Fish Species Richness in Polish Lakes
Abstract
1. Introduction
2. Materials and Methods
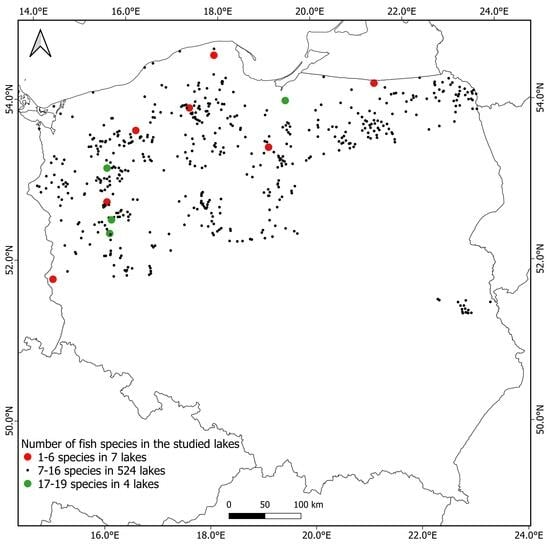
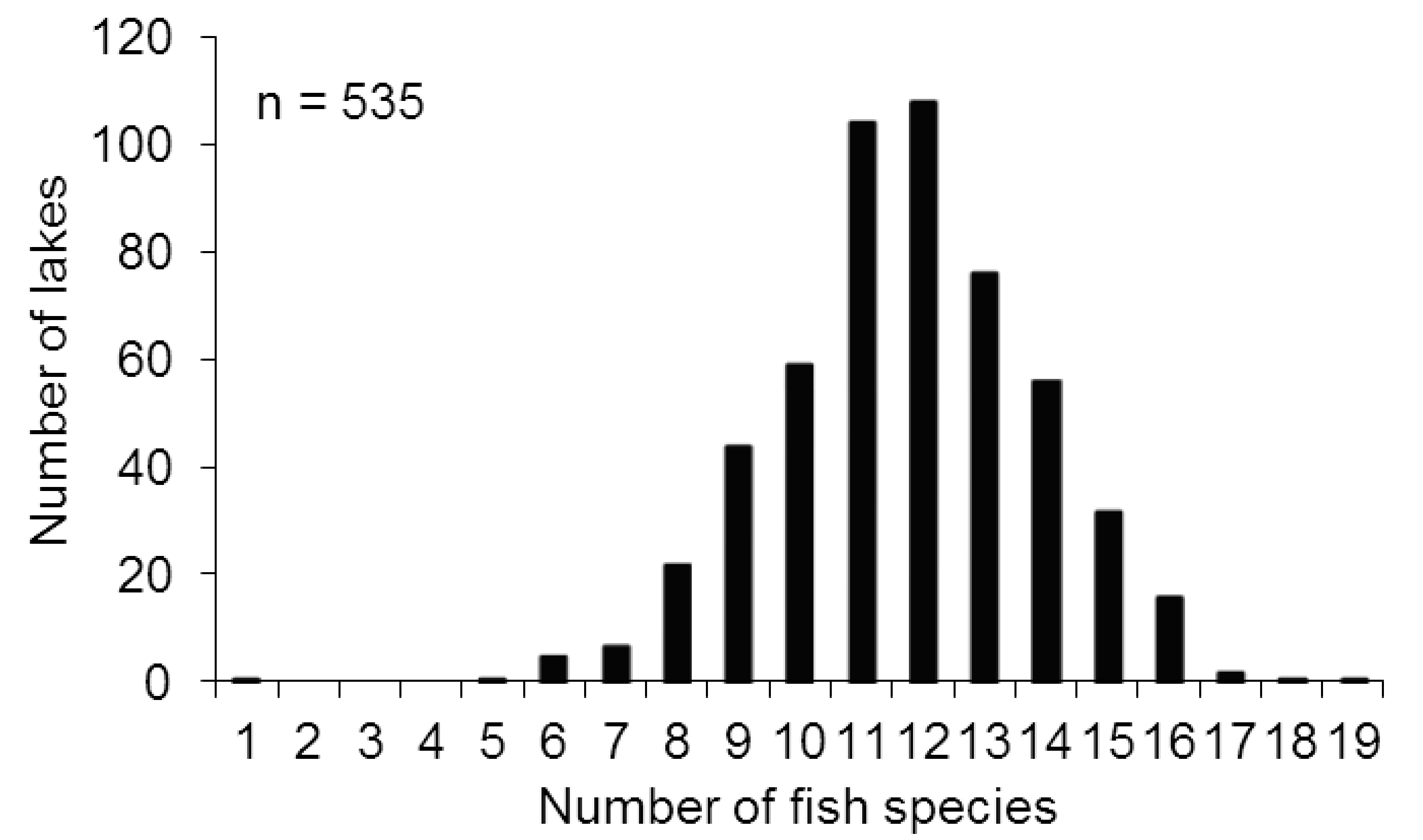
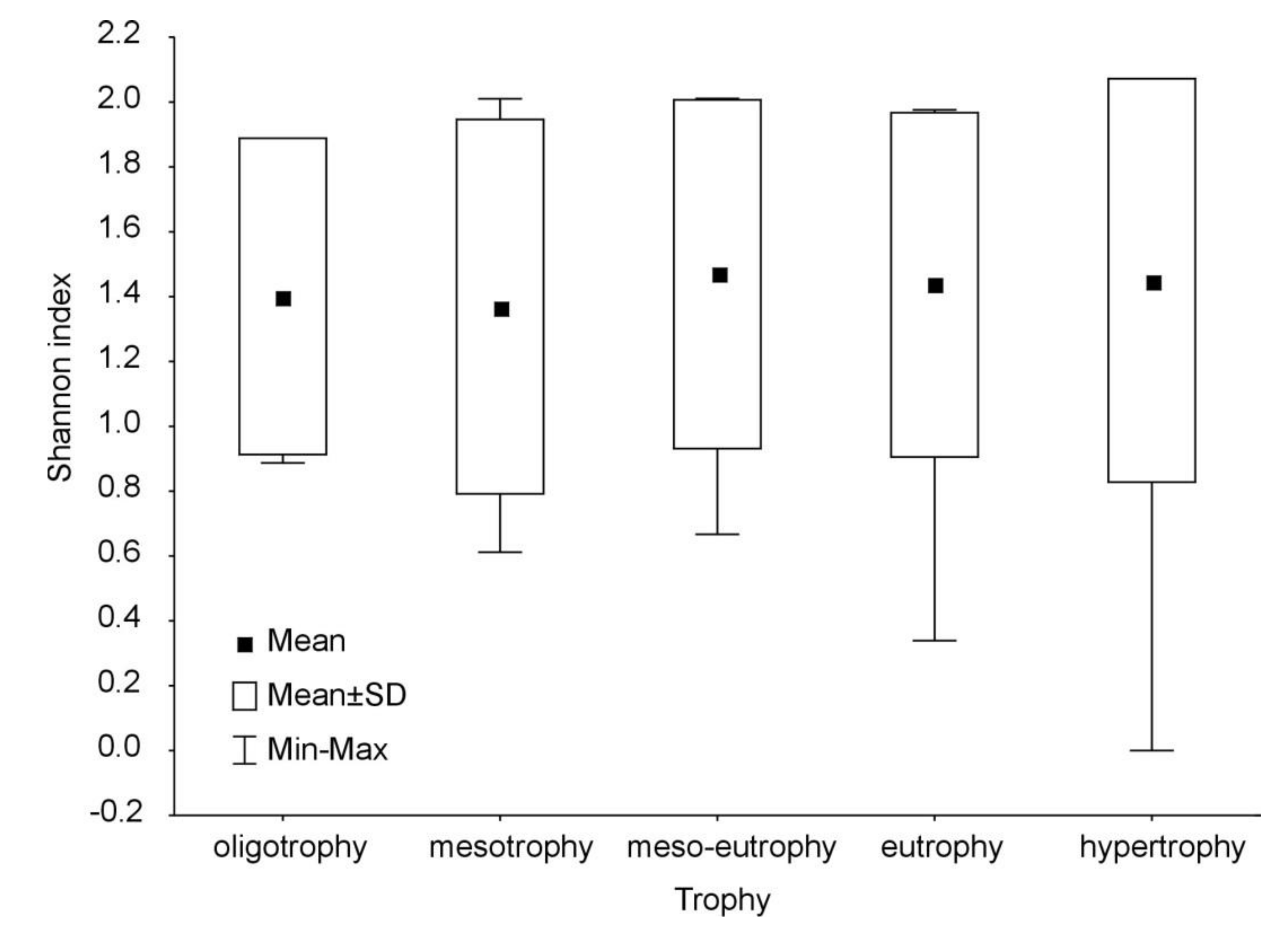
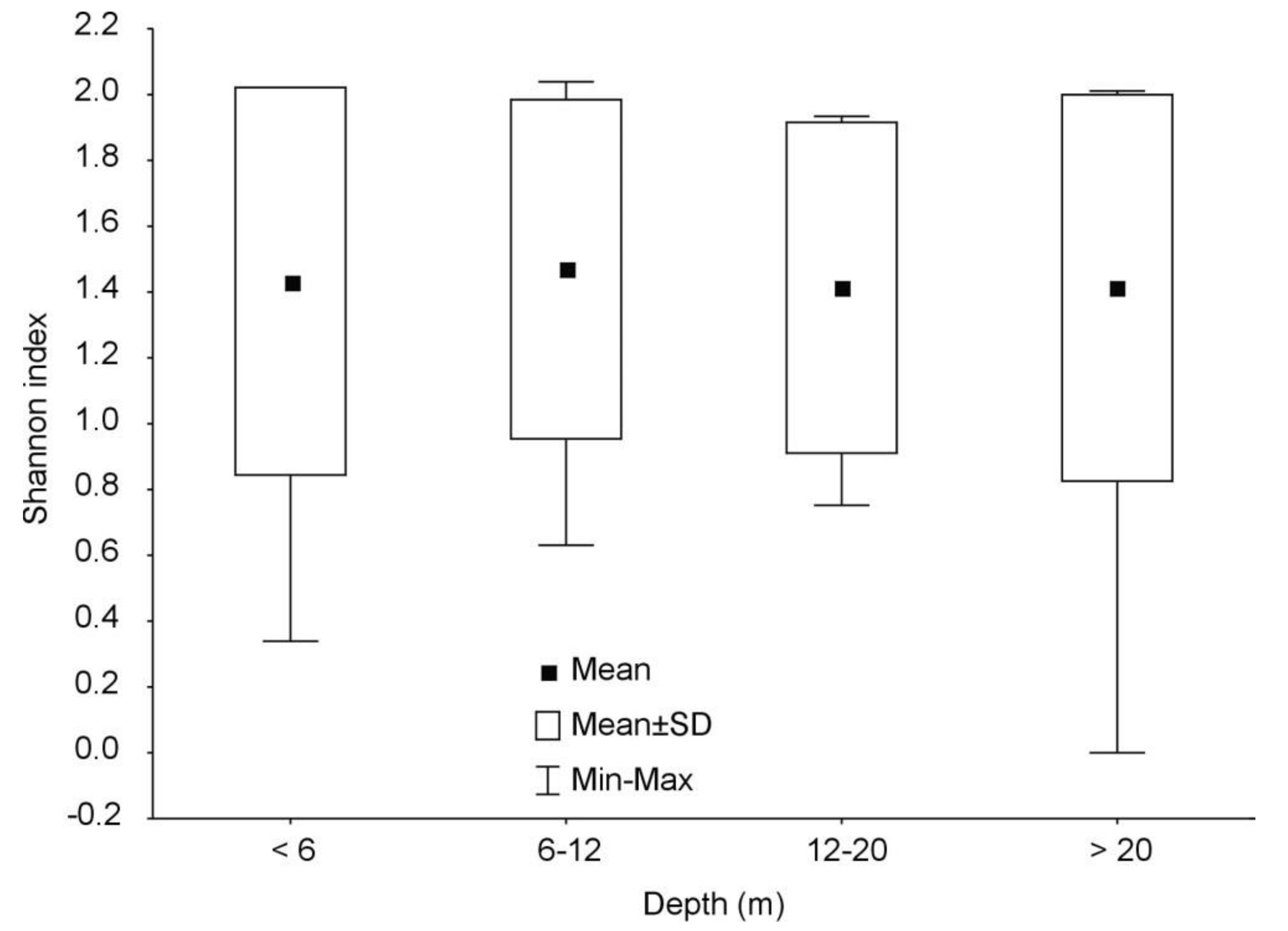
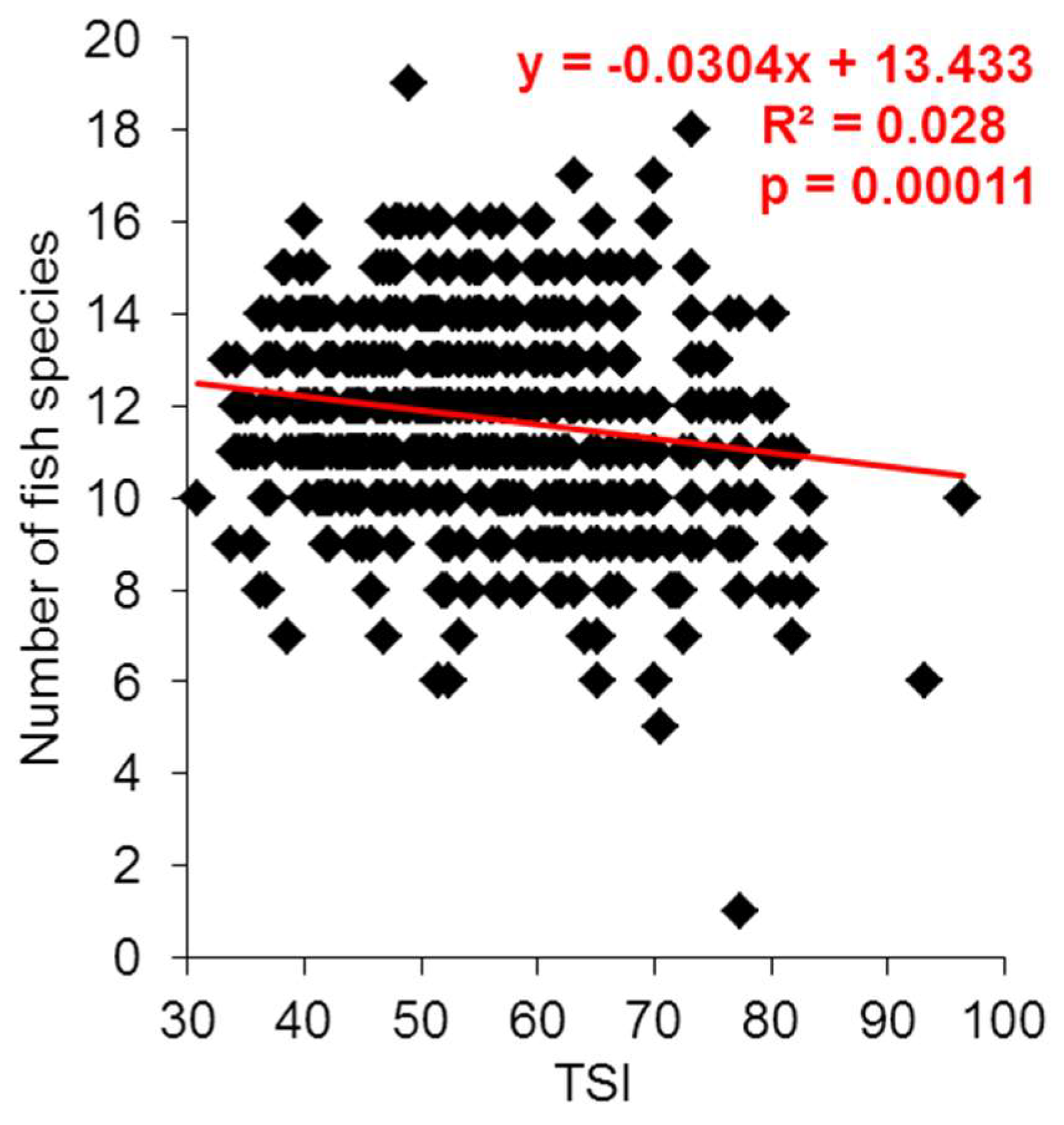
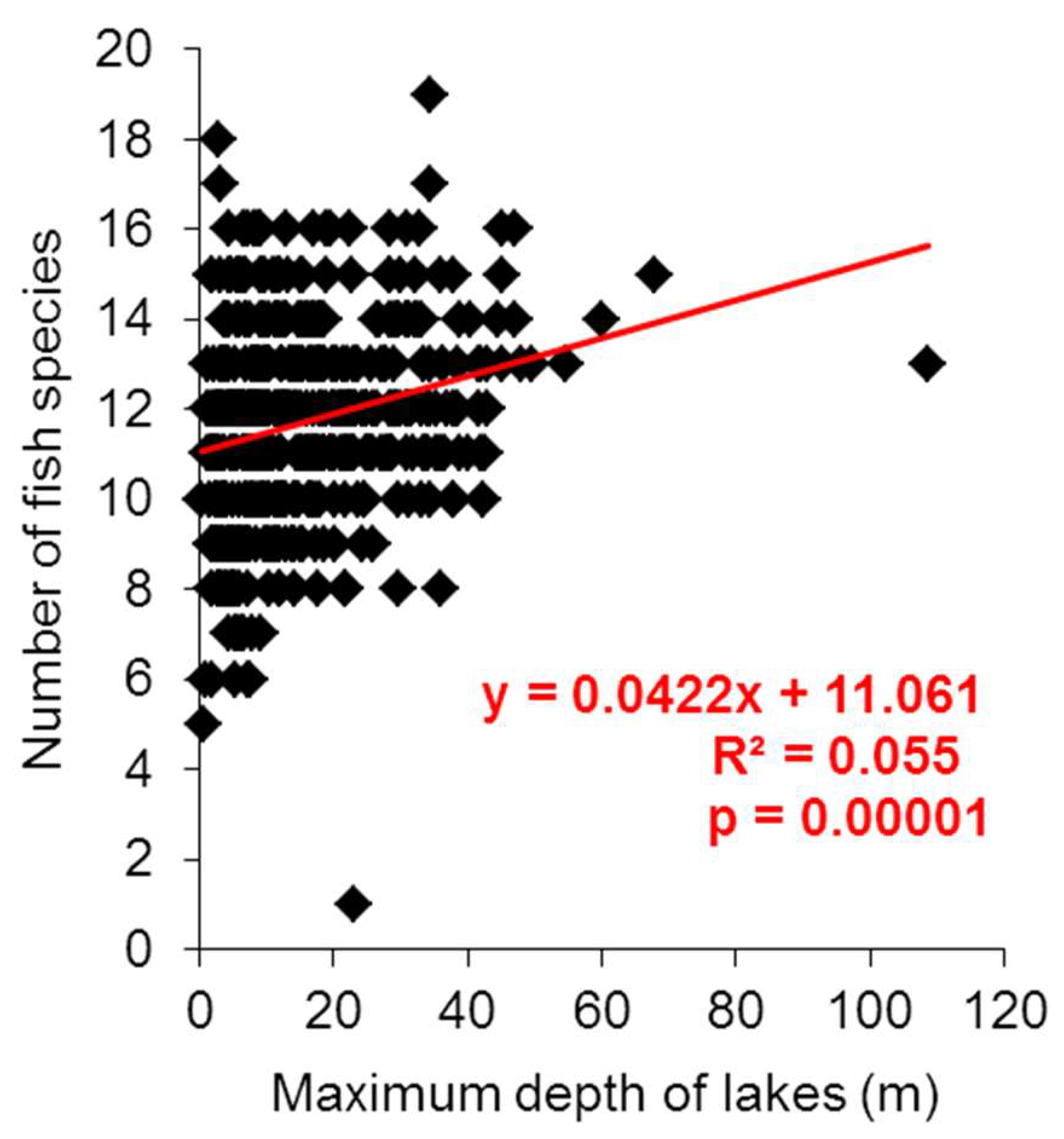
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Power, M.E.; Tilman, D.; Estes, J.A.; Menge, B.A.; Bond, W.J.; Mills, L.S.; Daily, G.; Castilla, J.C.; Lubchenco, J.; Paine, R.T. Challenges in the quest for keystones: Identifying keystone species is difficult—But essential to understanding how loss of species will affect ecosystems. BioScience 1996, 46, 609–620. [Google Scholar] [CrossRef]
- Carpenter, S.; Frost, T.; Persson, L.; Power, M.; Soto, D. Freshwater ecosystems: Linkages of complexity and processes. In Functional Roles of Biodiversity: A Global Perspective; Mooney, H.A., Cushman, J.H., Medina, E., Sala, O.E., Schulze, E.-D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1996; pp. 299–325. [Google Scholar]
- Kopylov, A.I.; Kosolapov, D.B.; Lazareva, V.I.; Mineeva, N.M.; Pryanichnikova, E.G. Structure, biomass and production of the biotic component of the ecosystem of an growing eutrophic reservoir. Biosyst. Divers. 2018, 26, 117–122. [Google Scholar] [CrossRef]
- Brucet, S.; Pédron, S.; Mehner, T.; Lauridsen, T.L.; Argillier, C.; Winfield, I.J.; Volta, P.; Emmrich, M.; Hesthagen, T.; Holmgren, K.; et al. Fish diversity in European lakes: Geographical factors dominate over anthropogenic pressures. Freshw. Biol. 2013, 58, 1779–1793. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Olin, M.; Rask, M.; Ruuhljärvi, J.; Kurkilahti, M.; Ala Opas, P.; Ylönen, O. Fish community structure in mesotrophic and eutrophic lakes of southern Finland: The relative abundances of percids and cyprinids along a trophic gradient. J. Fish Biol. 2002, 60, 593–612. [Google Scholar] [CrossRef]
