Diplosphaera elongata sp. nova: Morphology and Phenotypic Plasticity of This New Microalga Isolated from Lichen Thalli
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Origin and Culture Conditions
2.2. Microscopic Analyses
2.3. DNA Extraction
2.4. Phylogenetic Analysis
2.5. Analysis of the ITS2 Secondary Structure
2.6. Chlorophyll-Fluorescence Measurements
2.7. Statistical Analyses
3. Results
3.1. Taxonomic Assessment
- Family: Trebouxiaceae Friedl
- Genus: Diplosphaera Bialosuknia
- Diplosphaera elongata Chiva and Barreno sp. nova.
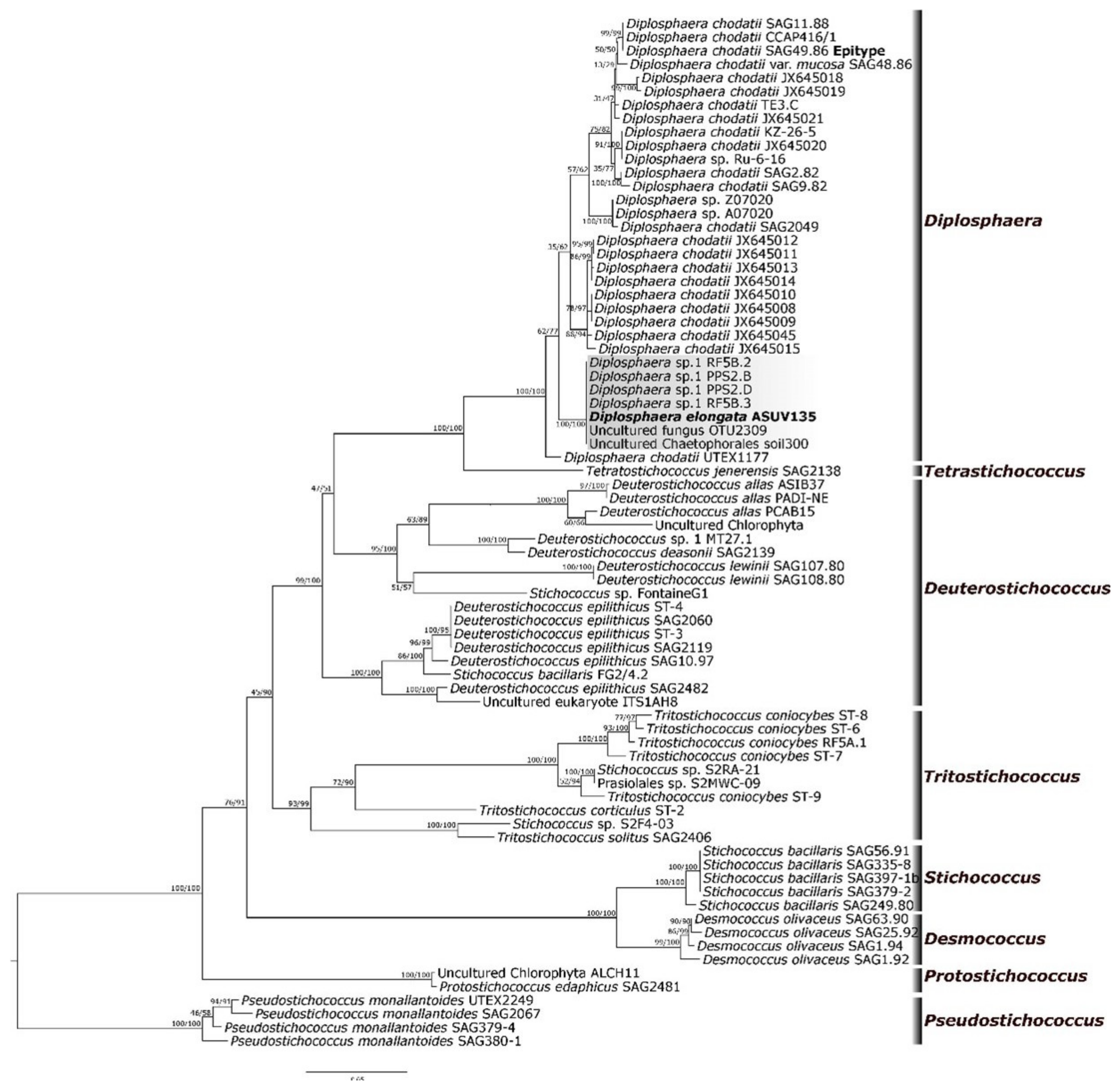
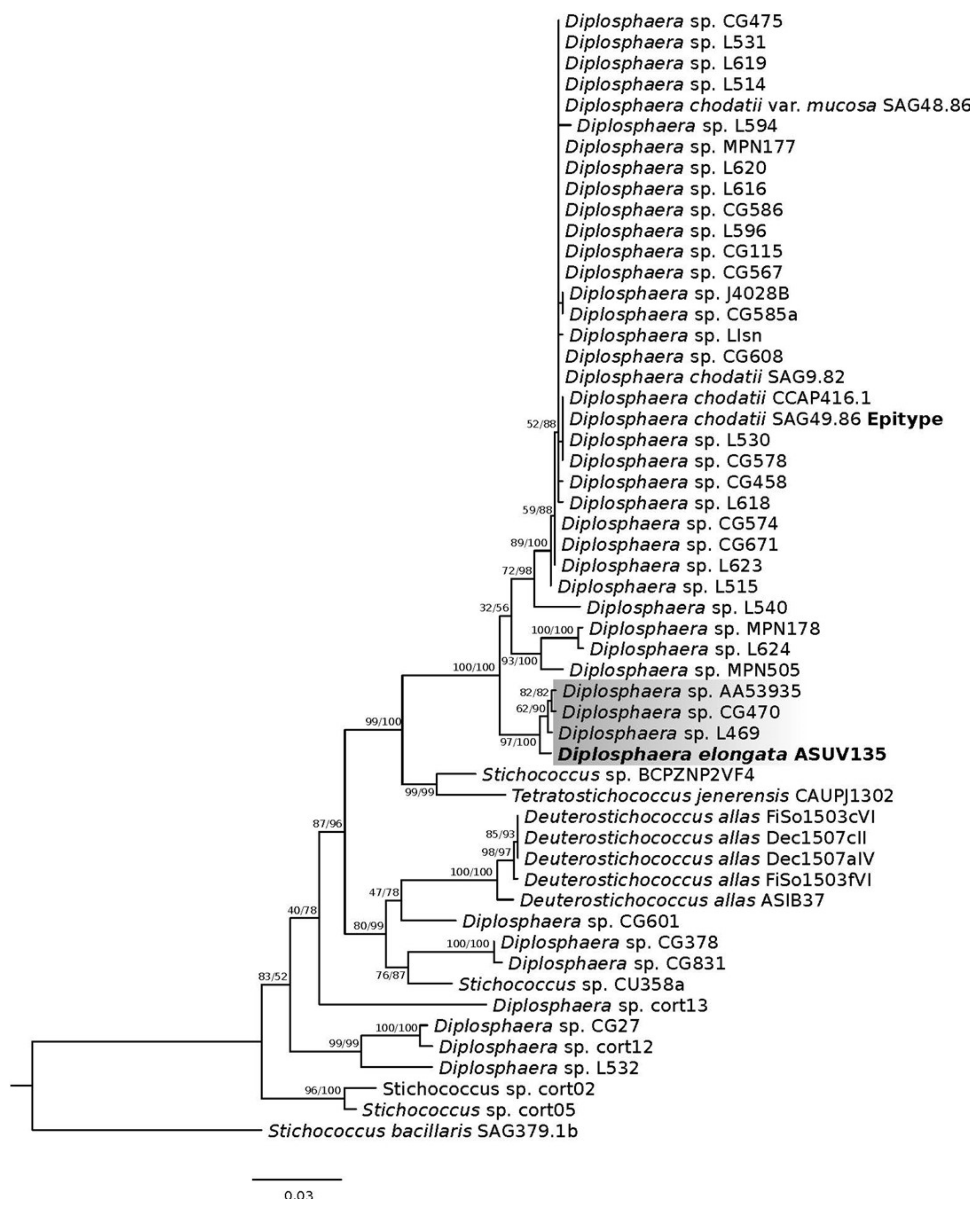
3.2. Molecular Phylogeny
3.3. ITS2 Secondary Structure
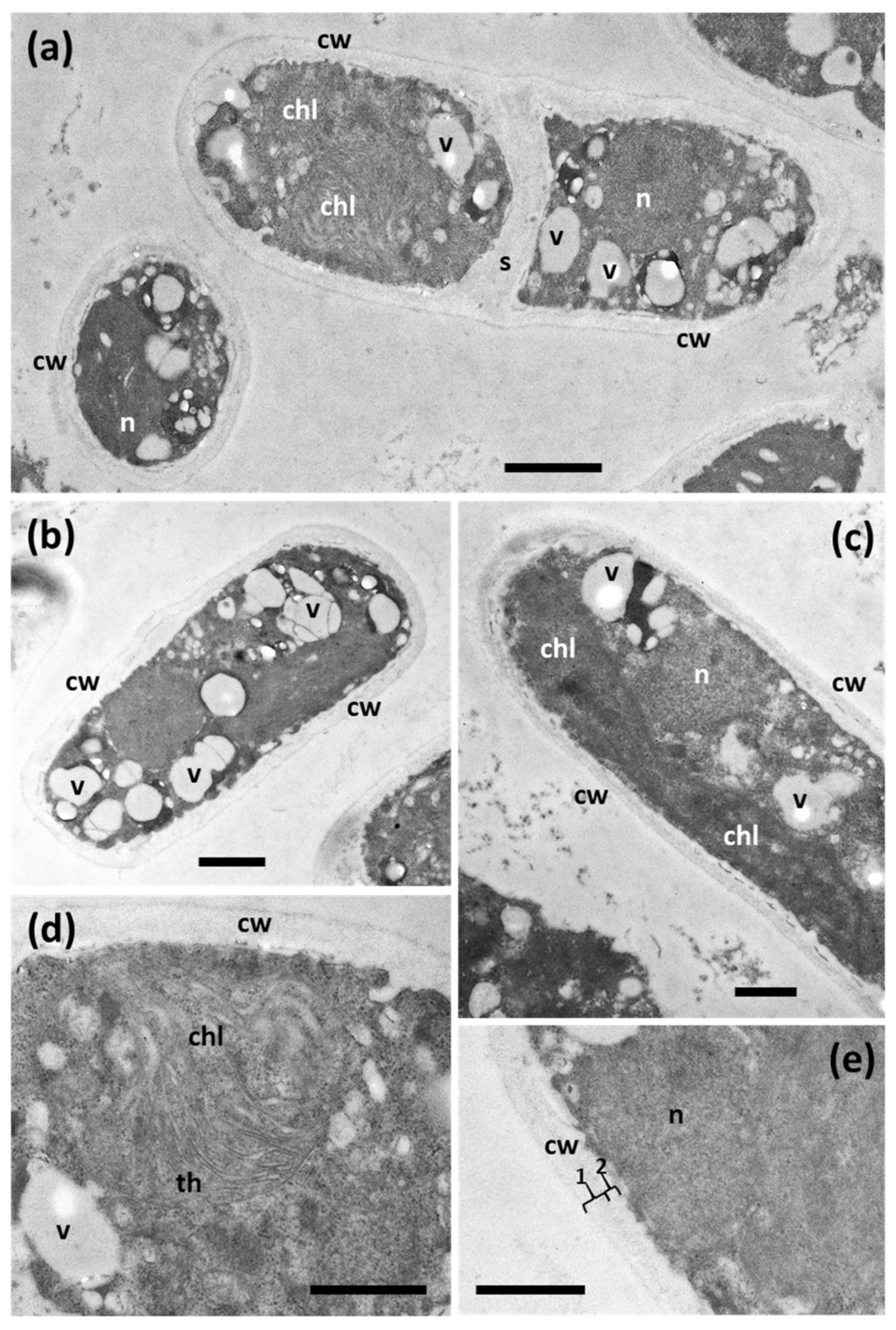
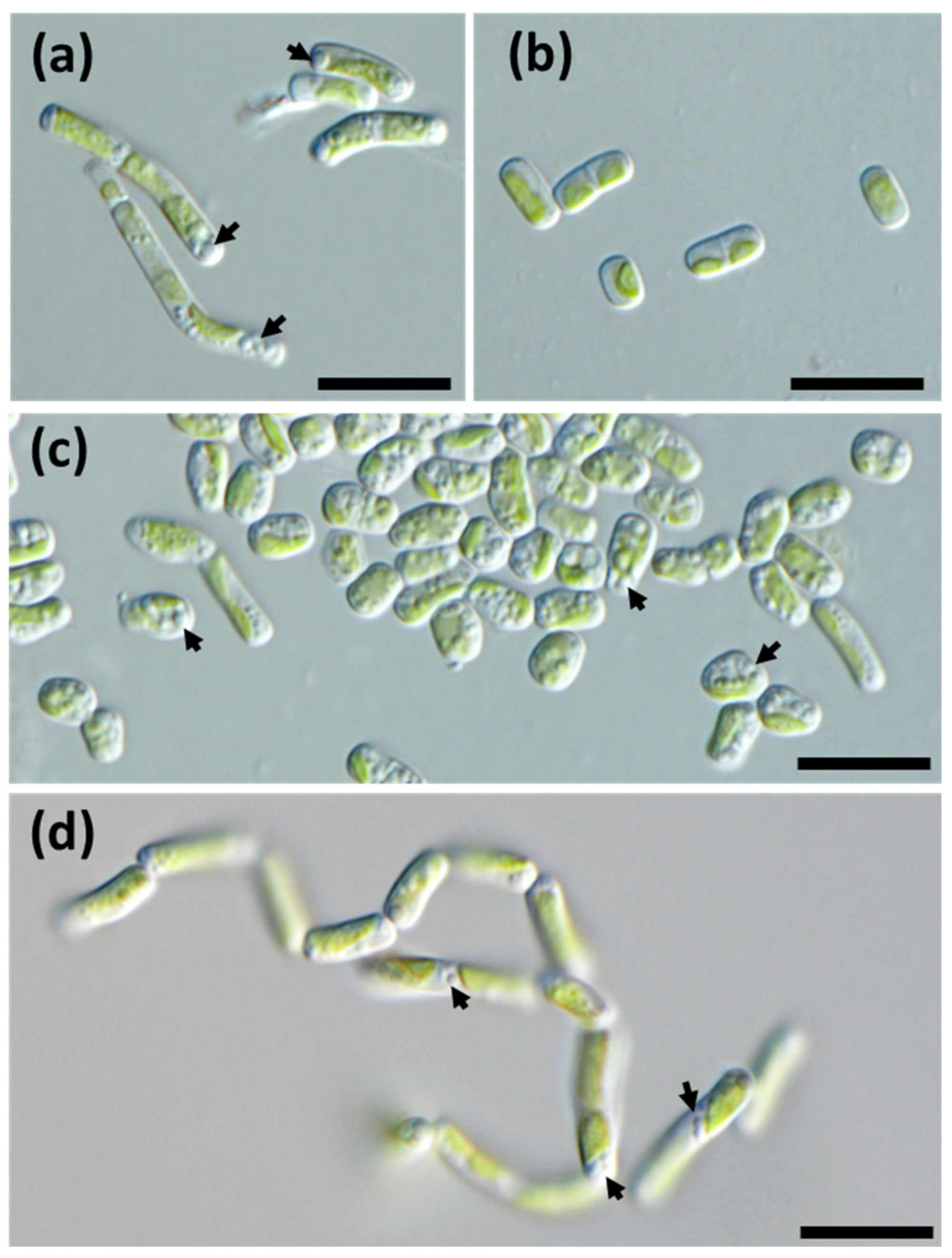
3.4. Ultrastructure and Morphology in Different Culture Media
3.5. Evaluation of Chlorophyll-Fluorescence Measurements
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ml | Stock Solution | g/400 mL dist. water | g/1000 mL dist. water |
|---|---|---|---|
| 10 | NaNO3 | 10 | 25 |
| 10 | CaCl2·2H2O | 1 | 2.5 |
| 10 | MgSO4·7H2O | 3 | 7.5 |
| 10 | K2HPO4·3 H2O | 3 | 7.5 |
| 10 | KH2PO4 | 7 | 17.5 |
| 10 | NaCl | 1 | 2.5 |
