Insights into Daily Dynamics of Fish Migration during Spring in the Konda River
Abstract
1. Introduction
2. Materials and Methods
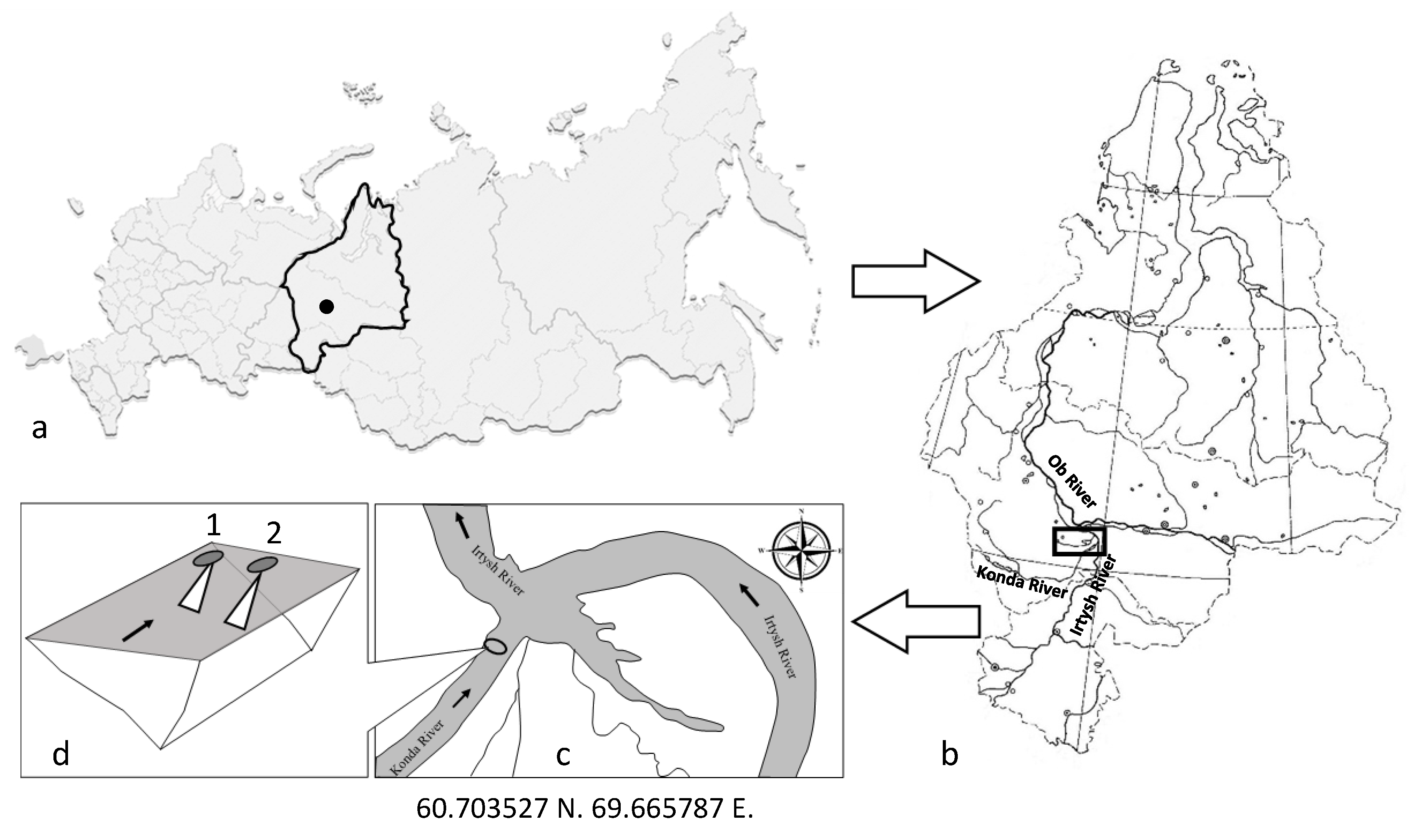
