Seasonal and Interannual Variability in the Phenolic Content of the Seagrass Nanozostera noltei: Characterization of Suitable Candidates for the Monitoring of Seagrass Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. General Experimental Procedures
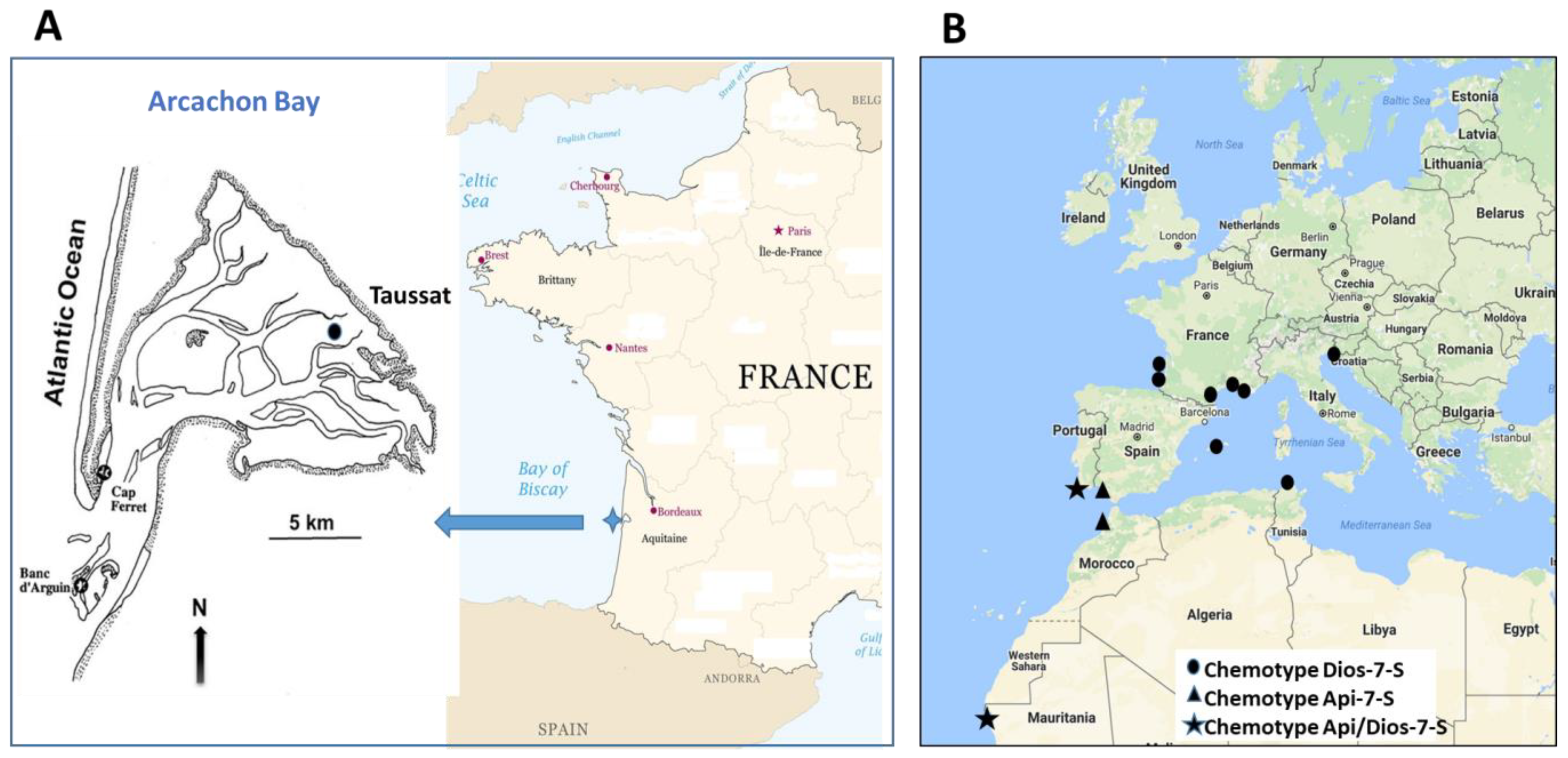
2.3. Study Sites
2.4. Collection
| Bioregion | Country | Sampling Site | Coordinates | Sampling Date |
|---|---|---|---|---|
| Atlantic Ocean | France | Arcachon Bay, Taussat | 44°42′ N, 1°4′ W | monthly from January 2017 to December 2020 |
| other sites | ||||
| France | Arcachon Bay, Arguin Bank | 44°34′ N, 1°14′ W | 20 November 2019 | |
| France | Hossegor Lake | 43°66′ N, 1°40′ W | 10 September 2011 | |
| Spain | Cadiz Bay | 36°23′ N, 6°10′ W | 24 June 2010 | |
| Morocco | Merja Zerga | 34°84′ N, 6°27′ W | 15 November 2011 | |
| Portugal | Ria Formosa | 37°01′ N, 7°51′ W | 27 June 2010 | |
| Mauritania | Pointe de l’Etoile | 21°01′ N, 17°00′ W | 29 April 2014 | |
| Mediterranean Sea | Slovenia | Strunjan | 45°53′ N, 13°60′ E | 30 October 2014 |
| Tunisia | Bizerte | 37°13′ N, 9°55′ E | 07 July 2011 | |
| France | Salses Leucate Lagoon | 42°50′ N, 3°37′ E | 16 August 2010 | |
| France | Thau Lagoon | 43°45′ N, 3°65′ E | 30 June 2010 | |
| France | Berres Lagoon | 43°28′ N, 5°10′ E | 31 August 2010 |
2.5. Extraction
2.6. Purification of Phenolic Compounds for Structural Assignment
2.7. Acid Hydrolysis of the Crude Extracts
2.8. Purification of the Aglycone Mixtures
2.9. Synthesis of the Sulfated Flavonoid Standards
2.10. Qualitative and Quantitative HPLC Analyses
2.11. LC/MS Analysis
2.12. Statistical Analyses
3. Results
3.1. Determination of the Phenolic Content of N. noltei from Arcachon Bay
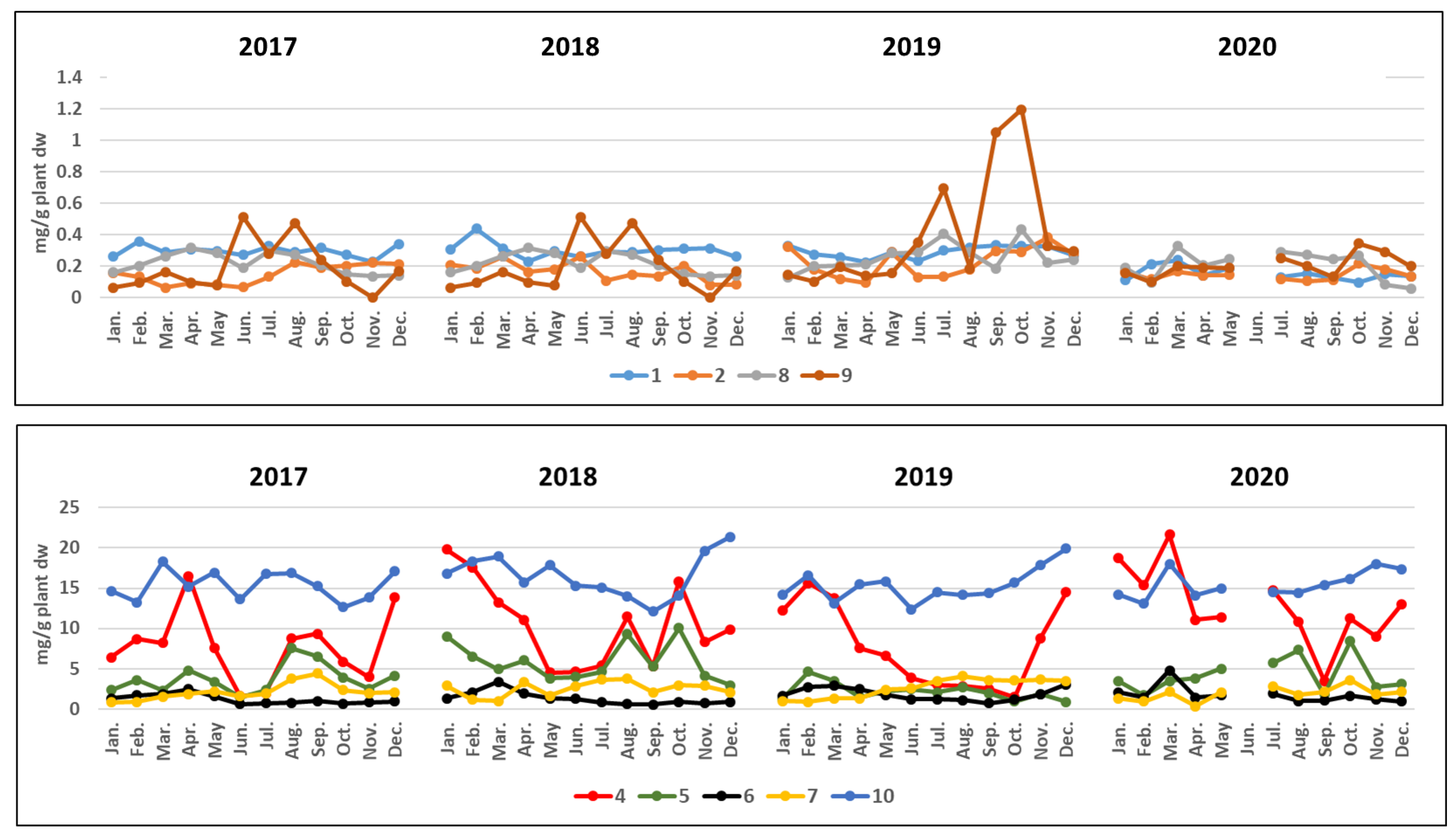
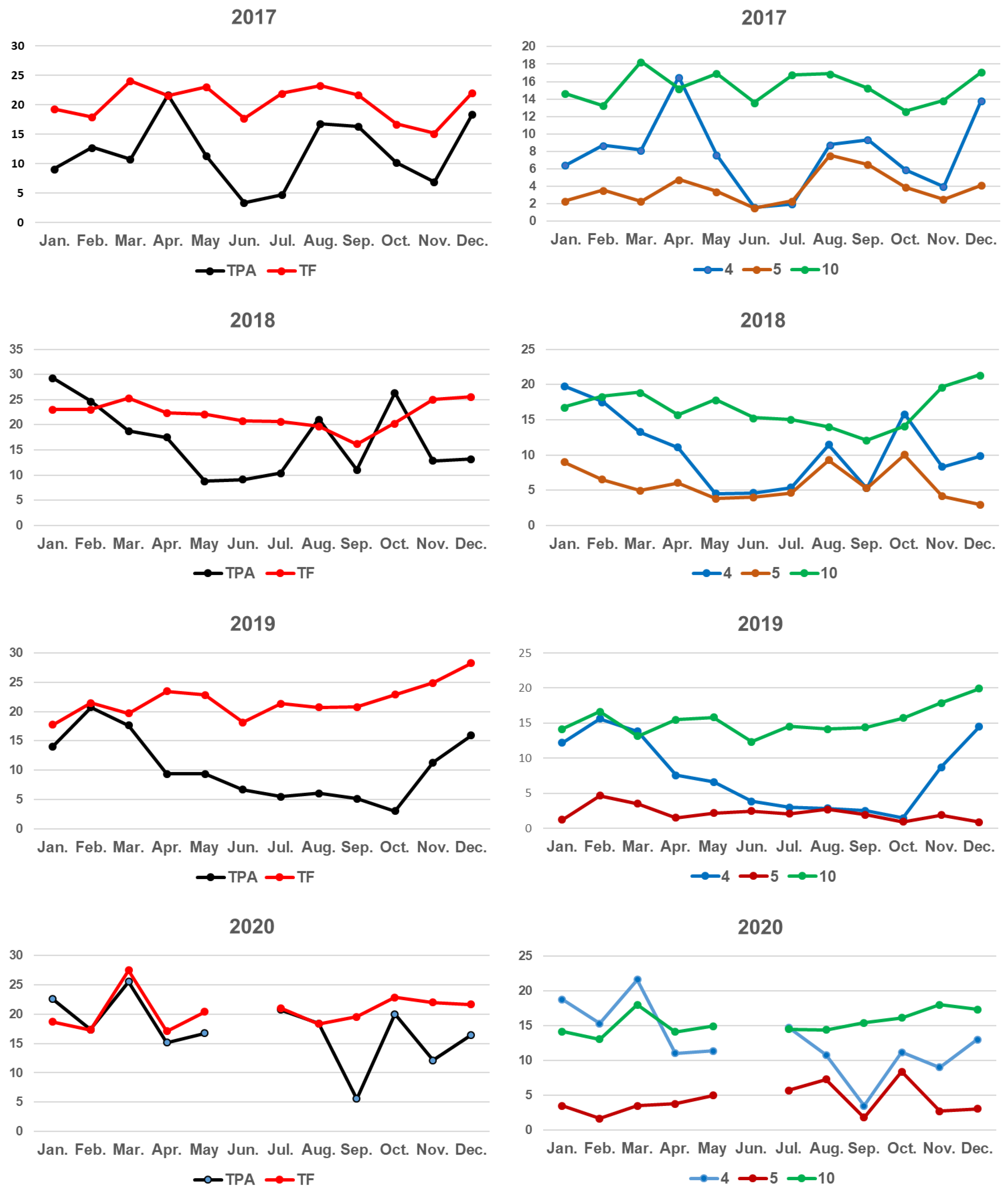
3.2. Seasonal and Interannual Dynamics of the Individual Phenolic Concentrations in N. Noltei
3.2.1. Phenolic Concentrations in N. noltei Leaves
| 2017 | Compounds | January | February | March | April | May | June | July | August | September | October | November | December | Concentration Ranges |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | 1 | 0.26 ± 0.04 | 0.36 ± 0.02 | 0.29 ± 0.01 | 0.31 ± 0.01 | 0.30 ± 0.01 | 0.27 ± 0.01 | 0.33 ± 0.01 | 0.29 ± 0.01 | 0.32 ± 0.01 | 0.27 ± 0.01 | 0.23 ± 0.01 | 0.34 ± 0.02 | 0.23–0.36 |
| 2 | 0.19 ± 0.01 | 0.13 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.13 ± 0.01 | 0.22 ± 0.01 | 0.19 ± 0.01 | 0.20 ± 0.02 | 0.22 ± 0.01 | 0.22 ± 0.02 | 0.07–0.22 | |
| 3 | 0 | 0 | 0 | 0.03 ± 0.01 | 0 | 0 | 0.02 ± 0.01 | 0 | 0 | 0 | 0 | 0 | 0–0.03 | |
| 4 | 6.42 ± 0.09 | 8.72 ± 0.02 | 8.18 ± 0.01 | 16.51 ± 0.01 | 7.59 ± 0.12 | 1.61 ± 0.01 | 1.98 ± 0.02 | 8.76 ± 0.01 | 9.35 ± 0.02 | 5.90 ± 0.01 | 3.97 ± 0.01 | 13.82 ± 0.03 | 1.61–16.51 | |
| 5 | 2.33 ± 0.03 | 3.58 ± 0.02 | 2.28 ± 0.02 | 4.78 ± 0.01 | 3.41 ± 0.14 | 1.51 ± 0.01 | 2.34 ± 0.01 | 7.56 ± 0.01 | 6.52 ± 0.01 | 3.90 ± 0.01 | 2.53 ± 0.15 | 4.13 ± 0.01 | 1.51–7.56 | |
| TPA | 9.20 | 12.79 | 10.82 | 21.72 | 11.38 | 3.46 | 4.80 | 16.83 | 16.38 | 10.27 | 6.95 | 18.51 | 3.46–18.51 | |
| % of TP | 32% | 42% | 31% | 50% | 33% | 16% | 18% | 42% | 43% | 38% | 28% | 46% | 16–50% | |
| Flavonoids | 6 | 1.31 ± 0.01 | 1.73 ± 0.05 | 1.88 ± 0.01 | 2.46 ± 0.02 | 1.61 ± 0.01 | 0.63 ± 0.01 | 0.73 ± 0.01 | 0.79 ± 0.01 | 1.01 ± 0.01 | 0.70 ± 0.01 | 0.86 ± 0.01 | 0.96 ± 0.01 | 0.7–2.46 |
| 7 | 0.85 ± 0.03 | 0.86 ± 0.02 | 1.56 ± 0.01 | 1.86 ± 0.02 | 2.21 ± 0.01 | 1.60 ± 0.01 | 1.90 ± 0.01 | 3.75 ± 0.01 | 4.40 ± 0.01 | 2.34 ± 0.01 | 2.0 ± 0.02 | 2.10 ± 0.01 | 0.85–4.40 | |
| 8 | 0.16 ± 0.01 | 0.20 ± 0.01 | 0.27 ± 0.01 | 0.32 ± 0.01 | 0.28 ± 0.01 | 0.19 ± 0.01 | 0.30 ± 0.01 | 0.27 ± 0.01 | 0.21 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.14–0.32 | |
| 9 | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.16 ± 0.01 | 0.10 ± 0.01 | 0.08 ± 0.02 | 0.51 ± 0.01 | 0.28 ± 0.02 | 0.48 ± 0.01 | 0.24 ± 0.01 | 0.10 ± 0.01 | 0 | 0.17 ± 0.01 | 0–0.51 | |
