Effects of Clothianidin Pesticide Application on the Strength of Honey Bee Colonies and Stress-Related Genes in the Vicinity of Rice Fields in the Republic of Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Landscape Analysis
2.3. Analysis of Plant Species Composition in Pollen
2.4. Analysis of Pesticide Residual in Pollen
2.5. Monitoring the Strength of Honey Bee Colonies
2.6. Quantitative Real-Time PCR
2.7. Data Analysis
3. Results
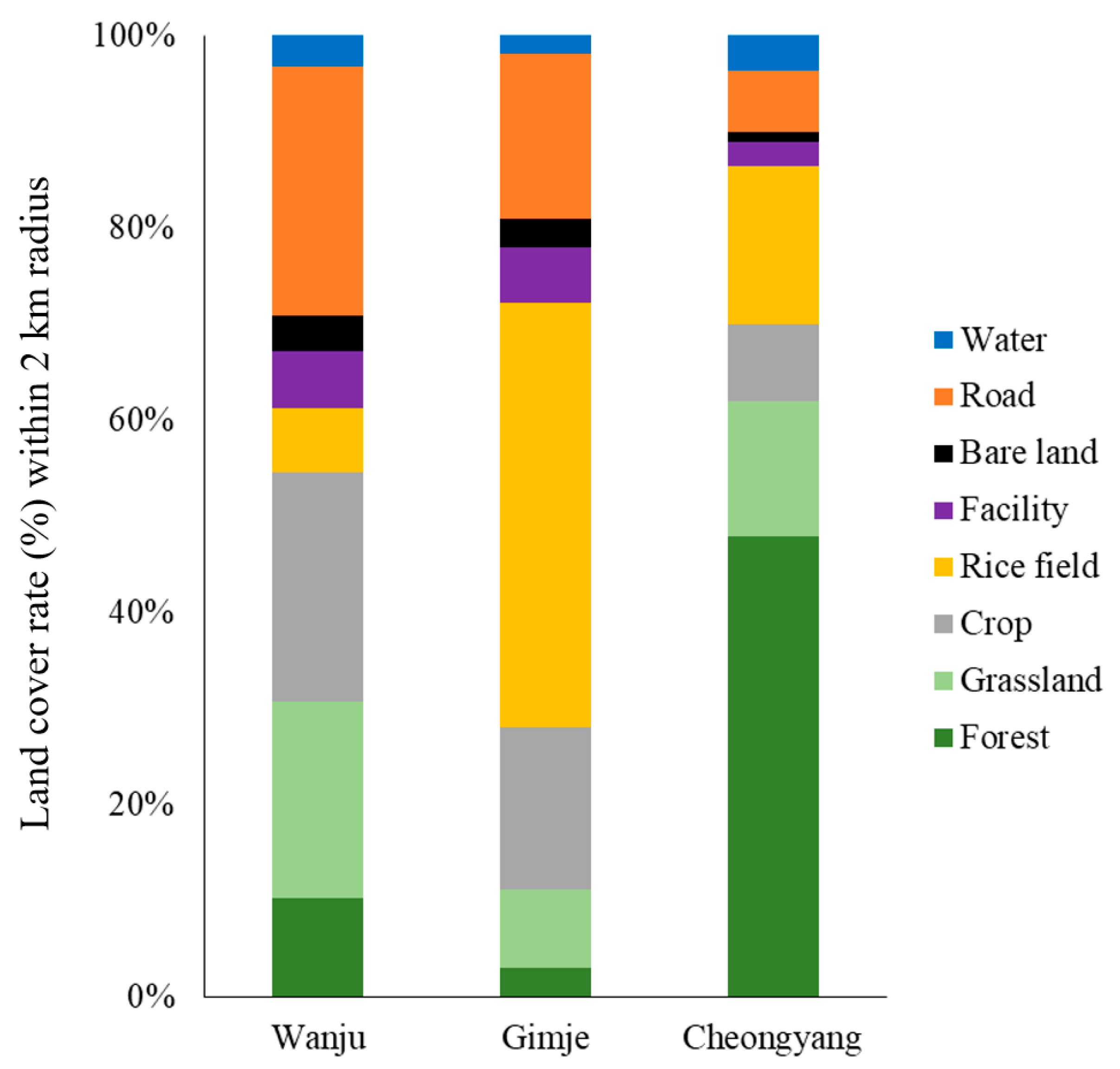
3.1. Analysis of Landscape Factors within a 2 km Radius of the Honey Bee Colonies’ Establishment Sites
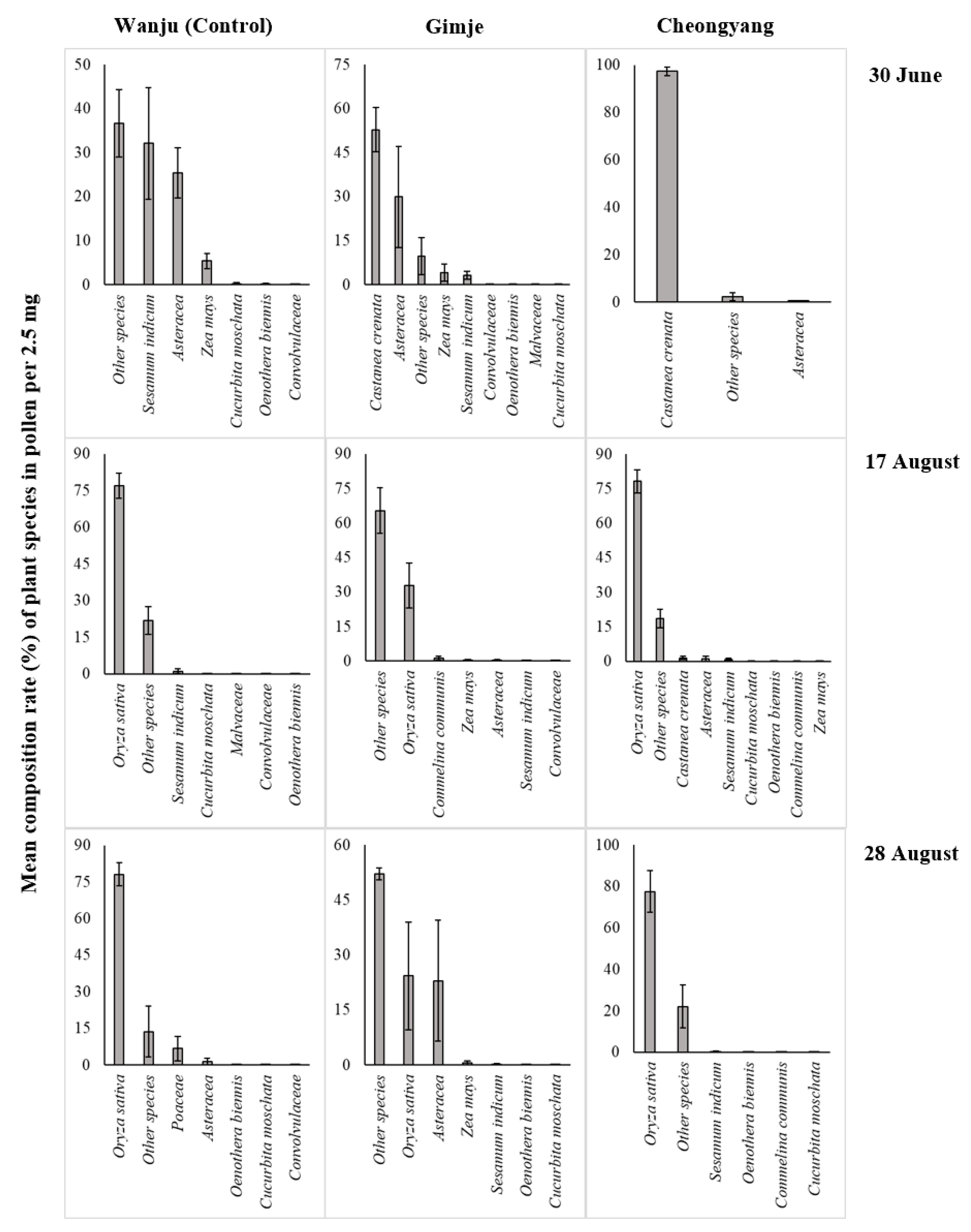
3.2. Composition of Plant Species and Residual Pesticides in Pollen
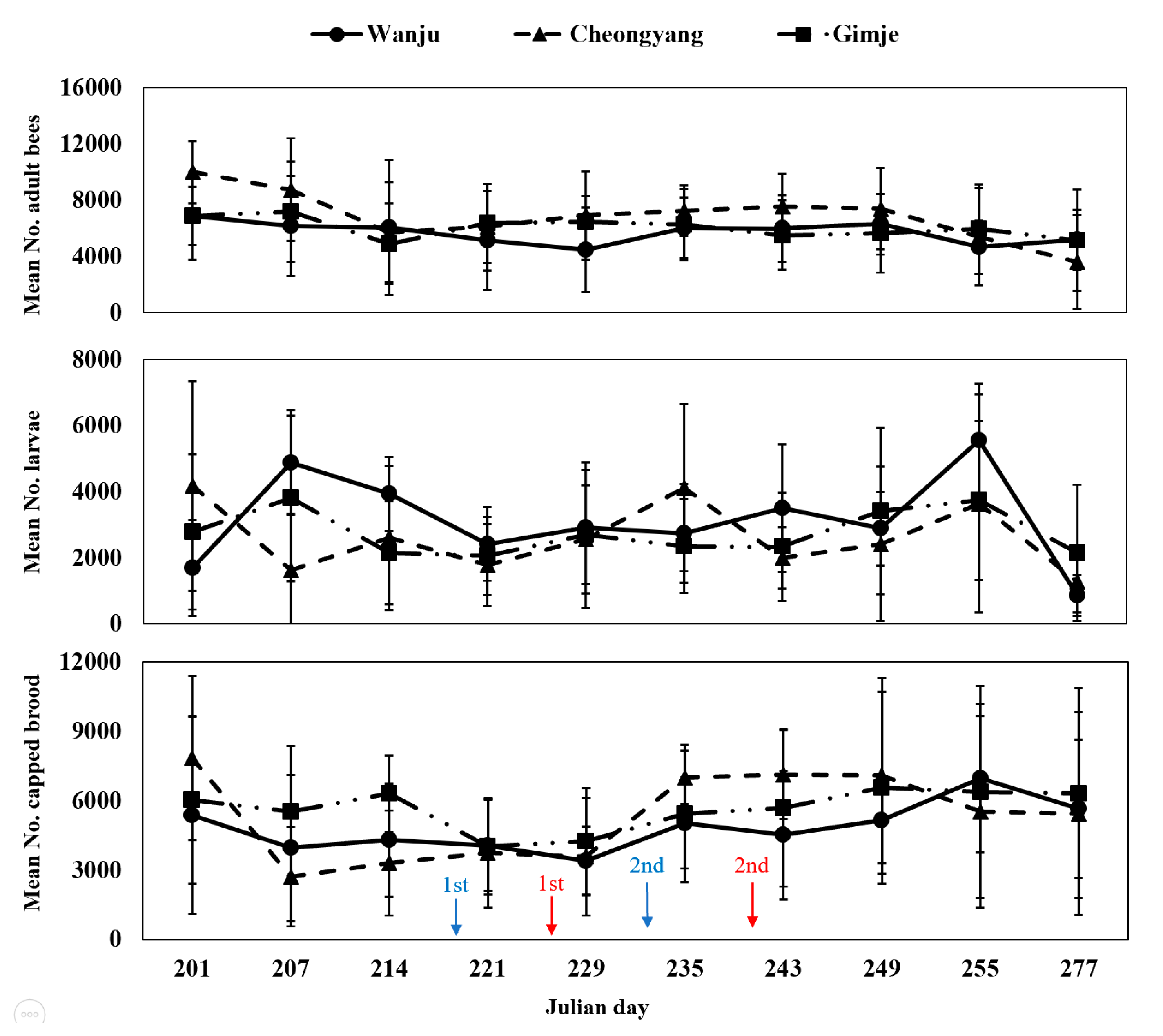
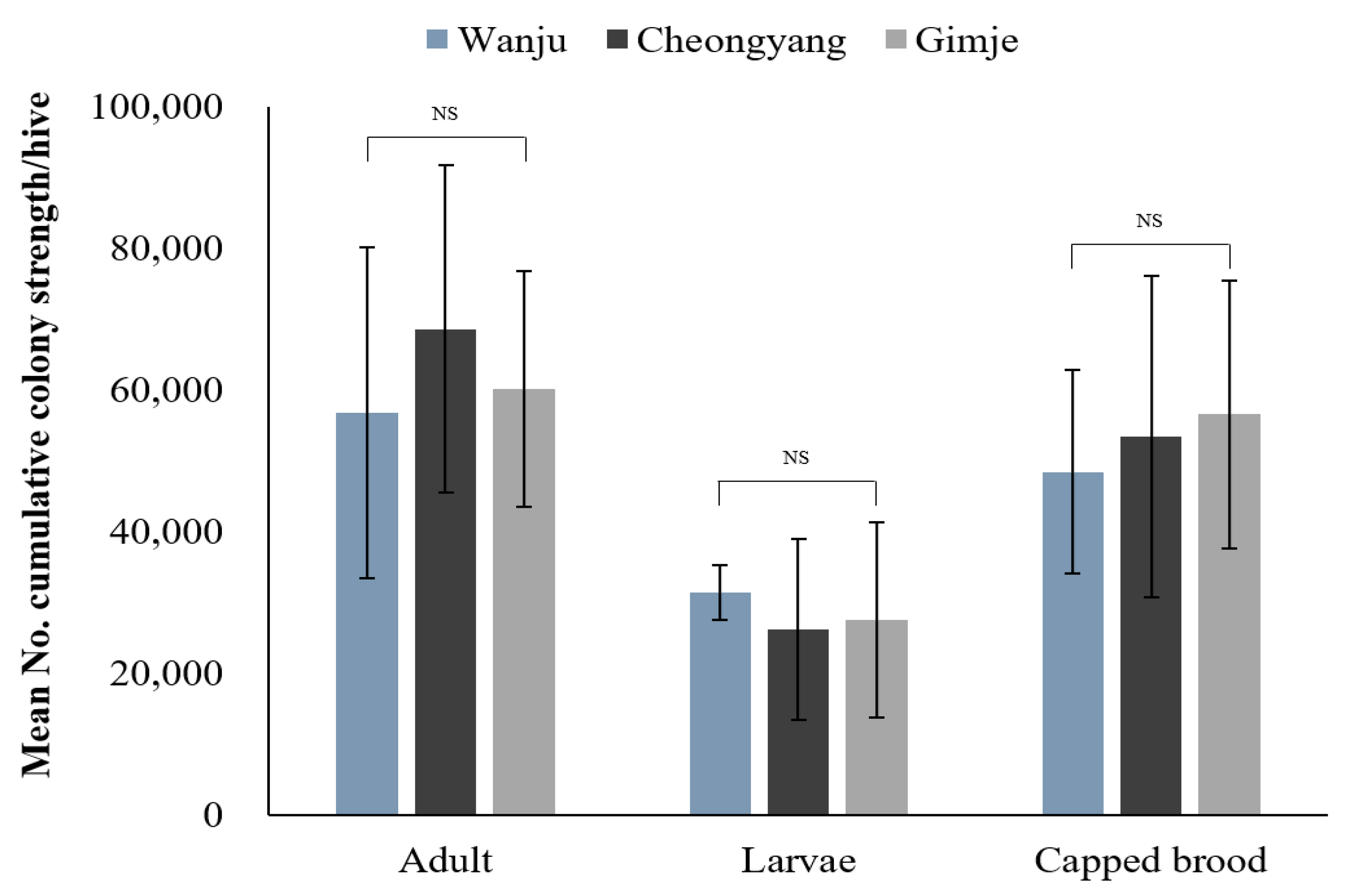
3.3. Monitoring the Strength of Honey Bee Colonies in Nearby Rice Fields
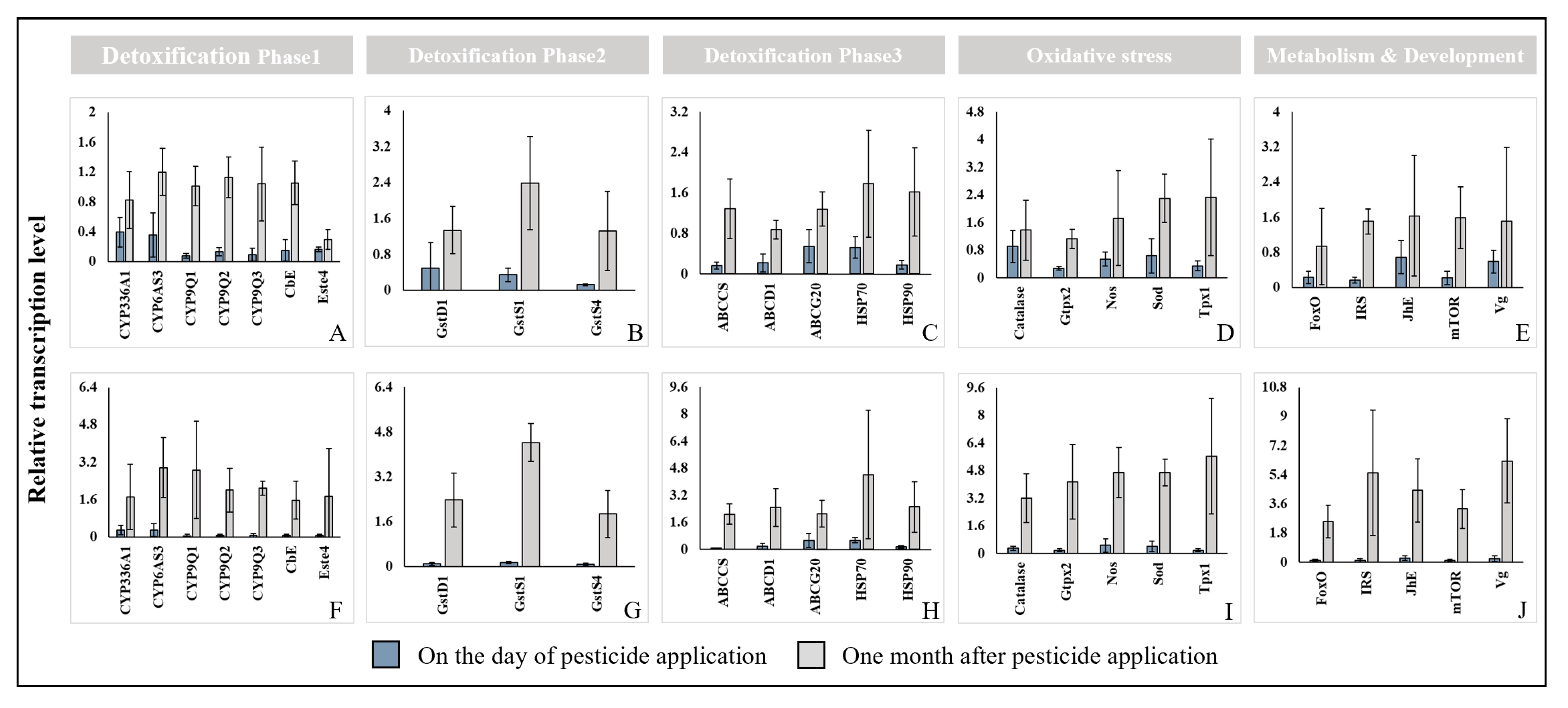
3.4. Genomic Analysis of Honey Bee Stress Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, V.; Zoller, L.; Bennett, J.M.; Knight, T.M. Pollinator dependence but no pollen limitation for eight plants occurring north of the Arctic Circle. Ecol. Evol. 2020, 10, 13664–13672. