Low Temperature Scanning Electron Microscopy (LTSEM) Findings on the Ultrastructure of Trebouxia lynnae (Trebouxiophyceae, Lichenized Microalgae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Strains and Growth Conditions
2.2. Low Temperature Scanning Electron Microscopy (LTSEM)
2.3. Transmission Electron Microscopy (TEM)
3. Results and Discussion
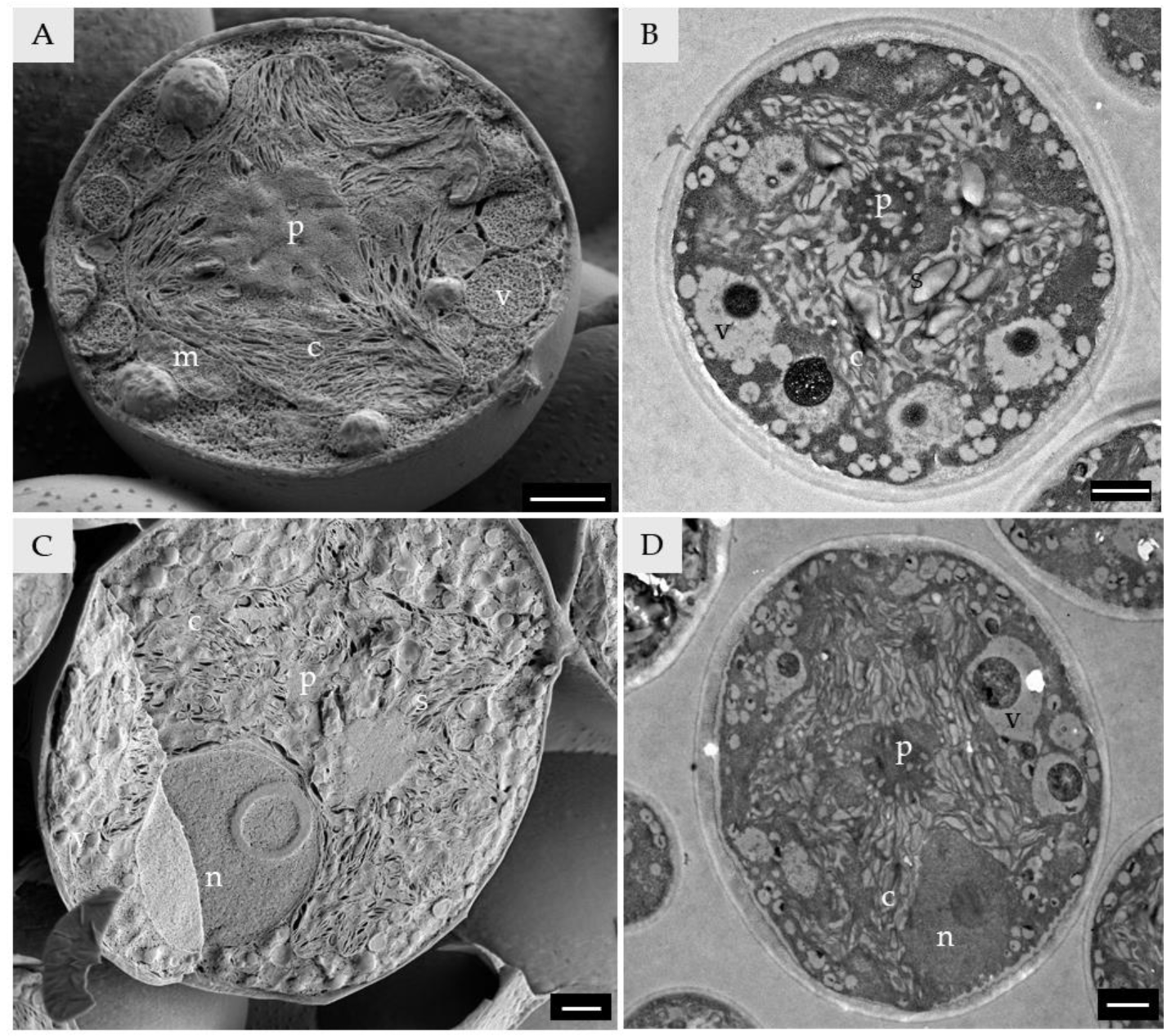
3.1. General Aspects of Axenically Cultured Trebouxia lynnae LTSEM for Cell Ultrastructure Analysis
3.2. Chloroplast and Pyrenoid
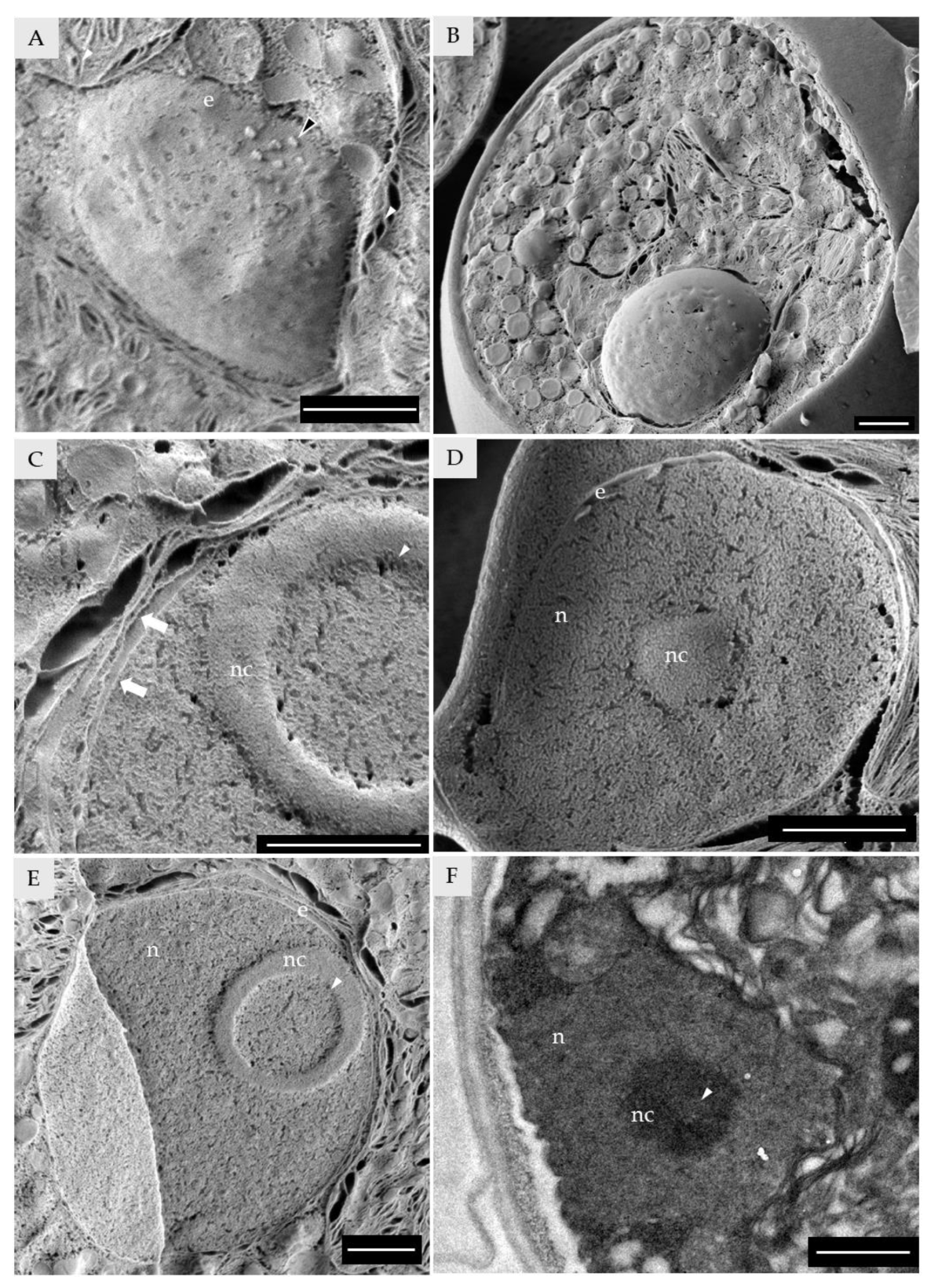
3.3. Nucleus
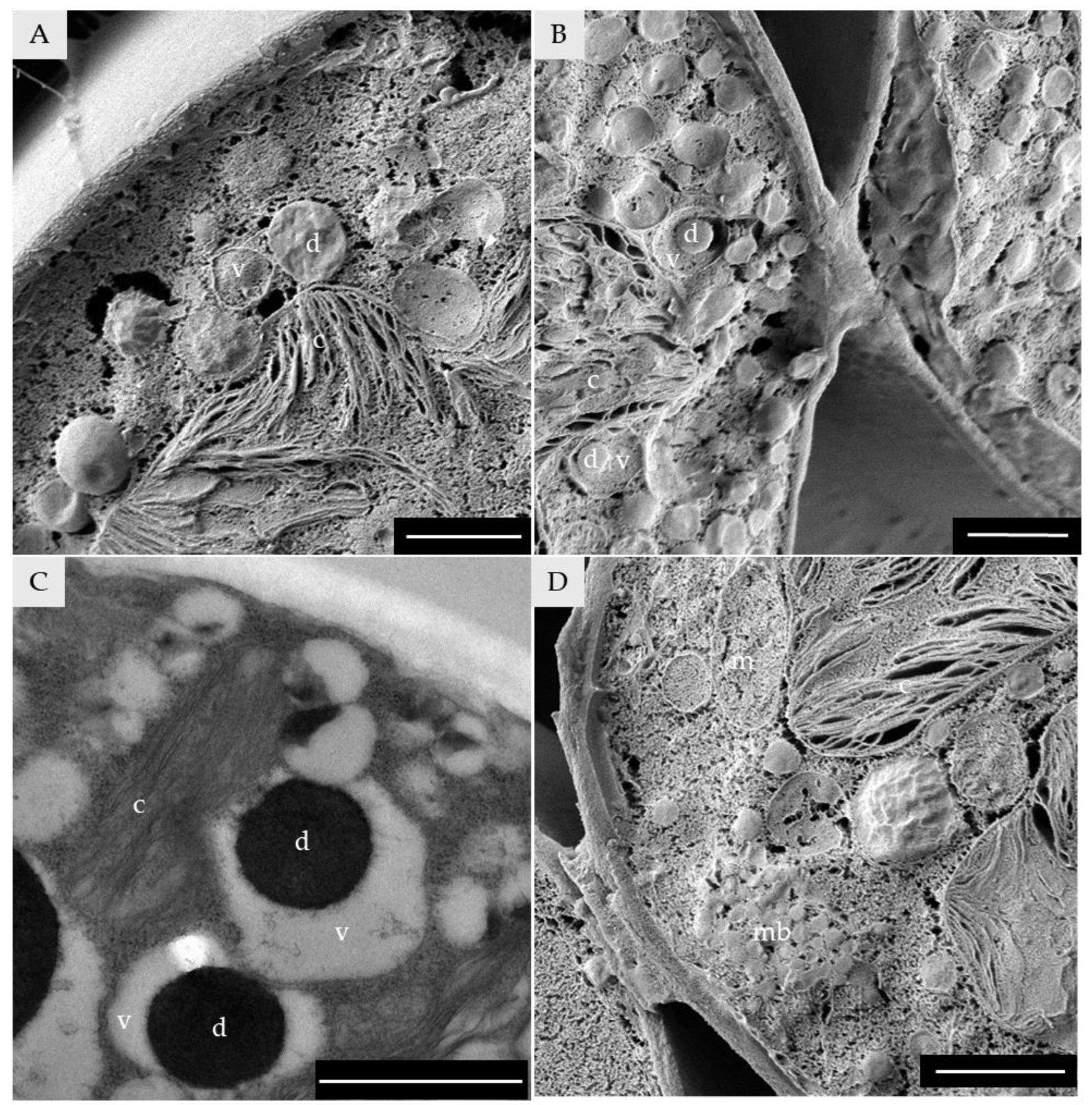
3.4. Cytosol and Cytosolic Organelles
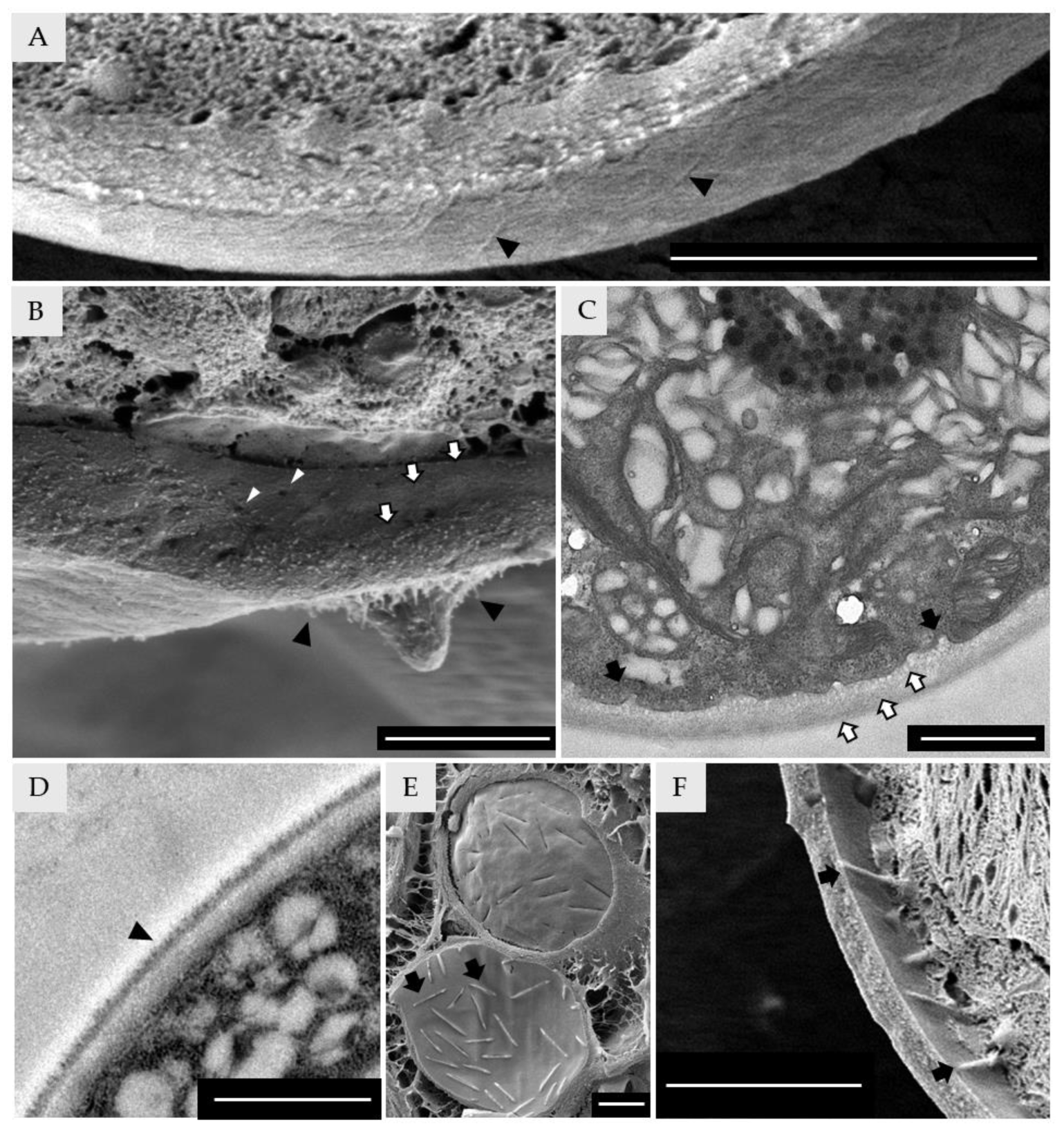
3.5. Cell-Wall
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Friedl, T. Systematik und biologie von Trebouxia (Microthamniales, Chlorophyta) als phycobiont der Parmeliaceae (lichenisierte Ascomyceten). Ph.D. Thesis, Universitat Bayreuth, Bayreuth, Germany, 1989. [Google Scholar]
- Tschermak-Woess, E. The Algal Partner. In Handbook of Lichenology; CRC Press: Boca Raton, FL, USA, 2019; pp. 39–92. ISBN 0429291787. [Google Scholar]
- Friedl, T. Comparative ultrastructure of pyrenoids in Trebouxia (Microthamniales, Chlorophyta). Plant Syst. Evol. 1989, 164, 145–159. [Google Scholar] [CrossRef]
- Muggia, L.; Leavitt, S.; Barreno, E. The hidden diversity of lichenised Trebouxiophyceae (Chlorophyta). Phycologia 2018, 57, 503–524. [Google Scholar] [CrossRef]
- Leavitt, S.D.; Kraichak, E.; Nelsen, M.P.; Altermann, S.; Divakar, P.K.; Alors, D.; Esslinger, T.L.; Crespo, A.; Lumbsch, T. Fungal specificity and selectivity for algae play a major role in determining lichen partnerships across diverse ecogeographic regions in the lichen-forming family Parmeliaceae (Ascomycota). Mol. Ecol. 2015, 24, 3779–3797. [Google Scholar] [CrossRef] [PubMed]
- Muggia, L.; Nelsen, M.P.; Kirika, P.M.; Barreno, E.; Beck, A.; Lindgren, H.; Lumbsch, H.T.; Leavitt, S.D. Formally described species woefully underrepresent phylogenetic diversity in the common lichen photobiont genus Trebouxia (Trebouxiophyceae, Chlorophyta): An impetus for developing an integrated taxonomy. Mol. Phylogenet. Evol. 2020, 149, 106821. [Google Scholar] [CrossRef]