| 10 | 14.65 ± 0.04 | 13.25 ± 0.03 | 18.31 ± 0.01 | 15.21 ± 0.01 | 16.94 ± 0.03 | 13.62 ± 0.02 | 16.80 ± 0.02 | 16.89 ± 0.02 | 15.26 ± 0.01 | 12.63 ± 0.01 | 13.84 ± 0.01 | 17.09 ± 0.02 | 12.63–18.31 | |
| 11 | 0.27 ± 0.01 | 0.22 ± 0.01 | 0.46 ± 0.01 | 0.37 ± 0.01 | 0.58 ± 0.01 | 0.30 ± 0.01 | 0.46 ± 0.01 | 0.34 ± 0.01 | 0.17 ± 0.01 | 0.16 ± 0.02 | 0.40 ± 0.01 | 0.26 ± 0.01 | 0.16–0.58 | |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 13 | 2.04 ± 0.02 | 1.6 ± 0.02 | 1.45 ± 0.02 | 1.27 ± 0.02 | 1.37 ± 0.01 | 0.88 ± 0.01 | 1.54 ± 0.02 | 0.80 ± 0.01 | 0.43 ± 0.01 | 0.68 ± 0.01 | 0.97 ± 0.01 | 1.39 ± 0.02 | 0.43–2.04 | |
| TF | 19.34 | 17.95 | 24.09 | 21.59 | 23.07 | 17.73 | 22.01 | 23.32 | 21.72 | 16.76 | 18.21 | 22.11 | 18.21–24.09 | |
| % of TP | 68% | 58% | 69% | 50% | 67% | 84% | 82% | 58% | 57% | 62% | 72% | 54% | 50–84% | |
| TP | 28.54 | 30.74 | 34.91 | 43.31 | 34.45 | 21.19 | 26.81 | 40.15 | 38.10 | 27.03 | 25.16 | 40.62 | 21.19–43.31 | |
| 2018 | Compounds | January | February | March | April | May | June | July | August | September | October | November | December | Concentration ranges |
| Phenolic acids | 1 | 0.31 ± 0.01 | 0.44 ± 0.01 | 0.31 ± 0.01 | 0.23 ± 0.01 | 0.29 ± 0.01 | 0.26 ± 0.02 | 0.29 ± 0.02 | 0.29 ± 0.01 | 0.30 ± 0.02 | 0.31 ± 0.02 | 0.31 ± 0.02 | 0.26 ± 0.02 | 0.23–0.44 |
| 2 | 0.21 ± 0.01 | 0.19 ± 0.01 | 0.26 ± 0.01 | 0.16 ± 0.01 | 0.18 ± 0.02 | 0.26 ± 0.02 | 0.11 ± 0.01 | 0.15 ± 0.02 | 0.14 ± 0.01 | 0.20 ± 0.02 | 0.08 ± 0.02 | 0.08 ± 0.02 | 0.08–0.26 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 19.82 ± 0.02 | 17.57 ± 0.16 | 13.26 ± 0.02 | 11.06 ± 0.02 | 4.51 ± 0.01 | 4.63 ± 0.02 | 5.36 ± 0.01 | 11.44 ± 0.20 | 5.30 ± 0.02 | 15.87 ± 0.02 | 8.33 ± 0.03 | 9.86 ± 0.03 | 4.51–24.74 | |
| 5 | 9.0 ± 0.02 | 6.52 ± 0.01 | 4.94 ± 0.02 | 6.03 ± 0.01 | 3.82 ± 0.03 | 4.0 ± 0.03 | 4.63 ± 0.02 | 9.27 ± 0.03 | 5.29 ± 0.02 | 10.05 ± 0.02 | 4.14 ± 0.01 | 2.95 ± 0.02 | 3.82–10.05 | |
| TPA | 29.34 | 24.72 | 18.77 | 17.48 | 8.80 | 9.15 | 10.39 | 21.15 | 11.03 | 26.43 | 12.86 | 13.15 | 8.80–29.34 | |
| % of TP | 56% | 52% | 43% | 44% | 28% | 31% | 33% | 52% | 41% | 56% | 34% | 34% | 28–56% | |
| Flavonoids | 6 | 1.34 ± 0.01 | 2.11 ± 0.01 | 3.38 ± 0.01 | 1.93 ± 0.02 | 1.35 ± 0.02 | 1.29 ± 0.02 | 0.82 ± 0.01 | 0.63 ± 0.02 | 0.55 ± 0.02 | 0.90 ± 0.02 | 0.71 ± 0.01 | 0.89 ± 0.02 | 0.55–3.38 |
| 7 | 2.92 ± 0.02 | 1.14 ± 0.01 | 1.01 ± 0.01 | 3.35 ± 0.02 | 1.66 ± 0.02 | 2.82 ± 0.02 | 3.64 ± 0.02 | 3.77 ± 0.02 | 2.10 ± 0.02 | 2.96 ± 0.02 | 2.87 ± 0.02 | 2.06 ± 0.02 | 1.01–3.77 | |
| 8 | 0.17 ± 0.01 | 0.09 ± 0.01 | 0.41 ± 0.02 | 0.32 ± 0.02 | 0.19 ± 0.01 | 0.29 ± 0.01 | 0.21 ± 0.01 | 0.16 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.09 ± 0.02 | 0.13 ± 0.02 | 0.09–0.41 | |
| 9 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.12 ± 0.01 | 0.08 ± 0.01 | 0.16 ± 0.01 | 0.25 ± 0.02 | 0.21 ± 0.01 | 0.13 ± 0.02 | 0.21 ± 0.02 | 0.13 ± 0.02 | 0.10 ± 0.02 | 0.13 ± 0.01 | 0.08–0.25 | |
| 10 | 16.84 ± 0.02 | 18.35 ± 0.02 | 18.91 ± 0.01 | 15.73 ± 0.02 | 17.88 ± 0.02 | 15.29 ± 0.02 | 15.08 ± 0.03 | 14.0 ± 0.02 | 12.10 ± 0.02 | 14.07 ± 0.02 | 19.65 ± 0.17 | 21.37 ± 0.13 | 12.10–21.37 | |
| 11 | 0.31 ± 0.01 | 0.12 ± 0.01 | 0.17 ± 0.01 | 0.31 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.02 | 0.19 ± 0.01 | 0.41 ± 0.01 | 0.37 ± 0.01 | 1.71 ± 0.01 | 0.72 ± 0.02 | 0.12 ± 0.01 | 0.12–1.71 | |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 13 | 1.40 ± 0.01 | 1.21 ± 0.01 | 1.36 ± 0.01 | 0.70 ± 0.02 | 0.73 ± 0.01 | 0.65 ± 0.02 | 0.51 ± 0.02 | 0.62 ± 0.02 | 0.74 ± 0.01 | 0.51 ± 0.02 | 0.92 ± 0.02 | 0.92 ± 0.01 | 0.51–1.40 | |
| TF | 23.09 | 23.12 | 25.36 | 22.42 | 22.19 | 20.81 | 20.66 | 19.72 | 16.18 | 20.39 | 25.06 | 25.62 | 16.18–25.62 | |
| % of TP | 44% | 48% | 57% | 56% | 72% | 69% | 67% | 48% | 59% | 44% | 66% | 66% | 44–72% | |
