Abstract
Temperature is an important factor determining the abundance, distribution and diversity of termite species. Thus, termites are affected by changing climate and have to adopt different means of surviving in order to avoid extinction. Using termite occurrence data, bioclimatic variables and vegetation cover, we modelled and predicted the current and future habitat suitability for mound-building termites in Nigeria. Of the 19 bioclimatic variables and the vegetation index (NDVI) tested, only six were significant and eligible as predictors of habitat suitability for the mound-building termites Macrotermes subhyalinus and M. bellicosus. Under current climatic conditions (2022), the northwest, northeast and central regions are highly suitable for M. subhyalinus, while the distribution of M. bellicosus decreased in the North West, North East and in the Central region. However, regarding habitat suitability for the future (2050), there was a predicted range expansion into suitable areas for the two termite species. The increase in temperature due to global warming has an effect which can either result in migration or sometimes extinction of termite species within an ecosystem. Here, we have predicted habitat suitability for the two mound-building termite species under current and future climatic scenarios, and how the change in climatic variables would lead to an expansion in their range across Nigeria.
1. Introduction
Increasing environmental changes, caused either through anthropogenic activities or due to effects of global warming, result directly or indirectly in changes affecting the distribution of both plant and animal species [1,2,3]. Generally, arthropods are the most affected by fluctuating temperatures due to their physiology and body sizes (surface area), which in turn affects both their diversity and abundance [4,5,6]. Change in temperature due to global warming brings about a decline in arthropod abundance and diversity, which has a great impact on the ecosystem and services they provide [3,7]. Termites are among soil macroinvertebrates that contribute to soil development [8]. Species within the genus Macrotermes, which are mound builders and fungus growers, are mostly found in tropical and subtropical regions of the world [9,10]. The presence of termites in an environment means the habitat is suitable for the termites to inhabit, with temperature having a key role in defining the type of species found in the habitat [11,12,13,14,15]. Termites are no exception to the effects caused by the changing climate as they have to adopt different means of surviving the changes, either through actively adjusting their physiological requirements [16] or, passively, by means of modifying the structures of their mounds and the location where they build them [17,18,19]. Those species unable to adjust or adapt to the changes caused by temperature rises will move to new habitats or become extinct [20,21,22]. Termite species may be able to shift their ranges due to the pressure exerted by the changing microclimatic conditions. This is because termites are delicate and sensitive to temperature fluctuations within the environment [23,24].
Nigeria has four climatic zones: (i) the warm desert in the northeast, (ii) the warm semiarid region in the other parts of the north (northwest and central), (iii) the monsoon region in the Niger Delta, and (iv) the tropical savannahs in the middle belt and parts of the southwest [25]. The main ecological zones in Nigeria are the tropical rainforest in the south, savannah in the middle belt, and semiarid zones in the north [26]. With the various types of climate, different termite species are found in the different ecological zones [27,28]. Termites in Nigeria mostly belong to the families: Termitidae, Kalotermitidea and Rhinotermitidae [29,30], and the species Macrotermes bellicosus, M. subhyalinus, Odontotermes sudanensis, O. magdalenae and O. smeathmani are amongst the most dominant [29,31,32,33]. Given the economic and the ecological impacts of mound-building termites in an ecosystem and their susceptibility to climate changes, it is crucial to predict habitat suitability for M. bellicosus and M. subhyalinus in Nigeria. Predicting the habitat suitability for these species will provide relevant information for understanding their ecological niche under current and future climatic conditions, and hence assist in future conservation planning.
Different models such as ecological niche modeling (ENM) and spatially explicit stochastic individual-based simulation have been used to predict the current and future distribution of termite species found in an environment [13,34,35,36]. These ENMs depend on the occurrence of a species and environmental variables to predict the habitat suitability of the species. Due to the fact that it is difficult to assess a species’ presence in a wide area, ENM uses the known occurrence points (GPS records) of the species obtained from sampled areas to predict the probability of occurrence in unsampled areas. These models correlate the occurrence points with the environmental variables to compute a conditional probability (habitat suitability) of where a species could occur. In this study, we seek to answer the following question. Will an increase in temperature bring about the redistribution or extinction of the two mound-building termite species (M. bellicosus and M. subhyalinus) within Nigeria? Similarly, we predicted the current and future (2050) habitat suitability of the two mound-building termite species using the simulated bioclimatic variables of the Hadley Centre Global Environmental Model version 2-Earth System (HADGEM2-ES) [37].
2. Materials and Methods
2.1. Study Area
The four climatic zones in Nigeria are characterized by varying amounts of rainfall. The equatorial zone in the southern region has an annual rainfall varying between 2400 to 4000 mm, the tropical rainforest in the central region has annual rainfall of 1500 to 2000 mm, while the arid regions in the north are drier with 500 to 750 mm of rainfall. Temperature and humidity remain relatively constant throughout the year in the south, while the seasons vary considerably in the north where daily average summer temperatures range between 38 °C to 40 °C. On the coast (southern and western regions), the mean monthly maximum temperatures are lower at approximately 20 °C. [25,38].
