An Annotated Checklist of Algae from the Order Synurales (Chrysophyceae) of Viet Nam
Abstract
1. Introduction
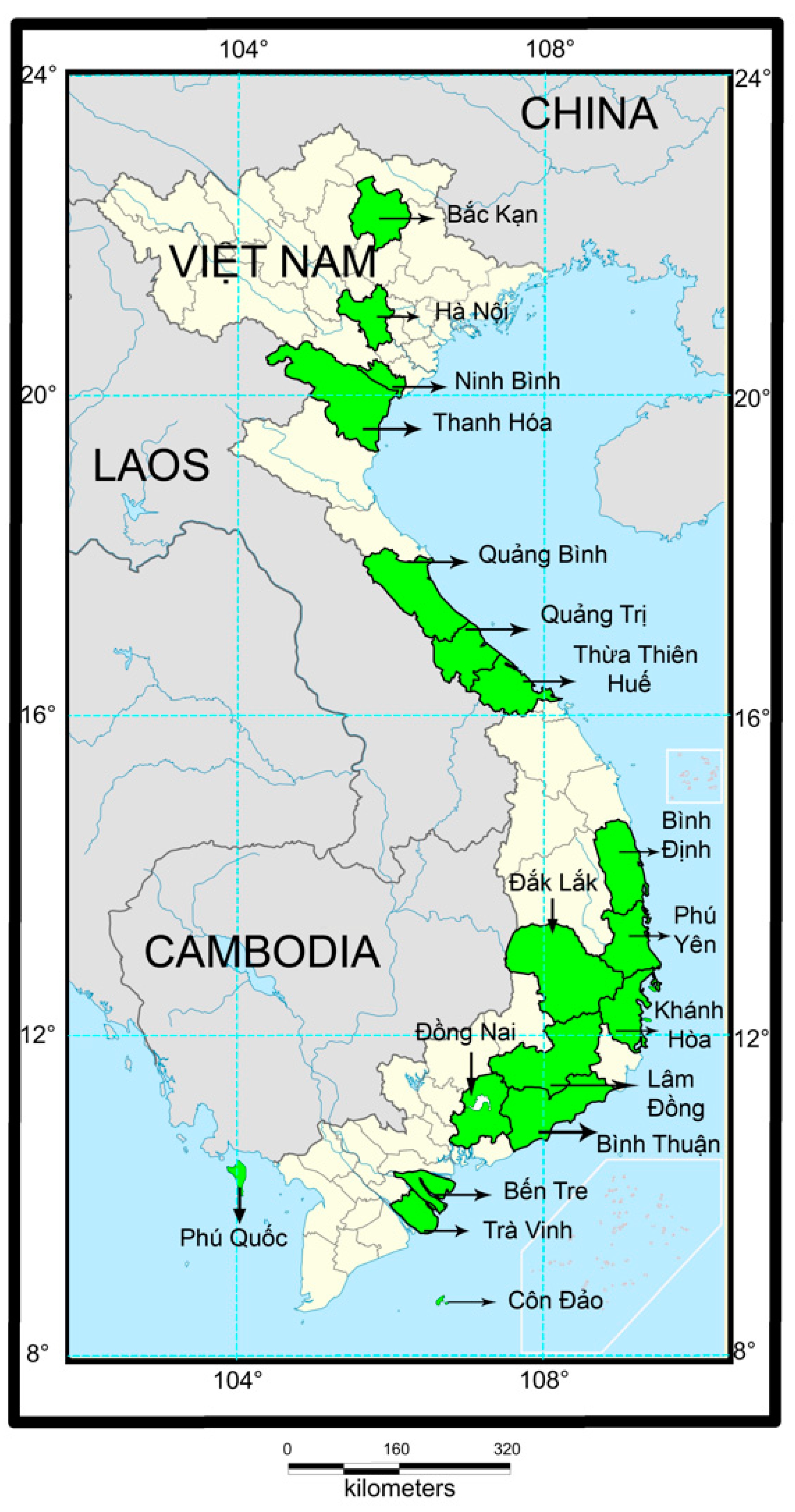
2. Materials and Methods
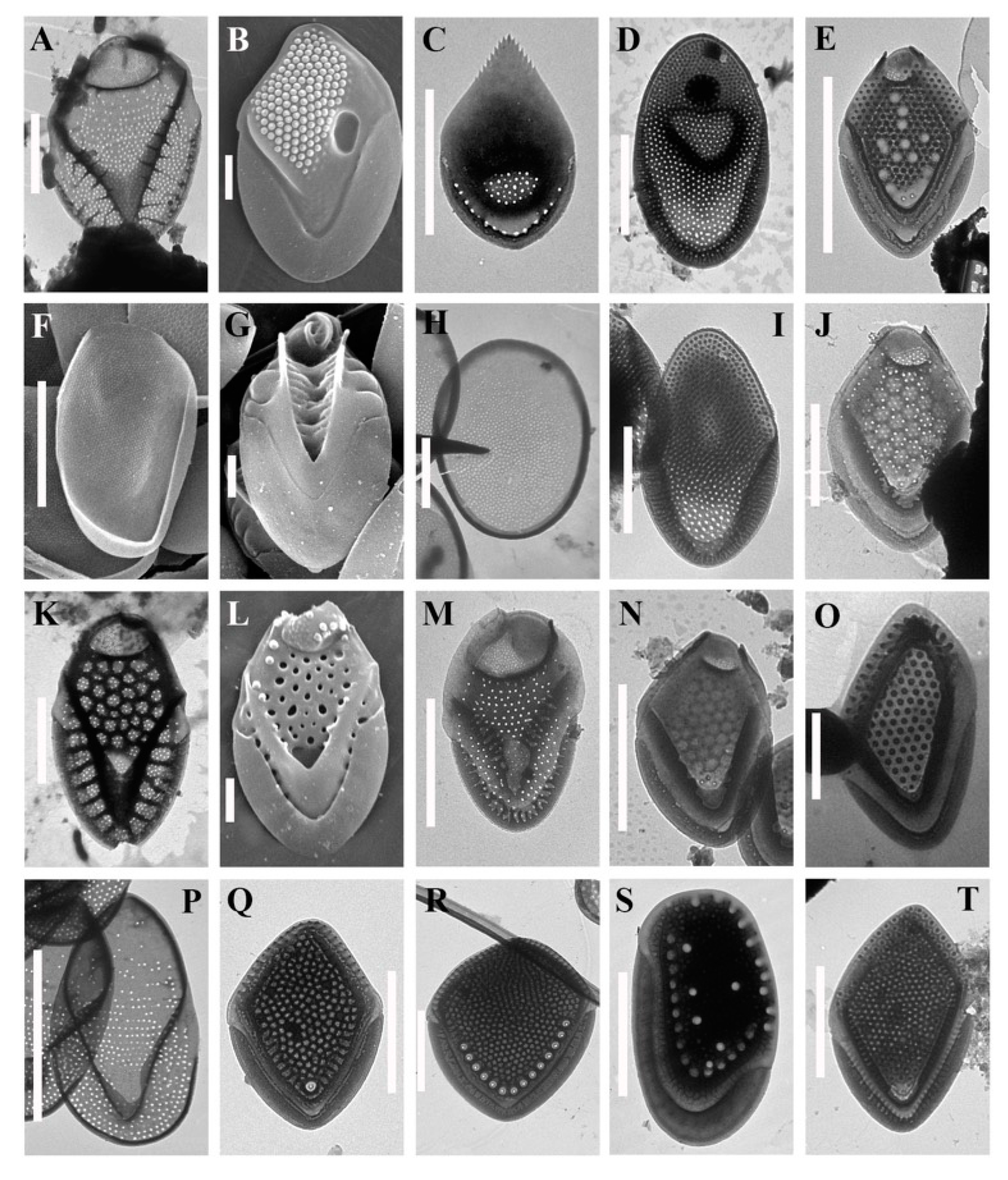
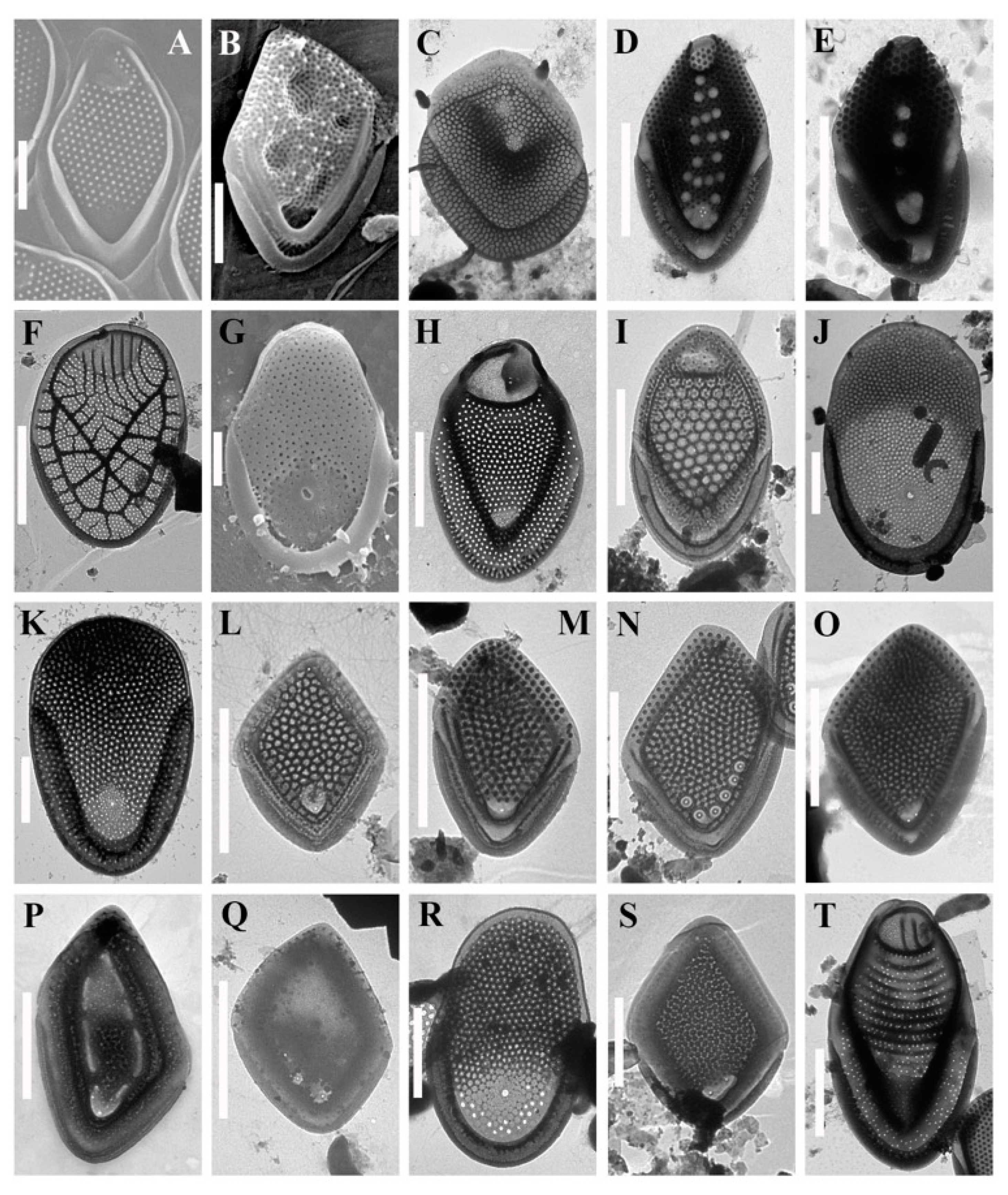
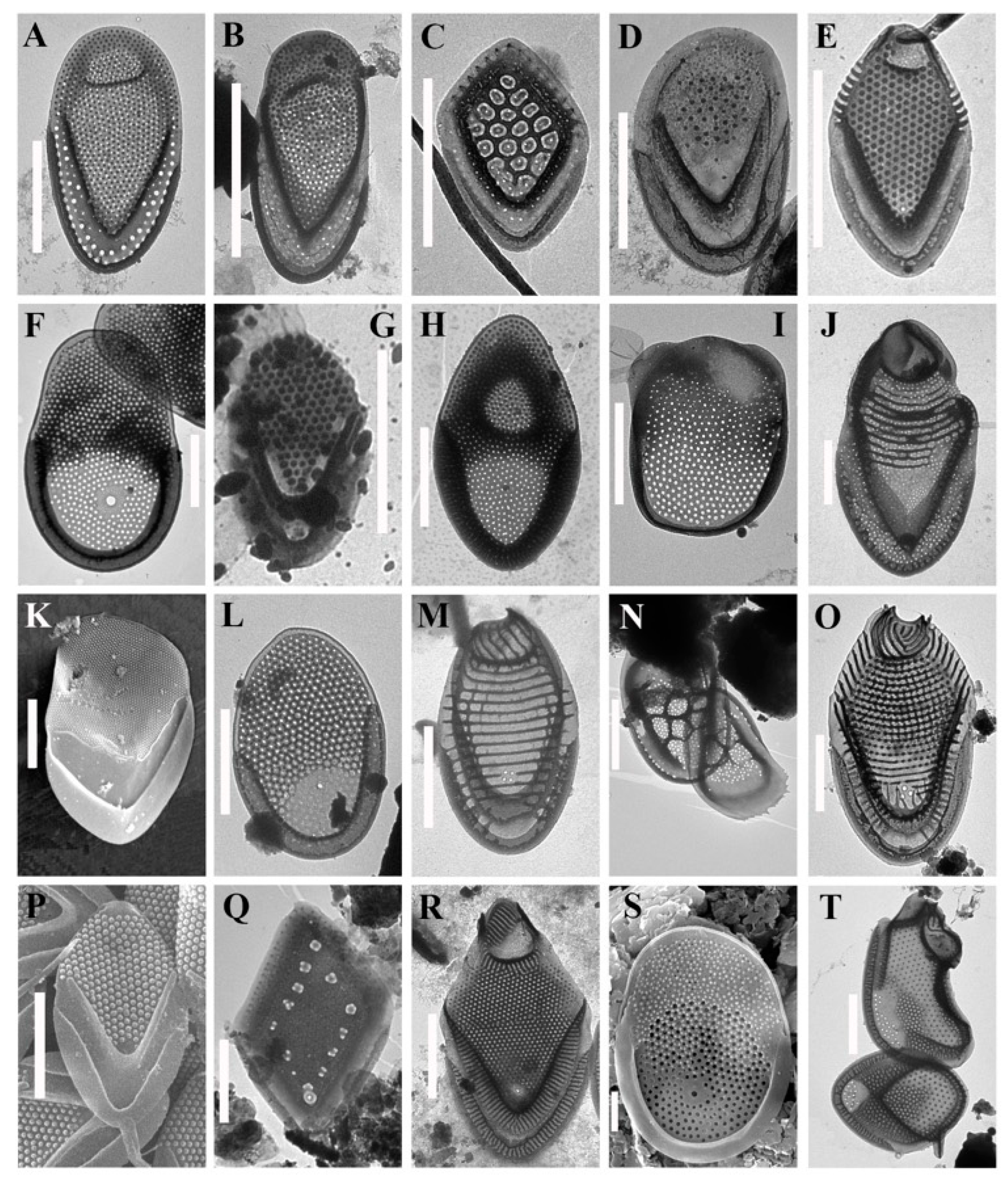
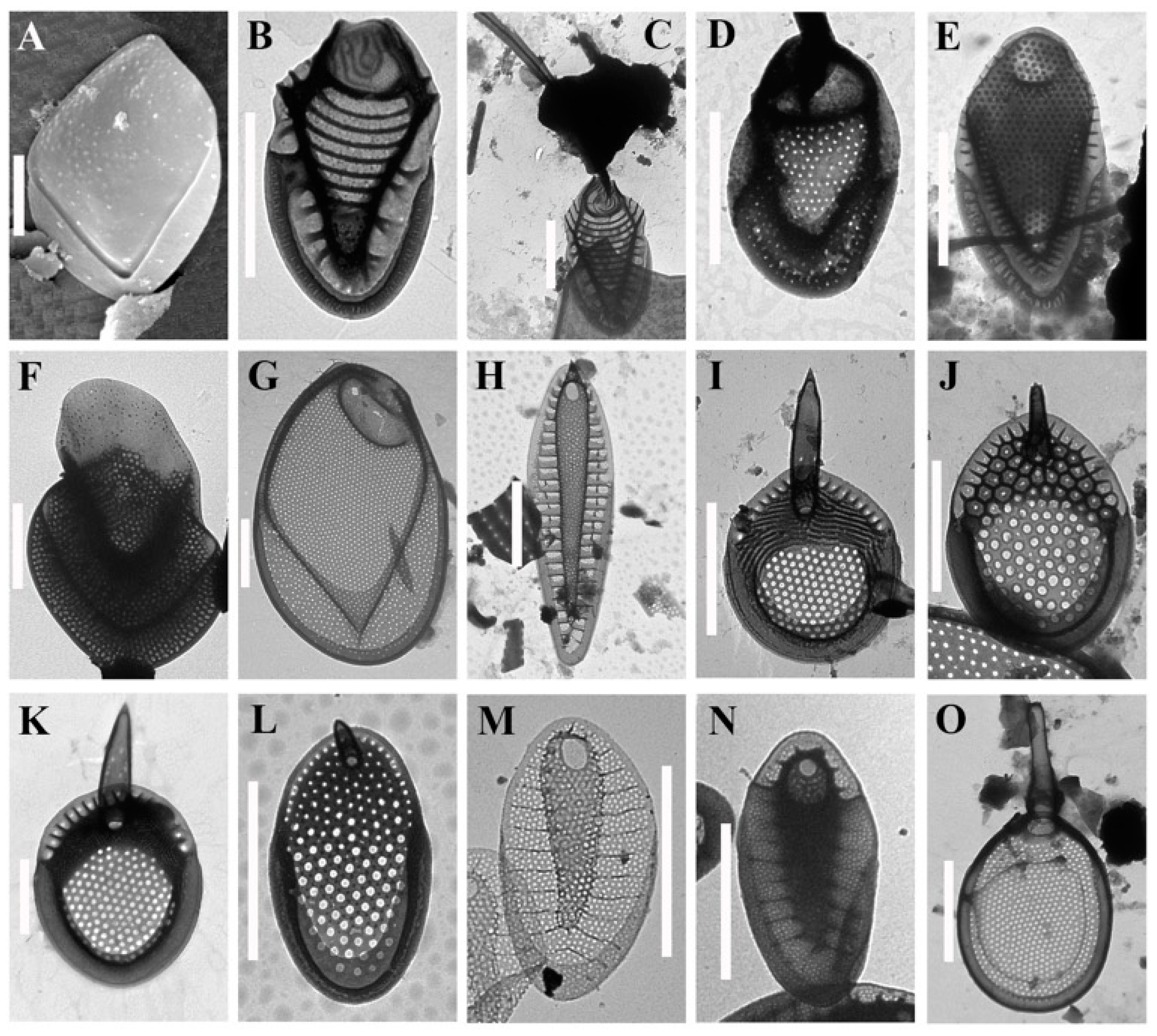
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kristiansen, J.; Preisig, H.R. Chrysophyta and Haptophyta Algae, 2nd part: Synurophyceae. In Süsswasserflora Von Mitteleuropa (Freshwater Flora of Central Europe); Büdel, B., Gärtner, G., Krienitz, L., Preisig, H.R., Schagerl, M., Eds.; Spektrum Akademisher Verlag, Springer: Berlin, Germany, 2007; pp. 1–252. ISBN 978-3-8274-1701-5. [Google Scholar]
- Kristiansen, J.; Škaloud, P. Chrysophyta. In Handbook of the Protists, 2nd ed.; Archibald, J.M., Simpson, A.G.B., Slamovits, C.H., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 331–366. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 6 December 2022).