| TP | 52.43 | 47.84 | 44.13 | 39.90 | 30.99 | 29.96 | 31.05 | 40.87 | 27.21 | 46.82 | 37.92 | 38.77 | 27.21–52.43 | |
| 2019 | Compounds | January | February | March | April | May | June | July | August | September | October | November | December | Concentration ranges |
| Phenolic acids | 1 | 0.33 ± 0.02 | 0.28 ± 0.02 | 0.26 ± 0.02 | 0.23 ± 0.01 | 0.29 ± 0.01 | 0.23 ± 0.02 | 0.30 ± 0.02 | 0.32 ± 0.02 | 0.33 ± 0.02 | 0.33 ± 0.02 | 0.33 ± 0.02 | 0.27 ± ±0.02 | 0.23–0.33 |
| 2 | 0.32 ± 0.01 | 0.18 ± 0.02 | 0.12 ± 0.02 | 0.10 ± 0.02 | 0.29 ± 0.01 | 0.13 ± 0.02 | 0.13 ± 0.02 | 0.18 ± 0.02 | 0.30 ± 0.02 | 0.29 ± 0.02 | 0.38 ± 0.01 | 0.28 ± 0.02 | 0.10–0.38 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0.04 ± 0.01 | 0 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0–0.047 | |
| 4 | 12.22 ± 0.03 | 15.63 ± 0.02 | 13.79 ± 0.03 | 7.56 ± 0.02 | 6.63 ± 0.02 | 3.85 ± 0.03 | 3.00 ± 0.02 | 2.86 ± 0.02 | 2.53 ± 0.02 | 1.47 ± 0.02 | 8.73 ± 0.02 | 14.47 ± 0.01 | 1.47–15.63 | |
| 5 | 1.20 ± 0.02 | 4.64 ± 0.02 | 3.49 ± 0.02 | 1.51 ± 0.01 | 2.19 ± 0.01 | 2.45 ± 0.02 | 2.08 ± 0.02 | 2.69 ± 0.02 | 1.96 ± 0.02 | 0.95 ± 0.02 | 1.87 ± 0.02 | 0.87 ± 0.02 | 0.87–4.64 | |
| TPA | 14.07 | 20.73 | 17.66 | 9.40 | 9.40 | 6.70 | 5.51 | 6.09 | 5.17 | 3.08 | 11.36 | 15.93 | 3.08–20.73 | |
| % of TP | 44% | 49% | 47% | 29% | 29% | 27% | 21% | 23% | 20% | 12% | 31% | 36% | 21–49% | |
| Flavonoids | 6 | 1.64 ± 0.01 | 2.74 ± 0.02 | 2.90 ± 0.02 | 2.48 ± 0.02 | 1.74 ± 0.01 | 1.19 ± 0.02 | 1.24 ± 0.01 | 1.13 ± 0.01 | 0.72 ± 0.02 | 1.18 ± 0.02 | 1.79 ± 0.02 | 3.06 ± 0.02 | 0.72–3.06 |
| 7 | 1.02 ± 0.02 | 0.91 ± 0.02 | 1.31 ± 0.01 | 1.30 ± 0.02 | 2.43 ± 0.02 | 2.55 ± 0.03 | 3.5 ± 0.03 | 4.11 ± 0.02 | 3.61 ± 0.02 | 3.53 ± 0.02 | 3.67 ± 0.02 | 3.49 ± 0.02 | 0.91–4.11 | |
| 8 | 0.13 ± 0.01 | 0.20 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.28 ± 0.02 | 0.29 ± 0.02 | 0.41 ± 0.02 | 0.29 ± 0.01 | 0.19 ± 0.02 | 0.43 ± 0.03 | 0.22 ± 0.01 | 0.24 ± 0.02 | 0.13–0.43 | |
| 9 | 0.15 ± 0.02 | 0.10 ± 0.02 | 0.20 ± 0.02 | 0.14 ± 0.01 | 0.16 ± 0.01 | 0.35 ± 0.02 | 0.69 ± 0.01 | 0.19 ± 0.02 | 1.05 ± 0.02 | 1.20 ± 0.02 | 0.33 ± 0.02 | 0.29 ± 0.02 | 0.10–1.20 | |
| 10 | 14.16 ± 0.03 | 16.61 ± 0.03 | 13.16 ± 0.04 | 15.47 ± 0.03 | 15.83 ± 0.02 | 12.36 ± 0.02 | 14.52 ± 0.02 | 14.16 ± 0.02 | 14.4 ± 0.03 | 15.71 ± 0.02 | 17.87 ± 0.02 | 19.92 ± 0.02 | 12.36–19.92 | |
| 11 | 0.06 ± 0.02 | 0.09 ± 0.02 | 0.28 ± 0.01 | 0.75 ± 0.02 | 0.65 ± 0.01 | 0.51 ± 0.02 | 0.32 ± 0.02 | 0.28 ± 0.01 | 0.17 ± 0.02 | 0.19 ± 0.02 | 0.18 ± 0.02 | 0.33 ± 0.01 | 0.06–0.75 | |
| 12 | 0 | 0 | 0 | 0.05 ± 0.01 | 0 | 0.18 ± 0.01 | 0.08 ± 0.010 | 0 | 0.04 ± 0.01 | 0.08 ± 0.01 | 0.14 ± 0.01 | 0 | 0–0.18 | |
| 13 | 0.59 ± 0.03 | 0.83 ± 0.02 | 1.64 ± 0.02 | 3.03 ± 0.02 | 1.76 ± 0.02 | 0.72 ± 0.02 | 0.59 ± 0.01 | 0.53 ± 0.02 | 0.61 ± 0.01 | 0.57 ± 0.02 | 0.68 ± 0.02 | 0.90 ± 0.01 | 0.59–3.03 | |
| TF | 17.75 | 21.48 | 19.70 | 23.43 | 22.85 | 18.15 | 21.35 | 20.69 | 20.79 | 22.89 | 24.88 | 28.23 | 17.75–28.23 | |
| % of TP | 56% | 51% | 53% | 71% | 71% | 73% | 79% | 77% | 80% | 88% | 69% | 64% | 51–88% | |
| TP | 31.82 | 42.21 | 37.36 | 32.83 | 32.25 | 24.85 | 26.86 | 26.78 | 25.96 | 25.97 | 36.24 | 44.16 | 24.85–44.16 | |
| 2020 | Compounds | January | February | March | April | May | June | July | August | September | October | November | December | Concentration ranges |
| Phenolic acids | 1 | 0.11 ± 0.01 | 0.21 ± 0.02 | 0.24 ± 0.02 | 0.14 ± 0.01 | 0.19 ± 0.01 | 0.13 ± 0.02 | 0.15 ± 0.02 | 0.13 ± 0.02 | 0.10 ± 0.02 | 0.15 ± 0.02 | 0.14 ± 0.02 | 0.10–0.24 | |
| 2 | 0.17 ± 0.01 | 0.12 ± 0.01 | 0.17 ± 0.01 | 0.14 ± 0.01 | 0.15 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.02 | 0.11 ± 0.01 | 0.22 ± 0.02 | 0.18 ± 0.01 | 0.14 ± 0.02 | 0.11–0.22 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 18.78 ± 0.02 | 15.34 ± 0.02 | 21.63 ± 0.02 | 11.08 ± 0.01 | 11.41 ± 0.01 | 14.79 ± 0.01 | 10.82 ± 0.02 | 3.52 ± 0.02 | 11.23 ± 0.02 | 9.04 ± 0.01 | 13.05 ± 0.03 | 9.04–21.63 | ||