- Barreno, E.; Muggia, L.; Chiva, S.; Molins, A.; Bordenave, C.; García-Breijo, F.; Moya, P. Trebouxia lynnae sp. nov. (former Trebouxia sp. TR9): Biology and biogeography of an epitome lichen symbiotic microalga. Biology 2022, 11, 1196. [Google Scholar] [CrossRef]
- Bordenave, C.D.; Muggia, L.; Chiva, S.; Leavitt, S.D.; Carrasco, P.; Barreno, E. Chloroplast morphology and pyrenoid ultrastructural analyses reappraise the diversity of the lichen phycobiont genus Trebouxia (Chlorophyta). Algal. Res. 2022, 61, 102561. [Google Scholar] [CrossRef]
- Hinojosa-Vidal, E.; Marco, F.; Martínez-Alberola, F.; Escaray, F.J.; García-Breijo, F.J.; Reig-Armiñana, J.; Carrasco, P.; Barreno, E. Characterization of the responses to saline stress in the symbiotic green microalga Trebouxia sp. TR9. Planta 2018, 248, 1473–1486. [Google Scholar] [CrossRef]
- del Hoyo, A.; Álvarez, R.; del Campo, E.M.; Gasulla, F.; Barreno, E.; Casano, L.M. Oxidative stress induces distinct physiological responses in the two Trebouxia phycobionts of the lichen Ramalina farinacea. Ann. Bot. 2011, 107, 109–118. [Google Scholar] [CrossRef]
- Casano, L.M.; del Campo, E.M.; García-Breijo, F.J.; Reig-Armiñana, J.; Gasulla, F.; del Hoyo, A.; Guéra, A.; Barreno, E. Two Trebouxia algae with different physiological performances are ever-present in lichen thalli of Ramalina farinacea. Coexistence versus competition? Environ. Microbiol. 2011, 13, 806–818. [Google Scholar] [CrossRef]
- Casano, L.M.; Braga, M.R.; Álvarez, R.; del Campo, E.M.; Barreno, E. Differences in the cell walls and extracellular polymers of the two Trebouxia microalgae coexisting in the lichen Ramalina farinacea are consistent with their distinct capacity to immobilize extracellular Pb. Plant Sci. 2015, 236, 195–204. [Google Scholar] [CrossRef]
- Martínez-Alberola, F.; Barreno, E.; Casano, L.M.; Gasulla, F.; Molins, A.; del Campo, E.M. Dynamic evolution of mitochondrial genomes in Trebouxiophyceae, including the first completely assembled mtDNA from a lichen-symbiont microalga (Trebouxia sp. TR9). Sci. Rep. 2019, 9, 8209. [Google Scholar] [CrossRef]