- Škaloud, P.; Škaloudová, M.; Jadrná, I.; Bestová, H.; Pusztai, M.; Kapustin, D.; Siver, P.A. Comparing morphological and molecular estimates of species diversity in the freshwater genus Synura (Stramenopiles): A model for understanding diversity of eukaryotic microorganisms. J. Phycol. 2020, 56, 574–591. [Google Scholar] [CrossRef]
- Škaloud, P.; Kristiansen, J.; Škaloudová, M. Developments in The Taxonomy of Silica-Scaled Chrysophytes—From Morphological and Ultrastructural to Molecular Approaches. Nord. J. Bot. 2013, 31, 385–402. [Google Scholar] [CrossRef]
- Jo, B.Y.; Shin, W.; Boo, S.M.; Kim, H.S.; Siver, P.A. Studies on Ultrastructure and Three-Gene Phylogeny of the Genus Mallomonas (Synurophyceae). J. Phycol. 2011, 47, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Jo, B.Y.; Shin, W.; Kim, H.S.; Siver, P.A.; Andersen, R.A. Phylogeny of the Genus Mallomonas (Synurophyceae) and Descriptions of Five New Species on the Basis of Morphological Evidence. Phycologia 2013, 52, 266–278. [Google Scholar] [CrossRef]
- Jo, B.Y.; Kim, J.I.; Škaloud, P.; Siver, P.A.; Shin, W. Multigene Phylogeny of Synura (Synurophyceae) and Descriptions of Four New Species Based on Morphological and DNA Evidence. Eur. J. Phycol. 2016, 51, 413–430. [Google Scholar] [CrossRef]
- Siver, P.A.; Jo, B.Y.; Kim, J.I.; Shin, W.; Lott, A.M.; Wolfe, A.P. Assessing the Evolutionary History of The Class Synurophyceae (Heterokonta) Using Molecular, Morphometric, and Paleobiological Approaches. Am. J. Bot. 2015, 102, 921–941. [Google Scholar] [CrossRef] [PubMed]
- Čertnerová, D.; Čertner, M.; Škaloud, P. Molecular Phylogeny and Evolution of Phenotype in Silica-Scaled Chrysophyte Genus Mallomonas. J. Phycol. 2019, 55, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Hayakawa, T. The Synuraceae (Chrysophyceae) in Bangladesh. Phykos 1979, 18, 129–147. [Google Scholar]
- Dürrschmidt, M.; Croome, R. Mallomonadaceae (Chrysophyceae) from Malaysia and Australia. Nord. J. Bot. 1985, 5, 285–298. [Google Scholar] [CrossRef]
- Neustupa, J.; Řezáčová, M. The genus Mallomonas (Mallomonadales, Synurophyceae) in Several Southeast Asian Urban Water Bodies—The Biogeographical Implications. Nova Hedwig. 2007, 84, 249–259. [Google Scholar] [CrossRef]
- Dürrschmidt, M.; Cronberg, G. Contribution to The Knowledge of Tropical Chrysophytes: Mallomonadaceae and Paraphysomonadaceae from Sri Lanka. Algol. Stud. 1989, 54, 15–37. [Google Scholar]
- Wei, Y.X.; Yuan, X.P. Studies on Silica-Scaled Chrysophytes from the Tropics and Subtropics of China. Nova Hedwig. 2001, 122, 169–187. [Google Scholar]
- Wei, Y.X.; Yuan, X.P.; Kristiansen, J. Silica-Scaled Chrysophytes from Hainan, Guangdong Provinces and Hong Kong Special Administrative Region, China. Nord. J. Bot. 2014, 32, 881–896. [Google Scholar] [CrossRef]
- Saha, L.C.; Wujek, D.E. Scale-bearing Chrysophytes from Tropical Northeast India. Nord. J. Bot. 1990, 10, 343–355. [Google Scholar] [CrossRef]
- Wujek, D.E.; Saha, L.C. Scale-Bearing Chrysophytes (Chrysophyceae and Synurophyceae) from India. II. Nova Hedwig. 1996, 112, 367–377. [Google Scholar]
- Wujek, D.E.; Adesalut, A.; Nwankwo, D.I. Silica scaled Chrysophyceae and Synurophyceae (Chrysophyta) from Nigeria. II. Lake Lekki. Trop. Freshwat. Biol. 2003, 12, 99–103. [Google Scholar] [CrossRef]
- Wujek, D.E.; Kadiri, M.O.; Dziedzic, R.M. Silica-scaled Chrysophyceae and Synurophyceae from Nigeria. III. Chrysophytes from rivers of Edo State. Fottea 2010, 10, 93–98. [Google Scholar] [CrossRef]
- Vyverman, W.; Cronberg, G. Scale Bearing Chrysophytes from Papua New Guinea. Nord. J. Bot. 1993, 13, 111–120. [Google Scholar] [CrossRef]
- Kapustin, D.A.; Gusev, E.S. Silica-Scaled Chrysophytes from West Java (Indonesia) Including Description of a New Chrysosphaerella Species. Nova Hedwig. 2019, 148, 11–20. [Google Scholar] [CrossRef]
- Gusev, E.; Kapustin, D.; Martynenko, N.; Kulikovskiy, M. Diversity of Silica-Scaled Chrysophytes (Stramenopiles: Chrysophyceae) from Indonesian Papua. Diversity 2022, 14, 726. [Google Scholar] [CrossRef]
- Cronberg, G.; Hickel, B. Mallomonas fenestrata sp. nov. and M. perforata sp. nov. (Chrysophyceae, Mallomonadaceae) from Tropical Lakes. Nord. J. Bot. 1985, 5, 105–110. [Google Scholar] [CrossRef]
- Hansen, P. Silica-scaled Chrysophyceae and Synurophyceae from Madagascar. Arch. Protistenkd. 1996, 147, 145–172. [Google Scholar] [CrossRef]
- Wujek, D.E.; Asmund, B. Mallomonas cyathellata sp. nov. and Mallomonas cyathellata var. kenyana var. nov. (Chrysophyceae) studied by means of scanning and transmission electron microscopy. Phycologia 1979, 18, 115–119. [Google Scholar] [CrossRef]
- Compère, P. Mallomonas bronchartiana, Chrysophycée nouvelle du lac Tchad. Bull. Jard. Bot. Nat. Belg. Bull. Nat. Plantentuin Belg. 1974, 44, 61–63. [Google Scholar] [CrossRef]
- Cronberg, G. Scaled Chrysophytes from the Tropics. Nova Hedwig. 1989, 95, 191–232. [Google Scholar]