| 5 | 3.52 ± 0.01 | 1.69 ± 0.01 | 3.51 ± 0.02 | 3.82 ± 0.02 | 5.01 ± 0.01 | 5.73 ± 0.01 | 7.33 ± 0.02 | 1.82 ± 0.01 | 8.44 ± 0.02 | 2.74 ± 0.01 | 3.09 ± 0.02 | 1.69–8.44 | ||
| TPA | 22.58 | 17.36 | 25.55 | 15.18 | 16.76 | 20.77 | 18.41 | 5.58 | 19.99 | 12.11 | 16.42 | 5.58–25.55 | ||
| % of TP | 55% | 50% | 48% | 47% | 45% | 50% | 50% | 22% | 47% | 36% | 43% | 22–55% | ||
| Flavonoids | 6 | 2.06 ± 0.01 | 1.45 ± 0.01 | 4.81 ± 0.01 | 1.41 ± 0.01 | 1.71 ± 0.02 | 1.94 ± 0.02 | 0.98 ± 0.01 | 1.06 ± 0.02 | 1.67 ± 0.01 | 1.20 ± 0.01 | 0.97 ± 0.02 | 0.97–4.81 | |
| 7 | 1.31 ± 0.02 | 0.95 ± 0.02 | 2.12 ± 0.01 | 0.32 ± 0.01 | 2.07 ± 0.02 | 2.86 ± 0.02 | 1.78 ± 0.02 | 2.15 ± 0.01 | 3.63 ± 0.02 | 1.84 ± 0.02 | 2.16 ± 0.02 | 0.32–3.63 | ||
| 8 | 0.19 ± 0.02 | 0.10 ± 0.01 | 0.33 ± 0.01 | 0.21 ± 0.01 | 0.24 ± 0.02 | 0.29 ± 0.01 | 0.27 ± 0.02 | 0.25 ± 0.02 | 0.27 ± 0.01 | 0.08 ± 0.02 | 0.06 ± 0.01 | 0.06–0.33 | ||
| 9 | 0.16 ± 0.02 | 0.10 ± 0.02 | 0.20 ± 0.01 | 0.19 ± 0.02 | 0.19 ± 0.01 | 0.25 ± 0.02 | 0.20 ± 0.01 | 0.13 ± 0.01 | 0.35 ± 0.02 | 0.29 ± 0.02 | 0.20 ± 0.02 | 0.10–0.35 | ||
| 10 | 14.20 ± 0.02 | 13.07 ± 0.02 | 18.02 ± 0.02 | 14.12 ± 0.02 | 14.96 ± 0.02 | 14.52 ± 0.03 | 14.43 ± 0.02 | 15.42 ± 0.02 | 16.16 ± 0.01 | 18.02 ± 0.02 | 17.35 ± 0.02 | 13.07–18.02 | ||
| 11 | 0.09 ± 0.01 | 0.28 ± 0.02 | 0.30 ± 0.01 | 0.17 ± 0.01 | 0.27 ± 0.01 | 0.45 ± 0.02 | 0.21 ± 0.01 | 0.15 ± 0.02 | 0.33 ± 0.02 | 0.11 ± 0.01 | 0.19 ± 0.02 | 0.09–0.45 | ||
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 13 | 0.71 ± 0.01 | 1.36 ± 0.02 | 1.68 ± 0.02 | 0.71 ± 0.02 | 0.96 ± 0.01 | 0.73 ± 0.02 | 0.51 ± 0.02 | 0.40 ± 0.02 | 0.45 ± 0.01 | 0.43 ± 0.03 | 0.74 ± 0.01 | 0.40–1.68 | ||
| TF | 18.72 | 17.31 | 27.46 | 17.13 | 20.40 | 21.04 | 18.38 | 19.56 | 22.86 | 21.97 | 21.67 | 17.13–27.46 | ||
| % of TP | 45% | 50% | 52% | 53% | 55% | 50% | 50% | 78% | 53% | 64% | 57% | 45–78% | ||
| TP | 41.30 | 34.67 | 53.01 | 32.31 | 37.16 | 41.81 | 36.79 | 25.14 | 42.85 | 34.08 | 38.09 | 25.14–53.01 |
3.2.2. Phenolic Concentrations in N. noltei Rhizomes
| Compounds | May 2019 | June 2019 | July 2019 | April 2020 | August 2020 | October 2020 | Concentration Ranges | |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids | 1 | 0.72 ± 0.02 | 0.26 ± 0.02 | 0.24 ± 0.01 | 0.13 ± 0.02 | 0.62 ± 0.02 | 0.50 ± 0.02 | 0.13–0.72 |
| 2 | 0.08 ± 0.02 | 0.09 ± 0.01 | 0.05 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.02 | 0.02–0.09 | |
| 3 | 0.21 ± 0.02 | 1.14 ± 0.02 | 0.20 ± 0.01 | 0.22 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.06–1.14 | |
| 4 | 3.28 ± 0.02 | 1.85 ± 0.01 | 1.65 ± 0.02 | 2.35 ± 0.02 | 1.39 ± 0.01 | 1.79 ± 0.02 | 1.39–3.28 | |
| 5 | 0.92 ± 0.02 | 0.74 ± 0.02 | 1.21 ± 0.02 | 1.57 ± 0.02 | 1.47 ± 0.01 | 1.75 ± 0.01 | 0.74–1.75 | |
| TPA | 5.21 | 4.08 | 3.35 | 4.29 | 3.59 | 4.18 | 3.35–5.21 | |
| Flavonoids | 6 | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.01 | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.05–0.09 |
| 7 | 0.11 ± 0.02 | 0.05 ± 0.02 | 0 | 0 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0–0.05 | |
| 8 | 0 | 0 | 0 | 0 | 0.04 ± 0.01 | 0 | 0–0.04 | |
| 9 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0 | 0 | 0 | 0–0.05 | |
| 10 | 0.71 ± 0.02 | 0.20 ± 0.01 | 0.17 ± 0.01 | 0 | 0.86 ± 0.02 | 0.26 ± 0.01 | 0–0.86 | |
| 11 | 0.11 ± 0.02 | 0.11 ± 0.01 | 0 | 0.04 ± 0.02 | 0 | 0 | 0–0.11 | |
| 12 | 0 | 0 | 0 | 0 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0–0.01 | |
| 13 | 1.22 ± 0.02 | 0.79 ± 0.02 | 0.59 ± 0.02 | 0.67 ± 0.01 | 0.34 ± 0.02 | 0.15 ± 0.02 | 0.59–1.221 | |
| TF | 2.29 | 1.26 | 0.83 | 0.76 | 1.36 | 0.51 | 0.51–2.29 | |
| TP | 7.50 | 5.34 | 4.18 | 5.05 | 4.95 | 4.69 | 4.18–7.50 |
| Compounds | May 2019 | June 2019 | July 2019 | April 2020 | August 2020 | October 2020 | |
|---|---|---|---|---|---|---|---|