| 6 | Microelement solution | ||
| 5 | Lichen extract (5%) |
| Ingredients | Quantity |
|---|---|
| FeCl3·6H2O | 97 mg |
| MnCl2·4H2O | 41 mg |
| ZnCl2·6H2O | 5 mg |
| CoCl2·6H2O | 2 mg |
| Na2MoO4·2H2O | 4 mg |
| Ingredients | Quantity |
|---|---|
| NaCl | 2.25 g (dissolve in 200 mL) |
| KCl | 0.105 g (dissolve in 100 mL) |
| CaCl2 | 0.06 g (dissolve in 100 mL) * |
| NaHCO3 | 0.05 g (dissolve in 100 mL) |
| Distilled water | Up to 1000 mL |
References
- Leliaert, F.; Smith, D.R.; Moreau, H.; Herron, M.D.; Verbruggen, H.; Delwiche, C.F.; De Clerck, O. Phylogeny and molecular evolution of the green algae. Crit. Rev. Plant Sci. 2012, 31, 1–46. [Google Scholar] [CrossRef]
- Heesch, S.; Pažoutová, M.; Moniz, M.B.; Rindi, F. Prasiolales (Trebouxiophyceae, Chlorophyta) of the Svalbard Archipelago: Diversity, biogeography and description of the new genera Prasionella and Prasionema. Eur. J. Phycol. 2016, 51, 171–187. [Google Scholar] [CrossRef]
- Nelson, W.A.; Sutherland, J.E. Prasionema heeschiae sp. nov. (Prasiolales, Chlorophyta) from Campbell Island, New Zealand: First record of Prasionema in the southern hemisphere. Eur. J. Phycol. 2018, 53, 198–207. [Google Scholar] [CrossRef]