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts, and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Aslan, C.E.; Liang, C.T.; Galindo, B.; Hill, K.; Topete, W. The role of honey bees as pollinators in natural areas. Nat. Areas J. 2016, 36, 478–488. [Google Scholar] [CrossRef]
- Hung, K.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef] [PubMed]
- Klatt, B.K.; Holzschuh, A.; Westphal, C.; Clough, Y.; Smit, I.; Pawelzik, E.; Tscharntke, T. Bee pollination improves crop quality, shelf life and commercial value. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132440. [Google Scholar] [CrossRef] [PubMed]
- Francuski, L.; Beukeboom, L.W. Insects in production—An introduction. Entomol. Exp. Appl. 2020, 168, 422–431. [Google Scholar] [CrossRef]
- Osterman, J.; Aizen, M.A.; Biesmeijer, J.C.; Bosch, J.; Howlett, B.G.; Inouye, D.W.; Jung, C.; Martins, D.J.; Medel, R.; Pauw, A.; et al. Global trends in the number and diversity of managed pollinator species. Agric. Ecosyst. Environ. 2021, 322, 107653. [Google Scholar] [CrossRef]
- Brodschneider, R.; Moosbeckhofer, R.; Crailsheim, K. Surveys as a tool to record winter losses of honey bee colonies: A two-year case study in Austria and South Tyrol. J. Apic. Res. 2010, 49, 23–30. [Google Scholar] [CrossRef]
- vanEngelsdorp, D.; Traynor, K.S.; Andree, M.; Lichtenberg, E.M.; Chen, Y.; Saegerman, C.; Cox-Foster, D.L. Colony collapse disorder (CCD) and bee age impact honey bee pathophysiology. PLoS ONE 2017, 12, e0179535. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, S.; Jung, C.; Burgett, M. Acute oral toxicity of neonicotinoid insecticides to four species of honey bee Apisflorea, A. cerana, A. mellifera, and A. dorsata. J. Apic. 2016, 31, 51–58. [Google Scholar]
- Highfield, A.C.; Nagar, A.E.; Mackinder, L.C.M.; Noel, L.M.-L.J.; Hall, M.J.; Martin, S.J.; Schroeder, D.C. Deformed wing virus implicated in overwintering honeybee colony losses. Appl. Environ. Microbiol. 2009, 75, 7212–7220. [Google Scholar] [CrossRef]
- Staveley, J.P.; Law, S.A.; Fairbrother, A.; Menzie, C.A. A causal analysis of observed declines in managed honey bees (Apis mellifera). Hum. Ecol. Risk Assess. 2014, 20, 566–591. [Google Scholar] [CrossRef]
- Doke, M.A.; Frazier, M.; Grozinger, C.M. Overwintering honey bees: Biology and management. Curr. Opin. Insect Sci. 2015, 10, 185–193. [Google Scholar] [CrossRef]