| Phenolic acids | 1 | 14% | 6% | 7% | 3% | 17% | 12% |
| 2 | 1% | 2% | 1% | 1% | 2% | 2% | |
| 3 | 4% | 28% | 6% | 5% | 2% | 2% | |
| 4 | 63% | 45% | 49% | 55% | 39% | 43% | |
| 5 | 18% | 18% | 36% | 36% | 41% | 42% | |
| Flavonoids | 6 | 4% | 6% | 6% | 6% | 6% | 12% |
| 7 | 5% | 4% | 2% | 5% | |||
| 8 | 3% | ||||||
| 9 | 2% | 3% | 3% | ||||
| 10 | 31% | 16% | 20% | 63% | 52% | ||
| 11 | 5% | 9% | 5% | ||||
| 12 | 1% | 1% | |||||
| 13 | 53% | 63% | 71% | 89% | 25% | 30% | |
| TPA | 69% | 76% | 80% | 85% | 72% | 89% | |
| TF | 31% | 24% | 20% | 15% | 28% | 11% |
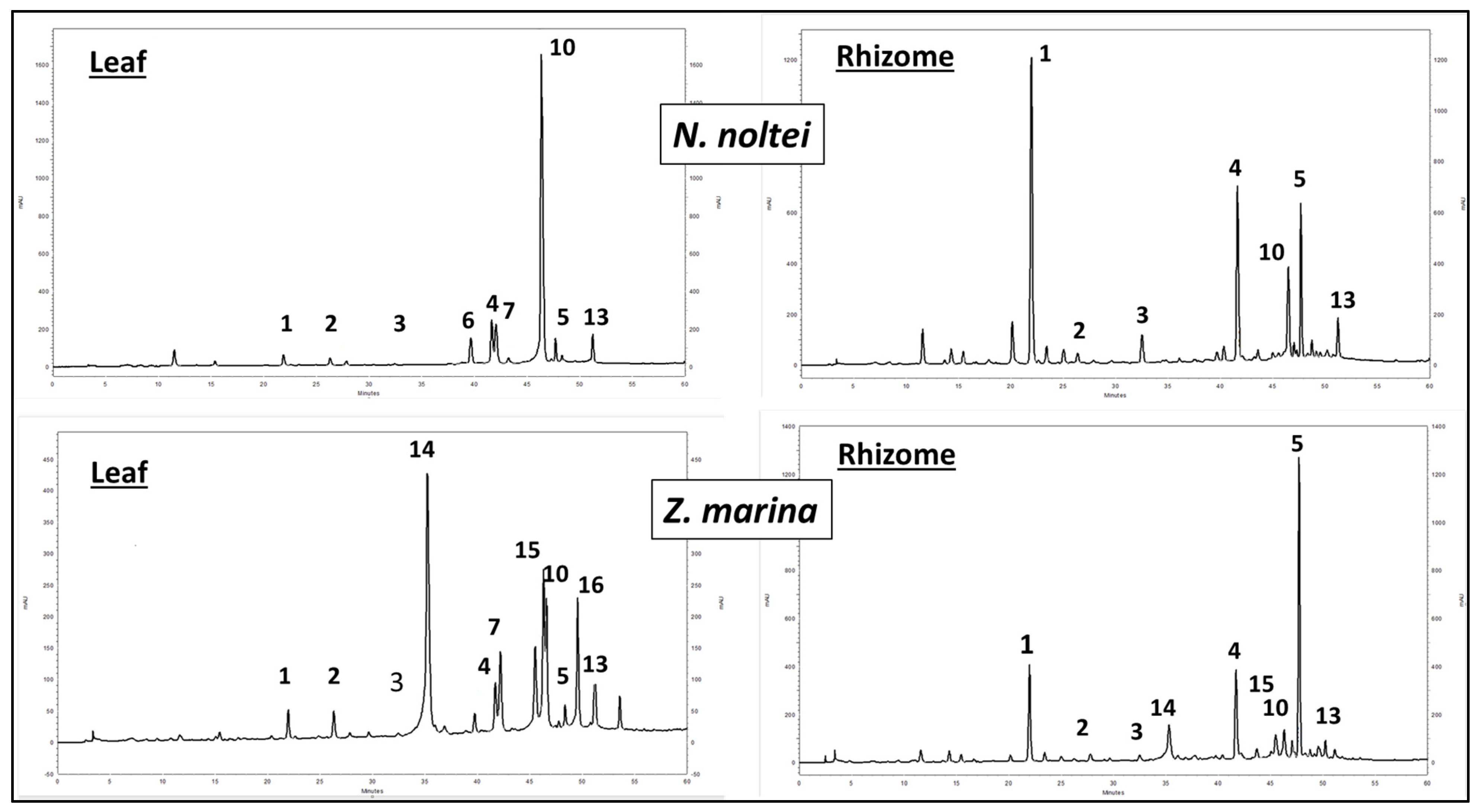
3.2.3. Phenolic Concentrations in Z. marina Rhizomes and N. noltei Samples from Other Sites in the Atlantic and Mediterranean
| Sites | N. noltei Leaves | N. noltei Rhizomes | Z. marina Rhizomes |
|---|---|---|---|
| Arguin | 3.48 ± 0.02 | 3.07 ± 0.02 | |
| Hossegor | 1.08 ± 0.01 | 1.37 ± 0.02 | 7.54 ± 0.02 |
| Thau | 2.59 ± 0.02 | 1.13 ± 0.01 | 5.47 ± 0.02 |
| Berres | 1.86 ± 0.02 | ||
| Salses | 0.22 ± 0.01 | ||
| Strunjan | 1.80 ± 0.02 | ||
| Bizerte | 1.57 ± 0.02 | ||
| Ria Formosa | 3.16 ± 0.02 | 0.54 ± 0.01 | |
| Pointe de l’Etoile | 3.31 ± 0.02 | ||
| Cadiz bay | 2.37 ± 0.02 | ||
| Merja Zerga | 1.03 ± 0.02 | ||
| Concentration ranges | 0.22–3.48 | 0.54–1.37 | 3.07–7.54 |
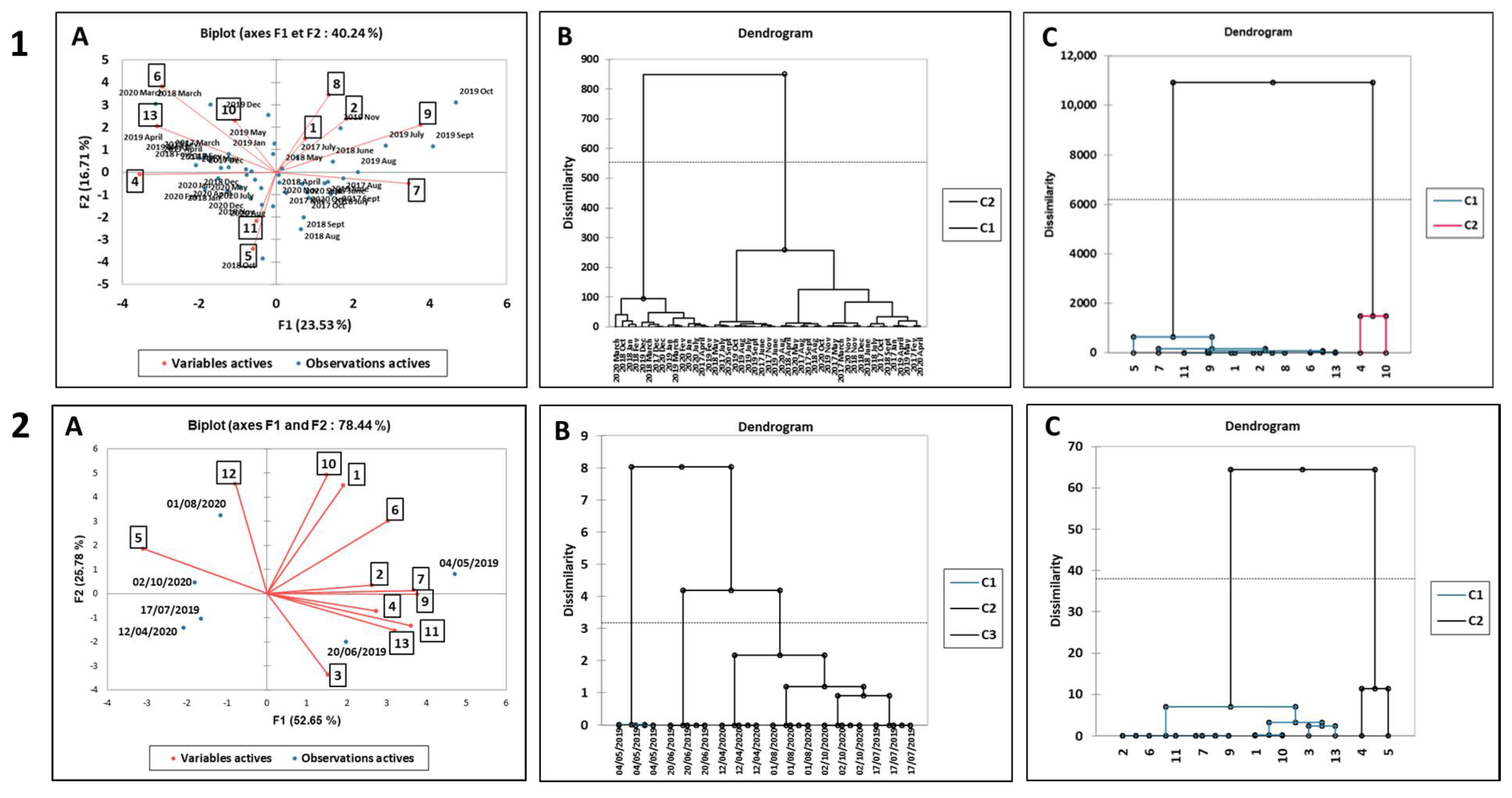
3.3. Principal Component Analysis and Hierarchical Cluster Analysis
3.3.1. N. noltei Leaves from Arcachon Bay
3.3.2. N. noltei Rhizomes from Arcachon Bay
4. Discussion
- -
- Phenolic acids: Rosmarinic > Zosteranoic >>> Zosteric ~ Caffeic >> Coumaric.
- -
- Flavonoids: Diosmetin 7-sulfate >>> Luteolin 7-sulfate ~ Luteolin 7-O-glucoside > Apigenin 7-O-glucoside ~ Apigenin 7-sulfate.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Short, F.T.; Short, C.A.; Novak, A. Seagrasses. In The Wetland Book: II: Distribution, Description and Conservation; Finlayson, C.M., Milton, G.R., Prentice, R.C., Davidson, N.C., Eds.; Springer Science: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Mtwana Nordlund, L.; Koch, E.W.; Barbier, E.B.; Creed, J.C. Seagrass ecosystem services and their variability across genera and geographical regions. PLoS ONE 2016, 11, e0163091. [Google Scholar] [CrossRef] [PubMed]
- Apostoloumi, C.; Malea, P.; Kevrekidis, T. Principles and concepts about seagrasses: Towards a sustainable future for seagrass ecosystems. Mar. Pollut. Bull. 2021, 173, 112936. [Google Scholar] [CrossRef] [PubMed]
- do Amaral Camara Lima, M.; Bergamo, T.F.; Ward, R.D.; Joyce, C.B. A review of seagrass ecosystem services: Providing nature-based solutions for a changing world. Hydrobiologia 2023, 850, 2655–2670. [Google Scholar] [CrossRef]
- Duarte, C.M. Reviews and syntheses: Hidden forests, the role of vegetated coastal habitats in the ocean carbon budget. Biogeosciences 2017, 14, 301–310. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Ricart, A.M.; York, P.H.; Bryant, C.; Rasheed, M.A.; Ierodiaconou, D.; Macreadie, P.I. High variability of Blue Carbon storage in seagrass meadows at the estuary scale. Sci. Rep. 2020, 10, 5865. [Google Scholar] [CrossRef]
- Orth, R.J.; Luckenbach, M.L.; Marion, S.R.; Moore, K.A.; Wilcox, D.J. Seagrass recovery in the Delmarva coastal bays, USA. Aquat. Bot. 2006, 84, 26–36. [Google Scholar] [CrossRef]
- Short, F.T.; Wyllie-Echeverria, S. Natural and human-induced disturbance of seagrasses. Environ. Conserv. 1996, 23, 17–27. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L., Jr.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- Duffy, J.E.; Benedetti-Cecchi, L.; Trinanes, J.; Muller-Karger, F.E.; Ambo-Rappe, R.; Boström, C.; Buschmann, A.H.; Byrnes, J.; Coles, R.G.; Creed, J.; et al. Toward a coordinated global observing system for seagrasses and marine macroalgae. Front. Mar. Sci. 2019, 6, 317. [Google Scholar] [CrossRef]