- Mehner, T.; Holmgren, K.; Lauridsen, T.L.; Jeppesen, E.; Diekmann, M. Lake depth and geographical position modify lake fish assemblages of the European ‘Central Plains’ ecoregion. Freshw. Biol. 2007, 52, 2285–2297. [Google Scholar] [CrossRef]
- Kozłowski, J.; Chybowski, Ł.; Poczyczyński, P. Ichtiofauna jeziora Hańcza. In Środowisko i Ichtiofauna Jeziora Hańcza; Kozłowski, J., Poczyczyński, P., Zdanowski, B., Eds.; IRS: Olsztyn, Poland, 2008; pp. 121–124. [Google Scholar]
- Kapusta, A.; Bogacka-Kapusta, E.; Czarkowski, T.K.; Czarnecki, B.; Duda, A.; Hutorowicz, J.; Jarmołowicz, S.; Napiórkowska-Krzebietke, A.; Prusińska, M.; Stawecki, K.; et al. Czy typ użytkowania rybackiego ma wpływ na strukturę zespołów ryb w jeziorach północnej Polski? In Działalność Podmiotów Rybackich i Wędkarskich w 2020 Roku w Świetle Uwarunkowań Gospodarczych, Ekonomicznych i Środowiskowych; Kowalska, A., Wołos, A., Eds.; IRS: Olsztyn, Poland, 2021; pp. 99–109. [Google Scholar]
- Białokoz, W.; Krzywosz, T. Struktura ichtiofauny w jeziorach Wigierskiego Parku Narodowego. In Jeziora Wigierskiego Parku Narodowego. Stan Eutrofizacji i Kierunki Ochrony; Zdanowski, B., Ed.; Ossolineum: Wrocław, Poland, 1992; pp. 153–162. [Google Scholar]
- Colby, P.J.; Spangler, G.R.; Hurley, D.A.; McCombie, A.M. Effects of eutrophication on salmonid communities in oligotrophic lakes. Can. J. Fish. Aquat. Sci. 1972, 29, 975–983. [Google Scholar] [CrossRef]