- Genersch, E.; Ohe, W.V.D.; Kaatz, H.; Schroeder, A.; Otten, C.; Buchler, R.; Berg, S.; Ritter, W.; Muhlen, W.; Gisder, S.; et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Lee, J.; Kang, J.; Lee, S.; Park, H.J.; Nam, J.; Jung, C. Impact of ambient temperature variability on the overwintering failure of honeybees in South Korea. J. Apic. 2022, 37, 331–347. [Google Scholar]
- Johnson, R.M.; Ellis, M.D.; Mullin, C.A.; Frazier, M. Pesticides and honey bee toxicity—USA. Apidologie 2010, 41, 312–331. [Google Scholar] [CrossRef]
- Calatayud-Vernich, P.; Calatayud, F.; Simo, E.; Suarez-Varela, M.M.; Pico, Y. Influence of pesticide use in fruit orchards during blooming on honeybee mortality in 4 experimental apiaries. Sci. Total Environ. 2016, 541, 33–41. [Google Scholar] [CrossRef]
- McArt, S.H.; Fersch, A.A.; Milano, N.J.; Truitt, L.L.; Boroczky, K. High pesticide risk to honey bees despite low focal crop pollen collection during pollination of a mass blooming crop. Sci. Rep. 2017, 7, 46554. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Yuan, X.; Kim, M.; Kyung, K.S.; Noh, H.H. Monitoring and risk analysis of residual pesticides drifted by unmanned aerial spraying. Sci. Rep. 2023, 13, 10834. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhu, Y.C.; Adamczyk, J. Responses of honey bees to lethal and sublethal doses of formulated clothianidin alone and mixtures. J. Econ. Entomol. 2018, 111, 1517–1525. [Google Scholar] [CrossRef]
- Gross, M. EU ban puts spotlight on complex effects of neonicotinoids. Curr. Biol. 2013, 23, R462–R464. [Google Scholar] [CrossRef]
- Epstein, Y.; Chapron, G.; Verheggen, F. What is an emergency? Neonicotinoids and emergency situations in plant protection in the EU. Ambio 2022, 51, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.C.; Scott-Dupree, C.D. Exposure to clothianidin seed-treated canola has no long-term impact on honey bees. Ecotoxicology 2007, 100, 765–772. [Google Scholar] [CrossRef]
- Choi, Y.; Kwon, C.; Yun, T.; Lee, Y. Persistence of the insecticide clothianidin in paddy and upland soils. Korean J. Environ. Agric. 2014, 33, 290–297. [Google Scholar] [CrossRef]
- Kim, S.K.; Seong, M.; Lee, S.H. The effects of Flupyradifurone exposure on honey bee physiology. J. Apic. 2023, 38, 33–40. [Google Scholar]
- Prisco, G.D.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2018, 110, 18466–18471. [Google Scholar] [CrossRef] [PubMed]
- Tison, L.; Rößner, A.; Gerschewski, S.; Menzel, R. The neonicotinoid clothianidin impairs memory processing in honey bees. Ecotox. Environ. Saf. 2019, 180, 139–145. [Google Scholar] [CrossRef]
- Ministry of Environment. Environmental Geographic Information Service. Available online: https://egis.me.go.kr/ (accessed on 2 October 2023).
- QGIS Development Team. QGIS Geographic Information Systems. Available online: https://www.qgis.org (accessed on 15 September 2023).
- Chang, N.M.; Rim, Y.D. Morphological Studies on the Pollen of Flowering Plants in Korea; Seoul National University Press: Seoul, Republic of Korea, 1979. [Google Scholar]
- Delaplane, K.S.; Van Der Steen, J.; Guzman-Novoa, E. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Cho, S.; Lee, S.H.; Kim, S. Determination of the optimal maturation temperature for adult honey bee toxicity testing. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 257, 109359. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 3 September 2023).