- Piątek, J. Mallomonas camerunensis sp. nov. (Chrysophyceae, Stramenopiles) from a shallow puddle in the Guineo Congolian rainforest (Cameroon). Pol. Bot. J. 2015, 60, 119–126. [Google Scholar] [CrossRef]
- Piątek, J.; Łukaszek, M. Mallomonas cronbergiae (Chrysophyceae, Stramenopiles), a new species from the Guineo-Congolian rainforest in Cameroon. Pol. Bot. J. 2016, 61, 199–204. [Google Scholar] [CrossRef]
- Němcová, Y.; Bulant, P.; Kristiansen, J. Mallomonas solea-ferrea and Mallomonas siveri (Stramenopiles): Two new taxa from the Western Cape (South Africa). Nova Hedwig. 2011, 93, 375–384. [Google Scholar] [CrossRef]
- Němcová, Y.; Kreidlová, J. Two new species of Mallomonas (Chrysophyceae: Synurales): Mallomonas temonis and Mallomonas divida. Phytotaxa 2013, 87, 11–18. [Google Scholar] [CrossRef]
- Vuuren, S.J.; Levanets, A.; Kapustin, D.; Swanepoel, A. Morphology, ecology and geographic distribution of three Mallomonas (Phylum Ochrophyta) species from the Vaal River, South Africa. S. Afr. J. Bot. 2022, 149, 160–169. [Google Scholar] [CrossRef]
- Wujek, D.E. Scale bearing Chrysophyceae (Mallomonadaceae) from North-Central Costa Rica. Brenesia 1984, 22, 309–313. [Google Scholar]
- Wujek, D.E.; Dziedic, R.M. Silica-scaled Chrysophytes from Ecuador. Gayana Bot. 2005, 62, 1–8. [Google Scholar] [CrossRef]
- Wujek, D.E. Scale bearing Chrysophyceae from the Panama Canal. Jpn. J. Phycol. 1986, 34, 83–86. [Google Scholar]
- Carty, S.; Wujek, D.E. A new species of Peridinium and new records of dinoflagellates and silica-scaled chrysophytes from Belize. Caribbean J. Sci. 2003, 39, 136–139. [Google Scholar]
- Vigna, M.S.; Duque, S.R.; Nunez-Avellaneda, M. Tropical silica-scaled chrysophyte flora (Chrysophyceae and Synurophyceae) from Colombia. Nova Hedwig. 2005, 128, 151–166. [Google Scholar]
- Franceschini, I.M.; Couté, A. Quelques Chrysophycées (Algae, Chromophyta) à écailles siliceuses de l’extrême sud-est du Brésil. Algol. Stud. Arch. Hydrobiol. 1991, 62, 31–45. [Google Scholar]
- Kristiansen, J.; Menezes, M. Silica-scaled Chrysophytes from an Amazonian flood-plain lake, Mussurá Lake, northern Brazil. Algol. Stud. Arch. Hydrobiol. 1998, 90, 97–118. [Google Scholar] [CrossRef]
- Couté, A.; Franceschini, I.M. Scale-Bearing Chrysophytes from Acid Water of Florianopolis, Santa Catarina Island, South Brazil. Algol. Stud. 1998, 88, 37–61. [Google Scholar] [CrossRef]
- Franceschini, I.M.; Kristiansen, J.M. New records of scale-bearing chrysophytes for Florianópolis, Santa Catarina Island, Southern Brazil. Algol. Stud. Arch. Hydrobiol. 2004, 111, 63–77. [Google Scholar] [CrossRef]
- Gusev, E.; Martynenko, N.; Tran, H. Studies on Algae from the Order Synurales (Chrysophyceae) in Northern Vietnam. Diversity 2021, 13, 602. [Google Scholar] [CrossRef]
- Gusev, E.S.; Nguyen, T.H.T. Silica-Scaled Chrysophytes (Chrysophyceae and Synurophyceae) from Vietnam (Khanh Hoa and Quang Nam provinces). Nova Hedwig. 2011, 93, 191–199. [Google Scholar] [CrossRef]
- Gusev, E.S. Studies on Synurophycean Algae from Mangrove Wetlands (Vietnam). Nova Hedwig. 2013, 142, 87–95. [Google Scholar]
- Gusev, E.S.; Doan-Nhu, H.; Nguyen-Ngoc, L.; Guseva, E.E.; Phan Tan, L. Silica-Scaled Chrysophytes from Cam Ranh Peninsula (Khanh Hoa Province, Vietnam). Nova Hedwig. 2019, 148, 63–76. [Google Scholar] [CrossRef]
- Doan-Nhu, H.; Thi-Tinh, T.; Gusev, E.; Kulikovskiy, M.; Phan-Tan, L.; Nguyen-Ngoc, L. Taxonomic Composition of Silica-Scaled Chrysophytes in a Tropical Mountain Reservoir. Inland Wat. Biol. 2021, 14, 490–499. [Google Scholar] [CrossRef]
- Gusev, E.; Martynenko, N. Diversity of Silica-Scaled Chrysophytes in Central Vietnam. Water 2022, 14, 65. [Google Scholar] [CrossRef]
- Gusev, E.S.; Doan-Nhu, H.; Nguyen-Ngoc, L. Silica-Scaled Chrysophytes from Cat Tien National Park (Dong Nai Province, Vietnam). Nova Hedwig. 2017, 105, 347–364. [Google Scholar] [CrossRef]
- Gusev, E.S.; Gusakov, V.A.; Guseva, E.E.; Kulikovskiy, M.S.; Tsvetkov, A.I.; Dịnh, C.N. Flora of Silica-Scaled Chrysophytes (Chrysophyceae: Synurales, Paraphysomonadales) of the Mekong Delta. Inland Wat. Biol. 2020, 13, 349–357. [Google Scholar] [CrossRef]
- Gusev, E.S.; Guseva, E.E.; Dien, T.D.; Kulikovskiy, M.S. Flora of Silica-Scaled Chrysophytes (Chrysophyceae) of Two Provinces in Southern Viet Nam. Inland Wat. Biol. 2022, 15, 205–216. [Google Scholar] [CrossRef]
- Gusev, E.; Martynenko, N.; Kapustin, D.; Doan-Nhu, H.; Nguyen-Ngoc, L. Diversity of Silica-Scaled Chrysophytes of Two Tropical Islands: Phu Quoc and Con Son (Viet Nam). Life 2022, 12, 1611. [Google Scholar] [CrossRef]
- Řezáčová, M.; Škaloud, P. A contribution to the knowledge of the silica-scaled chrysophytes in eastern Hungary. Czech Phycol. 2004, 4, 67–73. [Google Scholar]
- Calado, A.J.; Craveiro, S.C. Notes on the ecology of Synurophycean algae found in Portugal. Nord. J. Bot. 1995, 15, 641–654. [Google Scholar] [CrossRef]
