Seed Morphology, Life Form and Distribution in Three Bromheadia Species (Epidendroideae, Orchidaceae)
Abstract
1. Introduction
2. Materials and Methods
3. Results
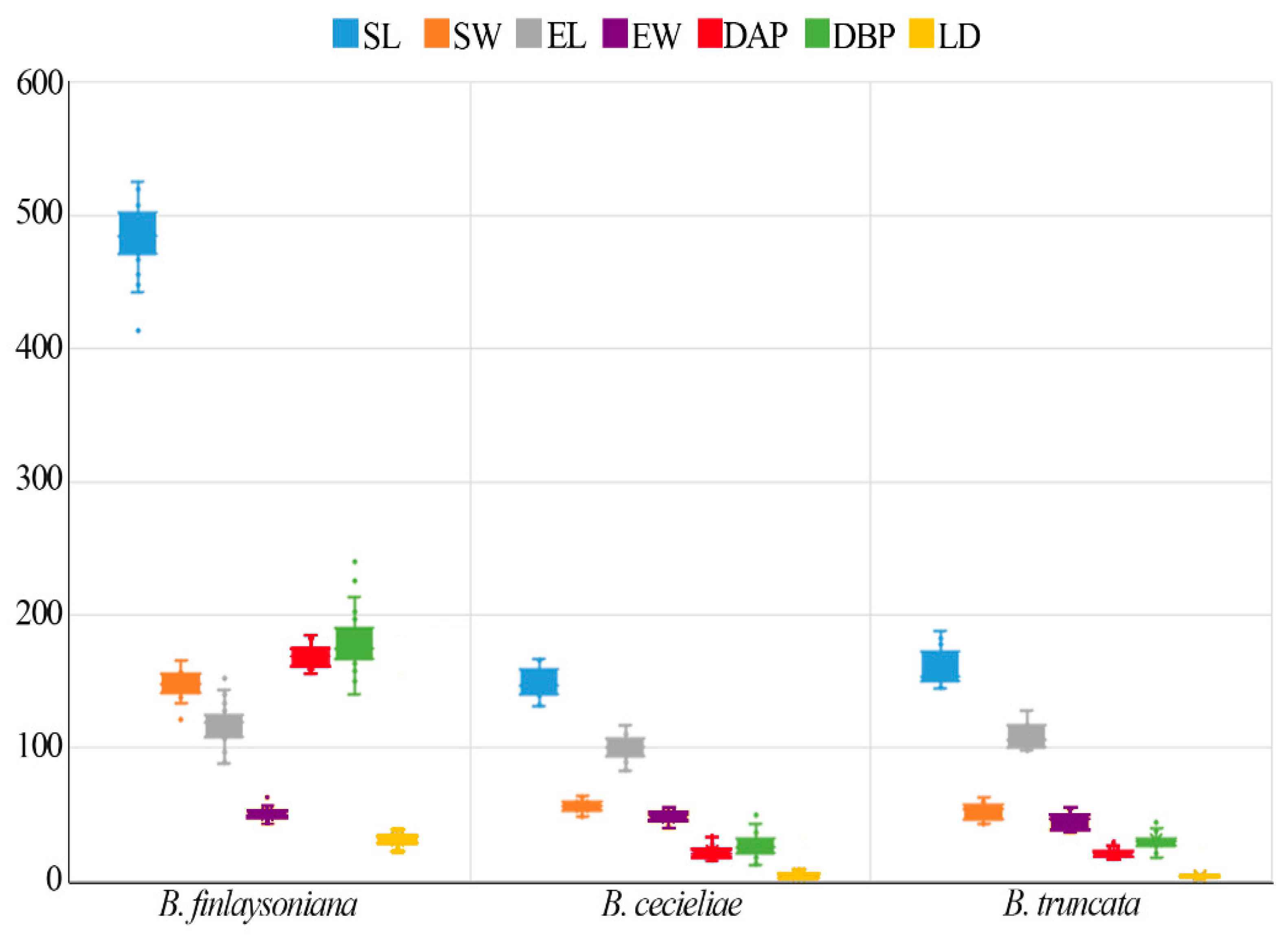
3.1. Seed Morphology
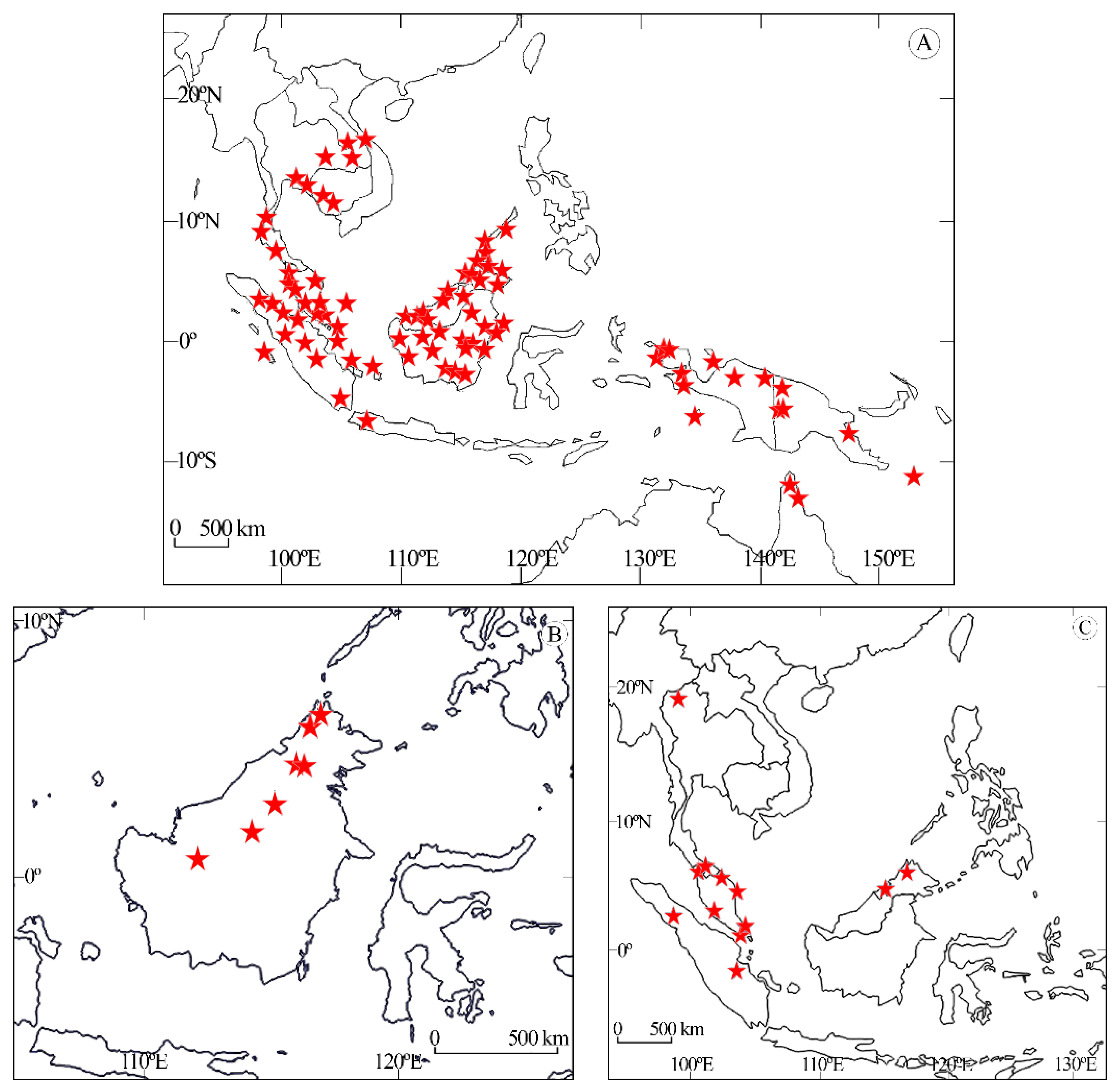
3.2. Distribution of the Species
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Country | Locality | Collector and Number | Voucher |
|---|---|---|---|---|
| B. cecieliae | Indonesia | Borneo, W Kalimantan, Serawai, Sungai Merah | A.C. Church 2114 et al. | NY03998648 |
| Malaysia | Borneo, Mount Kinabalu, Gurulau Spur | J. and M.S. Clemens 50541 | NY03998647 | |
| Malaysia | Tambunan District, Crocker Range | J.H. Beaman 10414 et al. | L0283052 | |
| Malaysia | Sabah, S. Rurun headwaters | J.J. Vermeulen and H. Duistermaet 1067 | L1488365 | |
| Malaysia | Sabah, Long Pa Sia to Long Samadoh | E.F. de Vogel 8524 et al. | L1488364 | |
| Malaysia | Sarawak, Hose Mountains | E.F. de Vogel 1174 | L1488361 | |
| Malaysia | Sarawak, Batu Lawi | Y. Mahmud et al. S.88176 | K000718611 | |
| Malaysia | Sarawak, Kelabit Highlands, Bukit Batu Buli | A. Vogel et al. | L1488362 | |
| B. finlaysoniana | Australia | Queensland, Brown Creek, Iron range | Sine coll. | CANB |
| Australia | Queensland, Cape York | Sine coll. | CANB | |
| Brunei | Belait Bukit | M.J.S. Sands 5477 | K | |
| Cambodia | Nord Kampot, Knai | M. Poilane 14685 | P00460027 | |
| Cambodia | Mulu Prey | Dr. Normand | NY03998645 | |
| Indonesia | Lampung, Bangka Island, G. Maras | A.J. Kostermans 1328 and Anta | K000482120 | |
| Indonesia | Sumatra, Barat Kota, Siberut Island | J.J. Smith | K000482123 | |
| Indonesia | Jambi province, Batanghari Kabupaten Harapan Rainforest | Wardi et al. | K000734779 | |
| Indonesia | Sumatra, Langga Pajoeng, Soengei Kanan | R. Si Toroes 3844 | NY03998654 | |
| Indonesia | Sumatra, Tigapulu Mts, Talang Lakat, Bukit Karampal area | J.S. Burley 1475a et al. | MO | |
| Indonesia | Riau Rengat, Tigapulu Mts., Karampal area | J.S. Burley and Tukirin 1475 | K000482121 | |
| Indonesia | Blitoeng | Teysmann | L1488250 | |
| Indonesia | Borneo, Kalimantan, Banjarmasin | J. Motley 809 | K000482115 | |
| Indonesia | Borneo, Kalimantan, Maruwai | P.J.A. Kessler 2707 | K000482114 | |
| Indonesia | Borneo, Kalimantan, Haruwu | J.S. Burley and Tukirin 602 | K000482116 | |
| Indonesia | Borneo, Kalimantan, S. Kahayan, Haruwu | J.S. Burley 602 et al. | NY00009461 | |
| Indonesia | Moluccas, Aroe Island, Sia | P. Buwalda 5508 | K000482118 | |
| Indonesia | Irian Jaya, Wasabori, Seroei | L.J. van Dijk 473 | K000482104 | |
| Indonesia | Irian Jaya, Barat Babo | Lundquist 648 | K000482102 | |
| Indonesia | Irian Jaya, Barat Fak, Mimika Timur | E.A. Widjaja 2177 | K000482107 | |
