Abstract
Bats are reservoirs for various pathogens, including SARS-like coronaviruses (CoVs). Understanding the distribution of bat species is crucial to identifying areas where viral spillover from bats to other animals or humans might occur. In this study, we performed species distribution modeling to predict suitable habitats within Thailand under current and predicted future climate conditions for Rhinolophus acuminatus, a bat species that has been found to host SARS-CoV-2-related viruses. Our assessment of current conditions revealed that temperature seasonality had the greatest impact on habitat suitability and that suitable habitats were primarily restricted to the southern and eastern regions of Thailand. Over time, the projections indicate a diminishing availability of suitable habitats, suggesting a potential trend toward migration into neighboring areas. We next combined modeled bat distribution with urbanization data to estimate regions in Thailand where bat–human interactions might occur. The resulting map highlighted regions of heightened interaction risk, encompassing approximately 46,053.94 km2 across 58 provinces and representing approximately 9.24% of Thailand’s total area. These risk concentrations are prominently situated in the southern, central, and eastern Thai regions, with extensions into neighboring border areas. Our findings will significantly aid future risk surveillance efforts and enhance the effectiveness of monitoring and managing emerging diseases within the country and in contiguous regions.
1. Introduction
The ongoing COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has profoundly affected millions of people worldwide [1,2].
It has caused significant social, economic, and health-related damage, and over six million fatalities. This pandemic has disrupted economies leading to reduced productivity across various sectors and GDPs globally, as well as extensive job losses. This has exacerbated the strain on human welfare and adversely affected mental health [3,4].
Bat species, and particularly horseshoe bat species (Rhinolophidae: Rhinolophus), have been identified as natural reservoirs of coronaviruses [5,6]. Phylogenetic analyses strongly suggest that SARS-CoV-2 originates from bat hosts [1,2,7,8]. Rhinolophus species, such as R. affinis (virus RaTG13: Yunnan, China), R. malayanus (virus RmYN02: Yunnan, China), R. pusillus (virus RpYN06: Yunnan, China), and R. shameli (virus RshSTT200: Cambodia), carry coronaviruses that bear genetic similarities to SARS-CoV-2 [8,9,10]. These findings highlight the possible existence of coronaviruses that can infect humans in Southeast Asia, including Thailand [11]. While direct transmission of the SARS-CoV-2 virus from bats to humans has not been demonstrated [12,13], continuous surveillance and research on potential hosts are essential for understanding the dynamics of animal-to-human disease transmission (“spillover”).
Recently, a novel SARS-CoV-2-related coronavirus (SC2r-CoV), RacCS203, was detected in a population of R. acuminatus (Figure 1) in eastern Thailand [14]. Commonly known as the acuminate horseshoe bat, R. acuminatus is native to various regions of Southeast Asia, including Thailand, Malaysia, Indonesia, and the Philippines. This insectivorous species plays a crucial role in ecosystems by controlling insect populations and contributing to biodiversity [15,16,17]. R. acuminatus is listed as “Least Concern” on the IUCN Red List of Threatened Species (last assessed in 2018) and is protected by law in Thailand [17,18].

Figure 1.
Rhinolophus acuminatus from eastern Thailand. Photo by N. Sirichan.
Understanding the distribution of reservoir hosts is crucial for predicting and preventing zoonotic spillover. The likelihood of zoonotic transmission is determined by several factors, including the presence and propagation of the virus in the host species [19,20]. Monitoring host populations, identifying their habitats, and studying their interactions with humans and the environment are crucial to mitigating the risk of zoonotic transmission. Species distribution models (SDMs) are invaluable tools for predicting regions of environmental suitability for wildlife species and for assessing potential distribution changes in response to environmental alterations [21,22,23]. The spatial assessment of habitat associations can substantially inform wildlife disease surveillance.
Disease outbreaks often hinge on specific drivers, such as climate patterns, economic development, human–wildlife interactions, and ecosystem changes, all of which can affect ecological dynamics, increase host vulnerability, and facilitate pathogen transmission [24,25]. Emerging infectious disease events are often associated with urban land use; this connection is attributed to the human–ecosystem interaction, characterized by heightened environmental encroachment and changes in land use [26,27]. In particular, the urbanization of previously forested areas can increase wildlife–human interactions, potentially facilitating the transmission of zoonotic diseases. In urban environments in Thailand, activities such as hunting and consuming bats, collecting or cleaning bat guano, and encountering bat carcasses facilitate close contact between bats and humans [28]. Additionally, the use of caves in Thailand for tourism, religious practices, and bat guano collection increases the proximity of humans to bats. It is vital to manage these potential risks to effectively mitigate zoonotic disease transmission.
In this study, we used species distribution modeling to map the potential geographic distribution of R. acuminatus under current and predicted future climate conditions. We further identified areas with both high habitat suitability for R. acuminatus and high urbanization, thereby providing valuable insights into regions where bat–human contact, and potential viral transmission, could occur. These findings may aid in the prioritization of surveillance and control efforts and provide a reference for effective public health strategies for mitigating zoonotic disease transmission.
2. Materials and Methods
Our objective was to map regions in Thailand of potential contact between humans and R. acuminatus, a species in which a novel SC2r-CoV was detected [14]. This process included the construction of an SDMs using species occurrence data and environmental variables to predict habitat suitability for R. acuminatus across Thailand. Next, we created an urbanization layer to evaluate the potential impact on human–bat interactions, generated a risk map by overlaying bat habitat suitability and urbanization, and provided a visual representation of the potential risk areas (Figure 2). We provide further details on this process below.
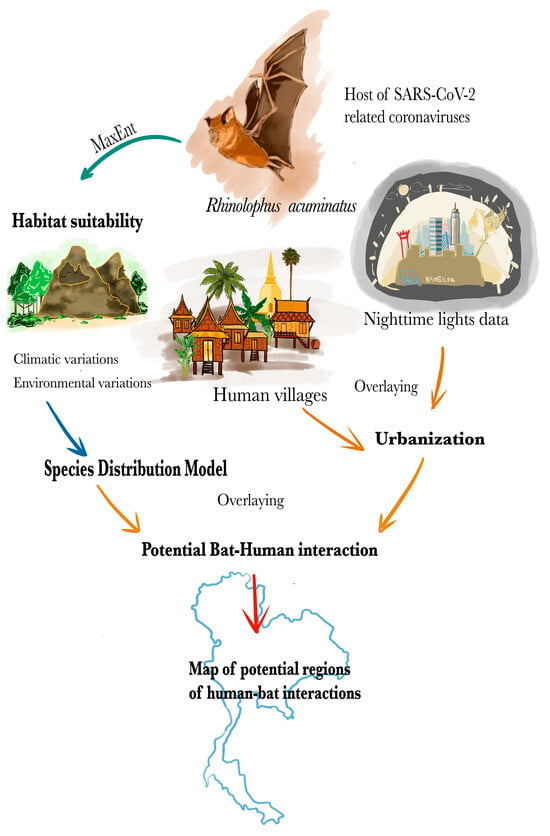
Figure 2.