2.2. Termite Occurrence
The termite occurrence points used in this study were obtained from field sampling conducted in Kebbi state, in northwestern Nigeria [39] (Figure 1). The sampling was conducted in two ecological zones, namely Sahel and Sudan savannah [17,39]. The Sahel is characterized by the long dry season that lasts for seven–eight months and receives an annual rainfall between 250 mm and 510 mm. On the other hand, the Sudan savannah has a shorter dry season which lasts between five–seven months in a year, and receives an annual rainfall of between 510 mm–1140 mm. Sampling was conducted during the rainy season between the months of July and December. A random sampling procedure was applied to locate the presence of mound-building termites and their mounds [17,39]. During sampling, termites were collected from each mound for identification [17,39], and GPS coordinates were recorded at each locality where termites were present. To identify the termite species, representative samples of the soldier castes were collected from each mound, and stored in 70% ethanol and identified using morphometric keys compiled by Uys [40] and those by Ruelle [41] at species level. In total, 152 mounds of M. subhyalinus and M. bellicosus were identified during the field sampling and used in the study. Each of the 152 mounds were also georeferenced and their GPS coordinates noted. Among these mounds, M. subhyalinus was the most abundant with 126 occurrences followed by M. bellicosus which inhabited 26 mounds. The georeferenced points for these mounds were filtered to remove mounds that fall within 1 km of each other to reduce spatial autocorrelation between the data [42]. This process resulted in 29 and 26 georeferenced points for M. subhyalinus and M. bellicosus, respectively, which were used for modelling habitat suitability for the two termite species.
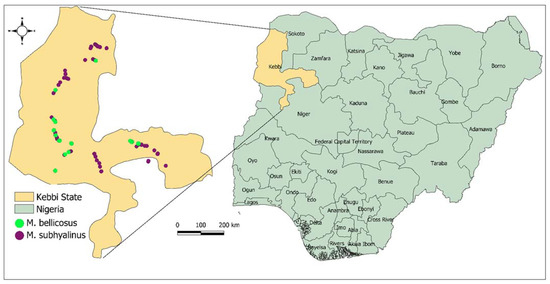
Figure 1.
The sampling site and the occurrence points used to predict habitat suitability for two termite species, Macrotermes subhyalinus and M. bellicosus, in Nigeria.
2.3. Bioclimatic Variables and Vegetation Cover
A set of 19 bioclimatic variables were obtained from WorldClim database and the normalized difference vegetation index (NDVI) was obtained from DIVA-GIS [43,44] (Table 1). The NDVI quantifies the vegetation greenness and is useful in understanding vegetation density and assessing changes in plant health within a location. These data were clipped into Nigerian boundaries and then resampled to a 30 m spatial resolution using R to allow the layers to have the same geographical extent and resolution. The acquired data was used to predict habitat suitability for the two termite species under current climatic conditions. To predict the habitat suitability for the species under future conditions (2050), we used the simulated bioclimatic variables of the Hadley Centre Global Environmental Model version 2-Earth System (HADGEM2-ES) [37]. We used the representative concentration pathway 4.5 (RCP4.5) to predict the potential impact of climate change on habitat suitability for the two termite species. RCP4.5 is a moderate scenario that stabilizes greenhouse gas emissions by the year 2100, using different technologies and mitigation measures. It has a radiative force of 4.5 W m−2, a carbon dioxide (CO2) emission of 538 ppm and global temperature rise between 2 °C and 3 °C by 2100 [45]. Due to the fact that there are no data available for NDVI under future climatic scenarios, we use the same NDVI layer to predict the habitat suitability for termites under both current and future climatic conditions.

Table 1.
Bioclimatic variables and vegetation cover (NDVI) used to predict habitat suitability for two termite species: Macrotermes subhyalinus, and M. bellicosus. The variables in bold are those that were selected based on variance inflation factor (VIF) for the modelling process.
2.4. Variables Selection
The multicollinearity between predictor variables results in overfitting in a model, hence affecting its performance and providing uncertain results [46]. Therefore, to have a robust output, it is recommended to exclude correlated predictor variables from the model fitting [46,47]. As such, to examine multicollinearity between the predictor variables, we performed a variance inflation factor (VIF) between all predictors [46]. The VIF reflects how much the standard errors are inflated due to the multicollinearity of the predictor variables. This method compares the variables that have high linear correlation and then removes the ones with high VIF [46]. All variables that had a VIF of >10 were eliminated from the modelling procedure because they were highly correlated with other variables [46]. Only the variables that met the selection criterion (uncorrelated variables) were used in our modelling (Table 1).
2.5. Model Development and Validation
Habitat suitability for the two termite species was predicted using the Maxent algorithm in R software. Maxent is a machine-learning algorithm that uses presence-only data for predicting the suitable habitat for species occurrence with the highest entropy. It computes the conditional probability of species occurrence based on the environmental conditions at known occurrence records of the species. In this study, we used a 10-fold cross-validation approach to fit and validate the model using the occurrence records and predictor variables for each species separately. The cross-validation method provides a robust output and minimizes selection bias and overfitting because it fits and validates the model performance in multiple ways (at least 10-fold) using the same dataset. The model performance and accuracy were assessed using the area under the curve (AUC) and true skill statistic (TSS) [48]. The values for AUC range between 0 and 1, with 1 demonstrating the highest performance and accuracy of the model [49]. On the other hand, TSS combines both sensitivity and specificity to account for both omission and commission errors [48]. It had values ranging between −1 and +1, where +1 is the ideal agreement between the observed and the predicted occurrence of the species, while −1 is the opposite [48]. As a rule of thumb, models that have AUC and TSS values of ≥0.7 are said to be highly accurate in predicting the distribution of a species [50].