| Indonesia | Irian Jaya, Samberbaba, Seroei | L.J. van Dijk 829 | K000482106 | |
| Indonesia | Irian Jaya, Cyclops mountains above Hollandia | C. Koster 4303 | K000482098 | |
| Indonesia | Irian Jaya, Biak Island, Paieri | A.J. Kostermans 936 and Soegeng | K000482110 | |
| Indonesia | Irian Jaya, Tablasoefoe | P. van Royen and H. Sleumer 6446 | K000482095 | |
| Indonesia | Irian Jaya, Barat Fak Fak, Borowai district | C.J. Stefels 3157 | K000482097 | |
| Laos | Sé-moun | F.J. Harmand 314 | P00436675 | |
| Malaysia | Sabah, Leila Forest Reserve, Distr. Sandakan | K. Murch s.n. | K | |
| Malaysia | Kepong | Sine coll. | C | |
| Malaysia | Sarawak, Bako National Park | J.W. Purseglove 4899 | K000482133 | |
| Malaysia | Sarawak, Sungai Likau, Similajau National Park | A.B.G. Mohtar and H.J. Othman 59456 | K000482139 | |
| Malaysia | Sarawak, Kuching District, Mount Serapi | J.H. Beaman 11540 et al. | K000482136 | |
| Malaysia | Sarawak, Serian | H.J. Othman and A. Munting 61607 | K000482135 | |
| Malaysia | Sarawak, Lundu Kampung Biawak | A. Munting 56380 | K000482138 | |
| Malaysia | Sarawak, Bario | P. Sie 35387 | K000482132 | |
| Malaysia | Sarawak, between Bario and Pa Umor | J.H. Beaman and G. Ismail 11234 | K000482137 | |
| Malaysia | Sarawak, Kelabit Highlands, Kalimantan | H. Christiansen and F.L. Apu 8 | K000482134 | |
| Malaysia | Perak Larut, Kampong | Kiah 299 | K000482088 | |
| Malaysia | Johor, Bukit Tinggi | Sine coll. | K000482094 | |
| Malaysia | Sabah, Long Pasia | A. Hoare and L. Beliau 36 | K000482130 | |
| Malaysia | Sabah, Keningau | S. Sazana et al. | K000342079 | |
| Malaysia | Anambas Islands, Telok Padang, Jemaja | M.R. Henderson 20439 | K000482092 | |
| Malaysia | Penang Hill | A.F.G. Kerr | K000597047 | |
| Malaysia | Perak Kampong Pokok Assam | L. Wray 3121 | K000482090 | |
| Malaysia | Pahang Rompin Leban Chondong | J.H.R. Evans | K000492089 | |
| Malaysia | Sabah, Sandakan | Keith 6717 | K000482127 | |
| Malaysia | Sabah, Keningau Nabawan Syarikat | K. Fidilis 128074 | K000482129 | |
| Malaysia | Johor Kampong, Hubong, Endau | Kadim bin Tassim and Noor 372 | K000482093 | |
| Malaysia | Sabah, Lahad DAtu, Mount Silam | J.H. Beaman 11621 | K000482131 | |
| Malaysia | Negeri Sembilan, Pulau Rumbia, Sembilan Islands | C. Boden-Kloss | K000482086 | |
| Malaysia | Sabah, Sipitang | A. Cuadra 4063 | K000482128 | |
| Malaysia | Keningau Distr. | J.J. Wood 753 | K | |
| Malaysia | Perak Larut and Matang Larut Hao | Sine coll. | K000482087 | |
| Malaysia | Sabah Beluran, Sg. Tungud | Sine coll. | K000482126 | |
| Malaysia | Johore Bahru | C.W. Franck 284 | P00436678 | |
| Malaysia | Kampong Pulau Domar | J. Sinclair | P00436679 | |