Species distribution modeling was used to determine potential regions where humans might interact with Rhinolophus acuminatus, a bat species identified as a possible host for SC2r-CoVs in Thailand. The green arrow displays the modeling approach used, which is MaxEnt. The blue arrow indicates the determination of variables related to the ecological niche of the bat. The orange arrow represents potential transmission pathways, whereas the red arrow displays the mapping of potential regions of human interaction with R. acuminatus.
2.1. Study Area
According to the IUCN Red List, R. acuminatus is widely distributed across various regions of Thailand, with a notable presence in the southern, central, and eastern parts of the country [18] (Figure 3). The region has a tropical climate and is seasonally influenced by monsoon winds [29,30]. The annual average temperature is 26.3 °C, with a seasonal variation of 5.7 °C. The highest rainfall occurs in August and September, with approximately 255 mm recorded during these months [31].
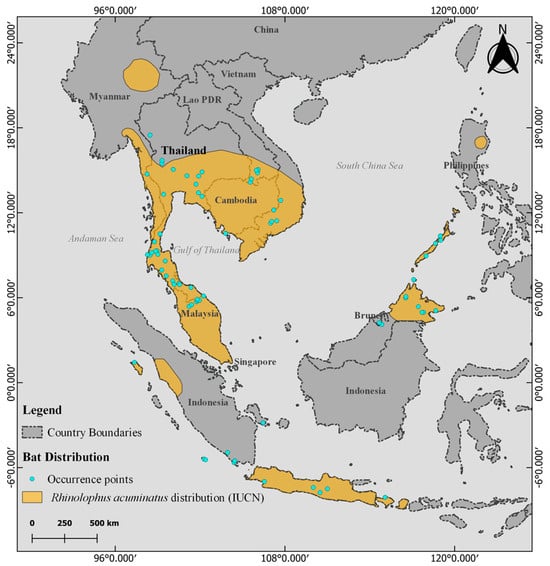
Figure 3.
The IUCN Red List distribution of Rhinolophus acuminatus (yellow) is overlaid on a map of Thailand and surrounding countries. The turquoise dots denote R. acuminatus occurrence locations sourced from the literature and used in model construction.
Thailand, which comprises 47% agricultural land, has undergone remarkable social and economic development, shifting from low-income to upper-middle-income status [32]. The population is estimated to be ~71.8 million people in mid-2023 [33].
2.2. Data Sources
2.2.1. Bat Occurrence Data
To inform the SDMs of R. acuminatus, we searched for known occurrence records in Southeast Asia: Thailand, Lao PDR, Myanmar, Vietnam, Cambodia, Malaysia, Indonesia, Brunei, and the Philippines. In total, 101 occurrence records were obtained from secondary sources, including the Global Biodiversity Information Facility database (75 records) [34], Cave-Dwelling Bats of Thailand (1 record) [18], Wildlife Yearbook by the Department of National Parks, Wildlife and Plant Conservation (11 records) [35], Horseshoe Bats of the World (Chiroptera: Rhinolophidae) (4 records) [15], and 10 records from previous studies [14,36,37,38,39,40]. To address potential sampling bias, we spatially thinned species occurrence records using the spThin package [41] in the R statistical environment (v4.2.3) [42]; after thinning, 71 occurrence records remained (Table S1).
2.2.2. Environmental Variables
To map the current and future distributions of the target species, we considered environmental variables that are thought to significantly affect bats [43]. Because R. acuminatus inhabit lowland dipterocarp forests, primary and secondary tropical forests, urban areas, caves, tree hollows, and buildings [15,16], we incorporated elevation, slope, land cover (19 categories), forest canopy cover height, and cave presence as predictor variables [21,44]. Elevation and slope data were obtained from the Shuttle Radar Topography Mission 90-m Digital Elevation Database v4.1. Land cover data and global forest canopy height data were obtained from Global Land Analysis and Discovery [45,46]. Cave locations were downloaded from the Caves and Caving in Thailand websites [47] and Global database for bats in karsts and caves [48], which were then customized to fit Thailand and Southeast Asia. The Euclidean distance (km) from each cave point vector was calculated to create a distance raster using ArcGIS.
Because bats may be sensitive to climate change, which could lead to shifts in habitat requirements and distribution patterns [49,50], our analysis also included climatic variables. Bioclimatic variable layers (n = 19) for current conditions (ca. 1970–2000) and future scenarios were obtained from the WorldClim website v2.1 [51]. Representative Concentration Pathway 4.5 (RCP4.5) was utilized to predict the climate for 2050 (representing 2041–2060) and 2070 (representing 2061–2080) using the HadGEM2-ES model, which was created by the Met Office Hadley Centre for Coupled Model Intercomparison Project Phase 5 (CMIP5) centennial simulations. HadGEM2-ES was selected for its detailed representation of climatic variation, which makes it suitable for mapping species distributions [44]. We excluded four data layers (BIO8: mean temperature in the wettest quarter; BIO9: mean temperature in the driest quarter; BIO18: precipitation in the warmest quarter; and BIO19: precipitation in the coldest quarter) that demonstrated inconsistent climatic variations among neighboring pixels because they combined information on temperature and precipitation [52,53]. This left twenty layers remaining (Table S2). All spatial layers were clipped to match the geography of Thailand and Southeast Asia. All variable grids utilized the WGS84 datum and latitude–longitude coordinate reference system at the highest resolution (30 arcsec, ~1 km) [54].