3. Results
3.1. Variable Selection and Model Performance
Amongst the 20 temperature- and precipitation-related variables that were tested, only six were eligible for modelling habitat suitability for the two termite species, namely mean diurnal range (Bio2), mean temperature of the wettest quarter (Bio8), annual precipitation (Bio12), precipitation of the wettest month (Bio13), precipitation of the warmest quarter (Bio18) and normalized difference vegetation index (NDVI) (Table 1). These variables had a variance inflation factor lower than ten, indicating that they are not correlated with other variables, hence their independence and eligibility in the modelling procedure (Table 1). The results of the receiver operating curves (ROC) for the 10-fold cross-validation and AUC are shown in Figure 2. Although the models showed a slight variation for the 10 replicates (light blue lines), they predicted the occurrence of the two species with high accuracy (Figure 2). The tested AUC ranged between 0.92 and 0.97 and TSS between 0.85 and 0.91 indicating high preferences of the models in predicting the occurrence of M. subhyalinus and M. bellicosus.
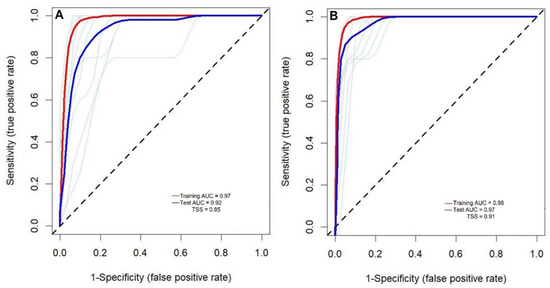
Figure 2.
The receiver operating curves (ROC) of Maxent algorithm used to predict habitat suitability for two termite species: (A) Macrotermes subhyalinus and (B) Macrotermes bellicosus. The red and the dark blue lines represent the mean area under the curve (AUC) for training and testing data, respectively, while the light blue lines represent the 10 replications of the training data.
3.2. Variable Importance
Among the six variables that were used, the precipitation of the warmest quarter (Bio18) was the most important variable in predicting the occurrence of the two termite species (Table 2; Figure 3), whereas the bioclimatic variables Bio2, Bio8 and Bio18 were the most important in predicting the occurrence of M. subhyalinus, with a contribution of 33.9, 23 and 26%, respectively (Table 2; Figure 3A). However, for M. bellicosus, Bio2, Bio18 and NDVI were the most important variables for predicting the occurrence of this species contributing 15, 36.8 and 28.4%, respectively, to the model performance (Table 2; Figure 3B).

Table 2.
Contribution of each variable to occurrence of Macrotermes subhyalinus and M. bellicosus.
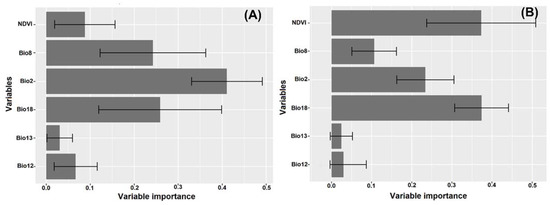
Figure 3.
Variables and their importance for predicting habitat suitability for two termite species using Maxent algorithm. (A) Macrotermes subhyalinus and (B) Macrotermes bellicosus. The bars represent a 95% confidence interval.
3.3. Predicting Habitat Suitability for Macrotermes Subhyalinus and M. bellicosus under Current Climatic Conditions
Under current climatic conditions, the model prediction showed that northwestern states of Nigeria, comprising Kebbi, Sokoto, Katsina, Zamfara and Kano, are highly suitable for M. subhyalinus to thrive (Figure 4A). Similarly, the northeastern states of Bauchi, Gombe, Adamawa, Taraba, Nasarawa and Niger were predicted to be highly suitable for this termite species to occur. The model prediction also showed a highly favorable climate suitability for M. bellicosus to occur in the northwestern states of Kebbi, Zamfara, Katsina and Kano, and northeastern states of Bauchi, Gombe and parts of Adamawa, as well as the central states of Niger and Kogi (Figure 4B).
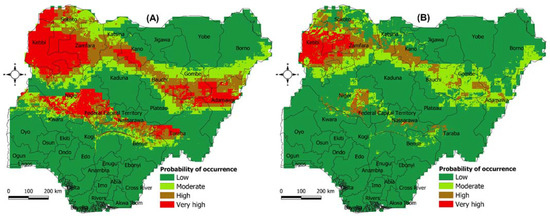
Figure 4.
The probability of occurrence of two termite species under current (YEAR 2022) climate scenario predicted using the Maxent algorithm. (A) Macrotermes subhyalinus and (B) Macrotermes bellicosus. The probability was classified as follows: (i) Low (0.0–0.20), (ii) Moderate (0.21–0.40), (iii) High (0.41–0.60), and (iv) Very high suitability (0.61–1.0).