| Malaysia | Sabah, Leila Forest Reserve, Distr. Sandakan | K. Murch | K | |
| Malaysia | Sarawak, Bako National Park, Telok Pandan | B.C. Stone 684 | PH00594440 | |
| Malaysia | Sarawak, Marudi District, Bario | T.E. Beaman 184 and R. Repin | NY03998652 | |
| Malaysia | Sarawak, Kuching | M. and J. Clemens 6678 | NY03998655 | |
| Malaysia | Sarawak, Sengghai | Sine coll. | NY03998659 | |
| Malaysia | Sarawak, Bako National Park, Telok Asam | J.W. Purseglove 4899 | NY03998660 | |
| Malaysia | Sarawak, Kuching District, Mount Serapi | J.H. Beaman 11540 et al. | NY03998653 | |
| Malaysia | Mount Matang | J. and M. Clemens 22401 | MO | |
| Malaysia | Malacca, Pulau Besar | B.C. Stone 11494 | PH00594439 | |
| Malaysia | Kedah, G. Jerai | B.C. Stone 12701a | PH00594441 | |
| Malaysia | Terengganu, P. Redang | K.C. Liew 172 | PH00594442 | |
| Malaysia | Sabah, Kota Kinabalu District, Bukit Padang | J.H. and R.S. Beaman 6836 | MO | |
| Papua New Guinea | Kaiser-Wilhelmsland, Jaduna | R. Schlechter 19288 | G00165063 | |
| Papua New Guinea | Kiunga subdistrict, Ingembit | Ridsdale 33240 et al. | K000482100 | |
| Papua New Guinea | Western Amanab, W. Sepik | R. Brown 1882 | K000482101 | |
| Philippines | Tagalinog Island, Palawan | D.R. Mendoza and R. Espiritu 91317 | K000482124 | |
| Singapore | Pasir Panjang | M. Togasi | K000482091 | |
| Thailand | Ranong prov., Muang Lan | G. Seidenfaden and Smitinand GT6137 | C | |
| Thailand | Ban Na, Surat | A.F.G. Kerr 0427 | K000597044 | |
| Thailand | Tako, Langsuan | A.F.G. Kerr 0380 | K000597039 | |
| Thailand | Bangsak, Trang | A.F.G. Kerr 0831 | K000597043 | |
| Thailand | Satul | A.F.G. Kerr 0467 | K000597042 | |
| Thailand | Saba Yoi, Songkla | A.F.G. Kerr | K000597045 | |
| Thailand | Kampengpet, Songkla | A.F.G. Kerr | K000597048 | |
| Thailand | Sangka, Surin | A.F.G. Kerr 0129 | K000597049 | |
| Vietnam | Lang-Than | Thorel | P00436677 | |
| Vietnam | Quang Nam Prov., Dai Loc Distr., Dai Hong | L. Averyanov et al. | P01019665 | |
| B. truncata | Indonesia | Sumatra: Sicikeh-Cikeh Forest | Hartini [43] | |
| Indonesia | Sumatra, Jambi | Hartini [43] | ||
| Malaysia | Johor, Gunung Panti area | Sine coll. | L1488171 | |
| Malaysia | Terengganu | G.P. Lewis 100 | K | |
| Malaysia | Selangor | Segerbäck 2128 | C | |
| Malaysia | Penang | A.C. Maingay 1680 | K | |
| Malaysia | Borneo, Kinabalu, Gurulau spur | C.E. Carr | HUH02341030 | |
| Malaysia | Sabah, Ulu Kalang | A. Lamb 2004/1116 | L1488170 | |
| Singapore | Chawchu Kang | H.N. Ridley | BM000629516 | |
| Thailand | Doi Suthep | G. Seidenfaden and Smitinand GT2691 | C | |
| Thailand | Waeng Forest Station | G. Seidenfaden and Smitinand GT7535 | C |
References