2.2.3. Urbanization
The spread of zoonotic pathogens to humans is often triggered by increased interactions between humans and wildlife [19] and the encroachment of urban areas onto wildlife habitats [25]. Therefore, by merging data on human settlement locations with nighttime light data and urbanization dynamics [55,56], we aimed to identify regions where bats and humans might be most likely to interact [57,58,59], facilitating an evaluation of the impact of urban development on potential disease transmission pathways [24,25]. We used data on village locations provided by the Thailand Land Development Department to compute the Euclidean distances (km) from these locations. We procured annual global Visible and Infrared Imaging Suite (VIIRS) nighttime light data for 2021 from the Earth Observation Group [60]. The degree of urbanization is often correlated with the intensity of light, symbolizing the extent of economic activity occurring during nighttime [61]. After clipping these data to align with Thailand’s boundaries, we obtained a representation of urbanization within the country. We then re-projected the data into grid cells of the same resolution and applied the classification method reported by Levin and Zhang [62] to categorize the light values into seven classes: 2, 5, 10, 25, 50, 100, and 250 nanoWatts/cm2/sr.
2.3. Methods
2.3.1. Species Distribution Modeling
To ascertain the distribution of R. acuminatus, we performed species distribution modeling using the maximum entropy method (MaxEnt v3.4.1) [63]. This method employs a probability density estimation approach [64,65]. Bat occurrence data and environmental variables were used as model inputs to predict the current and future distribution ranges of R. acuminatus. We applied a threshold approach to analyze the SDM results, maximizing test sensitivity and specificity. The final models were constructed using a complete set of species occurrence records and selected parameterizations. Test points were then randomly selected from the species’ presence locations using a “random test percentage” option, with the entered value of “25” instructing the program to allocate 25% of the occurrence records for testing [66]. Model performance was evaluated using 10 replicates with cross-validation, 500 iterations, and 10,000 background data added from samples for predictive accuracy testing [44]. Model accuracy was determined using the area under the curve (AUC) of the receiver operating characteristic (ROC) plot. The AUC value, which ranges from 0 to 1, was computed based on comparisons of the model predictions of species occurrence and actual occurrence data. AUC = 0.5 indicates an accuracy level no higher than random chance, whereas AUC ≥ 0.7 indicates a well-fitted model with high predictive accuracy [67]. We used permutation importance as an indicator of variable importance [66]. We reclassified the model output (a raster of bat habitat suitability values ranging from 0 to 1) into four habitat suitability classes: unsuitable (0–0.2), less suitable (0.2–0.4), moderately suitable (0.4–0.6), and highly suitable (0.6–1.0) [49]. After processing datasets to obtain a consistent resolution grid and projection, ensuring compatibility for the overlay method, we overlaid the datasets to capture urbanization levels, where higher levels were interpreted as greater potential for human–bat interaction [68,69] and disease transmission.
2.3.2. Mapping the Potential Risk of Human–Bat Interactions Based on Bat Distribution
To generate a risk map that explored the potential for interactions between humans and R. acuminatus, we employed bat habitat suitability and urbanization layers. After generating the raster of bat habitat suitability (under current climate conditions) and then customizing it to fit Thailand, we overlaid and masked the habitat suitability data with the urbanization layer, representing areas of human development and activity. This process allowed us to evaluate the potential risk levels related to human–bat interactions by highlighting areas where human activities intersect with suitable bat habitats. To classify the risk levels, we implemented Jenks natural break classification [70] on the combined data, which yielded a map consisting of three levels (low, medium, and high) for potential interaction. Low-risk areas show a minimal overlap of human activities and suitable bat habitats, medium-risk areas display medium overlap, and high-risk areas indicate substantial overlap.
3. Results
3.1. Evaluation of the Model Accuracy and Variable Contribution
The R. acuminatus model yielded high accuracy. The average test AUC values for the replicate run were 0.83 ± 0.08, 0.83 ± 0.05, and 0.82 ± 0.06 for the current, 2050, and 2070 distributions, respectively. These values indicate good species distribution prediction accuracy for both present and future climate scenarios, as well as good model reliability for estimating R. acuminatus habitat suitability.
Our study revealed that several environmental variables contributed highly to the model, as assessed by permutation importance. Under current climate conditions, temperature seasonality (BIO4) had the highest permutation importance (41.1%), followed by distance from cave locations (14.7%), precipitation in the driest month (BIO14, 10.5%), and annual temperature range (BIO7, 8.4%) (Table 1). Temperature seasonality (2050: 35.0%; 2070: 36.2%) and distance from cave locations (2050: 13.3%; 2070: 16.5%) also had the two highest permutation importance values for predicted future climate scenarios (Table S3).

Table 1.
Permutation importance of the variables in the species distribution model for Rhinolophus acuminatus in Thailand.
3.2. Habitat Suitability Modeling of Rhinolophus acuminatus
Climate variables under current and future conditions were employed to assess the suitability levels of the distribution. Regions exhibiting high suitability for R. acuminatus were constrained to southern Thailand and certain areas of southern Myanmar. Additionally, moderate suitability was observed in Malaysia, Cambodia, Vietnam, the Philippines, and specific parts of Indonesia (Figure S1).
The results of our assessment of habitat suitability for R. acuminatus in Thailand under current climate conditions are shown in Figure 4 and Table 2. In the current (present-day) scenario, almost all of southern Thailand was identified as highly suitable for the species, as were parts of the southeastern and southwestern borders and a discontinuous region in central Thailand. In total, these highly suitable regions accounted for 25.44% of the country’s total area. Additionally, 9.94% and 26.76% of the total area were classified as moderately and less suitable across central Thailand and some parts of lower northern and northeastern Thailand. The northern and northeastern regions were mostly unsuitable habitats for R. acuminatus (Figure 4a).
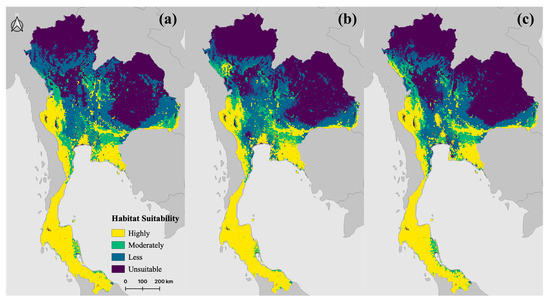
Figure 4.
Potential habitat suitability for Rhinolophus acuminatus in Thailand under (a) present conditions and future climate scenarios for (b) 2050 and (c) 2070.

Table 2.
The area and percent of habitat predicted to be unsuitable, less suitable, moderately suitable, or highly suitable for Rhinolophus acuminatus under current and future (2050 and 2070) climatic scenarios in Thailand.