3.4. Predicting Habitat Suitability for Macrotermes subhyalinus and M. bellicosus under Future (Year 2050) Climatic Conditions
Under future climate conditions, the model predicted a range expansion in suitable areas for the two termite species (Figure 5). For M. subhyalinus, most areas that are predicted to be suitable under current conditions will be very highly suitable in the future, indicating an optimal climate for this termite species to occur (Figure 5A). Besides current suitable areas, M. subhyalinus will expand its geographical range to cover Yobe and Borno states in the future (Figure 5A). Future climate projections also showed that M. bellicosus will expand its geographical range to cover all the states in the northwestern and northeastern parts of Nigeria (Figure 5B).
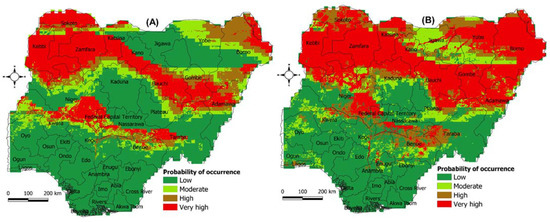
Figure 5.
The probability of occurrence of two termite species under future climate scenario (2050s) predicted using the Maxent algorithm. (A) Macrotermes subhyalinus and (B) Macrotermes bellicosus. The probability was classified as follows: (i) Low (0.0–0.20), (ii) Moderate (0.21–0.40), (iii) High (0.41–0.60) and (iv) Very high suitability (0.61–1.0).
4. Discussion
Climate is a major factor in determining the diversity, distribution and abundance of both plants and animals in an environment. Insects, especially termites, are delicate and susceptible to changes in their microhabitats which can either be of advantage or disadvantage to their physiological state [51,52,53]. When the changes work in their favor, they become diverse and widely distributed and also experience a surge in population size. However, when the changes are drastic, it affects their physiology and results in population decline which may lead to migration or extinction [54]. Modelling is one of the tools used in predicting the presence or absence of an organism based on available data [55,56]. We modelled the distributions of two mound-building termite species, Macrotermes bellicosus and M. subhyalinus, and predicted the current and future habitat suitability for them in the different regions of Nigeria. We also predicted the effects of rising temperatures on their habitat due to global warming, which usually brings about changes in temperature which plays a major role in the distribution and abundance of termite species [39,57,58].
Of the 19 bioclimatic variables and one vegetation layer that were used, only six were eligible for modelling and predicting habitat suitability for Macrotermes subhyalinus and M. bellicosus. These variables were able to present the current and future status in terms of habitat suitability of the two termite species [56]. Among the six variables that were used, the precipitation of the warmest quarter was the most important variable in predicting the occurrence of the two termite species. Precipitation and temperature explain the variation in plant species richness which was critical in determining the distribution of termite species within an environment [59,60]. Plants are the primary producers in most food chains and are usually considered as indicators for many climatic elements within an ecosystem. Thus, any shift in these climatic variables tends to affect the species richness of both the producers and the consumers [61]. Ayuke et al. [62] reported that the presence of termites in a region depends on the vegetation cover found in that region. Termites can either be generalist or specialist feeders within a habitat. Both Macrotermes bellicosus and M. subhyalinus consume dead plant materials by cultivating a basidiomycete fungus of the genus Termitomyces inside their mounds. This fungus is maintained on plant materials which only survive under the optimal micro climatic conditions within the mound [63]. Precipitation of the warmest quarter was among the conditions necessary for proper plant growth and the presence of plants usually attracts termites to the area in search of food, which brings about the redistribution of termites to new areas where they were not found in the past [53,59].
Two bioclimatic variables, mean temperature of the wettest quarter and precipitation of the warmest quarter, were important in predicting the occurrence of M. subhyalinus, indicating that both variables have an impact on predicting the current and future climate conditions on species distribution. However, for M. bellicosus, mean diurnal range, precipitation of warmest quarter and vegetation cover were the most important variables for predicting the occurrence of this species. Plant species richness increases with increasing precipitation of the warmest quarter and increase in plant richness also brings about an increase in termite numbers [59]. Similar to our findings, Salas et al. [64] used bioclimatic variables to show the future distribution of species in an environment; their model projected losses and gains in suitable bioclimatic envelopes for the future within their study area. Nevertheless, our future prediction used the same vegetation layer as the current scenario to predict the habitat subtility of M. subhyalinus and M. bellicosus under future climatic conditions. The vegetation cover is highly correlated with climatic variables, especially rainfall, thus the change in the amount of rainfall as a result of climate change is expected to shift the vegetation cover, consequently affecting habitat suitability for both termite species. Therefore, the predicted habitat suitability under future climatic conditions should be interpreted with caution as vegetation cover might be changed in the future as a results of climate change.
The model predicts that M. subhyalinus is suitable in most areas under current conditions and will continue to be with increasing temperatures in the future. This indicates that besides currently suitable areas, M. subhyalinus might expand its geographical distribution to cover Yobe and Borno states in the future. Similarly, M. bellicosus might expand its geographical range to cover all the states in the northwestern and northeastern parts of Nigeria [27]. Similar to our findings, Suppo et al. [57] show that increase in temperatures favors the spread of the eastern subterranean termite Reticulitermes flavipes commonly found in North America. To further support the prediction by our model for the current distribution of Macrotermes species in Nigeria, their presence in the North Central region was reported by Abe et al. [65], Longhurst et al. [66] and Wood et al. [28]. Similarly, Ntukuyoh et al. [67] reported the presence of M. bellicosus in the South South, and most recently Ejomah et al. [68] reported the presence of M. bellicosus in the southern region of Nigeria, while Bandiya et al. [69], Alamu et al. [27] and Collins [70] all reported the presence of M. bellicosus in the northwestern region. Macrotermes subhyalinus was also reported in the North Central region of the country by Longhurst et al. [66] and Wood et al. [28], while Alamu et al. [27] also reported the presence of M. subhylinus in the northwestern region. In their study of species richness, abundance and diversity of termites, Kemabonta and Balogun [71] reported the presence of M. subhyalinus in southwestern Nigeria.
