 0.021 | 0.060 | 0.030 | 0.020 |
| Density | Biomass | |||
|---|---|---|---|---|
| r | p | r | p | |
| WT | 0.80 | 0.016 | 0.74 | 0.037 |
| pH | 0.53 | 0.175 | 0.70 | 0.056 |
| DO | −0.49 | 0.220 | −0.29 | 0.489 |
| EC | 0.30 | 0.469 | 0.13 | 0.756 |
| SD | 0.92 | 0.001 | 0.86 | 0.006 |
| WD | 0.99 | <0.001 | 1.00 | <0.001 |
| Turb | −0.84 | 0.010 | −0.93 | 0.001 |
| NH4+-N | −0.82 | 0.012 | −0.92 | 0.001 |
| TN | 0.45 | 0.264 | 0.46 | 0.248 |
| TP | −0.60 | 0.118 | −0.43 | 0.287 |
| Chl.a | −0.91 | 0.002 | −0.86 | 0.006 |
| Cov | 0.49 | 0.216 | 0.53 | 0.174 |
| Sunshine duration | 0.75 | 0.032 | 0.65 | 0.078 |
| Density | Biomass | |||
|---|---|---|---|---|
| r | p | r | p | |
| WT | 0.80 | 0.016 | 0.82 | 0.013 |
| pH | 0.72 | 0.045 | 0.62 | 0.105 |
| DO | −0.63 | 0.098 | −0.56 | 0.146 |
| EC | 0.13 | 0.766 | 0.10 | 0.817 |
| SD | 0.97 | <0.001 | 0.95 | <0.001 |
| WD | 0.18 | 0.664 | 0.31 | 0.458 |
| Turb | 0.03 | 0.944 | −0.06 | 0.887 |
| NH4+-N | 0.53 | 0.180 | 0.39 | 0.334 |
| TN | −0.17 | 0.692 | −0.30 | 0.477 |
| TP | −0.88 | 0.004 | −0.93 | 0.001 |
| Chl.a | −0.71 | 0.048 | −0.62 | 0.102 |
| Cov | 0.45 | 0.268 | 0.44 | 0.274 |
| Sunshine duration | 0.77 | 0.025 | 0.70 | 0.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Zhou, Z.; Chen, W. Effects of Floods on Zooplankton Community Structure in the Huayanghe Lake. Diversity 2023, 15, 250. https://doi.org/10.3390/d15020250
Zhou M, Zhou Z, Chen W. Effects of Floods on Zooplankton Community Structure in the Huayanghe Lake. Diversity. 2023; 15(2):250. https://doi.org/10.3390/d15020250
Chicago/Turabian StyleZhou, Mengmeng, Zhongze Zhou, and Wenwen Chen. 2023. "Effects of Floods on Zooplankton Community Structure in the Huayanghe Lake" Diversity 15, no. 2: 250. https://doi.org/10.3390/d15020250
APA StyleZhou, M., Zhou, Z., & Chen, W. (2023). Effects of Floods on Zooplankton Community Structure in the Huayanghe Lake. Diversity, 15(2), 250. https://doi.org/10.3390/d15020250