- Vij, S.P.; Kaur, P.; Kaur, S.; Kaushal, P.S. The orchid seeds: Taxonomic, evolutionary and functional aspects. J. Orchid Soc. India 1992, 6, 91–107. [Google Scholar]
- Arditti, J.; Michaud, J.D.; Healey, P.L. Morphometry of orchid seeds. I. Paphiopedilum and native California and related species of Cypripedium. Am. J. Bot. 1979, 66, 1128–1137. [Google Scholar] [CrossRef]
- Arditti, J.; Ghani, A.K.A. Tansley Review No. 110. Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef] [PubMed]
- Gamarra, R.; Ortúñez, E.; Galán Cela, P.; Guadaño, V. Anacamptis versus Orchis (Orchidaceae): Seed micromorphology and its taxonomic significance. Plant Syst. Evol. 2012, 298, 597–607. [Google Scholar] [CrossRef]
- Barthlott, W.; Große-Veldmann, B.; Korotkova, N. Orchid Seed Diversity: A Scanning Electron Microscopy Survey. Englera 2014, 32, 3–245. [Google Scholar]
- Vafaee, Y.; Mohammadi, G.; Nazari, F.; Fatahi, M.; Kaki, A.; Gholami, S.; Ghorbani, A.; Khadivi, A. Phenotypic characterization and seed-micromorphology diversity of the threatened terrestrial orchids: Implications for conservation. S. Afr. J. Bot. 2021, 137, 386–398. [Google Scholar] [CrossRef]
- Healey, P.L.; Michaud, J.D.; Arditti, J. Morphometry of orchid seeds. III. Native California and related species of Goodyera, Piperia, Platanthera and Spiranthes. Am. J. Bot. 1980, 67, 508–518. [Google Scholar] [CrossRef]
- Chase, M.W.; Pippen, J.S. Seed morphology in the Oncidiinae and related subtribes (Orchidaceae). Syst. Bot. 1988, 13, 313–323. [Google Scholar] [CrossRef]
- Kurzweil, H. Seed morphology in Southern African Orchidoideae (Orchidaceae). Plant Syst. Evol. 1993, 185, 229–247. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Yukawa, T.; Lee, N.S.; Lee, C.S.; Kato, M. Phylogeny and comparative seed morphology of epiphytic and terrestrial species of Liparis (Orchidaceae) in Japan. J. Plant Res. 2007, 120, 405–412. [Google Scholar] [CrossRef]
- Gamarra, R.; Galán, P.; Pedersen, H.A.; Ortúñez, E.; Sanz, E. Seed micromorphology in Dactylorhiza Necker ex Nevski (Orchidaceae) and allied genera. Turk. J. Bot. 2015, 39, 298–309. [Google Scholar] [CrossRef]
- Fan, X.-L.; Chomicki, G.; Hao, K.; Liu, Q.; Xiong, Y.-Z.; Renner, S.S.; Gao, J.-Y.; Huang, S.-Q. Transitions between the terrestrial and epiphytic habit drove the evolution of seed-aerodynamic traits in orchids. Am. Nat. 2020, 195, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.N. Terrestrial Orchids: From Seed to Mycotrophic Plant; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Gamarra, R.; Ortúñez, E.; Galán Cela, P.; Merencio, Á. Seed micromorphology of Orchidaceae in the Gulf of Guinea (West Tropical Africa). Plant Syst. Evol. 2018, 304, 665–677. [Google Scholar] [CrossRef]