Our model shows that by 2050 (future climate conditions), the ranges of the two termite species would expand. For M. subhyalinus, most areas that are predicted to be suitable under current conditions will be very highly suitable in the future, indicating optimal climate for this termite species to occur. Future climate projections also showed that M. bellicosus will expand its geographical range to cover all the states in the northwestern and northeastern parts of Nigeria. Generally, both M. subhyalinus, and M. bellicosus will be well distributed within the different regions across the country, despite the climatic changes that will be experienced in their current geographical range. Tonini et al. [34] predicted the geographical distribution of two invasive termite species, Coptotermes gestroi and Coptotermes formosanus, and showed that future global warming seems to affect their projected probability, thereby decreasing the population growth of the two studied termite species. Similarly, Gull et al. [72] modelled the geographic distribution of the Ponerine ant Brachyponera nigrita and indicated that it would expand its range in the future, in comparison with its current distribution. Similar to our observations on the two termite species, Zhao et al. [73] modeled the potential geographical distribution of the corn earworm Helicoverpa zea in China and predicted its future suitability in areas outside their current distribution due to future (in the 2030s and 2050s) climate change. Confirming the predictions for habitat suitability on the future distribution of the two termite species, Tonini et al. [35] argue that only if the climatic conditions are suitable will the introduced species flourish in an area; they modelled an invasive termite species Nasutitermes corniger to determine the suitability of the species in a new environment. They also believe that modelling will assist regulatory agencies to be prepared and also to develop successful early detection of invasive species.
This study showed the current and predicted future distribution of the two termite species, M. subhyalinus and M. bellicosus, based on temperature and vegetation suitability. The results showed the suitability of the two termite species in all of the states found in the North West, North Central and North East part of the country. Considering the economic and the ecological impacts of the two mound-building termites species, it is imperative that we predict their current and future distribution in Nigeria. Such a study is important in the light of climate change and its effects on ecosystem services and will assist in future conservation planning.
Author Contributions
Conceptualization, A.P.I., C.W.W.P., A.A.Y.; methodology, A.P.I., A.G.A.A.; formal analysis, A.P.I., A.G.A.A.; investigation and data curation, A.P.I., C.W.W.P., A.A.Y.; writing—original draft preparation, A.P.I., A.G.A.A.; writing—review and editing, A.P.I., A.G.A.A., C.W.W.P., A.A.Y., funding; C.W.W.P., A.A.Y. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research was provided in part by: The University of Pretoria, The South African National Research Foundation (NRF) Incentive Funding for Rated Researchers (IFRR) to CWWP and AAY, Y-Rated Research Grant, PI grant from South African Research Chair in Mathematical Methods in Bioengineering and Biosciences (M2B3) and Alexander von Humboldt’s Georg Foster HERMES Experienced Research Fellowship (grant nos. ZAF-1164298 –GFHERMES-E) to AAY. AIP was supported by a University of Pretoria Postgraduate Bursary and the Nigerian Tertiary Education Trust Fund (TETFund).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study were included in this published article.
Acknowledgments
We thank Robin Crewe for his fruitful discussions and for editing the manuscript for language. Our appreciation goes to members of the Social Insects Research Group (SIRG) University of Pretoria for their support during this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chunco, A.J. Hybridization in a warmer world. Ecol. Evol. 2014, 4, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Nechols, J.R.; Hough, A.R.; Margolies, D.C.; Ruberson, J.R.; Mccornack, B.P.; Sandercock, B.K.; Murray, L. Effect of Temperature on Plant Resistance to Arthropod Pests. Environ. Entomol. 2020, 49, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.M.; Khan, M.A.; Arshad, M.; Sagheer, M.; Sattar, Z.; Shafi, J.; Haq, E.U.; Ali, A.; Aslam, U.; Mushtaq, A.; et al. Impact of global warming on insects. Arch. Phytopathol. Plant Prot. 2015, 48, 84–94. [Google Scholar] [CrossRef]