- Martínez-Alberola, F.; Barreno, E.; Casano, L.M.; Gasulla, F.; Molins, A.; Moya, P.; González-Hourcade, M.; Campo, E.M. The chloroplast genome of the lichen-symbiont microalga Trebouxia sp. Tr9 (Trebouxiophyceae, Chlorophyta) shows short inverted repeats with a single gene and loss of the rps4 gene, which is encoded by the nucleus. J. Phycol. 2020, 56, 170–184. [Google Scholar] [CrossRef]
- Nelsen, M.P.; Leavitt, S.D.; Heller, K.; Muggia, L.; Lumbsch, H.T. Contrasting patterns of climatic niche divergence in Trebouxia—A clade of lichen-forming algae. Front. Microbiol. 2022, 13, 791546. [Google Scholar] [CrossRef]
- Molins, A.; Moya, P.; García-Breijo, F.J.; Reig-Armiñana, J.; Barreno, E. Molecular and morphological diversity of Trebouxia microalgae in sphaerothallioid Circinaria spp. lichens. J. Phycol. 2018, 54, 494–504. [Google Scholar] [CrossRef]
- Dal Grande, F.; Rolshausen, G.; Divakar, P.K.; Crespo, A.; Otte, J.; Schleuning, M.; Schmitt, I. Environment and host identity structure communities of green algal symbionts in lichens. New Phytol. 2018, 217, 277–289. [Google Scholar] [CrossRef]
- Molins, A.; Moya, P.; Muggia, L.; Barreno, E. Thallus growth stage and geographic origin shape microalgal diversity in Ramalina farinacea lichen holobionts. J. Phycol. 2021, 57, 975–987. [Google Scholar] [CrossRef]
- Moya, P.; Molins, A.; Škaloud, P.; Divakar, P.K.; Chiva, S.; Dumitru, C.; Molina, M.C.; Crespo, A.; Barreno, E. Biodiversity patterns and ecological preferences of the photobionts associated with the lichen-forming genus Parmelia. Front. Microbiol. 2021, 12, 3950. [Google Scholar] [CrossRef]
- Moya, P.; Chiva, S.; Molins, A.; Garrido-Benavent, I.; Barreno, E. Unravelling the symbiotic microalgal diversity in Buellia zoharyi (lichenized Ascomycota) from the Iberian Peninsula and Balearic Islands using dna metabarcoding. Diversity 2021, 13, 220. [Google Scholar] [CrossRef]
- Moya, P.; Molins, A.; Chiva, S.; Bastida, J.; Barreno, E. Symbiotic microalgal diversity within lichenicolous lichens and crustose hosts on Iberian Peninsula gypsum biocrusts. Sci. Rep. 2020, 10, 14060. [Google Scholar] [CrossRef]