- Swamy, K.K.; Kumar, H.N.K.; Ramakrishna, T.M.; Ramaswamy, S.N. Studies on seed morphometry of epiphytic orchids from Western Ghats of Karnataka. Taiwania 2004, 49, 124–140. [Google Scholar] [CrossRef]
- Verma, J.; Kusum; Thakur, K.; Sembi, J.K.; Vij, S.P. Study on seed morphometry of seven threatened Himalayan orchids exhibiting varied life modes. Acta Bot. Gall. 2012, 159, 443–449. [Google Scholar] [CrossRef]
- Galán Cela, P.; Seligrat, I.; Ortúñez, E.; Gamarra, R.; Vivar, A.; Scrugli, A. A study of seed micromorphology in the genus Ophrys (Orchidaceae). An. Jard. Bot. Madrid 2014, 71, e008. [Google Scholar] [CrossRef]
- Zotz, G.; Weichgrebe, T.; Happatz, H.; Einzmann, H.J.R. Measuring the terminal velocity of tiny diaspores. Seed Sci. Res. 2016, 26, 222–230. [Google Scholar] [CrossRef]
- Chaudhary, B.; Chattopadhyay, P.; Banerjee, N. Modulations in seed micromorphology reveal signature of adaptive species-diversification in Dendrobium (Orchidaceae). Open J. Ecol. 2014, 4, 33–42. [Google Scholar] [CrossRef]
- Diantina, S.; McGill, C.; Millner, J.; Nadarajan, J.; Pritchard, H.W.; Clavijo McCormick, A. Comparative seed ecology of tropical and temperate orchid species with different growth habits. Plants 2020, 9, 161. [Google Scholar] [CrossRef]
- Pridgeon, A.M.; Cribb, P.J.; Chase, M.W.; Rasmussen, F.N. Genera Orchidacearum—Volume 6: Epidendroideae (Part Three); Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Kruizinga, J.; Van Scheindelen, H.J.; De Vogel, E.F. Revision of the genus Bromheadia (Orchidaceae). Orchid. Monogr. 1997, 8, 79–118. [Google Scholar]
- Repetur, C.P.; Van Welzen, P.C.; De Vogel, E.F. Phylogeny and historical biogeography of the genus Bromheadia (Orchidaceae). Syst. Bot. 1997, 22, 465–477. [Google Scholar] [CrossRef]
- Puspitaningtyas, D.M. Orchid exploration in Mount Bintan Besar protected forest, Bintan Island, Riau Islands Province, Sumatra, Indonesia. Biodiversitas 2018, 19, 1081–1088. [Google Scholar] [CrossRef]
- Nordin, F.A.; Othman, A.S.; Zainudin, N.A.; Khalil, N.; Asi, N.; Azmi, A.; Mangsor, K.N.; Harun, M.S.; Zin, K.F. The Orchid Flora of Gunung Ledang (Mount Ophir), Malaysia—120 years after Ridley. Pertanika J. Trop. Agric. Sci. 2021, 44, 369–387. [Google Scholar] [CrossRef]
- Chong, K.Y.; Tan, H.T.W.; Corlett, R.T. A Checklist of the Total Vascular Plant Flora of Singapore. Native, Naturalized and Cultivated Species; National University of Singapore: Singapore, 2009. [Google Scholar]
- Brummitt, N. Bromheadia finlaysoniana. The IUCN Red List of Threatened Species 2013, e.T44393543A44410760. Available online: https://doi.org/10.2305/IUCN.UK.2013-1.RLTS.T44393543A44410760.en (accessed on 16 January 2023).