- Siver, P.A. Two new taxa in the Section Papillosae of the genus Mallomonas (Synurophyceae) from the Ocala National Forest, Florida, USA. Nord. J. Bot. 2002, 22, 123–128. [Google Scholar] [CrossRef]
- Siver, P.A.; Lott, A.M. The Scaled Chrysophyte Flora from the Pinelands National Preserve of Southern New Jersey, U.S.A. Nova Hedwig. 2010, 136, 167–180. [Google Scholar] [CrossRef]
- Gusev, E.S.; Siver, P.A.; Shin, W. Mallomonas bronchartiana Compère Revisited: Two New Species Described from Asia. Cryptogam. Algol. 2017, 38, 3–16. [Google Scholar] [CrossRef]
- Cronberg, G. Scaled chrysophytes from the Okavango Delta, Botswana, Africa. Nova Hedwig. 1996, 114, 91–108. [Google Scholar]
- Gusev, E.S.; Doan-Nhu, H.; Nguyen-Ngoc, L. Mallomonas cattiensis sp. nov. (Synurales, Chrysophyceae), a New Species from Viet Nam. Phytotaxa 2015, 221, 188–192. [Google Scholar] [CrossRef]
- Gusev, E.S.; Kulikovskiy, M.S. Two new species of genus Mallomonas from swamp localities in Vietnam. Phytotaxa 2020, 468, 121–129. [Google Scholar] [CrossRef]
- Siver, P.A.; Voloshko, L.N.; Gavrilova, O.V.; Getsen, M.V. The scaled chrysophyte flora of the Bolshezemelskaya tundra (Russia). Nova Hedwig. 2005, 128, 125–150. [Google Scholar]
- Siver, P.A.; Skogstad, A. Morphological variation and ecology of Mallomonas crassisquama (Chrysophyceae). Nord. J. Bot. 1988, 8, 99–107. [Google Scholar] [CrossRef]
- Bessudova, A.Y.; Tomberg, I.V.; Firsova, A.D.; Kopyrina, L.I.; Likhoshway, Y.V. Silica-scaled chrysophytes in lakes Labynkyr and Vorota of the Sakha (Yakutia) Republic, Russia. Nova Hedwig. 2019, 148, 35–48. [Google Scholar] [CrossRef]
- Dürrschmidt, M. Mallomonas cristata sp. nov. (Chrysophyceae, Synuraceae) from South Chilean Inland Waters. Phycologia 1981, 20, 298–302. [Google Scholar] [CrossRef]
- Asmund, B.; Kristiansen, J. The Genus Mallomonas (Chrysophyceae). A Taxonomic Survey Based on The Ultrastructure of Silica Scales and Bristles. Opera Bot. 1986, 85, 1–128. [Google Scholar]
- Němcová, Y.; Rott, E. Diversity of Silica-Scaled Chrysophytes in High-Altitude Alpine Sites (North Tyrol, Austria) Including a Description of Mallomonas pechlaneri sp. nov. Cryptogam. Algol. 2018, 39, 63–83. [Google Scholar] [CrossRef]
- Gusev, E.; Němcová, Y.; Kulikovskiy, M. Mallomonas voloshkoae sp. nov. (Synurales, Chrysophyceae) and distribution of M. pechlaneri in mountain lakes of Siberia. Phytotaxa 2022, 530, 221–229. [Google Scholar] [CrossRef]
- Gusev, E.S.; Doan-Nhu, H.; Nguyen-Ngoc, L.; Kapustin, D.A. Two New Species of the Genus Mallomonas from the Cat Tien National Park (Viet Nam): Mallomonas distinguenda and Mallomonas skvortsovii. Phytotaxa 2016, 273, 59–64. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H.; Shin, W.; Jo, B.Y. Mallomonas elevata sp. nov. (Synurophyceae), a new scaled Chrysophyte from Jeju Island, South Korea. Nova Hedwig. 2014, 98, 89–102. [Google Scholar] [CrossRef]
- Asmund, B. Electron microscope observations on Mallomonas species and remarks on their occurrence in some Danish ponds and lakes. III. Dan. Bot. Ark. 1959, 18, 1–50. [Google Scholar]
- Kristiansen, J. Cosmopolitan chrysophytes. Syst. Geog. Plants 2000, 70, 291–300. [Google Scholar] [CrossRef]
- Nicholls, K.H. Four new Mallomonas species of the Torquatae series (Chrysophyceae). Canadian J. Bot. 1984, 62, 1583–1591. [Google Scholar] [CrossRef]
- Kim, J.; Hee Park, Y.; Jung Kim, H. Silica-Scaled Chrysophytes (Synurophyceae) from Jeju Island, Korea. Nova Hedwig. 2009, 89, 201–218. [Google Scholar] [CrossRef]
- Gusev, E.S. A new species of the genus Mallomonas (Synurales, Chrysophyceae), Mallomonas fimbriata, sp. nov. Phytotaxa 2015, 195, 291–296. [Google Scholar] [CrossRef]
- Gusev, E.; Kapustin, D.; Skurina, N.; Martynenko, N.; Phan Trong, H. Mallomonas fragariformis sp. nov. (Synurales, Chrysophyceae), a new tropical chrysophyte species. Phytotaxa 2022, 576, 123–130. [Google Scholar] [CrossRef]
- Gusev, E.S.; Čertnerová, D.; Škaloudová, M.; Škaloud, P. Exploring Cryptic Diversity and Distribution Patterns in the Mallomonas kalinae/rasilis Species Complex with a Description of a New Taxon—Mallomonas furtiva sp. nov. J. Eukaryot. Microbiol. 2018, 65, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E. Studies on genera Mallomonas, Synura and other plankton in fresh-water with electron microscope. IV. On two new species of Mallomonas found in ditches at Tsuruoka in North-East of Japan. Bull. Yamagata Univ. Agric. Sci. 1963, 4, 169. [Google Scholar]
- Kim, J.H.; Kim, H.S. Seasonal occurrence of silica-scaled chrysophytes in a small eutrophic swamp, South Korea. Nova Hedwig. 2011, 93, 411–436. [Google Scholar] [CrossRef]
- Gusev, E.S.; Kapustin, D.A.; Martynenko, N.A.; Guseva, E.E.; Kulikovskiy, M.S. Mallomonas gusakovii sp. nov. (Chrysophyceae, Synurales), a New Species from Phu Quoc Island, Vietnam. Phytotaxa 2019, 406, 199–205. [Google Scholar] [CrossRef]