- Darienko, T.; Gustavs, L.; Pröschold, T. Species concept and nomenclatural changes within the genera Elliptochloris and Pseudochlorella (Trebouxiophyceae) based on an integrative approach. J. Phycol. 2016, 52, 1125–1145. [Google Scholar] [CrossRef]
- Pröschold, T.; Darienko, T. The green puzzle Stichococcus (Trebouxiophyceae, Chlorophyta): New generic and species concept among this widely distributed genus. Phytotaxa 2020, 441, 113–142. [Google Scholar] [CrossRef]
- Karsten, U.; Friedl, T.; Schumann, R.; Hoyer, K.; Lembcke, S. Mycosporine-like amino acids and phylogenies in green algae: Prasiola and its relatives from the Trebouxiophyceae (Chlorophyta). J. Phycol. 2005, 41, 557–566. [Google Scholar] [CrossRef]
- Rindi, F.; McIvor, L.; Sherwood, A.R.; Friedl, T.; Guiry, M.D.; Sheath, R.G. Molecular phylogeny of the green algal order Prasiolales (Trebouxiophyceae, Chlorophyta). J. Phycol. 2007, 43, 811–822. [Google Scholar] [CrossRef]
- Medwed, C.; Holzinger, A.; Hofer, S.; Hartmann, A.; Michalik, D.; Glaser, K.; Karsten, U. Ecophysiological, morphological, and biochemical traits of free-living Diplosphaera chodatii (Trebouxiophyceae) reveal adaptation to harsh environmental conditions. Protoplasma 2021, 258, 1187–1199. [Google Scholar] [CrossRef]
- De Wever, A.; Leliaert, F.; Verleyen, E.; Vanormelingen, P.; Van der Gucht, K.; Hodgson, D.A.; Sabbe, K.; Vyverman, W. Hidden levels of phylodiversity in Antarctic green algae: Further evidence for the existence of glacial refugia. Proc. R. Soc. B Biol. Sci. 2009, 276, 3591–3599. [Google Scholar] [CrossRef]
- Vishnivetskaya, T.A. Viable cyanobacteria and green algae from the permafrost darkness. In Permafrost Soils; Soil Biology; Margesin, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 16, pp. 73–84. [Google Scholar]
- Khan, N.; Tuffin, M.; Stafford, W.; Cary, C.; Lacap, D.C.; Pointing, S.B.; Cowan, D. Hypolithic microbial communities of quartz rocks from Miers Valley, McMurdo Dry Valleys, Antarctica. Polar Biol. 2011, 34, 1657–1668. [Google Scholar] [CrossRef]
- Van, A.T.; Karsten, U.; Glaser, K. A chemosystematic investigation of selected Stichococcus-like organisms (Trebouxiophyta). Algae 2021, 36, 123–135. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2019; Available online: https://www.algaebase.org (accessed on 22 January 2023).