- Orcic, S.M.; Celic, T.V.; Purac, J.S.; Vukasinovic, E.L.; Kojic, D.K. Acute toxicity of sublethal concentrations of thiacloprid and clothianidin to immune response and oxidative status of honey bees. Apidologie 2022, 53, 50. [Google Scholar] [CrossRef]
- Iwasa, T.; Motoyama, N.; Ambrose, J.T.; Roe, R.M. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 2004, 25, 371–378. [Google Scholar] [CrossRef]
- Ulziibayar, D.; Jung, C. Comparison of acute toxicity of different groups of pesticides to honey bee workers (Apis mellifera L.). J. Apic. 2019, 34, 305–313. [Google Scholar] [CrossRef]
- Williams, G.R.; Troxler, A.; Retschnig, G.; Roth, K.; Yanez, O.; Shutler, D.; Neumann, P.; Gauthier, L. Neonicotinoid pesticides severely affect honey bee queens. Sci. Rep. 2015, 5, 14621. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Buchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, N.; Samson-Robert, O.; Sood, K.; Patel, H.S.; Malena, D.A.; Gajiwala, P.H.; Maciukiewicz, P.; Fournier, V.; Zayed, A. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 2017, 365, 1395–1397. [Google Scholar] [CrossRef]
- Tosi, S.; Burgio, G.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 2017, 7, 1201. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Demares, F.J.; Nicolson, S.W.; Medrzycki, P.; Pirk, C.W.W.; Human, H. Effects of a neonicotinoid pesticide on thermoregulation of African honey bees (Apis mellifera scutellate). J. Insect Physiol. 2016, 93, 56–63. [Google Scholar] [CrossRef]
- Meikle, W.G.; Corby-Harris, V.; Carroll, M.J.; Weiss, M.; Snyder, L.A.; Meador, C.A.D.; Beren, E.; Brown, N. Exposure to sublethal concentrations of methoxyfenozide disrupts honey bee colony activity and thermoregulation. PLoS ONE 2019, 14, e0204635. [Google Scholar] [CrossRef]
- Fahrenholz, L.; Lamprecht, I.; Schricker, B. Thermal investigations of a honey bee colony: Thermoregulation of the hive during summer and winter and heat production of members of different bee castes. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1989, 159, 551–560. [Google Scholar] [CrossRef]
- Chou, S.; Prince, T.; Gong, J.; Calderwood, S.K. mTOR is essential for the proteotoxic stress response, HSF1 activation, and heat shock protein synthesis. PLoS ONE 2012, 7, e39679. [Google Scholar] [CrossRef]
- Verghese, J.; Abrams, J.; Wang, Y.; Morano, K.A. Biology of the heat shock response and protein chaperone: Budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol. Mol. Biol. Rev. 2012, 76, 115–158. [Google Scholar] [CrossRef]
- Amdam, G.V.; Omholt, S.W. The hive bee to forager transition in honeybee colonies: The double repressor hypothesis. J. Theor. Biol. 2003, 223, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Seehuus, S.; Norberg, K.; Gimsa, U.; Amdam, G.V. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef]
- Gregorc, A.; Alburaki, M.; Sampson, B.; Knight, P.R.; Adamczyk, J. Toxicity of selected acaricides to honey bees (Apis mellifera) and varroa (Varroa destructor Anderson and Trueman) and their use in controlling varroa within honey bee colonies. Insects 2018, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.; Lee, J.H.; Han, S.H.; Lee, S.H. Differential expression of acetylcholinesterase 1 in response to various stress factors in honey bee workers. Sci. Rep. 2019, 9, 10342. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive markers of honey bee colony collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef]
- Even, N.; Devaud, J.; Barron, A.B. General stress responses in the honey bee. Insects 2012, 3, 1271–1298. [Google Scholar] [CrossRef] [PubMed]
- Alptekin, S.; Bass, C.; Nicholls, C.; Paine, M.J.I.; Clark, S.J.; Field, L.; Moores, G.D. Induced thiacloprid insensitivity in honeybees (Apis mellifera L.) is associated with up-regulation of detoxification genes. Insect Mol. Biol. 2016, 25, 171–180. [Google Scholar] [CrossRef]
- Wu, M.; Chang, Y.; Lu, K.; Yang, E. Gene expression changes in honey bees induced by sublethal imidacloprid exposure during the larval stage. Insect Biochem. Mol. Biol. 2017, 88, 12–20. [Google Scholar] [CrossRef]