- Nicholls, K.H. Descriptions of four new Mallomonas taxa (Mallomonadaceae, Chrysophyceae). J. Phycol. 1989, 25, 292–300. [Google Scholar] [CrossRef]
- Wei, Y.X.; Kristiansen, J. Studies on silica-scaled chrysophytes from Fujian Province, China. Chin. J. Ocean. Limnol. 1998, 16, 256–266. [Google Scholar] [CrossRef]
- Takahashi, E. Studies on Genera Mallomonas and Synura, and Other Plankton in Fresh Water with the Electron Microscope. IX. Mallomonas harrisae sp. nov. (Chrysophyceae). Phycologia 1975, 14, 41–44. [Google Scholar] [CrossRef]
- Wei, Y.-X.; Yuan, X.-P. Studies on Silica-Scaled Chrysophytes from Zhejiang, Jiangsu and Jiangxi Provinces, China. Nova Hedwig. 2013, 142, 163–179. [Google Scholar]
- Franceschini, I.M.; Couté, A.; Silva, A.J. Synurophyceae et Chrysophyceae à écailles siliceuses du rio dos Sinos, RS, Brésil. Algol. Stud. 1996, 80, 59–85. [Google Scholar]
- Wujek, D.E. Chrysophyceae (Mallomonadaceae) from Florida. Fla. Sci. 1984, 3, 160–170. [Google Scholar]
- Gusev, E.; Kulizin, P.; Guseva, E.; Shkurina, N.; Kulikovskiy, M. Mallomonas lamii sp. nov. (Synurales, Chrysophyceae), a New Species Bearing Large Scales Described from the Tropics. Phytotaxa 2019, 423, 266–272. [Google Scholar] [CrossRef]
- Gusev, E.; Shkurina, N.; Kulikovskiy, M. Mallomonas loricata sp. nov. (Synurales, Chrysophyceae), a New Tropical Species from Section Plantae. Phytotaxa 2021, 500, 225–233. [Google Scholar] [CrossRef]
- Gusev, E.; Kezlya, E. Mallomonas lusca sp. nov.—A rare species from Southeast Asia. Phytotaxa 2021, 529, 105–112. [Google Scholar] [CrossRef]
- Dürrschmidt, M. A Taxonomic Study of the Mallomonas mangofera Group (Mallomonadaceae, Chrysophyceae), Including the Description of four New Taxa. Plant Syst. Evol. 1983, 143, 175–196. [Google Scholar] [CrossRef]
- Harris, K.; Bradley, D.E. A taxonomic study of Mallomonas. J. Gen. Micr. 1960, 22, 750–777. [Google Scholar] [CrossRef]
- Péterfi, L.S.; Momeu, L.; Padisák, J.; Varga, V. Silica-scaled chrysophytes from permanent and temporary waters of Hortobágy, eastern Hungary. Hydrobiologia 1998, 369, 339–351. [Google Scholar] [CrossRef]
- Škaloud, P.; Škaloudová, M.; Pichrtová, M.; Němcová, Y.; Kreidlová, J.; Pusztai, M. www.chrysophytes.eu—A Database on Distribution and Ecology of Silica-Scaled Chrysophytes in Europe. Nova Hedwig. 2013, 142, 141–146. [Google Scholar]
- Wei, Y.X.; Yuan, X.P. Studies on silica-scaled chrysophytes from the Daxinganling Mountains and Wudalianchi Lake Regions, China. Nova Hedwig. 2015, 101, 299–315. [Google Scholar] [CrossRef]
- Kristiansen, J. Silica-scaled chrysophytes from China. Nord. J. Bot. 1989, 8, 539–552. [Google Scholar] [CrossRef]
- Wei, Y.X.; Kristiansen, J. Occurrence and Distribution of Silica-scaled Chrysophytes in Zhejiang, Jiangsu, Hubei, Yunnan and Shandong Provinces, China. Arch. Protistenk. 1994, 144, 433–449. [Google Scholar] [CrossRef]
- Němcová, Y.; Kreidlová, J.; Kosová, A.; Neustupa, J. Lakes and pools of Aquitaine region (France)—A biodiversity hotspot of Synurales in Europe. Nova Hedwig. 2012, 95, 1–24. [Google Scholar] [CrossRef]
- Hansen, A.F.; Johansen, J.E.; Skovgard, A. Floristik undersøgelse af skælbærende gulalger i Dyrehaven. Urt 1993, 17, 13–18. [Google Scholar]
- Hällfors, G.; Hällfors, S. Records of Chrysophytes with Siliceous Scales (Mallomonadaceae and Paraphysomonadaceae) from Finnish Inland Waters. In Flagellates in Freshwater Ecosystems; Jones, R., Ilmavirta, V., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 1988; pp. 1–29. [Google Scholar]
- Gusev, E.; Guseva, E.; Kezlya, E.; Kulikovskiy, M. Mallomonas minuscula sp. nov. (Synurales, Chrysophyceae), a new member in the section Torquatae from Vietnam. Fottea 2019, 19, 132–137. [Google Scholar] [CrossRef]
- Croome, R.L.; Tyler, P.A. Mallomonas morrisonensis (Chrysophyceae) a New Species from Australia. Br. Phycol. J. 1983, 18, 383–389. [Google Scholar] [CrossRef]
- Wujek, D.E.; Bicudo, C.E.M. Scale-bearing chrysophytes from the state of São Paulo, Brazil, 2: Additions to the flora. Braz. J. Bot. 2004, 64, 915–918. [Google Scholar] [CrossRef]
- Gusev, E.; Siver, P.A. Mallomonas neoampla sp. nov. from Vietnam, a New Species That Bridges the Gap Between Fossil and Modern Taxa. Nova Hedwig. 2017, 104, 521–528. [Google Scholar] [CrossRef]
- Ito, H. Chrysophytes in the southern part of Hyogo prefecture, Japan (I). Chrysophyte flora in three ponds and reservoir. Jpn. J. Phycol. 1990, 38, 327–332. [Google Scholar]
- Ito, H. Chrysophytes in the southern part of Hyogo prefecture, Japan (II). Mallomonas. Jpn. J. Phycol. 1991, 39, 253–262. [Google Scholar]
- Harris, K.; Bradley, D.E. Some Unusual Chrysophyceae Studied in the Electron Microscope. Microbiology 1958, 18, 71–83. [Google Scholar] [CrossRef]
- Gusev, E.S. A new species in genus Mallomonas Perty (Synurales, Chrysophyceae) from Vietnam. Int. J. Algae 2015, 17, 351–362. [Google Scholar] [CrossRef]