- Coppins, B.J. A taxonomic study of the lichen genus Micarea in Europe. Bull. Br. Mus. Nat. 1983, 11, 17–214. [Google Scholar]
- Fontaine, K.M.; Beck, A.; Stocker-Wörgötter, E.; Piercey-Normore, M.D. Photobiont relationships and phylogenetic history of Dermatocarpon luridum var. luridum and related Dermatocarpon species. Plants 2012, 1, 39–60. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E.; Türk, R. The resynthesis of thalli of Dermatocarpon miniatum under laboratory conditions. Symbiosis 1989, 7, 37–50. [Google Scholar]
- Orekhova, A.; Barták, M.; Özkar, A.; Elster, J. The effect of shock freezing on physiological properties and consequent growth of Antarctic filamentous (Stigeoclonium sp.) and coccal alga (Diplosphaera chodatii) on agar plates. Czech Polar Rep. 2019, 9, 37–48. [Google Scholar] [CrossRef]
- Bialosuknia, M.W. Sur un nouveau genre de Pleurococcacées. Bull. Soc. Bot. Genève 1909, 1, 101–104. [Google Scholar]
- Ettl, H.; Gärtner, G. Syllabus der Boden-, Luft- und Flechtenalgen.; Springer: Berlin/Heidelberg, Germany, 2014; p. 773. [Google Scholar]
- Sanders, W.B.; Masumoto, H. Lichen algae: The photosynthetic partners in lichen symbioses. Lichenologist 2021, 53, 347–393. [Google Scholar] [CrossRef]
- Fontaine, K.M.; Stocker-Wörgötter, E.; Booth, T.; Piercey-Normore, M.D. Genetic diversity of the lichen-forming alga, Diplosphaera chodatii, in North America and Europe. Lichenologist 2013, 45, 799–813. [Google Scholar] [CrossRef]
- Thüs, H.; Muggia, L.; Pérez-Ortega, S.; Favero-Longo, S.E.; Joneson, S.; O’Brien, H.; Nelsen, M.P.; Duque-Thüs, R.; Grube, M.; Friedl, T.; et al. Revisiting photobiont diversity in the lichen family Verrucariaceae (Ascomycota). Eur. J. Phycol. 2011, 46, 399–415. [Google Scholar] [CrossRef]
- Chiva, S.; Moya, P.; Barreno, E. Lichen phycobiomes as source of biodiversity for microalgae of the Stichococcus-like genera. Biologia 2022, 78, 389–397. [Google Scholar] [CrossRef]
- Pérez-Ortega, S.; Ríos, A.D.L.; Crespo, A.; Sancho, L.G. Symbiotic lifestyle and phylogenetic relationships of the bionts of Mastodia tessellata (Ascomycota, incertae sedis). Am. J. Bot. 2010, 97, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Benavent, I.; Pérez-Ortega, S.; de Los Ríos, A. From Alaska to Antarctica: Species boundaries and genetic diversity of Prasiola (Trebouxiophyceae), a foliose chlorophyte associated with the bipolar lichen-forming fungus Mastodia tessellate. Mol. Phylogenet. Evol. 2017, 107, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Bechteler, J.; Casanova-Katny, A.; Dzhilyanova, I. The pioneer lichen Placopsis in maritime Antarctica: Genetic diversity of their mycobionts and green algal symbionts, and their correlation with deglaciation time. Symbiosis 2019, 79, 1–24. [Google Scholar] [CrossRef]
- Chiva, S.; Dumitru, C.; Bordenave, C.D.; Barreno, E. Watanabea green microalgae (Trebouxiophyceae) inhabiting lichen holobiomes: Watanabea lichenicola sp. nova. Phycol. Res. 2021, 69, 226–236. [Google Scholar] [CrossRef]
- Bischoff, H.W.; Bold, H.C. Physiological Studies: IV. Some Soil Algae from Enchanted Rock and Related Algal Species; Publications No. 6318; University of Texas: Austin, TX, USA, 1963. [Google Scholar]
- Schlösser, U.C. Additions to the culture collection of algae since 1994. Bot. Acta 1997, 110, 424–429. [Google Scholar] [CrossRef]
- Ahmadjian, V. A guide to the algae occurring as lichen symbionts: Isolation, culture, cultural physiology, and identification. Phycologia 1967, 6, 127–160. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Bordenave, C.D.; Muggia, L.; Chiva, S.; Leavitt, S.D.; Carrasco, P.; Barreno, E. Chloroplast morphology and pyrenoid ultrastructural analyses reappraise the diversity of the lichen phycobiont genus Trebouxia (Chlorophyta). Algal Res. 2022, 61, 102561. [Google Scholar] [CrossRef]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef]
- Piercey-Normore, M.D.; DePriest, P.T. Algal switching among lichen symbioses. Am. J. Bot. 2001, 88, 1490–1498. [Google Scholar] [CrossRef]
- Kroken, S.; Taylor, J.W. Phylogenetic species, reproductive mode, and specificity of the green alga Trebouxia forming lichens with the fungal genus Letharia. Bryologist 2000, 103, 645–660. [Google Scholar] [CrossRef]
- Nelsen, M.P.; Plata, E.R.; Andrew, C.J.; Lücking, R.; Lumbsch, H.T. Phylogenetic diversity of Trentepohlialean algae associated with lichen-forming fungi 1. J. Phycol. 2011, 47, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree-Version 1.4.3, a Graphical Viewer of Phylogenetic Trees. 2017. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 22 January 2023).