- Belzunces, L.; Tchamitchian, S.; Brunet, J. Neural effects of insecticides in the honey bee. Apidologie 2012, 43, 348–370. [Google Scholar] [CrossRef]
- Gauthier, M.; Aras, P.; Paquin, J.; Boily, M. Chronic exposure to imidacloprid or thiamethoxam neonicotinoid causes oxidative damages and alters carotenoid-retinoid levels in caged honey bees (Apis mellifera). Sci. Rep. 2018, 8, 16274. [Google Scholar] [CrossRef] [PubMed]
- Chaimanee, V.; Evans, J.D.; Chen, Y.; Jackson, C.; Pettis, J.S. Sperm viability and gene expression in honey bee queens (Apis mellifera) following exposure to the neonicotinoid insecticide imidacloprid and the organophosphate acaricide coumaphos. J. Insect Physiol. 2016, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
| Collecting Days | Wanju (Control) | Gimje | Cheongyang |
|---|---|---|---|
| Clothianidin | |||
| 30 June | <LOQ | <LOQ | <LOQ |
| 17 August | <LOQ | 2.1 ± 1.51 | 1.3 ± 1.07 |
| 28 August | <LOQ | 1.5 ± 1.26 | 0.9 ± 0.64 |
| Flubendiamide | |||
| 30 June | <LOQ | <LOQ | <LOQ |
| 17 August | <LOQ | 1.8 ± 1.35 | 3.3 ± 0.71 |
| 28 August | <LOQ | 0.5 ± 0.73 | 2.5 ± 1.89 |
| Effect | Variable * | F Value | df | p Value |
|---|---|---|---|---|
| Location | A | 2.13 | 2 | 0.12 |
| L | 1.00 | 0.37 | ||
| C | 0.89 | 0.41 | ||
| Date | A | 1.59 | 9 | 0.12 |
| L | 1.76 | 0.13 | ||
| C | 1.80 | 0.07 | ||
| Location:Date | A | 0.47 | 18 | 0.97 |
| L | 1.21 | 0.26 | ||
| C | 0.54 | 0.94 |
| Wanju | Gimje | Cheongyang | χ2 | p | |
|---|---|---|---|---|---|
| Gimje 1st spray (8 August) | |||||
| Adult bee | −951.9 ± 942.4 b | 1456.7 ± 305.1 a | 383.3 ± 430.9 b | 7.2417 | 0.0268 |
| Larvae | 483.2 ± 1117.9 a | 650.0 ± 656.5 a | 783.3 ± 665.6 a | 0.1260 | 0.9389 |
| Capped brood | −666.7 ± 302.5 a | 216.7 ± 675.1 a | −150.0 ± 569.0 a | 0.8573 | 0.6514 |
| Gimje 2nd spray (21 August) | |||||
| Adult bee | 1552.5 ± 782.2 a | −172.5 ± 402.7 a | 325.8 ± 657.5 a | 1.9084 | 0.3851 |
| Larvae | −166.7 ± 892.1 a | −350.0 ± 592.9 a | 1550.0 ± 1506.8 a | 0.5743 | 0.7504 |
| Capped brood | 1633.3 ± 1027.0 a | 1200.0 ± 703.2 a | 3433.3 ± 1430.0 a | 0.7932 | 0.6726 |
| Cheongyang 1st spray (14 August) | |||||
| Adult bee | −651.7 ± 788.2 a | 95.8 ± 724.5 a | 824.2 ± 464.3 a | 2.4993 | 0.2866 |
| Larvae | 483.3 ± 1117.9 a | 650.0 ± 656.5 a | 783.3 ± 665.6 a | 0.1260 | 0.9389 |
| Capped brood | −666.7 ± 302.5 a | 216.7 ± 675.1 a | −150.0 ± 569.0 a | 0.8573 | 0.6514 |
| Cheongyang 2nd spray (28 August) | |||||
| Adult bee | −38.3 ± 371.3 a | −766.7 ± 564.5 a | 268.3 ± 587.4 a | 1.7258 | 0.4219 |
| Larvae | 766.74 ± 1163.2 a | −16.7 ± 398.2 a | −2116.7 ± 940.3 a | 4.6433 | 0.0981 |
| Capped brood | −516.7 ± 784.0 a | 250.0 ± 273.6 a | 116.7 ± 899.2 a | 0.7142 | 0.6997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, M.; Kim, J.; Kim, D.; Lee, C.-H.; Akongte, P.N.; Oh, D.; Choi, Y.-S.; Park, B.-S. Effects of Clothianidin Pesticide Application on the Strength of Honey Bee Colonies and Stress-Related Genes in the Vicinity of Rice Fields in the Republic of Korea. Diversity 2023, 15, 1217. https://doi.org/10.3390/d15121217
Son M, Kim J, Kim D, Lee C-H, Akongte PN, Oh D, Choi Y-S, Park B-S. Effects of Clothianidin Pesticide Application on the Strength of Honey Bee Colonies and Stress-Related Genes in the Vicinity of Rice Fields in the Republic of Korea. Diversity. 2023; 15(12):1217. https://doi.org/10.3390/d15121217
Chicago/Turabian StyleSon, Minwoong, Jisoo Kim, Dongwon Kim, Chang-Hoon Lee, Peter Njukang Akongte, Daegeun Oh, Yong-Soo Choi, and Bo-Sun Park. 2023. "Effects of Clothianidin Pesticide Application on the Strength of Honey Bee Colonies and Stress-Related Genes in the Vicinity of Rice Fields in the Republic of Korea" Diversity 15, no. 12: 1217. https://doi.org/10.3390/d15121217
APA StyleSon, M., Kim, J., Kim, D., Lee, C.-H., Akongte, P. N., Oh, D., Choi, Y.-S., & Park, B.-S. (2023). Effects of Clothianidin Pesticide Application on the Strength of Honey Bee Colonies and Stress-Related Genes in the Vicinity of Rice Fields in the Republic of Korea. Diversity, 15(12), 1217. https://doi.org/10.3390/d15121217