In the 2050 scenario, there was a slight decrease in highly suitable areas, accounting for 25.43%. The coverage of moderately suitable areas decreased to 8.72%, and the coverage of less suitable areas decreased to 23.75%. Consistent with the current scenario, the northern and northeastern regions remained mostly unsuitable habitats for R. acuminatus (Figure 4b).
In the 2070 scenario, the proportion of highly suitable areas was predicted to decrease compared to both the current and 2050 scenarios, covering 24.921%. The moderately suitable areas increased to 8.87%, and the less suitable areas decreased to 21.76%. As in the previous scenarios, the northern and northeastern regions were unsuitable habitats for R. acuminatus (Figure 4c).
3.3. Mapping the Potential Risk of Human–Bat Interaction
To evaluate the potential risk of contact between R. acuminatus and humans, we generated spatial risk maps by overlaying the species distribution model with urbanized areas based on the current habitat suitability. Areas of a high risk of human–bat interaction have been identified in regions exhibiting high human development and urbanization. These high-risk areas were identified across 56 provinces, 401 districts, and 1485 subdistricts, primarily in southern, central, western, and eastern Thailand. These high-risk regions cover an area of approximately 46,053.94 km2 and represent 9.24% of the country’s total area (Figure 5).
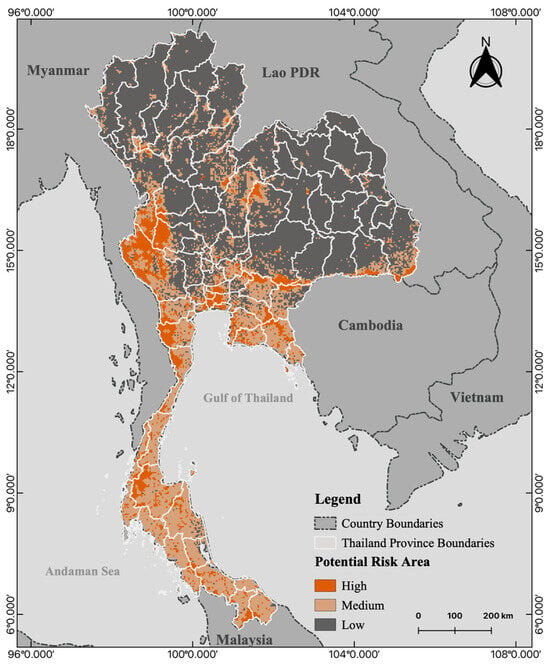
Figure 5.
Geographical map exhibiting the possible risk levels (high, medium, low) for interactions between Rhinolophus acuminatus and humans throughout Thailand.
4. Discussion
In this study, we used species distribution modeling to map the potential geographic distribution of R. acuminatus under current and future climate conditions. We found that temperature seasonality was the most important model variable, indicating that species distribution is influenced by temperature changes throughout the year. R. acuminatus may exhibit preferences for specific temperature ranges or demonstrate adaptations to cope with temperature fluctuations [49,71]. This reliance on temperature dynamics may be closely tied to the annual temperature range. The annual temperature range refers to the difference between the maximum and minimum temperatures recorded in a specific location over the course of a year. Temperature plays a vital role in bat distribution, as it affects their thermal tolerance, as their body temperature is influenced by the external environment [72]. The behavior of bats seeking cooler roosting sites to avoid overheating [73] is closely linked to the second most important variable, which is distance from caves. Caves, as natural structures, provide a reliable and long-lasting habitat, offering bats a stable microclimate. This environment safeguards them from harsh weather and potential predators. The enduring qualities of caves, including thermal stability and humidity, benefit bats by shielding them from adverse weather conditions and reducing the risk of dehydration [74,75,76].
Precipitation in the driest month, which measures the variability in precipitation during the driest month of the year in a specific location, is linked to the availability of water sources. The activity of insectivorous bats is positively correlated with water sources; this implies that a reliable water source could enhance habitat suitability for bats [77,78]. Access to water resources is crucial for bats, especially for successful reproduction and influencing factors, such as food abundance [44,79,80]. A study by Prajakjitr and Bumrungsri [81] in Thailand revealed that insects in the orders of Hymenoptera and Coleoptera are the most frequently consumed in the diet of R. acuminatus.
The current study revealed that under current climate conditions, regions in the southern, eastern, and western parts of Thailand are predicted to be highly suitable. However, there is potential concern regarding species habitat suitability in future climate change scenarios. The projections indicate a decrease in suitable habitat areas, predominantly in the western regions, especially the central and some parts of the eastern regions. There is a noticeable trend of shifting and transitioning toward areas categorized as moderately suitable, notably along the border of the country in the upper western region and the lower part of northeastern Thailand. This suggests a potential migration or population exchange with neighboring countries. This observation aligns with previous studies highlighting bats’ adaptive responses, including range shifts in potential habitats [44,50]. Similarly, fruit bats responding to climate change are anticipated to migrate to more suitable areas, potentially expanding into regions not historically inhabited by those species [82].
In addition to changing climate conditions, habitat loss due to deforestation has been occurring in Thailand, as indicated by a report from the Royal Forest Department and Global Forest Watch. The data reveal a troubling loss of 5.5% of humid primary forests in Thailand over the period from 2002 to 2022 [83,84]. Notably, the top five regions of tree cover loss (Surat Thani, Nakhon Si Thammarat, Songkhla, Krabi, and Trang) were all in the south of Thailand, a region with high predicted habitat suitability for R. acuminatus. Future studies could focus on the potential impact of forest loss to R. acuminatus or other forest-dwelling species. Deforestation is a result of development and urbanization, and it is a phenomenon evident in the ongoing increase in urbanization in Thailand, as indicated by Urban Population data reported by the World Bank. A study conducted by Bhandari et al. [85] emphasized the expansion of built-up spaces across Thai provinces and regions, leading to an increase in urban compactness. This potential habitat shrinkage raises concerns regarding increased human–bat interactions, primarily driven by escalating urbanization and the consequent loss of natural habitats [55]. Furthermore, some species of insectivorous bats forage near streetlamps that attract insects [86], thereby increasing the likelihood of interactions and crossings with humans.