- Catalá, S.; del Campo, E.M.; Barreno, E.; García-Breijo, F.J.; Reig-Armiñana, J.; Casano, L.M. Coordinated ultrastructural and phylogenomic analyses shed light on the hidden phycobiont diversity of Trebouxia microalgae in Ramalina fraxinea. Mol. Phylogenet. Evol. 2016, 94, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Moya, P.; Molins, A.; Martínez-Alberola, F.; Muggia, L.; Barreno, E. Unexpected associated microalgal diversity in the lichen Ramalina farinacea is uncovered by pyrosequencing analyses. PLoS ONE 2017, 12, e0175091. [Google Scholar] [CrossRef] [PubMed]
- Škaloud, P.; Moya, P.; Molins, A.; Peksa, O.; Santos-Guerra, A.; Barreno, E. Untangling the hidden intrathalline microalgal diversity in Parmotrema pseudotinctorum: Trebouxia crespoana sp. nov. Lichenologist 2018, 50, 357–369. [Google Scholar] [CrossRef]
- Molins, A.; Chiva, S.; Calatayud, Á.; Marco, F.; García-Breijo, F.; Reig-Armiñana, J.; Carrasco, P.; Moya, P. Multidisciplinary approach to describe Trebouxia diversity within lichenized fungi Buellia zoharyi from the Canary Islands. Symbiosis 2020, 82, 19–34. [Google Scholar] [CrossRef]
- Molins, A.; Moya, P.; García-Breijo, F.J.; Reig-Armiñana, J.; Barreno, E. Assessing lichen microalgal diversity by a multi-tool approach: Isolation, Sanger sequencing, HTS and ultrastructural correlations. Lichenologist 2018, 50, 123–138. [Google Scholar] [CrossRef]
- Chiva, S.; Garrido-Benavent, I.; Moya, P.; Molins, A.; Barreno, E. How did terricolous fungi originate in the Mediterranean region? A case study with a gypsicolous lichenized species. J. Biogeogr. 2019, 46, 515–525. [Google Scholar] [CrossRef]
- Moya, P.; Škaloud, P.; Chiva, S.; García-Breijo, F.J.; Reig-Armiñana, J.; Vančurová, L.; Barreno, E. Molecular phylogeny and ultrastructure of the lichen microalga Asterochloris mediterranea sp. nov. from mediterranean and Canary Islands ecosystems. Int. J. Syst. Evol. Microbiol. 2015, 65, 1838–1854. [Google Scholar] [CrossRef]
- Garrido-Benavent, I.; Chiva, S.; Bordenave, C.D.; Molins, A.; Barreno, E. Trebouxia maresiae sp. nov. (Trebouxiophyceae, Chlorophyta), a new lichenized species of microalga found in coastal environments. Cryptogam. Algol. 2022, 43, 135–145. [Google Scholar] [CrossRef]
- Schatten, H. Scanning Electron Microscopy for the Life Sciences; Cambridge University Press: Cambridge, UK, 2012; ISBN 1139851055. [Google Scholar]