- Miller, M.A. The CIPRES Science Gateway V. 3.3. 2012. Available online: http://www.phylo.org/index.php/portal (accessed on 22 January 2023).
- Koetschan, C.; Hackl, T.; Müller, T.; Wolf, M.; Förster, F.; Schultz, J. ITS2 database IV: Interactive taxon sampling for internal transcribed spacer 2 based phylogenies. Mol. Phylogenet. Evol. 2012, 63, 585–588. [Google Scholar] [CrossRef]
- Reuter, J.S.; Mathews, D.H. RNAstructure: Software for RNA secondary structure prediction and analysis. BMC Bioinform. 2010, 11, 129. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 279–319. [Google Scholar]
- Lazár, D. Parameters of photosynthetic energy partitioning. J. Plant Physiol. 2015, 175, 131–147. [Google Scholar] [CrossRef]
- Kasajima, I.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H. Estimation of the relative sizes of rate constants for Chlorophyll de-excitation processes through comparison of inverse fluorescence intensities. Plant Cell Physiol. 2009, 50, 1600–1616. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 22 January 2023).
- Addison, S.L.; Walbert, K.; Smaill, S.J.; Menkis, A. Edaphic properties related with changes in diversity and composition of fungal communities associated with Pinus radiata. Pedobiologia 2018, 66, 43–51. [Google Scholar] [CrossRef]
- Coleman, A.W. ITS2 is a double-edged tool for eukaryote evolutionary comparisons. Trends Genet. 2003, 19, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of Q A redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Doering, J.A. An Investigation of the Spatial Distribution and Inference of Dispersal Method in the Semi-Aquatic Lichenised Green Alga Diplosphaera chodatii around Payuk Lake, Manitoba. Master’s Thesis, Faculty of Graduate Studies of the University of Manitoba, Winnipeg, MB, Canada, 2017. Available online: https://mspace.lib.umanitoba.ca/xmlui/handle/1993/32334 (accessed on 22 January 2023).