Our analysis identified high-risk areas of human–bat interactions in southern and eastern Thailand. Eastern Thailand has previously been identified as a region where diverse coronaviruses have been sampled from bats [87]. Notably, the high-risk areas also extended near the borders of Thailand, particularly in the southern region neighboring Malaysia, certain parts of the eastern region connected with Cambodia, and some regions in the west connected with Myanmar. This highlights the need for increased attention, surveillance, and management strategies across countries to mitigate the risk of potential future zoonotic transmission. A comprehensive and coordinated approach to disease surveillance and management within Thailand and neighboring countries will be crucial.
Moreover, it is crucial to consider the possibility of “spillback” events, also known as reverse zoonosis, where diseases may be transmitted from humans to bats or free-ranging wildlife populations [88,89]. For example, the World Organization for Animal Health (OIE) reports evidence of SARS-CoV-2 spillover from infected humans to numerous animal species, including dogs, cats, gorillas, leopards, lions, minks, otters, pumas, and tigers. Thailand has also documented cases of SARS-CoV-2 infection in dogs and cats [90].
Finally, the importance of bat conservation in maintaining biodiversity and ecological balance cannot be overstated. Bats play critical roles as pollinators, seed dispersers, and insect controllers in ecosystems [91]. Protecting their habitats and ensuring their survival is essential not only for bat populations but also for overall ecosystem health. By acknowledging the significance of bats and their ecological contributions, we can promote their conservation along with public health initiatives.
While our study provides insight into habitat suitability and potential human–bat contact in Thailand, it has limitations. We found relatively few occurrence points for R. acuminatus in the literature; more field sampling to identify additional locations would likely improve future species distribution modeling efforts. The risk map that we generated lacks specifics on human activities influencing interactions. Essential actions include behavioral research and monitoring known bat roosting sites. Risk levels are relative, emphasizing potential rather than actual interaction frequencies. Examining public health records is valuable, but our study does not directly address health outcomes or pathogen prevalence. Addressing these limitations requires ongoing research and cautious interpretation.
5. Conclusions
This study provides valuable insight into habitat suitability for R. acuminatus in Thailand and the potential risks of human contact with this species, particularly in relation to possible pathogen transmission to humans. Our species distribution model and the environmental variables that we identified as important correlate to habitat suitability for R. acuminatus and will elucidate the ecology and potential impacts on the future habitat distribution of this species. Our map of the areas with a high predicted risk of human–bat interaction highlights the need for targeted interventions and strategies to mitigate zoonotic disease threats and aid in the surveillance of potential zoonotic disease outbreaks in Thailand. This information may be instrumental in formulating targeted interventions and strategies to reduce the risk of zoonotic spillover and safeguard human health.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d15121216/s1, Table S1: Occurrence locations of Rhinolophus acuminatus used for the modeling species distribution models (SDM) after spatial thinning; Table S2: Final climate variables and environmental variables that were used to construct species distribution models (SDM) for R. acuminatus; Table S3: Permutation importance (PI) values of variables used in the species distribution model for Rhinolophus acuminatus in Thailand under current and future climate scenarios based on the HadGEM2-ES model; Figure S1: Predicted habitat suitability (as determined by species distribution modeling) for Rhinolophus acuminatus within Thailand, Lao PDR, Myanmar, Vietnam, Cambodia, Malaysia, Indonesia, and the Philippines under (a) present conditions and future climate scenarios for (b) 2050 and (c) 2070.
Author Contributions
Conceptualization, N.S. and P.D.; resources, C.A.S.; methodology, N.S., A.C. and C.A.S.; formal analysis, N.S.; writing—original draft, N.S., P.D. and A.C.; writing—review and editing, N.S., P.D., A.C., C.A.S., S.W. and K.S.; supervision, P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Kasetsart University through the Graduate School Fellowship Program. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U01AI151797. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated in this study are presented in the article and its Supplementary, Data from published sources are available in Supplementary Tables.
Acknowledgments
We wish to thank the various people who contributed to the data and provided valuable and constructive recommendations for this project, with special thanks to Umphornpimon Prayoon, Paanwaris Paansri, and Andaman Chankhao.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Origin of SARS-CoV-2. Available online: https://www.who.int/publications-detail-redirect/origin-of-sars-cov-2 (accessed on 1 December 2022).
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A Novel Coronavirus Outbreak of Global Health Concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 1 December 2022).
- Naseer, S.; Khalid, S.; Parveen, S.; Abbass, K.; Song, H.; Achim, M.V. COVID-19 Outbreak: Impact on Global Economy. Front. Public Health 2023, 10, 1009393. [Google Scholar] [CrossRef] [PubMed]
- Latinne, A.; Hu, B.; Olival, K.J.; Zhu, G.; Zhang, L.; Li, H.; Chmura, A.A.; Field, H.E.; Zambrana-Torrelio, C.; Epstein, J.H.; et al. Origin and Cross-Species Transmission of Bat Coronaviruses in China. Nat. Commun. 2020, 11, 4235. [Google Scholar] [CrossRef]