- Wolf, B.M.; Niedzwiedzki, D.M.; Magdaong, N.C.M.; Roth, R.; Goodenough, U.; Blankenship, R.E. Characterization of a newly isolated freshwater Eustigmatophyte alga capable of utilizing far-red light as its sole light source. Photosynth. Res. 2018, 135, 177–189. [Google Scholar] [CrossRef]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef]
- Peled, E.; Pick, U.; Zarka, A.; Shimoni, E.; Leu, S.; Boussiba, S. Light-induced oil globule migration in Haematococcus pluvialis (chlorophyceae). J. Phycol. 2012, 48, 1209–1219. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Weesepoel, Y.; Bodenes, P.; Lamers, P.P.; Vincken, J.P.; Martens, D.E.; Gruppen, H.; Wijffels, R.H. Nitrogen-depleted Chlorella zofingiensis produces astaxanthin, ketolutein and their fatty acid esters: A carotenoid metabolism study. J. Appl. Phycol. 2015, 27, 125–140. [Google Scholar] [CrossRef]
- Polle, J.E.W.; Roth, R.; Ben-Amotz, A.; Goodenough, U. Ultrastructure of the green alga Dunaliella salina strain CCAP19/18 (Chlorophyta) as investigated by quick-freeze deep-etch electron microscopy. Algal. Res. 2020, 49, 101953. [Google Scholar] [CrossRef]
- Davidi, L.; Shimoni, E.; Khozin-Goldberg, I.; Zamir, A.; Pick, U. Origin of β-carotene-rich plastoglobuli in Dunaliella bardawil. Plant Physiol. 2014, 164, 2139–2156. [Google Scholar] [CrossRef]
- Lamers, P.P.; van de Laak, C.C.W.; Kaasenbrood, P.S.; Lorier, J.; Janssen, M.; de Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol. Bioeng. 2010, 106, 638–648. [Google Scholar] [CrossRef]
- Weiss, T.L.; Roth, R.; Goodson, C.; Vitha, S.; Black, I.; Azadi, P.; Rusch, J.; Holzenburg, A.; Devarenne, T.P.; Goodenough, U. Colony organization in the green alga Botryococcus braunii (Race B) is specified by a complex extracellular matrix. Eukaryot. Cell 2012, 11, 1424–1440. [Google Scholar] [CrossRef]
- Roth, R.; Goodenough, U. Lichen 1. Solo fungal and algal partners. Algal. Res. 2021, 58, 102334. [Google Scholar] [CrossRef]
- Lee, J.H.; Heuser, J.E.; Roth, R.; Goodenough, U. Eisosome ultrastructure and evolution in fungi, microalgae, and lichens. Eukaryot. Cell 2015, 14, 1017–1042. [Google Scholar] [CrossRef]
- González-Hourcade, M.; Braga, M.R.; del Campo, E.M.; Ascaso, C.; Patinõ, C.; Casano, L.M. Ultrastructural and biochemical analyses reveal cell wall remodelling in lichen-forming microalgae submitted to cyclic desiccation-rehydration. Ann. Bot. 2020, 125, 459–469. [Google Scholar] [CrossRef]