- Hodac, L.; Hallman, C.; Spitzer, K.; Elster, J.; Faßhauer, F.; Brinkmann, N.; Lepka, D.; Diwan, V.; Friedl, T. Widespread green algae Chlorella and Stichococcus exhibit polar-temperate and tropical-temperate biogeography. FEMS Microbiol. Ecol. 2016, 93, fiw122. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Masojídek, J.; Torzillo, G.; Koblížek, M. Photosynthesis in microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2013; pp. 21–36. [Google Scholar]
- White, S.; Anandraj, A.; Bux, F. PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids. Bioresour. Technol. 2011, 102, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Wilson, S. The mechanism of non-photochemical quenching in plants: Localization and driving forces. Plant Cell Physiol. 2021, 62, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Elshobary, M.E.; Osman, M.E.; Abushady, A.M.; Piercey-Normore, M.D. Comparison of lichen-forming cyanobacterial and green algal photobionts with free-living algae. Cryptogamie Algol. 2015, 36, 81–100. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, J. Survival analyses of symbionts isolated from Endocarpon pusillum Hedwig to desiccation and starvation stress. Sci. China Life Sci. 2011, 54, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Starr, R.C.; Zeikus, J.A. UTEX—The culture collection of algae at the University of Texas at Austin 1993 List of cultures 1. J. Phycol. 1993, 29, 1–106. [Google Scholar] [CrossRef]
- Biosca, E.G.; Flores, R.; Santander, R.D.; Díez-Gil, J.L.; Barreno, E. Innovative approaches using lichen enriched media to improve isolation and culturability of lichen associated bacteria. PLoS ONE 2016, 11, e0160328. [Google Scholar] [CrossRef]
- Schaad, N.W.; Süle, S.; van Vuurde, J.W.L.; Vruggink, H.; Alvarez, A.M.; Benedict, A.A. Serology. In Methods in Phytobacteriology; Klement, Z., Rudolph, K., Sands, D.C., Eds.; Akadémiai Kiadó: Budapest, Hungary, 1990; pp. 153–190. [Google Scholar]
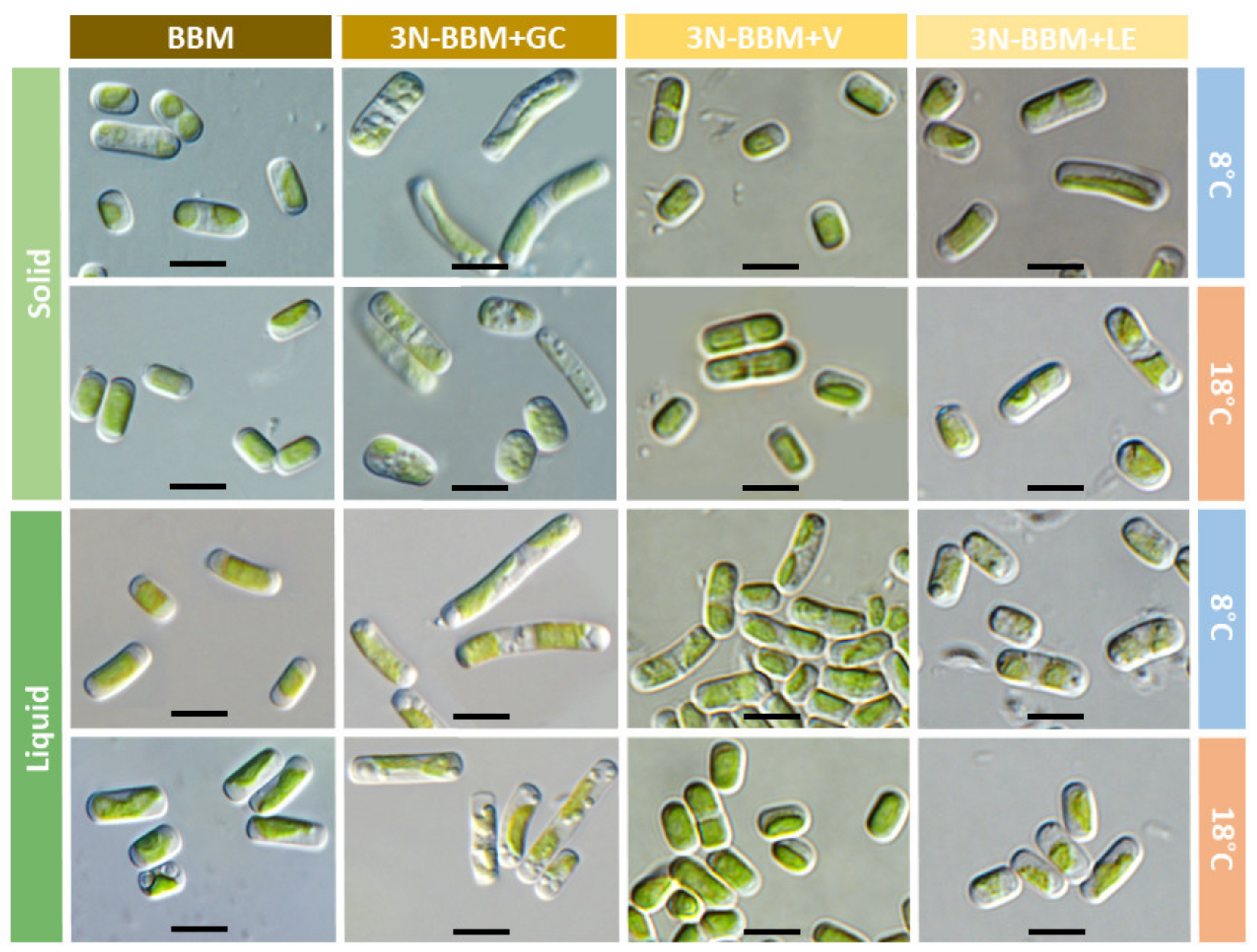