- Lytras, S.; Hughes, J.; Martin, D.; Swanepoel, P.; De Klerk, A.; Lourens, R.; Kosakovsky Pond, S.L.; Xia, W.; Jiang, X.; Robertson, D.L. Exploring the Natural Origins of SARS-CoV-2 in the Light of Recombination. Genome Biol. Evol. 2022, 14, evac018. [Google Scholar] [CrossRef]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats Are Natural Reservoirs of SARS-like Coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Zhou, H.; Ji, J.; Chen, X.; Bi, Y.; Li, J.; Wang, Q.; Hu, T.; Song, H.; Zhao, R.; Chen, Y.; et al. Identification of Novel Bat Coronaviruses Sheds Light on the Evolutionary Origins of SARS-CoV-2 and Related Viruses. Cell 2021, 184, 4380–4391.e14. [Google Scholar] [CrossRef]
- Delaune, D.; Hul, V.; Karlsson, E.A.; Hassanin, A.; Ou, T.P.; Baidaliuk, A.; Gámbaro, F.; Prot, M.; Tu, V.T.; Chea, S.; et al. A Novel SARS-CoV-2 Related Coronavirus in Bats from Cambodia. Nat. Commun. 2021, 12, 6563. [Google Scholar] [CrossRef]
- Hassanin, A.; Tu, V.T.; Curaudeau, M.; Csorba, G. Inferring the Ecological Niche of Bat Viruses Closely Related to SARS-CoV-2 Using Phylogeographic Analyses of Rhinolophus Species. Sci. Rep. 2021, 11, 14276. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Luk, H.K.H.; Wong, A.C.P.; Li, K.S.M.; Zhu, L.; He, Z.; Fung, J.; Chan, T.T.Y.; Fung, K.S.C.; Woo, P.C.Y. Possible Bat Origin of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg. Infect. Dis. 2020, 26, 1542–1547. [Google Scholar] [CrossRef]
- Letko, M.; Seifert, S.N.; Olival, K.J.; Plowright, R.K.; Munster, V.J. Bat-Borne Virus Diversity, Spillover and Emergence. Nat. Rev. Microbiol. 2020, 18, 461–471. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Tan, C.W.; Maneeorn, P.; Duengkae, P.; Zhu, F.; Joyjinda, Y.; Kaewpom, T.; Chia, W.N.; Ampoot, W.; Lim, B.L.; et al. Evidence for SARS-CoV-2 Related Coronaviruses Circulating in Bats and Pangolins in Southeast Asia. Nat. Commun. 2021, 12, 972. [Google Scholar] [CrossRef]
- Csorba, G.; Ujhelyi, P.; Thomas, N. Horseshoe Bats of the World (Chiroptera, Rhinolophidae); Alana Books: Shropshire, UK, 2003; ISBN 978-0-9536049-1-3. [Google Scholar]
- Burgin, C. Rhinolophus Acuminatus Peters 1871; Lynx Edicion: Barcelona, Spain, 2019. [Google Scholar] [CrossRef]
- Thong, V.D.; Thanh, H.T.; Soisook, P.; Csorba, G. The IUCN Red List of Threatened Species; IUCN Rhinolophus Acuminatus: Gland, Switzerland, 2019. [Google Scholar]
- Kanphan, S.; Wongwai, A.; Soisuk, P. Cave-Dwelling Bats of Thailand, 1st ed.; Wildlife Research Division, Department of National Parks, Wildlife and Plant Conservation: Bangkok, Thailand, 2015; ISBN 978-616-316-356-1. [Google Scholar]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to Zoonotic Spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Chies, J.A.B. Zoonotic Spillover: Understanding Basic Aspects for Better Prevention. Genet. Mol. Biol. 2021, 44, e20200355. [Google Scholar] [CrossRef]
- Jaberg, C.; Guisan, A. Modelling the Distribution of Bats in Relation to Landscape Structure in a Temperate Mountain Environment. J. Appl. Ecol. 2001, 38, 1169–1181. [Google Scholar] [CrossRef]
- Razgour, O.; Rebelo, H.; Di Febbraro, M.; Russo, D. Painting Maps with Bats: Species Distribution Modelling in Bat Research and Conservation. Hystrix Ital. J. Mammal. 2016, 27. [Google Scholar] [CrossRef]
- Peterson, A.T. (Ed.) Ecological Niches and Geographic Distributions; Monographs in Population Biology; Princeton Univ. Press: Princeton, NJ, USA, 2011; ISBN 978-1-4008-4067-0. [Google Scholar]
- Loh, E.H.; Zambrana-Torrelio, C.; Olival, K.J.; Bogich, T.L.; Johnson, C.K.; Mazet, J.A.K.; Karesh, W.; Daszak, P. Targeting Transmission Pathways for Emerging Zoonotic Disease Surveillance and Control. Vector Borne Zoonotic Dis. 2015, 15, 432–437. [Google Scholar] [CrossRef]
- Olson, S.H.; Benedum, C.M.; Mekaru, S.R.; Preston, N.D.; Mazet, J.A.K.; Joly, D.O.; Brownstein, J.S. Drivers of Emerging Infectious Disease Events as a Framework for Digital Detection. Emerg. Infect. Dis. 2015, 21, 1285–1292. [Google Scholar] [CrossRef]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Di Marco, M.; Breit, N.; Olival, K.J.; Daszak, P. Global Hotspots and Correlates of Emerging Zoonotic Diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and Disease Emergence: Dynamics at the Wildlife–Livestock–Human Interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef]
- Suwannarong, K.; Balthip, K.; Kanthawee, P.; Suwannarong, K.; Khiewkhern, S.; Lantican, C.; Ponlap, T.; Bupha, N.; Amonsin, A. Bats and Belief: A Sequential Qualitative Study in Thailand. Heliyon 2020, 6, e04208. [Google Scholar] [CrossRef]
- Thai Meteorological Department. Available online: http://www.aws-observation.tmd.go.th/main/main?menuId=&pageId= (accessed on 10 December 2022).
- Enfield, N.J.; Comrie, B. (Eds.) Mainland Southeast Asian Languages: State of the Art and New Directions. In Languages of Mainland Southeast Asia; De Gruyter: Berlin, Germany, 2015; pp. 1–28. ISBN 978-1-5015-0843-1. [Google Scholar]
- World Bank Group; Asian Development Bank. Climate Risk Country Profile: Thailand; World Bank: Washington, DC, USA, 2021. [Google Scholar]
- World Bank Climate Change Knowledge Portal. Available online: https://climateknowledgeportal.worldbank.org/ (accessed on 12 November 2023).
- World Population Prospects–Population Division–United Nations. Available online: https://population.un.org/wpp/ (accessed on 12 November 2023).
- GBIF.org. GBIF Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0001775-230918134249559 (accessed on 20 September 2023).
- Wildlife Yearbook. Available online: https://www.dnp.go.th/wildlife/indexpageall.htm (accessed on 10 December 2022).
- Sanborn, C.C. The Mammals of the Rush Watkins Zoological Expedition to Siam. Nat. Hist. Bull. Siam Soc. 1952, 15, 1–20. [Google Scholar]
- Hill, J.E. Kitti Thonglongya Bats from Thailand and Cambodia. Bull. Br. Mus. Nat. Hist. Zool. 1972, 22, 171–196. [Google Scholar]
- Harada, M.; Minezawa, M.; Takada, S.; Yenbutra, S.; Nunpakdee, P.; Ohtani, S. Karyological Analysis of 12 Species of Bats from Thailand. Caryologia 1982, 35, 269–278. [Google Scholar] [CrossRef]
- Hood, C.S.; Schlitter, D.A.; Iliopoulou-Georgudaki, J.; Yenbutra, S.; Baker, R.J. Chromosomal Studies of Bats (Mammalia: Chiroptera) from Thailand. Ann. Carnegie Mus. 1988, 57, 99–109. [Google Scholar] [CrossRef]
- Arnuphapprasert, A.; Riana, E.; Ngamprasertwong, T.; Wangthongchaicharoen, M.; Soisook, P.; Thanee, S.; Bhodhibundit, P.; Kaewthamasorn, M. First Molecular Investigation of Haemosporidian Parasites in Thai Bat Species. Int. J. Parasitol. Parasites Wildl. 2020, 13, 51–61. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 1 December 2022).