- Chybowski, Ł.; Białokoz, W. Dynamika zespołów ichtiofauny jeziora Wigry. In Funkcjonowanie i Ochrona Ekosystemów Wodnych na Obszarach Chronionych; Zdanowski, B., Kamiński, M., Martyniak, A., Eds.; IRS: Olsztyn, Poland, 1999; pp. 521–526. [Google Scholar]
- Brylińska, M. Ryby Słodkowodne Polski; PWN: Warszawa, Poland, 2000; p. 521. [Google Scholar]
- Penczak, T.; Kruk, A.; Marszał, L.; Zięba, G.; Galicka, W.; Tszydel, M.; Tybulczuk, S.; Pietraszewski, D. Monitoring ichtiofauny systemu rzeki Gwdy: Trzecia dekada badań. Rocz. Nauk. PZW 2008, 21, 61–89. [Google Scholar]
- Witkowski, A.; Kotusz, J. Stan ichtiofaunistycznych badań inwentaryzacyjnych rzek polski. Rocz. Nauk. PZW 2008, 21, 23–60. [Google Scholar]
- Witkowski, A.; Popiołek, M.; Kotusz, J.; Wawer, K.; Stefaniak, J. Ichtiofauna Dzikiej Orlicy (dorzecze Łaby, Polska)—dziesięć lat później. Rocz. Nauk. PZW 2015, 28, 5–25. [Google Scholar]
- Kapusta, A.; Chybowski, Ł.; Bogacka-Kapusta, E. Ichtiofauna Mazurskiego Parku Krajobrazowego. In Mazurski Park Krajobrazowy, Różnorodność Biologiczna i Kulturowa; Wittbrodt, K., Janecki, T., Eds.; Labrita: Kętrzyn, Poland, 2019; pp. 116–125. [Google Scholar]
- Kapusta, A.; Morzuch, J.; Bogacka-Kapusta, E.; Pająkowski, J. Ichtiofauna jeziora Mukrz w rezerwacie przyrody „Cisy Staropolskie im. Leona Wyczółkowskiego” w Wierzchlesie. Chrońmy Przyr. Ojczystą 2012, 68, 435–442. [Google Scholar]
- Czarkowski, T.K.; Martyniak, A.; Kapusta, A.; Wójcik, A.; Bowszys, M.; Wziątek, B.; Szymańska, U.; Kozłowski, J. Feeding ecology of vendace, Coregonus albula (L.), in Lake Wigry (Northeastern Poland). Arch. Pol. Fish. 2007, 15, 117–128. [Google Scholar]