- Bischoff, H.W.; Bold, H.C. Phycological studies. IV. In Some Soil Algae from Enchanted Rock and Related Algal Species; University of Texas Publications 6318: Austin, TX, USA, 1963. [Google Scholar]
- Bold, H.C.; Parker, B.C. Some supplementary attributes in the classification of Chlorococcum species. Arch. Mikrobiol. 1962, 42, 267–288. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Muggia, L.; Pérez-Ortega, S.; Kopun, T.; Zellnig, G.; Grube, M. Photobiont selectivity leads to ecological tolerance and evolutionary divergence in a polymorphic complex of lichenized fungi. Ann. Bot. 2014, 114, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Hayles, M.F.; de Winter, D.A.M. An introduction to cryo-FIB-SEM cross-sectioning of frozen, hydrated Life Science samples. J. Microsc. 2021, 281, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Gasulla, F.; de Nova, P.G.; Esteban-Carrasco, A.; Zapata, J.M.; Barreno, E.; Guéra, A. Dehydration rate and time of desiccation affect recovery of the lichenic algae Trebouxia erici: Alternative and classical protective mechanisms. Planta 2009, 231, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Candotto Carniel, F.; Zanelli, D.; Bertuzzi, S.; Tretiach, M. Desiccation tolerance and lichenization: A case study with the aeroterrestrial microalga Trebouxia sp. (Chlorophyta). Planta 2015, 242, 493–505. [Google Scholar] [CrossRef]
- Banchi, E.; Candotto Carniel, F.; Montagner, A.; Petruzzellis, F.; Pichler, G.; Giarola, V.; Bartels, D.; Pallavicini, A.; Tretiach, M. Relation between water status and desiccation-affected genes in the lichen photobiont Trebouxia gelatinosa. Plant Physiol. Biochem. 2018, 129, 189–197. [Google Scholar] [CrossRef]
- Gasulla, F.; Jain, R.; Barreno, E.; Guéra, A.; Balbuena, T.S.; Thelen, J.J.; Oliver, M.J. The response of Asterochloris erici (Ahmadjian) Skaloud et Peksa to desiccation: A proteomic approach. Plant Cell Environ. 2013, 36, 1363–1378. [Google Scholar] [CrossRef]
- Scheidegger, C.; Schroeter, B. Structural and Functional Processes during Water Vapour Uptake and Desiccation in Selected Lichens with Green Algal Photobionts. Planta 1995, 197, 399–409. [Google Scholar] [CrossRef]