- Ziegler, B. Mikromorphologie der Orchideensamen unter Berücksichtigung Taxomonischer Aspekte. Ph.D. Thesis, Ruprecht-Karl Universität, Heidelberg, Germany, 1981. [Google Scholar]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2022. Available online: http://www.plantsoftheworldonline.org/ (accessed on 19 December 2022).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria. Version 14. Prepared by the Standards and Petitions Committee. Available online: http://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 18 September 2019).
- Tohda, H. Seed morphology in Orchidaceae II. Tribe Cranichidae. Sci. Rep. Tohoku Univ. 4th Ser. Biol. 1985, 39, 21–43. [Google Scholar]
- Molvray, M.; Kores, P.J. Character analysis of the seed coat in Spiranthoideae and Orchidoideae, with special reference to the Diurideae (Orchidaceae). Am. J. Bot. 1995, 82, 1443–1454. [Google Scholar] [CrossRef]
- Ormerod, P.; Kurzweil, H.; Watthana, S. Annotated list of Orchidaceae from Myanmar. Phytotaxa 2021, 481, 1. [Google Scholar] [CrossRef]
- Gamarra, R.; Ortúñez, E. Endocarpic trichomes in Vandeae (Orchidaceae). Flora 2021, 280, 151844. [Google Scholar] [CrossRef]
- Tongbram, J.; Rao, A.N.; Vij, S.P. Seed morphometric studies in some orchids from Manipur. J. Orchid Soc. India 2012, 26, 25–29. [Google Scholar]
- Davies-Colley, R.; Payne, G.W.; Elswijk, M. Microclimate gradients across a forest edge. N. Z. J. Ecol. 2000, 24, 111–121. [Google Scholar]
- Kiyohara, S.; Fukunaga, H.; Sawa, S. Characteristics of the falling speed of Japanese orchid seeds. Int. J. Biol. 2012, 4, 10–12. [Google Scholar] [CrossRef]
- Shimizu, N.; Sawa, Y.; Sawa, S. Adaptation and evolution of seed shape on breeding area in Japanese orchids. Int. J. Biol. 2012, 4, 47–53. [Google Scholar] [CrossRef]