- Green, A.E.; Unsworth, R.K.F.; Chadwick, M.A.; Jones, P.J.S. Historical analysis exposes catastrophic seagrass loss for the United Kingdom. Front. Plant Sci. 2021, 12, 629962. [Google Scholar] [CrossRef] [PubMed]
- de los Santos, C.B.; Krause-Jensen, D.; Alcoverro, T.; Marbà, N.; Duarte, C.M.; van Katwijk, M.M.; Pérez, M.; Romero, J.; Sánchez-Lizaso, J.L.; Roca, G. Recent trend reversal for declining European seagrass meadows. Nat. Commun. 2019, 10, 3356. [Google Scholar] [CrossRef] [PubMed]
- Marbà, N.; Krause-Jensen, D.; Alcoverro, T.; Birk, S.; Pedersen, A.; Neto, J.M.; Orfanidis, S.; Garmendia, J.M.; Muxika, I.; Borja, A. Diversity of European seagrass indicators: Patterns within and across regions. Hydrobiologia 2013, 704, 265–278. [Google Scholar] [CrossRef]
- Roca, G.; Alcoverro, T.; Krause-Jensen, D.; Balsby, T.J.S.; van Katwijk, M.M.; Marbà, N.; Santos, R.; Arthur, R.; Mascaró, O.; Fernández-Torquemada, Y.; et al. Response of seagrass indicators to shifts in environmental stressors: A global review and management synthesis. Ecol. Indic. 2016, 63, 310–323. [Google Scholar] [CrossRef]
- Roca, G.; Alcoverro, T.; de Torres, M.; Manzanera, M.; Martínez-Crego, B.; Bennett, S.; Farina, S.; Pérez, M.; Romero, J. Detecting water quality improvement along the Catalan Coast (Spain) using stress-specific biochemical seagrass indicators. Ecol. Indic. 2015, 54, 161–170. [Google Scholar] [CrossRef]
- Griffiths, L.L.; Melvin, S.D.; Connolly, R.M.; Pearson, R.M.; Brown, C.J. Metabolomic indicators for low-light stress in seagrass. Ecol. Indic. 2020, 114, 106316. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of Phenolic Compounds in Plant-Defensive Mechanisms. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; pp. 517–532. [Google Scholar] [CrossRef]
- Sieg, R.D.; Kubanek, J. Chemical ecology of marine angiosperms: Opportunities at the interface of marine and terrestrial systems. J. Chem. Ecol. 2013, 39, 687–711. [Google Scholar] [CrossRef]
- Subhashini, P.; Dilipan, E.; Thangaradjou, T.; Papenbrock, J. Bioactive natural products from marine angiosperms: Abundance and functions. Nat. Prod. Bioprospect. 2013, 3, 129–136. [Google Scholar] [CrossRef]
- Zidorn, C. Secondary metabolites of seagrasses (Alismatales and Potamogetonales; Alismatidae): Chemical diversity, bioactivity, and ecological function. Phytochemistry 2016, 124, 5–28. [Google Scholar] [CrossRef]
- Vergeer, L.H.T.; Develi, A. Phenolic acids in healthy and infected leaves of Zostera marina and their growth-limiting properties towards Labyrinthula zosterae. Aquat. Bot. 1997, 58, 65–72. [Google Scholar] [CrossRef]
- Martinez-Crego, B.; Arteaga, P.; Tomas, F.; Santos, R. The role of seagrass traits in mediating Zostera noltei vulnerability to mesograzers. PLoS ONE 2016, 11, e0156848. [Google Scholar] [CrossRef]
- Guan, C.; Parrot, D.; Wiese, J.; Sönnichsen, F.D.; Saha, M.; Tasdemir, D.; Weinberger, F. Identification of rosmarinic acid and sulfated flavonoids as inhibitors of microfouling on the surface of eelgrass Zostera marina. Biofouling 2017, 33, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Laabir, M.; Grignon-Dubois, M.; Masseret, E.; Rezzonico, B.; Soteras, G.; Rouquette, M.; Rieuvilleneuve, F.; Cecchi, P. Algicidal effects of Zostera marina L. and Zostera noltii Hornem. extracts on the neuro-toxic bloom-forming dinoflagellate Alexandrium catenella. Aquat. Bot. 2013, 111, 16–25. [Google Scholar] [CrossRef]
- Papazian, S.; Parrot, D.; Burýšková, B.; Weinberger, F.; Tasdemir, D. Surface chemical defence of the eelgrass Zostera marina against microbial foulers. Sci. Rep. 2019, 9, 3323. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.; Zimmerman, R.C.; Crews, P.; Alberte, R.S. The antifouling activity of natural and synthetic phenolic acid sulfate esters. Phytochemistry 1993, 34, 401–404. [Google Scholar] [CrossRef]
- Sullivan, B.K.; Short, F.T. Taxonomic revisions in Zosteraceae (Zostera, Nanozostera, Heterozostera and Phyllospadix). Aquat. Bot. 2023, 187, 103636. [Google Scholar] [CrossRef]