- Kozłowski, K.; Kozłowski, J.; Poczyczyński, P.; Martyniak, A. Age and growth of vendace, Coregonus albula (L.), from Lake Wigry (northeast Poland). Arch. Pol. Fish. 2010, 18, 239–245. [Google Scholar] [CrossRef]
- Hutorowicz, A.; Kapusta, A.; Krzywosz, T.; Hutorowicz, J. The ichthyofauna of the dystrophic Lake Smolak (northern Poland) in light of selected physical and chemical water conditions thirty years after conclusion of liming and fertilization. Arch. Pol. Fish. 2005, 13, 207–225. [Google Scholar]
- Rechulicz, J.; Płaska, W.; Tarkowska-Kukuryk, M. The ichthyofauna of littoral of two shallow lakes on background of fishery management and angling pressure. Teka Kom. Ochr. Kszt. Środ. Przyr. 2014, 11, 163–172. [Google Scholar]
- Kapusta, A.; Perkowski, J.; Traczuk, P.; Heese, T.; Woźniak, J.; Strzałkowski, Ł.; Dobrowolski, M. Porównanie różnych metod połowu ryb do określenia składu gatunkowego oraz struktury ilościowej ichtiofauny jezior. In Działalność Podmiotów Rybackich i Wędkarskich w 2018 roku. Uwarunkowania Ekonomiczne i Środowiskowe; Mickiewicz, M., Wołos, A., Eds.; IRS: Olsztyn, Poland, 2019; pp. 65–73. [Google Scholar]
- Carlson, R.F. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Rudnicki, A.; Waluga, J.; Waluś, T. Rybactwo Jeziorowe; PWRiL: Warszawa, Poland, 1971; p. 403. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; p. 125. [Google Scholar]
- Robak, S.; Białokoz, W.; Chybowski, Ł. Ichtiofauna. In Ekosystemy Wodne Parku Narodowego „Bory Tucholskie”; Zdanowski, B., Hutorowicz, A., Białokoz, W., Eds.; IRS: Olsztyn, Poland, 2004; pp. 233–240. [Google Scholar]
- Mehner, T.; Diekmann, M.; Brämick, U.; Lemcke, R. Composition of fish communities in German lakes as related to lake morphology, trophic state, shore structure and human-use intensity. Freshw. Biol. 2005, 50, 70–85. [Google Scholar] [CrossRef]