- Thakur, K.K.; Verma, J. Study on distribution, habitat characteristics and seed morphometry of a medicinal orchid, Eulophia herbacea Lindl. Vegetos 2013, 26, 121–126. [Google Scholar] [CrossRef]
- Mytnik-Ejsmont, J. A Monograph of the Subtribe Polystachyinae Schltr. (Orchidaceae); University of Gdansk: Gdansk, Poland, 2011. [Google Scholar]
- Delforge, P. Orchids of Europe, North Africa and the Middle East, 3rd ed.; A&C Black: London, UK, 2006. [Google Scholar]
- Hartini, S. Orchids diversity in the Sicikeh-Cikeh Forest, North Sumatra, Indonesia. Biodiversitas 2019, 20, 1087–1096. [Google Scholar] [CrossRef]
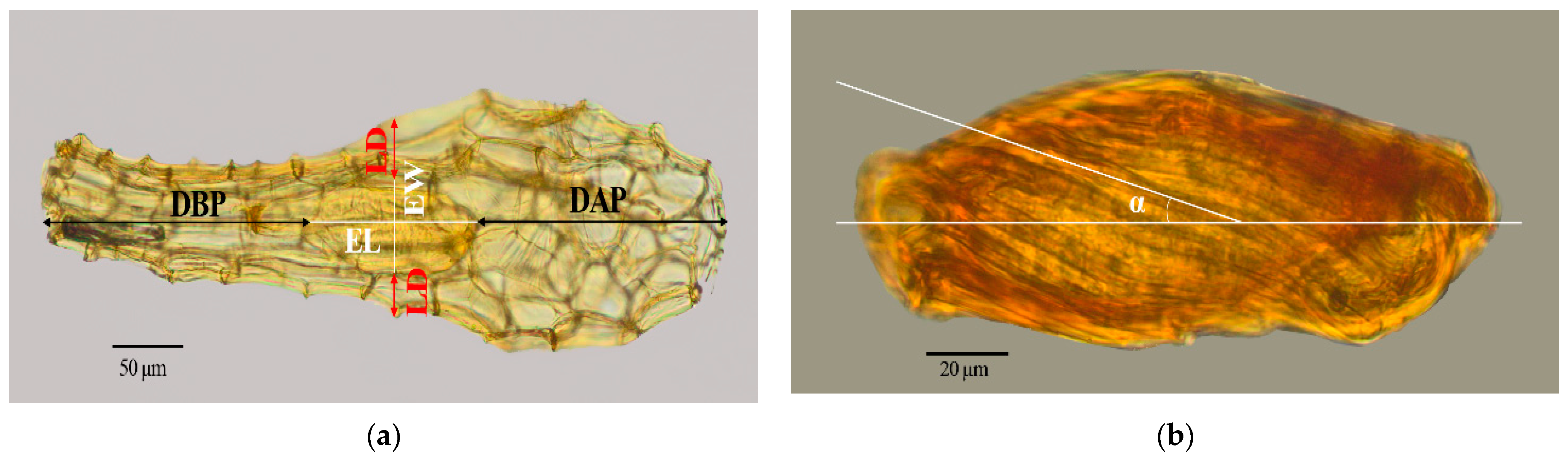
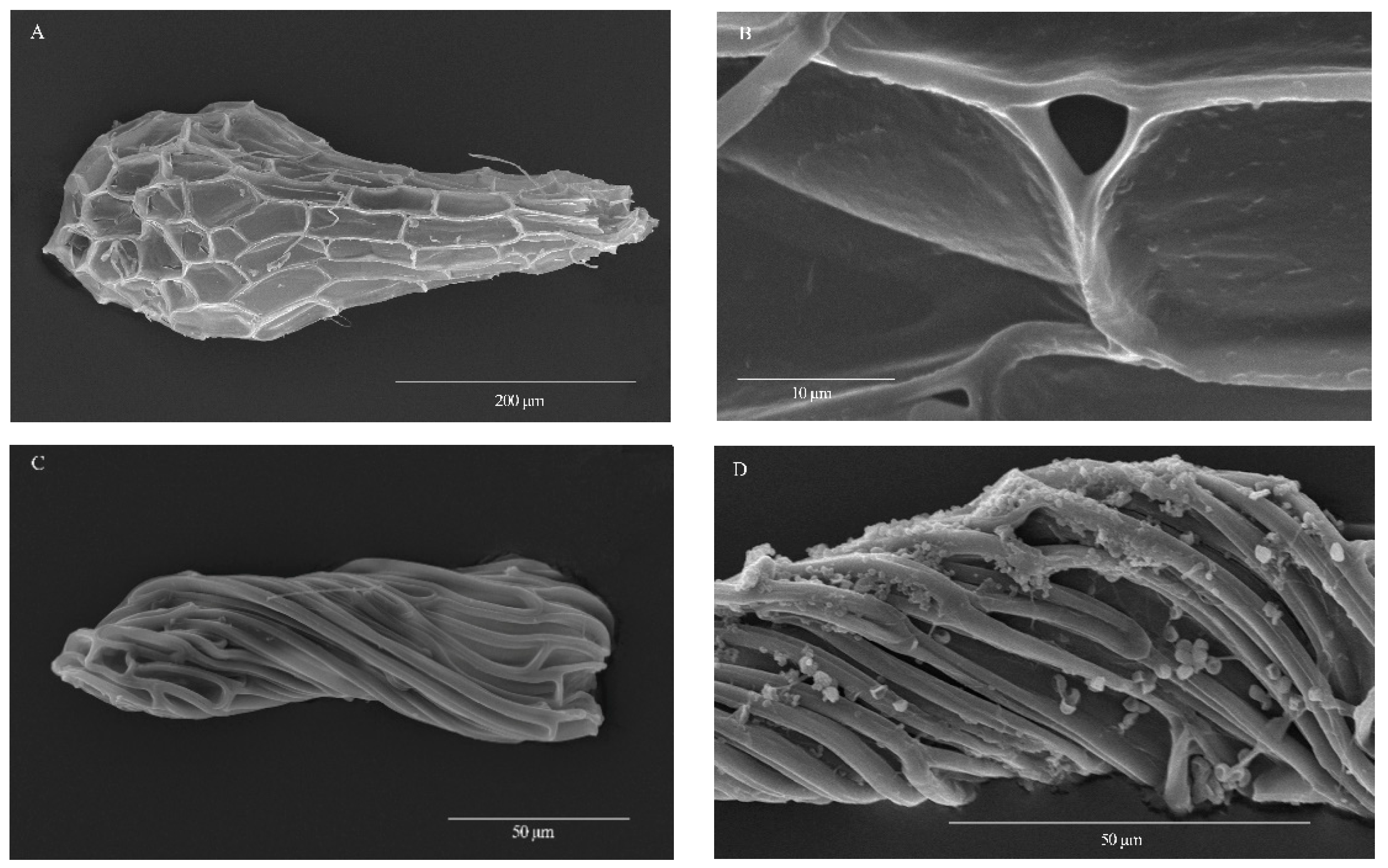


| Species | Life Form | Locality | Collector and Number | Voucher |
|---|---|---|---|---|
| B. finlaysoniana | T | Malaysia, Sabah: Distr. Sandakan, Leila Forest Reserve, 17-VIII-1971 | K. Murch s.n. | K |
| Papua New Guinea: Amanab, W Sepita, disturbed growth near road, semi-shade terrestrial, 3-IV-1928 | R. Brown 1882 | K000482101 | ||
| Thailand: Sangka, Surin, 300 m, by a stream in open evergreen forest, 15-I-1924 | A.F.G. Kerr 0129 | K000594041 | ||
| B. cecieliae | E | Malaysia, Sarawak: Batu Lawi, 1050 m, near the river, hill slope, 6-V-2002 | Y. Mahmud et al. S.88176 | K000718611 |
| B. truncata | E | Malaysia: Penang, s.f. | A.C. Maingay 1680 | K |
| Taxa | Seed Shape | Medial Cell Shape | Orient. Testa Cells | Long. Anticl. Walls | Pericl. Walls | Intercellular Gaps | Waxes |
|---|---|---|---|---|---|---|---|
| B. finlaysoniana | Fusiform to clavate | Rectangular | Parallel | Thin | Visible | Present | Absent |
| B. cecieliae | Fusiform | Elongated | Twisted | Thickened | Narrow-to-not visible | Absent | Present |