2.1. Research Area
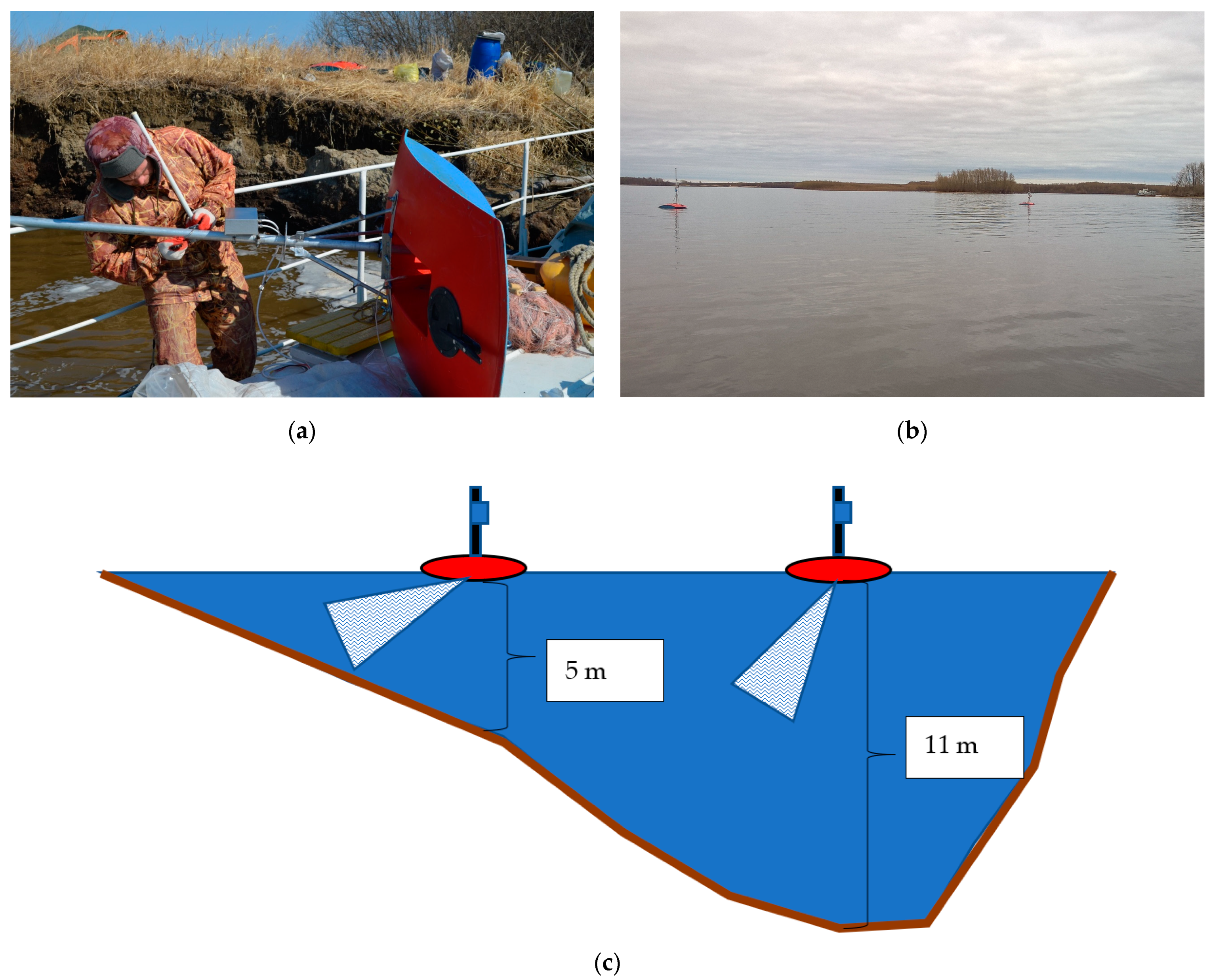
2.2. Field Work
2.3. Analyses