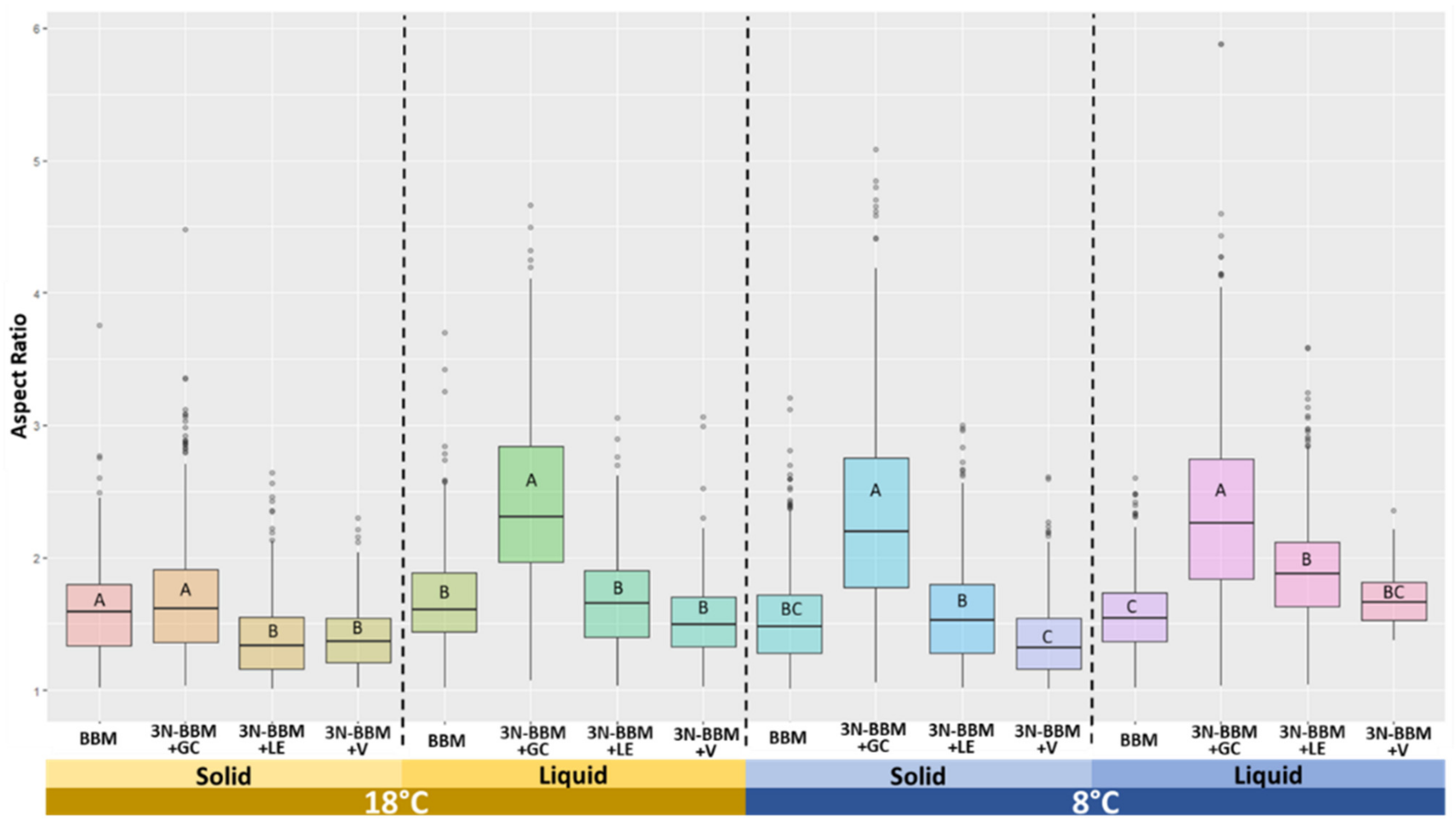

| Treatment | Standard Condition | Poor Condition | Rich Condition | Lichen Condition |
|---|---|---|---|---|
| Solid 8 °C | 3N-BBM + V | BBM | 3N-BBM + GC | 3N-BBM + LE |
| Solid 18 °C | 3N-BBM + V | BBM | 3N-BBM + GC | 3N-BBM + LE |
| Liquid 8 °C | 3N-BBM + V | BBM | 3N-BBM + GC | 3N-BBM + LE |
| Liquid 18 °C | 3N-BBM + V | BBM | 3N-BBM + GC | 3N-BBM + LE |
| Trait | Diplosphaera chodatii | Diplosphaera elongata |
|---|---|---|
| Morphological | Individual cells are rather oval [5]. | Individual cells are rod-shaped. |
| Ultrastructural | Cells show a pyrenoid, numerous electron-dense vacuoles and a chloroplast with regularly arranged thylakoid membranes [8]. | Cells without pyrenoids or electron-dense vacuoles. In addition, the thylakoids are not regularly organized. |
| Phenotypical | Main factor: Temperature.
| Main factor: Medium composition.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiva, S.; Bordenave, C.D.; Gázquez, A.; Barreno, E. Diplosphaera elongata sp. nova: Morphology and Phenotypic Plasticity of This New Microalga Isolated from Lichen Thalli. Diversity 2023, 15, 168. https://doi.org/10.3390/d15020168
Chiva S, Bordenave CD, Gázquez A, Barreno E. Diplosphaera elongata sp. nova: Morphology and Phenotypic Plasticity of This New Microalga Isolated from Lichen Thalli. Diversity. 2023; 15(2):168. https://doi.org/10.3390/d15020168
Chicago/Turabian StyleChiva, Salvador, César Daniel Bordenave, Ayelén Gázquez, and Eva Barreno. 2023. "Diplosphaera elongata sp. nova: Morphology and Phenotypic Plasticity of This New Microalga Isolated from Lichen Thalli" Diversity 15, no. 2: 168. https://doi.org/10.3390/d15020168
APA StyleChiva, S., Bordenave, C. D., Gázquez, A., & Barreno, E. (2023). Diplosphaera elongata sp. nova: Morphology and Phenotypic Plasticity of This New Microalga Isolated from Lichen Thalli. Diversity, 15(2), 168. https://doi.org/10.3390/d15020168