| B. truncata | Fusiform | Elongated | Twisted | Thickened | Narrow-to-not visible | Absent | Present |
| Taxa | SL (µm) ± SD | SW (µm) ± SD | NC | MCA | EL (µm) ± SD | EW (µm) ± SD | SV (mm3 × 10−3) ± SD | EV (mm3 × 10−3) ± SD | Air Space (%) | DAP (µm) ± SD | DBP (µm) ± SD | LD * (µm) ± SD | SM (µg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. finlaysoniana | 504.41 ± 24.71 | 144.41 ± 9.18 | 7–9 | 0° | 129.70 ± 14.77 | 52.60 ± 4.28 | 2.78 ± 3.94 | 1.56 ± 3.83 | 94.35 ± 1.11 | 175.35 ± 7.26 | 201.14 ± 21.86 | 32.19 ± 6.22 | 0.379 |
| B. cecieliae | 148.76 ± 10.36 | 56.25 ± 4.58 | 2–3 | 17–22° | 100.07 ± 9.30 | 48.58 ± 4.31 | 1.24 ± 2.5 | 1.25 ± 2.82 | −0.75 ± 12.21 | 21.43 ± 5.01 | 26.62 ± 8.24 | 3.88 ± 1.31 | 0.120 |
| B. truncata | 160.35 ± 13.27 | 51.58 ± 6.30 | 2–3 | 25–29° | 109.32 ± 9.07 | 45.32 ± 6.11 | 1.14 ± 3.15 | 1.14 ± 3.16 | −5.24 ± 8.26 | 20.86 ± 3.00 | 30.16 ± 6.58 | 3.12 ± 0.27 | 0.186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortúñez, E.; Gamarra, R. Seed Morphology, Life Form and Distribution in Three Bromheadia Species (Epidendroideae, Orchidaceae). Diversity 2023, 15, 195. https://doi.org/10.3390/d15020195
Ortúñez E, Gamarra R. Seed Morphology, Life Form and Distribution in Three Bromheadia Species (Epidendroideae, Orchidaceae). Diversity. 2023; 15(2):195. https://doi.org/10.3390/d15020195
Chicago/Turabian StyleOrtúñez, Emma, and Roberto Gamarra. 2023. "Seed Morphology, Life Form and Distribution in Three Bromheadia Species (Epidendroideae, Orchidaceae)" Diversity 15, no. 2: 195. https://doi.org/10.3390/d15020195
APA StyleOrtúñez, E., & Gamarra, R. (2023). Seed Morphology, Life Form and Distribution in Three Bromheadia Species (Epidendroideae, Orchidaceae). Diversity, 15(2), 195. https://doi.org/10.3390/d15020195