- Patrick, K.; Robert, D.S. The Effect of Decreasing Temperature on Arthropod Diversity and Abundance in Horse Dung Decomposition Communities of Southeastern Massachusetts. Psyche J. Entomol. 2012, 2012, 618701. [Google Scholar] [CrossRef]
- Klok, C.J.; Harrison, J.F. The temperature size rule in arthropods: Independent of macro-environmental variables but size dependent. Integr. Comp. Biol. 2013, 53, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Régnière, J.; Powell, J.; Bentz, B.; Nealis, V. Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. J. Insect Physiol. 2012, 58, 634–647. [Google Scholar] [CrossRef]
- Lister, B.C.; Garcia, A. Climate-driven declines in arthropod abundance restructure a rainforest food web. PNAS 2018, 115, E10397–E10406. [Google Scholar] [CrossRef]
- Gillison, A.N.; Jones, D.T.; Susilo, F.X.; Bignell, D.E. Vegetation indicates diversity of soil macroinvertebrates: A case study with termites along a land-use intensification gradient in lowland Sumatra. Org. Divers. Evol. 2003, 3, 111–126. [Google Scholar] [CrossRef]
- Maynard, D.S.; Crowther, T.W.; King, J.R.; Warren, R.J.; Bradford, M.A. Temperate forest termites: Ecology, biogeography, and ecosystem impacts. Ecol. Entomol. 2015, 40, 199–210. [Google Scholar] [CrossRef]
- Jones, D.T.; Eggleton, P. Global biogeography of termites: A compilation of sources. In Biology of Termites: A modern Synthesis; Springer: Berlin/Heidelberg, Germany, 2010; pp. 477–498. [Google Scholar]
- Woon, J.S.; Atkinson, D.; Adu-Bredu, S.; Eggleton, P.; Parr, C.L. Termites have wider thermal limits to cope with environmental conditions in savannas. J. Anim. Ecol. 2022, 91, 766–779. [Google Scholar] [CrossRef]
- Arango, R.A.; Schoville, S.D.; Currie, C.R.; Carlos-Shanley, C. Experimental Warming Reduces Survival, Cold Tolerance, and Gut Prokaryotic Diversity of the Eastern Subterranean Termite, Reticulitermes flavipes (Kollar). Front. Microbiol. 2021, 12, 632715. [Google Scholar] [CrossRef] [PubMed]
- Hyseni, C.; Garrick, R.C. Ecological drivers of species distributions and niche overlap for three subterranean termite species in the southern Appalachian Mountains, USA. Insects 2019, 10, 33. [Google Scholar] [CrossRef]
- Woon, J.; Boyle, M.; Ewers, R.; Chung, A.; Eggleton, P. Termite environmental tolerances are more linked to desiccation than temperature in modified tropical forests. Insectes Soc. 2019, 66, 57–64. [Google Scholar] [CrossRef]
- Sattar, A.; Naeem, M. Impact of environmental factors on the population dynamics, density and foraging activities of Odontotermes lokanandi and Microtermes obesi in Islamabad. Springerplus 2013, 2, 349. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Aiki, I.; Yusuf, A.; Pirk, C. Refuge in architecture: Mounds and diversity of termite species from a Sahel and Sudan savannah. Int. J. Trop. Insect Sci. 2021, 41, 1365–1371. [Google Scholar] [CrossRef]
- Yang, H.; Wu, M.; Liu, W.; Zhang, Z.; Zhang, N.; Wan, S. Community structure and composition in response to climate change in a temperate steppe. Glob. Chang. Biol. 2011, 17, 452–465. [Google Scholar] [CrossRef]
- Korb, J.; Linsenmair, K.E. Thermoregulation of termite mounds: What role does ambient temperature and metabolism of the colony play? Insectes Soc. 2000, 47, 357–363. [Google Scholar] [CrossRef]
- Alexander, L.; Allen, S.; Bindoff, N.L. Working group I contribution to the IPCC fifth assessment report climate change 2013: The physical science basis summary for policymakers; Cambridge Universty Press,: New York, NY, USA, 2013; pp. 1029–1136. Available online: https://www.ipcc.ch/working-group/wg1/ (accessed on 6 January 2023).
- Sinervo, B.; Mendez-De-La-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.; De Siqueira, M.F.; Grainger, A.; Hannah, L. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Ahmad, S.K.; Dawah, H.A.; Khan, M.A. Termites and Sustainable Management. Sustainability in Plant and Crop Protection. Ecol. Termit. 2018, 2, 47–68. [Google Scholar]
- Angert, A.; Crozier, L.; Rissler, L.; Gilman, S.; Tewksbury, J.; Chunco, A. Do species traits predict recent shifts at expanding range edges? Ecol. Lett. 2011, 14, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Akande, A.; Costa, A.C.; Mateu, J.; Henriques, R. Geospatial analysis of extreme weather events in Nigeria (1985–2015) using self-organizing maps. Adv. Meteorol. 2017, 2017, 8576150. [Google Scholar] [CrossRef]