- González-Hourcade, M.; del Campo, E.M.; Casano, L.M. The under-explored extracellular proteome of aero-terrestrial microalgae provides clues on different mechanisms of desiccation tolerance in non-model organisms. Microb. Ecol. 2021, 81, 437–453. [Google Scholar] [CrossRef]
- Bruñas Gómez, I.; Casale, M.; Barreno, E.; Catalá, M. Near-infrared metabolomic fingerprinting study of lichen thalli and phycobionts in culture: Aquaphotomics of Trebouxia lynnae dehydration. Microorganisms 2022, 10, 2444. [Google Scholar] [CrossRef]
- Kalinina, N.O.; Makarova, S.; Makhotenko, A.; Love, A.J.; Taliansky, M. The multiple functions of the nucleolus in plant development, disease and stress responses. Front. Plant Sci. 2018, 9, 132. [Google Scholar] [CrossRef]
- Stępiński, D. Functional ultrastructure of the plant nucleolus. Protoplasma 2014, 251, 1285–1306. [Google Scholar] [CrossRef]
- Tukaj, Z.; Baścik-Remisiewicz, A.; Skowroński, T.; Tukaj, C. Cadmium effect on the growth, photosynthesis, ultrastructure and phytochelatin content of green microalga Scenedesmus armatus: A study at low and elevated CO2 concentration. Environ. Exp. Bot. 2007, 60, 291–299. [Google Scholar] [CrossRef]
- Leonardo, T.; Farhi, E.; Pouget, S.; Motellier, S.; Boisson, A.-M.; Banerjee, D.; Rébeillé, F.; den Auwer, C.; Rivasseau, C. Silver accumulation in the green microalga Coccomyxa actinabiotis: Toxicity, in situ speciation, and localization investigated using synchrotron XAS, XRD, and TEM. Environ. Sci. Technol. 2016, 50, 359–367. [Google Scholar] [CrossRef]
- Pulich, W.M.; Ward, C.H. Physiology and ultrastructure of an oxygen-resistant Chlorella mutant under heterotrophic conditions. Plant Physiol. 1973, 51, 337–344. [Google Scholar] [CrossRef]
- Atkinson, A.W.; John, P.C.L.; Gunning, B.E.S. The growth and division of the single mitochondrion and other organelles during the cell cycle of Chlorella, studied by quantitative stereology and three dimensional reconstruction. Protoplasma 1974, 81, 77–109. [Google Scholar] [CrossRef]
- Dempsey, G.P.; Lawrence, D.; Cassie, V. The ultrastructure of Chlorella minutissima Fott et Nováková (Chlorophyceae, Chlorococcales). Phycologia 1980, 19, 13–19. [Google Scholar] [CrossRef]