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction, 1st ed.; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-521-87635-3. [Google Scholar]
- McGowan, N.E.; Roche, N.; Aughney, T.; Flanagan, J.; Nolan, P.; Marnell, F.; Reid, N. Testing Consistency of Modelled Predictions of the Impact of Climate Change on Bats. Clim. Change Ecol. 2021, 2, 100011. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Pickens, A.H.; Tyukavina, A.; Hernandez-Serna, A.; Zalles, V.; Turubanova, S.; Kommareddy, I.; Stehman, S.V.; Song, X.-P.; et al. Global Land Use Extent and Dispersion within Natural Land Cover Using Landsat Data. Environ. Res. Lett. 2022, 17, 034050. [Google Scholar] [CrossRef]
- Cave Database and Cave Locations|Caves & Caving in Thailand. Available online: https://www.thailandcaves.shepton.org.uk/cave-co-ordinates (accessed on 16 December 2022).
- Tanalgo, K.C.; Tabora, J.A.G.; De Oliveira, H.F.M.; Haelewaters, D.; Beranek, C.T.; Otálora-Ardila, A.; Bernard, E.; Gonçalves, F.; Eriksson, A.; Donnelly, M.; et al. DarkCideS 1.0, a Global Database for Bats in Karsts and Caves. Sci. Data 2022, 9, 155. [Google Scholar] [CrossRef]
- Bandara, A.P.M.J.; Madurapperuma, B.D.; Edirisinghe, G.; Gabadage, D.; Botejue, M.; Surasinghe, T.D. Bioclimatic Envelopes for Two Bat Species from a Tropical Island: Insights on Current and Future Distribution from Ecological Niche Modeling. Diversity 2022, 14, 506. [Google Scholar] [CrossRef]
- Festa, F.; Ancillotto, L.; Santini, L.; Pacifici, M.; Rocha, R.; Toshkova, N.; Amorim, F.; Benítez-López, A.; Domer, A.; Hamidović, D.; et al. Bat Responses to Climate Change: A Systematic Review. Biol. Rev. 2023, 98, 19–33. [Google Scholar] [CrossRef]
- Global Climate and Weather Data–WorldClim 1 Documentation. Available online: https://www.worldclim.org/data/index.html (accessed on 16 December 2022).
- Escobar, L.E.; Lira-Noriega, A.; Medina-Vogel, G.; Townsend Peterson, A. Potential for Spread of the White-Nose Fungus (Pseudogymnoascus Destructans) in the Americas: Use of Maxent and NicheA to Assure Strict Model Transference. Geospat. Health 2014, 9, 221. [Google Scholar] [CrossRef]
- Booth, T.H. Checking Bioclimatic Variables That Combine Temperature and Precipitation Data before Their Use in Species Distribution Models. Austral Ecol. 2022, 47, 1506–1514. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Tian, J.; Ma, Q. Urban Expansion Dynamics and Natural Habitat Loss in China: A Multiscale Landscape Perspective. Glob. Change Biol. 2014, 20, 2886–2902. [Google Scholar] [CrossRef]
- Ma, T.; Zhou, C.; Pei, T.; Haynie, S.; Fan, J. Quantitative Estimation of Urbanization Dynamics Using Time Series of DMSP/OLS Nighttime Light Data: A Comparative Case Study from China’s Cities. Remote Sens. Environ. 2012, 124, 99–107. [Google Scholar] [CrossRef]
- Wang, N.; Li, S.-Y.; Yang, X.-L.; Huang, H.-M.; Zhang, Y.-J.; Guo, H.; Luo, C.-M.; Miller, M.; Zhu, G.; Chmura, A.A.; et al. Serological Evidence of Bat SARS-Related Coronavirus Infection in Humans, China. Virol. Sin. 2018, 33, 104–107. [Google Scholar] [CrossRef]
- Menachery, V.D.; Yount, B.L.; Debbink, K.; Agnihothram, S.; Gralinski, L.E.; Plante, J.A.; Graham, R.L.; Scobey, T.; Ge, X.-Y.; Donaldson, E.F.; et al. A SARS-like Cluster of Circulating Bat Coronaviruses Shows Potential for Human Emergence. Nat. Med. 2015, 21, 1508–1513. [Google Scholar] [CrossRef]
- Ge, X.-Y.; Li, J.-L.; Yang, X.-L.; Chmura, A.A.; Zhu, G.; Epstein, J.H.; Mazet, J.K.; Hu, B.; Zhang, W.; Peng, C.; et al. Isolation and Characterization of a Bat SARS-like Coronavirus That Uses the ACE2 Receptor. Nature 2013, 503, 535–538. [Google Scholar] [CrossRef]
- Elvidge, C.D.; Zhizhin, M.; Ghosh, T.; Hsu, F.-C.; Taneja, J. Annual Time Series of Global VIIRS Nighttime Lights Derived from Monthly Averages: 2012 to 2019. Remote Sens. 2021, 13, 922. [Google Scholar] [CrossRef]
- Dou, Y.; Liu, Z.; He, C.; Yue, H. Urban Land Extraction Using VIIRS Nighttime Light Data: An Evaluation of Three Popular Methods. Remote Sens. 2017, 9, 175. [Google Scholar] [CrossRef]
- Levin, N.; Zhang, Q. A Global Analysis of Factors Controlling VIIRS Nighttime Light Levels from Densely Populated Areas. Remote Sens. Environ. 2017, 190, 366–382. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists: Statistical Explanation of MaxEnt. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Guidigan, M.L.G.; Azihou, F.; Idohou, R.; Okhimamhe, A.A.; Fandohan, A.B.; Sinsin, B.; Adet, L. Modelling the Current and Future Distribution of Kigelia Africana under Climate Change in Benin, West Africa. Model. Earth Syst. Environ. 2018, 4, 1225–1238. [Google Scholar] [CrossRef]
- Phillips, S.J. A Brief Tutorial on Maxent. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 29 August 2023).