- Compère, P. Mallomonas portae-ferreae: Peterfi & Asmund (Chrysophycée) au lac Tchad. Bull. Jard. Bot. Nat. Belg. Bull. Nat. Plantentuin Belg. 1973, 43, 236–237. [Google Scholar] [CrossRef]
- Siver, P.A. The Biology of Mallomonas: Morphology, Taxonomy and Ecology; Kluwer Academic: Dordrecht, Netherlands, 1991; pp. 1–230. [Google Scholar] [CrossRef]
- Siver, P.A.; Lott, A.M. Further observations on the scaled Chrysophycean and Synurophycean flora of the Ocala National Forest, Florida, USA. Nord. J. Bot. 2006, 24, 211–233. [Google Scholar] [CrossRef]
- Siver, P.A.; Lott, A.M. Do Scaled Chrysophytes Have a Biogeography? Evidence from the East Coast of North America. Freshw. Biol. 2012, 57, 451–467. [Google Scholar] [CrossRef]
- Gusev, E.; Kulikovskiy, M. Mallomonas siderea sp. nov. (Synurales, Chrysophyceae), a New Tropical Species from the Section Torquatae. Nova Hedwig. 2021, 113, 291–301. [Google Scholar] [CrossRef]
- Gusev, E.S. A New Species of the Genus Mallomonas (Synurophyceae), Mallomonas spinosa sp. nov., from Vietnam. Phytotaxa 2012, 66, 1–5. [Google Scholar] [CrossRef]
- Croome, R.; Diirrschmidt, M.; Tyler, P.A. Light and Electron Microscopical Investigation of Mallomonas splendens (G.S. West) Playfair (Mallomonadaceae, Chrysophyceae). Nova Hedwig. 1985, 41, 463–470. [Google Scholar]
- Kristiansen, J. The genus Mallomonas (Synurophyceae): A taxonomic survey based on the ultrastructure of silica scales and bristles. Opera Bot. 2002, 139, 1–218. [Google Scholar]
- Neustupa, J.; Němcová, Y. A geometric morphometric study of the variation in scales of Mallomonas striata (Synurophyceae, Heterokontophyta). Phycologia 2007, 46, 123–130. [Google Scholar] [CrossRef]
- Gusev, E.; Martynenko, N. Silica-Scaled Chrysophytes of Teletskoye Lake and Adjacent Area with a Description of a New Species from the Genus Mallomonas. Diversity 2022, 14, 1040. [Google Scholar] [CrossRef]
- Gusev, E.; Kezlya, E.; Tran, H.; Kulikovskiy, M. Mallomonas vietnamica sp. nov. (Synurales, Chrysophyceae), a New Species, That Shares Some Features with Fossil Taxa. Cryptogam. Algol. 2021, 42, 39–46. [Google Scholar] [CrossRef]
- Siver, P.A.; Kapustin, D.; Gusev, E. Investigations of two-celled colonies of Synura formerly described as Chrysodidymus with descriptions of two new species. Eur. J. Phycol. 2018, 53, 245–255. [Google Scholar] [CrossRef]
- Škaloud, P.; Kynčlová, A.; Benada, O.; Kofroňová, O.; Škaloudová, M. Toward a Revision of the Genus Synura, Section Petersenianae (Synurophyceae, Heterokontophyta): Morphological Characterization of Six Pseudo-Cryptic Species. Phycologia 2012, 51, 303–329. [Google Scholar] [CrossRef]
- Škaloud, P.; Škaloudová, M.; Procházková, A.; Němcová, Y. Morphological Delineation and Distribution Patterns of Four Newly Described Species within the Synura petersenii Species Complex (Chrysophyceae, Stramenopiles). Eur. J. Phycol. 2014, 49, 213–229. [Google Scholar] [CrossRef]
- Vyverman, W. A systematic account of the algal flora of the seasonal swamps in the southern part of Irian Jaya (Indonesia). In Recent Trends in Algal Taxonomy; Vidyavati Mahato, A.K., Ed.; Associated Publishing Co.: New Delhi, India, 2006; Volume 2, pp. 3–19. [Google Scholar]
- Le, T.P.Q.; Ho, T.C.; Duong, T.T.; Nguyen, T.B.N.; Vu, D.A.; Pham, Q.L.; Seidler, C. Water quality of the Red River system in the period 2012–2013. J. Vietnam. Environ. 2014, 6, 191–195. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Nguyen, T.K.; Le, P.Q.; Dang, D.K.; Duong, T.T. Assessment of the water quality downstream of Red River in 2015 (Vietnam). J. Vietnam. Environ. 2015, 8, 167–172. [Google Scholar] [CrossRef]
- Petrisor, A.; Hamma, W.; Nguyen, H.D.; Randazzo, G.; Muzirafuti, A.; Stan, M.; Tran, V.T.; Astefănoaiei, R.; Bui, Q.; Drago, V.D.; et al. Degradation of Coastlines under the Pressure of Urbanization and Tourism: Evidence on the Change of Land Systems from Europe, Asia and Africa. Land 2020, 9, 275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusev, E.; Martynenko, N.; Shkurina, N.; Huan, P.T.; Dien, T.D.; Thanh, N.T.H. An Annotated Checklist of Algae from the Order Synurales (Chrysophyceae) of Viet Nam. Diversity 2023, 15, 183. https://doi.org/10.3390/d15020183
Gusev E, Martynenko N, Shkurina N, Huan PT, Dien TD, Thanh NTH. An Annotated Checklist of Algae from the Order Synurales (Chrysophyceae) of Viet Nam. Diversity. 2023; 15(2):183. https://doi.org/10.3390/d15020183
Chicago/Turabian StyleGusev, Evgeniy, Nikita Martynenko, Nataliya Shkurina, Phan Trong Huan, Tran Duc Dien, and Nguyen Thi Hai Thanh. 2023. "An Annotated Checklist of Algae from the Order Synurales (Chrysophyceae) of Viet Nam" Diversity 15, no. 2: 183. https://doi.org/10.3390/d15020183
APA StyleGusev, E., Martynenko, N., Shkurina, N., Huan, P. T., Dien, T. D., & Thanh, N. T. H. (2023). An Annotated Checklist of Algae from the Order Synurales (Chrysophyceae) of Viet Nam. Diversity, 15(2), 183. https://doi.org/10.3390/d15020183