- Green, E.P.; Short, F.T. World Atlas of Seagrasses; University of California Press: Berkeley, CA, USA, 2003. [Google Scholar]
- Diekmann, O.E.; Gouveia, L.; Perez, J.A.; Gil-Rodriguez, C.; Serrão, E.A. The possible origin of Zostera noltii in the Canary Islands and guidelines for restoration. Mar. Biol. 2010, 157, 2109–2115. [Google Scholar] [CrossRef]
- Short, F.T.; Carruthers, T.J.R.; Waycott, M.; Kendrick, G.A.; Fourqurean, J.W.; Callabine, A.; Kenworthy, W.J.; Dennison, W.C. Zostera noltii. The IUCN Red List of Threatened Species. 2010: E.T173361A6999224. Available online: https://www.iucnredlist.org/species/173361/6999224 (accessed on 3 September 2023).
- Harborne, J.B.; Williams, C. Occurrence of sulphated flavones and caffeic acid esters in members of the fluviales. Biochem. Syst. Ecol. 1976, 4, 37–41. [Google Scholar] [CrossRef]
- McMillan, C.; Zapata, O.; Escobar, L. Sulphated phenolic compounds in seagrasses. Aquat. Bot. 1980, 8, 267–278. [Google Scholar] [CrossRef]
- Milkova, T.; Petkova, R.; Christov, R.; Popov, S.; Dimitrova-Konaklieva, S. Chemical composition of Zostera marina and Zostera nana Roth. from the Black Sea. Bot. Mar. 1995, 38, 99–101. [Google Scholar] [CrossRef]
- Males, Z.; Plazibat, M. Investigation of chemical composition of Zostera noltii Hornem. Farm. Glas. 2000, 56, 109–118. [Google Scholar]
- Grignon-Dubois, M.; Rezzonico, B. First phytochemical evidence of chemotypes for the seagrass Zostera noltii. Plants 2012, 1, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Grignon-Dubois, M.; Rezzonico, B. Phenolic chemistry of the seagrass Zostera noltei Hornem. Part 1: First evidence of three infraspecific flavonoid chemotypes in three distinctive geographical regions. Phytochemistry 2018, 146, 91–101. [Google Scholar] [CrossRef]
- Enerstvedt, K.H.; Lundberg, A.; Sjøtun, K.; Fadnes, P.; Jordheim, M. Characterization and seasonal variation of individual flavonoids in Zostera marina and Zostera noltii from Norwegian coastal waters. Biochem. Syst. Ecol. 2017, 74, 42–50. [Google Scholar] [CrossRef]
- Manck, L.; Quintana, E.; Rocio, S.; Brun, F.G.; Hernández, I.; Ortega, M.J.; Zubia, E. Profiling of phenolic natural products in the seagrass Zostera noltei by UPLC-MS. Nat. Prod. Commun. 2017, 12, 687–690. [Google Scholar] [CrossRef]
- Achamlale, S.; Rezzonico, B.; Grignon-Dubois, M. Rosmarinic acid from beach waste: Isolation and HPLC quantification in Zostera detritus from Arcachon lagoon. Food Chem. 2009, 113, 878–883. [Google Scholar] [CrossRef]
- Achamlale, A.; Rezzonico, B.; Grignon-Dubois, M. Evaluation of Zostera detritus as a potential new source of zosteric acid. J. Appl. Phyc. 2009, 21, 347–352. [Google Scholar] [CrossRef]
- Grignon-Dubois, M.; Rezzonico, B.; Alcoverro, T. Regional scale patterns in seagrass defences: Phenolic acid content in Zostera noltii. Estuar. Coast Shelf Sci. 2012, 114, 18–22. [Google Scholar] [CrossRef]
- Grignon-Dubois, M.; Rezzonico, B. Phenolic chemistry of the seagrass Zostera marina Linnaeus: First assessment of geographic variability among populations on a broad spatial scale. Phytochemistry 2023, 213, 113788. [Google Scholar] [CrossRef]
- Dybsland, S.C.; Bekkby, T.; Enerstvedt, H.K.; Kvalheim, O.M.; Rinde, E.; Jordheim, M. Variation in phenolic chemistry in Zostera marina seagrass along environmental gradients. Plants 2021, 10, 334. [Google Scholar] [CrossRef]
- Rigouin, L.; Trut, G.; Bajjouk, T.; Rebeyrol, S.; Liabot, P.O.; Ganthy, F.; Auby, I. Caractérisation de la Qualité Biologique des Masses d’Eau Côtières: Cartographie des Herbiers de Zostera noltei du Bassin d’Arcachon (MEC FRFC06–Arcachon Amont) par Imagerie Hyperspectrale; ODE/LITTORAL/LERAR/22.16; Ifermer: Plouzané, France, 2022; 72p, Available online: https://archimer.ifremer.fr/doc/00795/90675/ (accessed on 4 August 2023).