- Alamu, O.; Ewete, F.; Jimoh, S. Occurrence and diversity of termite species in Eucalyptus plantations in Afaka, Kaduna State. Nig. J. Res. For. Wildl. Environ. 2018, 10, 33–38. [Google Scholar]
- Wood, T.; Johnson, R.; Bacchus, S.; Shittu, M.; Anderson, J. Abundance and distribution of termites (Isoptera) in a riparian forest in the southern Guinea savanna vegetation zone of Nigeria. Biotropica 1982, 14, 25–39. [Google Scholar] [CrossRef]
- Krishna, K.; Grimaldi, D.A.; Krishna, V.; Engel, M.S. Treatise on the Isoptera of the World. Bull. Am. Mus. Nat. Hist. 2013, 377, 1–623. [Google Scholar] [CrossRef]
- Johnson, R.A.; Lamb, R.W.; Sands, W.A.; Shittu, M.O.; Williams, R.M.C.; Wood, T.G. A check list of Nigerian termites (Isoptera) with brief notes on their biology and distribution. Niger. Field 1980, 45, 50–64. [Google Scholar]
- Ndiaye, A.B.; Njie, E.; Correa, P.A. Termites (Blattodea Latreille 1810, Termitoidae Latreille 1802) of Abuko Nature Reserve, Nyambai Forest Park and Tanji Bird Reserve (The Gambia). Insects 2019, 10, 122. [Google Scholar] [CrossRef]
- Olagbemiro, T.; Lajide, L.; Sani, K.; Staddon, B. 2-Hydroxy-5-methyl-1, 4-benzoquinone from the salivary gland of the soldier termites Odontotermes magdalenae. Experientia 1988, 44, 1022–1024. [Google Scholar] [CrossRef]
- Lepage, M. Foraging of Macrotermes spp.(Isoptera: Mcrotermitinae) in the tropics. Soc. Insects Trop. 1983, 2, 205–218. [Google Scholar]
- Tonini, F.; Divino, F.; Lasinio, G.J.; Hochmair, H.H.; Scheffrahn, R.H. Predicting the geographical distribution of two invasive termite species from occurrence data. Environ. Entomol. 2014, 43, 1135–1144. [Google Scholar] [CrossRef]
- Tonini, F.; Hochmair, H.H.; Scheffrahn, R.H.; Deangelis, D.L. Simulating the spread of an invasive termite in an urban environment using a stochastic individual-based model. Environ. Entomol. 2013, 42, 412–423. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Walker, C.; Peterson, A.T.; Merow, C.; Papeş, M. A checklist for maximizing reproducibility of ecological niche models. Nat. Ecol. Evol. 2019, 3, 1382–1395. [Google Scholar] [CrossRef] [PubMed]
- Collins, W.J.; Bellouin, N.; Doutriaux-Boucher, M.; Gedney, N.; Halloran, P.; Hinton, T.; Woodward, S. Development and evaluation of an Earth-System model–HadGEM2. Geosci. Model Devt. 2011, 4, 1051–1075. [Google Scholar] [CrossRef]
- Daramola, M.; Eresanya, E.; Erhabor, S. Analysis of rainfall and temperature over climatic zones in Nigeria. J. Geogr. Environ. Earth Sci. Int. 2017, 11, 1–14. [Google Scholar] [CrossRef]
- Aiki, I.P.; Pirk, C.W.W.; Yusuf, A.A. Thermal regulatory mechanisms of termites from two different savannah ecosystems. J. Therm. Biol. 2019, 85, 102418. [Google Scholar] [CrossRef] [PubMed]
- Boria, A.R.; Link, E.O.; Steven, M.G.; Robert, P.A. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Modell. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. Q. J. R. Meteorol. Soc. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Guarino, L.; Bussink, C.; Mathur, P.; Cruz, M.; Barrantes, I.; Rojas, E. DIVA-GIS, Version 5. A Geographic Information System for the Analysis of Biodiversity Data. Manual 2005, 5.2, 1–79. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, A. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Tsoar, A.; Allouche, O.; Steinitz, O.; Rotem, D.; Kadmon, R. A comparative evaluation of presence-only methods for modelling species distribution. Divers. Distrib. 2007, 13, 397–405. [Google Scholar] [CrossRef]
- Dahlsjö, C.A.; Valladares Romero, C.S.; Espinosa Iñiguez, C.I. Termite Diversity in Ecuador: A Comparison of Two Primary Forest National Parks. J. Insect Sci. 2020, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, J.; Moeller, A.H. The effects of temperature on animal gut microbiomes. Front. Microbiol. 2020, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Dahlsjo, C.A.L.; Parr, C.L.; Malhi, Y.; Meir, P.; Eggleton, P. Describing termite assemblage structure in a Peruvian lowland tropical rain forest: A comparison of two alternative methods. Insectes Soc. 2015, 62, 141–150. [Google Scholar] [CrossRef]
- Wang, Z.; Li, P.; Li, L.; Huang, C.; Liu, M. Modeling and Forecasting Average Temperature for Weather Derivative Pricing. Adv. Meteorol. 2015, 2015, 837293. [Google Scholar] [CrossRef]
- Ramírez Villegas, J.; Bueno Cabrera, A. Working with climate data and niche modeling: I. Creation of bioclimatic variables; International Center for Tropical Agriculture (CIAT), 2009; pp. 1–6. Available online: https://hdl.handle.net/10568/90732 (accessed on 6 January 2023).