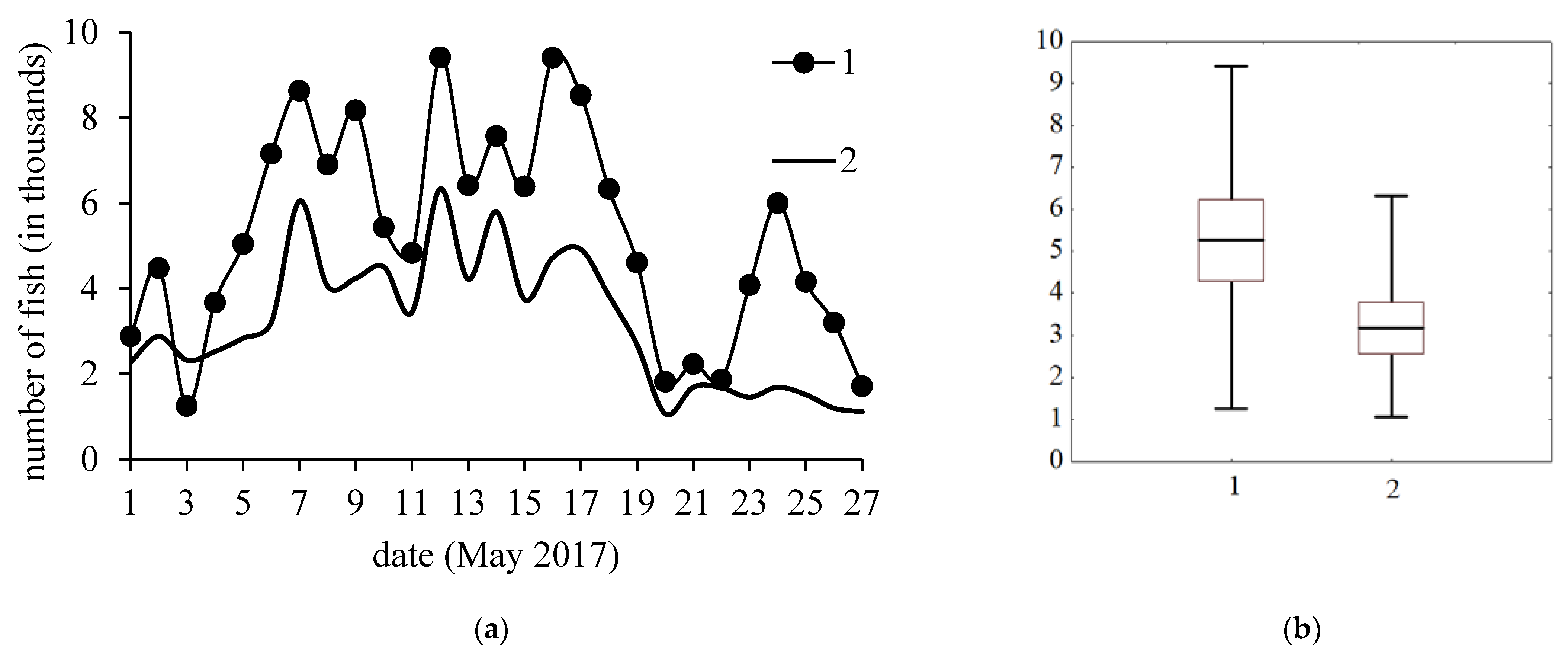
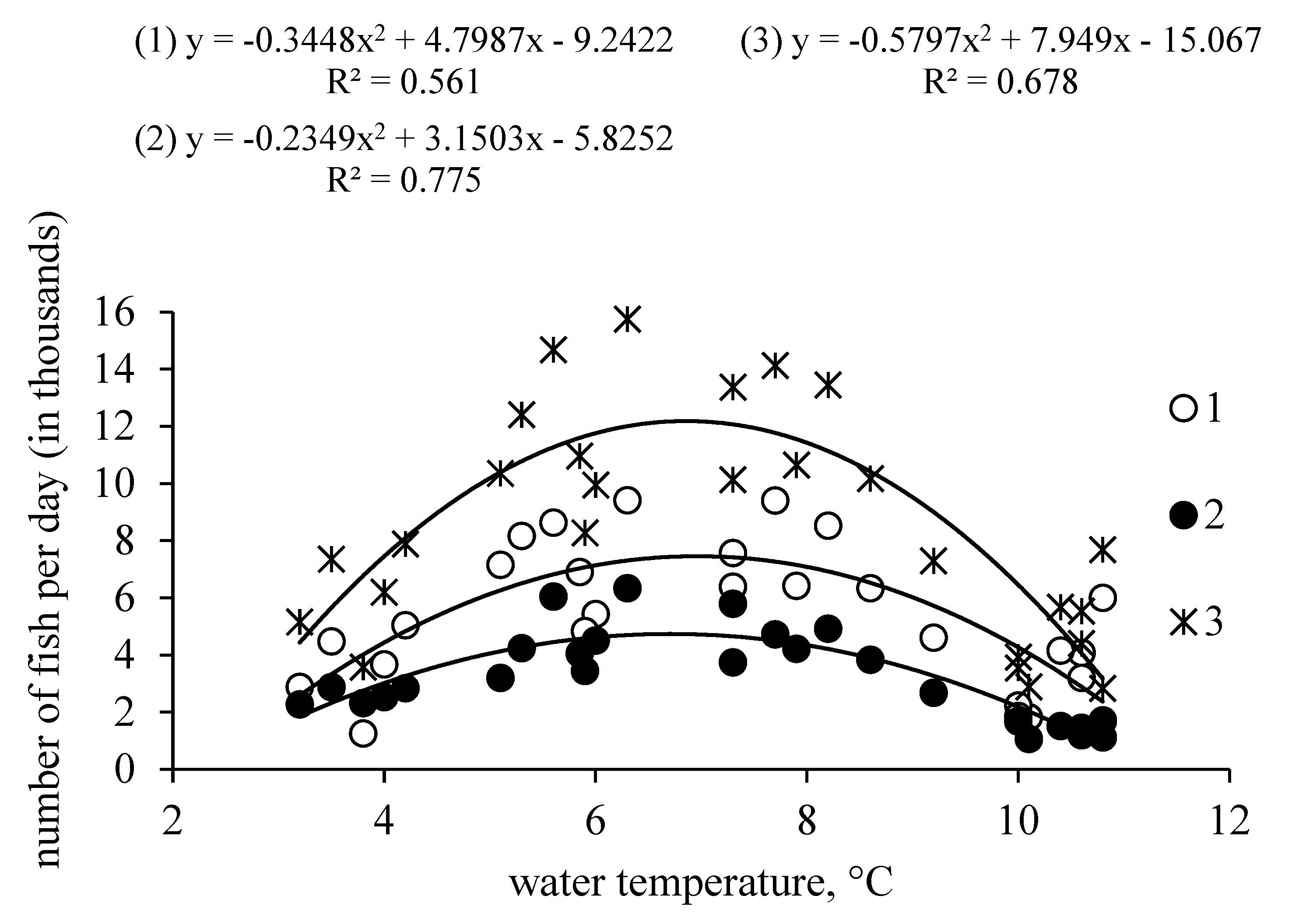