- Guo, W.; Feng, L.; Wang, Z.; Guo, J.; Park, D.; Carroll, B.L.; Zhang, X.; Liu, J.; Cheng, J. In-situ high-resolution 3D imaging combined with proteomics and metabolomics reveals enlargement of subcellular architecture and enhancement of photosynthesis pathways in nuclear-irradiated Chlorella pyrenoidosa. Chem. Eng. J. 2022, 430, 133037. [Google Scholar] [CrossRef]
- Wolf, J.M.; Espadas-Moreno, J.; Luque-Garcia, J.L.; Casadevall, A. Interaction of Cryptococcus neoformans extracellular vesicles with the cell wall. Eukaryot. Cell 2014, 13, 1484–1493. [Google Scholar] [CrossRef]
- Honegger, R. Cytological aspects of the mycobiont–phycobiont relationship in lichens. Lichenologist 1984, 16, 111–127. [Google Scholar] [CrossRef]
- Arakawa, S.; Kanaseki, T.; Wagner, R.; Goodenough, U. Ultrastructure of the foliose lichen Myelochroa leucotyliza and its solo fungal and algal (Trebouxia sp.) partners. Algal. Res. 2022, 62, 102571. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordenave, C.D.; García-Breijo, F.; Gazquez, A.; Muggia, L.; Carrasco, P.; Barreno, E. Low Temperature Scanning Electron Microscopy (LTSEM) Findings on the Ultrastructure of Trebouxia lynnae (Trebouxiophyceae, Lichenized Microalgae). Diversity 2023, 15, 170. https://doi.org/10.3390/d15020170
Bordenave CD, García-Breijo F, Gazquez A, Muggia L, Carrasco P, Barreno E. Low Temperature Scanning Electron Microscopy (LTSEM) Findings on the Ultrastructure of Trebouxia lynnae (Trebouxiophyceae, Lichenized Microalgae). Diversity. 2023; 15(2):170. https://doi.org/10.3390/d15020170
Chicago/Turabian StyleBordenave, César Daniel, Francisco García-Breijo, Ayelén Gazquez, Lucía Muggia, Pedro Carrasco, and Eva Barreno. 2023. "Low Temperature Scanning Electron Microscopy (LTSEM) Findings on the Ultrastructure of Trebouxia lynnae (Trebouxiophyceae, Lichenized Microalgae)" Diversity 15, no. 2: 170. https://doi.org/10.3390/d15020170
APA StyleBordenave, C. D., García-Breijo, F., Gazquez, A., Muggia, L., Carrasco, P., & Barreno, E. (2023). Low Temperature Scanning Electron Microscopy (LTSEM) Findings on the Ultrastructure of Trebouxia lynnae (Trebouxiophyceae, Lichenized Microalgae). Diversity, 15(2), 170. https://doi.org/10.3390/d15020170