- Volta, P.; Jeppesen, E.; Sala, P.; Galafassi, S.; Foglini, C.; Puzzi, C.; Winfield, I.J. Fish assemblages in deep Italian subalpine lakes: History and present status with an emphasis on non-native species. Hydrobiologia 2018, 824, 255–270. [Google Scholar] [CrossRef]
- Kapusta, A.; Błoniarz, W. Fish. In Animated Nature in the „Bory Tucholskie” National Park. Part I—Vertebrate Animals; Winiecki, A., Lubińska, K., Eds.; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2021; pp. 25–49. [Google Scholar]
- Kalinowska, K.; Napiórkowska-Krzebietke, A.; Ulikowski, D.; Bogacka-Kapusta, E.; Stawecki, K.; Traczuk, P. Under-ice environmental conditions, planktonic communities and ichthyofauna in dystrophic lakes. Eur. Zool. J. 2021, 88, 340–351. [Google Scholar] [CrossRef]
- Schilling, E.G.; Loftin, C.S.; Degoosh, K.E.; Huryn, A.D.; Webster, K.E. Predicting the locations of naturally fishless lakes. Freshw. Biol. 2008, 53, 1021–1035. [Google Scholar] [CrossRef]
- Galas, J. Pest fish in naturally fishless high mountain lakes of the national parks. Chrońmy Przyr. Ojcz. 2010, 66, 427–432. [Google Scholar]
- Kotusz, J.; Krappe, M.; Kusznierz, J.; Propiolek, M.; Riel, P.; Waterstraat, A.; Witkowski, A. Distribution, density and habitat of Cottus poecilopus (Heckel, 1836) in Lake Hancza (North East Poland) as compared with the situation in the Luzin lakes (North East Germany). Gesell. Ichtyol. 2004, 4, 91–105. [Google Scholar]
- Witkowski, A. Głowacz pręgopłetwy, Cottus poecilopus Heckel, 1836 w jeziorze Hańcza. Prz. Zool. 1975, 19, 224–227. [Google Scholar]