- Suppo, C.; Robinet, C.; Perdereau, E.; Andrieu, D.; Bagnères, A.G. Potential spread of the invasive North American termite, Reticulitermes flavipes, and the impact of climate warming. Biol. Invasions 2018, 20, 905–922. [Google Scholar] [CrossRef]
- Botkin, D.A.; Saxe, H.; Miguel, B.A.; Richard, B.; Richard, H.W.B.; Tomas, C.; Peter, C.; Terry, P.D.; Julie, R.E.; Daniel, P.F.; et al. Forecasting the effects of global warming on biodiversity. Bioscience 2007, 57, 227–236. [Google Scholar] [CrossRef]
- Gwitira, I.; Murwira, A.; Shekede, M.D.; Masocha, M.; Chapano, C. Precipitation of the warmest quarter and temperature of the warmest month are key to understanding the effect of climate change on plant species diversity in S outhern A frican savannah. Afr. J. Ecol. 2014, 52, 209–216. [Google Scholar] [CrossRef]
- Higgins, S.I.; O’Hara, R.B.; Römermann, C. A niche for biology in species distribution models. J. Biogeogr. 2012, 39, 2091–2095. [Google Scholar] [CrossRef]
- Shen, Z.; Fei, S.; Feng, J.; Liu, Y.; Liu, Z.; Tang, Z.; Wang, X.; Wu, X.; Zheng, C.; Zhu, B. Geographical patterns of community-based tree species richness in Chinese mountain forests: The effects of contemporary climate and regional history. Ecography 2012, 35, 1134–1146. [Google Scholar] [CrossRef]
- Ayuke, F.O.; Rao, M.; Swift, M.; Opondo-Mbai, M. Assessment of biomass transfer from green manure to soil macrofauna in agroecosystem-Soil macrofauna biomass. In Managing Nutrient Cycles to Sustain Soil Fertility in Sub-Saharan Africa; Academy Science Publishers: Nairobi, Kenya, 2004; pp. 411–422. [Google Scholar]
- Aanen, D.K.; Boomsma, J.J. Evolutionary dynamics of the mutualistic symbiosis between fungus-growing termites and Termitomyces fungi. In Insect-Fungal Associations: Ecology and Evolution; Oxford University Press: New York, NY, USA, 2005; pp. 191–210. [Google Scholar]
- Salas, E.; Seamster, V.; Harings, N.; Boykin, K.; Alvarez, G.; Dixon, K. Projected Future Bioclimate-Envelope Suitability for Reptile and Amphibian Species of Concern in South Central USA. Herpetol. Conserv. Biol. 2017, 12, 522–547. [Google Scholar]
- Abe, S.S.; Yamamoto, S.; Wakatsuki, T. Physicochemical and morphological properties of termite (Macrotermes bellicosus) mounds and surrounding pedons on a toposequence of an inland valley in the southern Guinea savanna zone of Nigeria. Soil Sci. Plant Nutr. 2009, 55, 514–522. [Google Scholar] [CrossRef]
- Longhurst, C.; Johnson, R.; Wood, T. Predation by Megaponera foetens (Fabr.) (Hymenoptera: Formicidae) on termites in the Nigerian southern Guinea savanna. Oecologia 1978, 32, 101–107. [Google Scholar] [CrossRef]
- Ntukuyoh, A.; Udiong, D.; Ikpe, E.; Akpakpan, A. Evaluation of nutritional value of termites (Macrotermes bellicosus): Soldiers, workers, and queen in the Niger Delta region of Nigeria. Int. J. Food Saf. Nutr. 2012, 1, 60–65. [Google Scholar]
- Ejomah, A.J.; Uyi, O.O.; Ekaye, S.O. Exposure of the African mound building termite, Macrotermes bellicosus workers to commercially formulated 2, 4-D and atrazine caused high mortality and impaired locomotor response. PLoS ONE 2020, 15, e0230664. [Google Scholar] [CrossRef] [PubMed]
- Bandiya, H.; Majeed, Q.; Ibrahim, N.; Yahaya, M.; Yahaya, M. Intra-colonial Population of Macrotermes bellicosus (Smeathman) [Isoptera: Termitidae] in Sokoto, Semi-Arid Zone of North-Western Nigeria. NJBAS 2013, 21, 55–59. [Google Scholar] [CrossRef]
- Collins, M.N. The nests of Macrotermes bellicosus (Smeathman) from Mokwa, Nigeria. Insectes Sociaux 1979, 26, 240–246. [Google Scholar] [CrossRef]
- Kemabonta, K.; Balogun, S. Species richness, diversity and relative abundance of termites (Insecta: Isoptera) in the University of Lagos, Lagos, Nigeria. FJRS 2014, 2, 188–197. [Google Scholar]
- Gull, E.F.; Mahmood, A.T.; Bodlah, I.; Rashid, A.; Khalid, A.; Mahmood, S. Modeling potential distribution of newly recorded ant, Brachyponera nigrita using Maxent under climate change in Pothwar region, Pakistan. PLoS ONE 2022, 17, e0262451. [Google Scholar]
- Zhao, H.; Xian, X.; Zhao, Z.; Zhang, G.; Liu, W.; Wan, F. Climate Change Increases the Expansion Risk of Helicoverpa zea in China According to Potential Geographical Distribution Estimation. Insects 2022, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Uys, V. A guide to the termites genera of of Southern Africa. In A Plant Protection Research Institute Handbook; ARC-Plant Protection Research Institute: Pretoria, South Africa, 2002; pp. 1–116. [Google Scholar]
- Ruelle, J.E. Revision of the termites of the genus Macrotermes from the Ethiopian region (Isoptera: Termitidae). Brit. Mus. Natur. Hist Bull. Entomol. 1970, 365–444. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201301208403 (accessed on 6 January 2023). [CrossRef]
- Thomson, A.M.; Calvin, K.V.; Smith, S.J.; Kyle, G.P.; Volke, A.; Patel, P.; Delgado-Arias, S.; Bond-Lamberty, B.; Wise, M.A.; Clarke, L.E.; et al. RCP4. 5: A pathway for stabilization of radiative forcing by 2100. Clim. Chang. 2011, 109, 77–94. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).