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Rulli, M.C.; D’Odorico, P.; Galli, N.; Hayman, D.T.S. Land-Use Change and the Livestock Revolution Increase the Risk of Zoonotic Coronavirus Transmission from Rhinolophid Bats. Nat. Food 2021, 2, 409–416. [Google Scholar] [CrossRef]
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic Host Diversity Increases in Human-Dominated Ecosystems. Nature 2020, 584, 398–402. [Google Scholar] [CrossRef]
- Jenks, G.F. The Data Model Concept in Statistical Mapping. Int. Yearb. Cartogr. 1967, 7, 186–190. [Google Scholar]
- Muylaert, R.L.; Kingston, T.; Luo, J.; Vancine, M.H.; Galli, N.; Carlson, C.J.; John, R.S.; Rulli, M.C.; Hayman, D.T.S. Present and Future Distribution of Bat Hosts of Sarbecoviruses: Implications for Conservation and Public Health. Proc. R. Soc. B 2022, 289, 20220397. [Google Scholar] [CrossRef]
- Kunz, T.H.; Fenton, M.B. Bat Ecology; University of Chicago Press: Chicago, IL, USA, 2005; ISBN 978-0-226-46207-3. [Google Scholar]
- Czenze, Z.J.; Naidoo, S.; Kotze, A.; McKechnie, A.E. Bat Thermoregulation in the Heat: Limits to Evaporative Cooling Capacity in Three Southern African Bats. J. Therm. Biol. 2020, 89, 102542. [Google Scholar] [CrossRef] [PubMed]
- Encyclopedia of Caves and Karst Science; Taylor and Francis: Chicago, IL, USA, 2014; ISBN 978-0-203-48385-5.
- Voigt, C.; Kingston, T. (Eds.) Bats in the Anthropocene: Conservation of Bats in a Changing World; Springer Open: Cham, Switzerland; New York, NY, USA, 2016; ISBN 978-3-319-25218-6. [Google Scholar]
- Leivers, S.J.; Meierhofer, M.B.; Pierce, B.L.; Evans, J.W.; Morrison, M.L. External Temperature and Distance from Nearest Entrance Influence Microclimates of Cave and Culvert-roosting Tri-colored Bats (Perimyotis Subflavus). Ecol. Evol. 2019, 9, 14042–14052. [Google Scholar] [CrossRef]
- Rainho, A.; Palmeirim, J.M. The Importance of Distance to Resources in the Spatial Modelling of Bat Foraging Habitat. PLoS ONE 2011, 6, e19227. [Google Scholar] [CrossRef]
- Lundy, M.; Montgomery, I.; Russ, J. Climate Change-linked Range Expansion of Nathusius’ Pipistrelle Bat, Pipistrellus Nathusii (Keyserling & Blasius, 1839). J. Biogeogr. 2010, 37, 2232–2242. [Google Scholar] [CrossRef]
- Frick, W.F.; Reynolds, D.S.; Kunz, T.H. Influence of Climate and Reproductive Timing on Demography of Little Brown Myotis Myotis lucifugus. J. Anim. Ecol. 2010, 79, 128–136. [Google Scholar] [CrossRef]
- Adams, R.A.; Hayes, M.A. Water Availability and Successful Lactation by Bats as Related to Climate Change in Arid Regions of Western North America. J. Anim. Ecol. 2008, 77, 1115–1121. [Google Scholar] [CrossRef]
- Prajakjitr, A.; Bumrungsri, S. The Relationship between the Proportion of Moths in Diet and Call Frequency of Bats in Superfamily Rhinolophoidea in Bala Forest, Hala-Bala Wildlife Sanctuary, Narathiwat Province; Prince of Songkla University: Songkhla, Thailand, 2008. [Google Scholar]
- Diengdoh, V.L.; Ondei, S.; Hunt, M.; Brook, B.W. Predicted Impacts of Climate Change and Extreme Temperature Events on the Future Distribution of Fruit Bat Species in Australia. Glob. Ecol. Conserv. 2022, 37, e02181. [Google Scholar] [CrossRef]
- Royal Forest Department. Thailand Forest Areas. Available online: http://forestinfo.forest.go.th/Content.aspx?id=1 (accessed on 15 July 2023).
- Vizzuality Thailand Deforestation Rates & Statistics|GFW. Available online: https://www.globalforestwatch.org/dashboards/country/THA (accessed on 15 July 2023).
- Bhandari, R.; Xue, W.; Virdis, S.G.P.; Winijkul, E.; Nguyen, T.P.L.; Joshi, S. Monitoring and Assessing Urbanization Progress in Thailand between 2000 and 2020 Using SDG Indicator 11.3.1. Sustainability 2023, 15, 9794. [Google Scholar] [CrossRef]
- Rydell, J. Exploitation of Insects around Streetlamps by Bats in Sweden. Funct. Ecol. 1992, 6, 744. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Duengkae, P.; Rodpan, A.; Kaewpom, T.; Maneeorn, P.; Kanchanasaka, B.; Yingsakmongkon, S.; Sittidetboripat, N.; Chareesaen, C.; Khlangsap, N.; et al. Diversity of Coronavirus in Bats from Eastern Thailand. Virol. J. 2015, 12, 57. [Google Scholar] [CrossRef]
- Pramod, R.K.; Nair, A.V.; Tambare, P.K.; Chauhan, K.; Kumar, T.V.; Rajan, R.A.; Mani, B.M.; Asaf, M.; Pandey, A.K. Reverse Zoonosis of Coronavirus Disease-19: Present Status and the Control by One Health Approach. Vet. World 2021, 2817–2826. [Google Scholar] [CrossRef]
- Olival, K.J.; Cryan, P.M.; Amman, B.R.; Baric, R.S.; Blehert, D.S.; Brook, C.E.; Calisher, C.H.; Castle, K.T.; Coleman, J.T.H.; Daszak, P.; et al. Possibility for Reverse Zoonotic Transmission of SARS-CoV-2 to Free-Ranging Wildlife: A Case Study of Bats. PLoS Pathog. 2020, 16, e1008758. [Google Scholar] [CrossRef]
- Crossing the Species Barrier: COVID-19, an Example of Reverse Zoonosis. Available online: https://www.woah.org/en/crossing-the-species-barriers-covid-19-an-example-of-reverse-zoonosis/ (accessed on 14 November 2023).
- Kunz, T.H.; Braun De Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem Services Provided by Bats: Ecosystem Services Provided by Bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).