- Brysiewicz, A.; Czerniejewski, P.; Dąbrowski, J.; Formicki, K.; Więcaszek, B. Fish diversity and abundance patterns in small watercourses of the Central European Plain Ecoregion in relation to environmental factors. Water 2022, 14, 2697. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E.; Jensen, J.P. Pond or lake: Does it make any difference? Arch. Hydrobiol. 2005, 162, 143–165. [Google Scholar] [CrossRef]
- Szlakowski, J.; Adamczyk, M.; Buras, P.; Ligięza, J.; Prus, P.; Wiśniewolski, W. Gatunki obce w monitoringu ichtiofauny w rzekach Polski w latach 2011–2018. In Działalność Podmiotów Rybackich i Wędkarskich w 2018 Roku. Uwarunkowania Ekonomiczne i Środowiskowe; Mickiewicz, M., Wołos, A., Eds.; IRS: Olsztyn, Poland, 2019; pp. 89–105. [Google Scholar]
- Holmgren, K.; Appelberg, M. Size structure of benthic freshwater fish communities in relation to environmental gradients. J. Fish Biol. 2000, 57, 1312–1330. [Google Scholar] [CrossRef]
- Jeppesen, E.; Mehner, T.; Winfield, I.J.; Kangur, K.; Sarvala, J.; Gerdeaux, D.; Rask, M.; Malmquist, H.J.; Holmgren, K.; Volta, P.; et al. Impacts of climate warming on the long-term dynamics of key fish species in 24 European lakes. Hydrobiologia 2012, 694, 1–39. [Google Scholar] [CrossRef]
- Jennings, M.J.; Bozek, M.A.; Hatzenbeler, G.R.; Emmons, E.E.; Staggs, M.D. Cumulative effects of incremental shoreline habitat modification on fish assemblages in north temperate lakes. N. Am. J. Fish. Manag. 1999, 19, 18–27. [Google Scholar] [CrossRef]
- Allan, D.J.; Abell, R.; Hogan, Z.; Revenga, C.; Taylor, B.W.; Welcomme, R.L.; Winemiller, K. Overfishing of inland waters. Biocience 2005, 55, 1041–1051. [Google Scholar] [CrossRef]


| Species | Status | Frequency (%) |
|---|---|---|