- Calleja, F.; Galván, C.; Silió-Calzada, A.; Juanes, J.A.; Ondiviela, B. Long-term analysis of Zostera noltei: A retrospective approach for understanding seagrasses’ dynamics. Mar. Environ. Res. 2017, 130, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, F. The Arcachon Bay estuary: A “collage” of landscapes. In Landscapes and Landforms of France; Fort, F.M., André, M.F., Eds.; World Geomorphological Landscapes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 71–80. [Google Scholar] [CrossRef]
- Plus, M.; Dumas, F.; Stanisière, J.Y.; Maurer, D. Hydrodynamic characterization of the Arcachon Bay using model-derived descriptors. Cont. Shelf Res. 2009, 29, 1008–1013. [Google Scholar] [CrossRef]
- den Hartog, C.; Kuo, J. Taxonomy and biogeography of seagrasses. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 1–23. [Google Scholar] [CrossRef]
- Barron, D.; Ibrahim, R.K. Hydrochloric acid and aryl-sulphatase as reagents for UV-spectral detection of 3- and 4′-sulphated flavonoids. Phytochemistry 1988, 27, 2335–2338. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimise on objective function. J. Amer. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Li, Y.; Rárová, L.; Scarpato, S.; Çiçek, S.S.; Jordheim, M.; Štenclová, T.; Strnad, M.; Mangoni, A.; Zidorn, C. Seasonal variation of phenolic compounds in Zostera marina (Zosteraceae) from the Baltic Sea. Phytochemistry 2022, 196, 113099. [Google Scholar] [CrossRef] [PubMed]
- Casado, M.A.; Ramírez-Sanz, L.; Castro, I.; de Miguel, J.M.; de Pablo, C.L. An Objective method for partitioning dendrograms based on entropy parameters. Plant Ecol. 1997, 131, 193–197. [Google Scholar] [CrossRef]
- Zapata, O.; McMillan, C. Phenolic acids in seagrasses. Aquat. Bot. 1997, 7, 307–317. [Google Scholar] [CrossRef]
- Grignon-Dubois, M.; Rezzonico, B.; Blanchet, H. Phenolic fingerprints of the Pacific seagrass Phyllospadix torreyi–Structural characterization and quantification of undescribed flavonoid sulfates. Phytochemistry 2022, 201, 113256. [Google Scholar] [CrossRef]
- Häusler, E.; Petersen, M.; Alfermann, A.W. Isolation of protoplasts and vacuoles from cell suspension cultures of Coleus blumei Benth. Plant Cell Rep. 1993, 12, 510–512. [Google Scholar] [CrossRef]
- Trócsányi, E.; György, Z.; Zámboriné-Németh, É. New insights into rosmarinic acid biosynthesis based on molecular studies. Curr. Plant Biol. 2020, 23, 100162. [Google Scholar] [CrossRef]
- Bais, H.P.; Walker, T.S.; Schweizer, H.P.; Vivanco, J.M. Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiol. Biochem. 2002, 40, 983–995. [Google Scholar] [CrossRef]
- McKone, K.L.; Tanner, C.E. Role of salinity in the susceptibility of eelgrass Zostera marina to the wasting disease pathogen Labyrinthula zosterae. Mar. Ecol. Progr. Ser. 2009, 377, 123–130. [Google Scholar] [CrossRef]
- Webb, T.J. Marine and terrestrial ecology: Unifying concepts, revealing differences. Trends Ecol. Evol. 2012, 27, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.H.; Brink, K.H.; Scott, B.E. Comparison of marine and terrestrial ecosystems: Suggestions of an evolutionary perspective influenced by environmental variation. ICES J. Mar. Sci. 2019, 76, 50–59. [Google Scholar] [CrossRef]
- Grosberg, R.K.; Vermeij, G.J.; Wainwright, P.C. Biodiversity in water and on land. Cur. Biol. 2012, 22, R900–R903. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Stafiniak, M.; Slusarczyk, S.; Pencakowski, B.; Matkowski, A.; Rahimmalek, M.; Bielecka, M. Seasonal variations of rosmarinic acid and its glucoside and expression of genes related to their biosynthesis in two medicinal and aromatic species of Salvia subg. Perovskia. Biology 2021, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Galasso, S.; Pacifico, S.; Kretschmer, N.; Pan, S.P.; Marciano, S.; Piccolella, S.; Monaco, P.; Bauer, R. Influence of seasonal variation on Thymus longicaulis C. Presl. Chemical composition and its antioxidant and anti-inflammatory properties. Phytochemistry 2014, 107, 80–90. [Google Scholar] [CrossRef]
- Raudone, L.; Zymone, K.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V.; Janulis, V. Phenological changes in triterpenic and phenolic composition of Thymus L. species. Ind. Crops. Prod. 2017, 109, 445–451. [Google Scholar] [CrossRef]
- Harborne, J.B. Flavonoid sulphates: A new class of sulphur compounds in higher plants. Phytochemistry 1975, 11, 1147–1155. [Google Scholar] [CrossRef]
- Barron, D.; Varin, L.; Ibrahim, R.K.; Harborne, J.B.; Williams, C.A. Sulphated flavonoids—An update. Phytochemistry 1988, 27, 2375–2395. [Google Scholar] [CrossRef]
- Teles, Y.C.F.; Souza, M.S.R.; Souza, M.F.V. Sulphated flavonoids: Biosynthesis, structures, and biological activities. Molecules 2018, 23, 480. [Google Scholar] [CrossRef] [PubMed]
- Varin, L.; Marsolais, F.; Richard, M.; Rouleau, M. Biochemistry and molecular biology of plant sulfotransferases. FASEB J. 1997, 11, 517e525. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. The Plant and Its Biochemical Adaptation to the Environment. In Introduction to Ecological Biochemistry; Academic Press: London, UK, 1997; pp. 1–32. [Google Scholar] [CrossRef]
- Jensen, P.R.; Jenkins, K.M.; Porter, D.; Fenical, W. Evidence that a new antibiotic flavone glycoside chemically defends the sea grass Thalassia testudinum against zoosporic Fungi. Appl. Environ. Microbiol. 1998, 64, 1490–1496. [Google Scholar] [CrossRef]
- Trevathan-Tacketta, S.M.; Lane, A.L.; Bishop, N.; Ross, C. Metabolites derived from the tropical seagrass Thalassia testudinum are bioactive against pathogenic Labyrinthula sp. Aquat. Bot. 2015, 122, 1–8. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grignon-Dubois, M.; Rezzonico, B. Seasonal and Interannual Variability in the Phenolic Content of the Seagrass Nanozostera noltei: Characterization of Suitable Candidates for the Monitoring of Seagrass Health. Diversity 2023, 15, 1210. https://doi.org/10.3390/d15121210
Grignon-Dubois M, Rezzonico B. Seasonal and Interannual Variability in the Phenolic Content of the Seagrass Nanozostera noltei: Characterization of Suitable Candidates for the Monitoring of Seagrass Health. Diversity. 2023; 15(12):1210. https://doi.org/10.3390/d15121210
Chicago/Turabian StyleGrignon-Dubois, Micheline, and Bernadette Rezzonico. 2023. "Seasonal and Interannual Variability in the Phenolic Content of the Seagrass Nanozostera noltei: Characterization of Suitable Candidates for the Monitoring of Seagrass Health" Diversity 15, no. 12: 1210. https://doi.org/10.3390/d15121210
APA StyleGrignon-Dubois, M., & Rezzonico, B. (2023). Seasonal and Interannual Variability in the Phenolic Content of the Seagrass Nanozostera noltei: Characterization of Suitable Candidates for the Monitoring of Seagrass Health. Diversity, 15(12), 1210. https://doi.org/10.3390/d15121210