| Rutilus rutilus (Linnaeus, 1758) | native | 99.8 |
| Perca fluviatilis Linnaeus, 1758 | native | 99.6 |
| Gymnocephalus cernua (Linnaeus, 1758) | native | 96.6 |
| Abramis brama (Linnaeus, 1758) | native | 94.4 |
| Alburnus alburnus (Linnaeus, 1758) | native | 92.7 |
| Blicca bjoerkna (Linnaeus, 1758) | native | 91.8 |
| Scardinius erythrophthalmus (Linnaeus, 1758) | native | 87.3 |
| Tinca tinca (Linnaeus, 1758) | native | 72.2 |
| Esox lucius Linnaeus, 1758 | native | 70.7 |
| Rhodeus amarus (Bloch, 1782) | native | 64.7 |
| Leucaspius delineatus (Heckel, 1843) | native | 47.5 |
| Cobitis taenia Linnaeus, 1758 | native | 47.3 |
| Carassius carassius (Linnaeus, 1758) | native | 44.1 |
| Sander lucioperca (Linnaeus, 1758) | native | 42.6 |
| Gobio gobio (Linnaeus, 1758) | native | 35.7 |
| Coregonus albula (Linnaeus, 1758) | native | 24.9 |
| Carassius gibelio (Bloch, 1782) | alien | 17.9 |
| Silurus glanis Linnaeus, 1758 | native | 15.3 |
| Gasterosteus aculeatus Linnaeus, 1758 | native | 9.0 |
| Coregonus maraena (Bloch, 1779) | native | 6.2 |
| Cyprinus carpio Linnaeus, 1758 | alien | 5.2 |
| Leuciscus aspius (Linnaeus, 1758) | native | 4.5 |
| Leuciscus idus (Linnaeus, 1758) | native | 4.5 |
| Ameiurus nebulosus (Lesueur, 1819) | alien | 4.3 |
| Misgurnus fossilis (Linnaeus, 1758) | native | 3.4 |
| Squalius cephalus (Linnaeus, 1758) | native | 3.2 |
| Osmerus eperlanus (Linnaeus, 1758) | native | 2.8 |
| Lota lota (Linnaeus, 1758) | native | 2.1 |
| Anguilla anguilla (Linnaeus, 1758) | native | 1.9 |
| Neogobius fluviatilis (Pallas, 1814) | alien | 0.9 |
| Acipenser baerii Brandt, 1869 | alien | 0.6 |
| Vimba vimba (Linnaeus, 1758) | native | 0.4 |
| Pseudorasbora parva (Temminck & Schlegel, 1846) | alien | 0.4 |
| Leuciscus leuciscus (Linnaeus, 1758) | native | 0.4 |
| Hypophthalmichthys molitrix (Valenciennes, 1844) | alien | 0.4 |
| Ctenopharyngodon idella (Valenciennes, 1844) | alien | 0.2 |
| Cottus poecilopus Heckel, 1837 | native | 0.2 |
| Ballerus ballerus (Linnaeus, 1758) | native | 0.2 |
| Salmo trutta Linnaeus, 1758 | native | 0.2 |
| Trophy | Number of Lakes | Number of Fish Species | |
|---|---|---|---|
| Min.–Max. | Mean ± SD | ||
| Oligotrophy | 37 | 7–15 | 12 ± 2 |
| Mesotrophy | 66 | 9–16 | 12 ± 2 |
| Meso-eutrophy | 68 | 7–19 | 12 ± 2 |
| Eutrophy | 281 | 6–17 | 12 ± 2 |
| Hypereutrophy | 83 | 1–18 | 11 ± 3 |
| Maximum Depth (m) | Number of Lakes | Number of Fish Species | |
|---|---|---|---|
| Min.–Max. | Mean ± SD | ||
| <6 | 120 | 5–18 | 11 ± 2 |
| 6–12 | 143 | 6–16 | 12 ± 2 |
| 12–20 | 122 | 8–16 | 12 ± 2 |
| >20 | 150 | 1–19 | 12 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinowska, K.; Ulikowski, D.; Traczuk, P.; Kozłowski, M.; Kapusta, A. Fish Species Richness in Polish Lakes. Diversity 2023, 15, 164. https://doi.org/10.3390/d15020164
Kalinowska K, Ulikowski D, Traczuk P, Kozłowski M, Kapusta A. Fish Species Richness in Polish Lakes. Diversity. 2023; 15(2):164. https://doi.org/10.3390/d15020164
Chicago/Turabian StyleKalinowska, Krystyna, Dariusz Ulikowski, Piotr Traczuk, Michał Kozłowski, and Andrzej Kapusta. 2023. "Fish Species Richness in Polish Lakes" Diversity 15, no. 2: 164. https://doi.org/10.3390/d15020164
APA StyleKalinowska, K., Ulikowski, D., Traczuk, P., Kozłowski, M., & Kapusta, A. (2023). Fish Species Richness in Polish Lakes. Diversity, 15(2), 164. https://doi.org/10.3390/d15020164