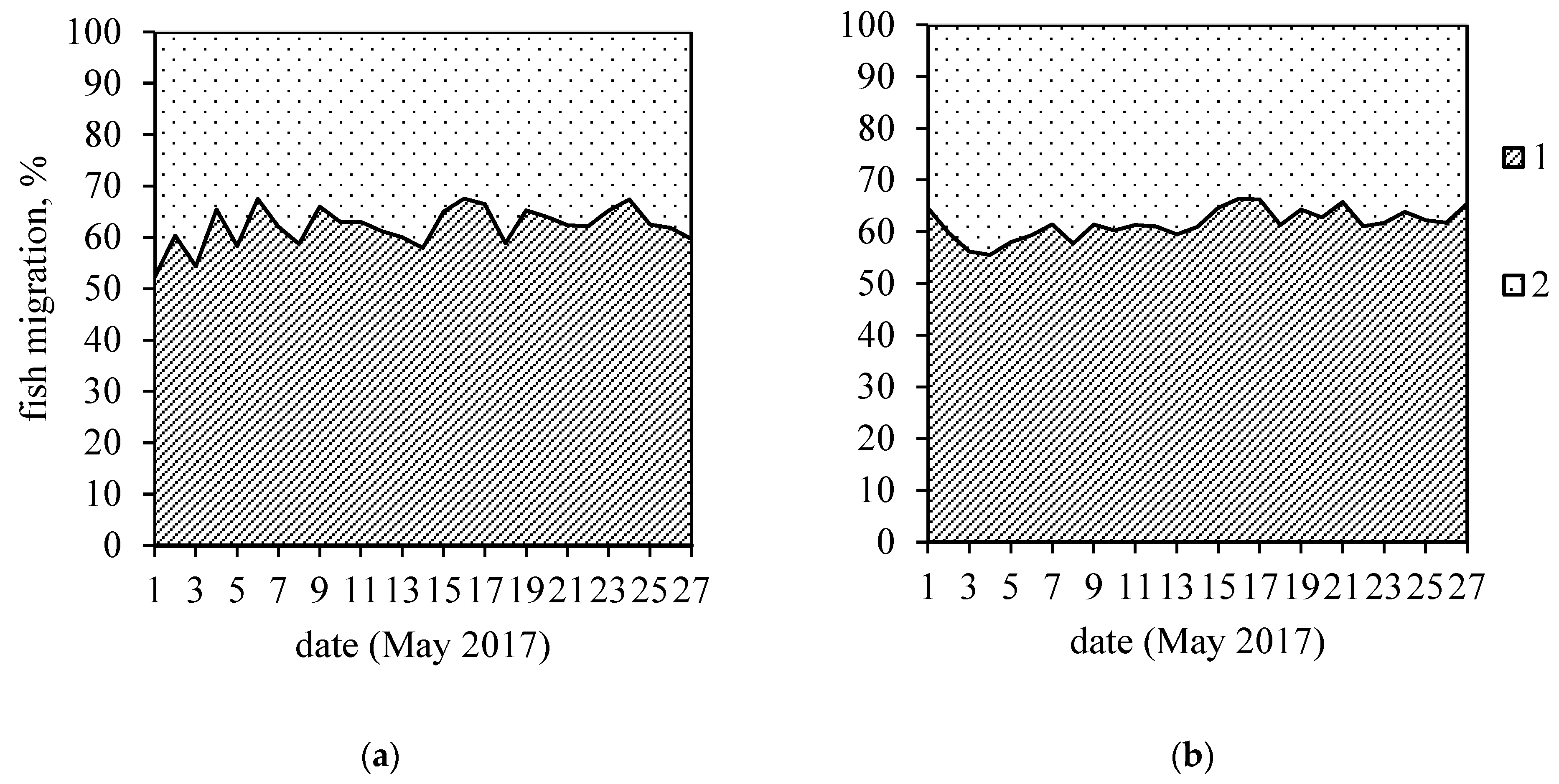
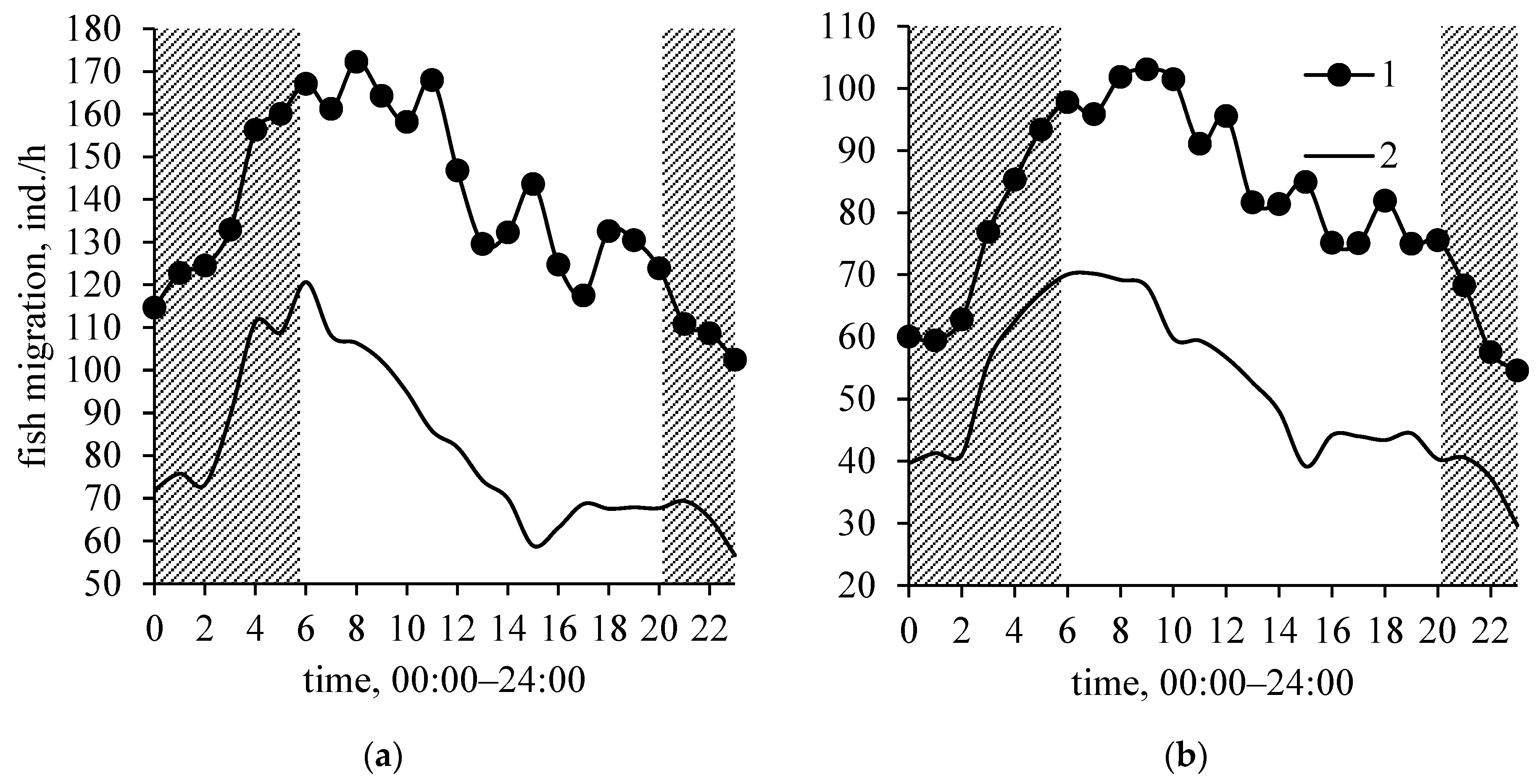
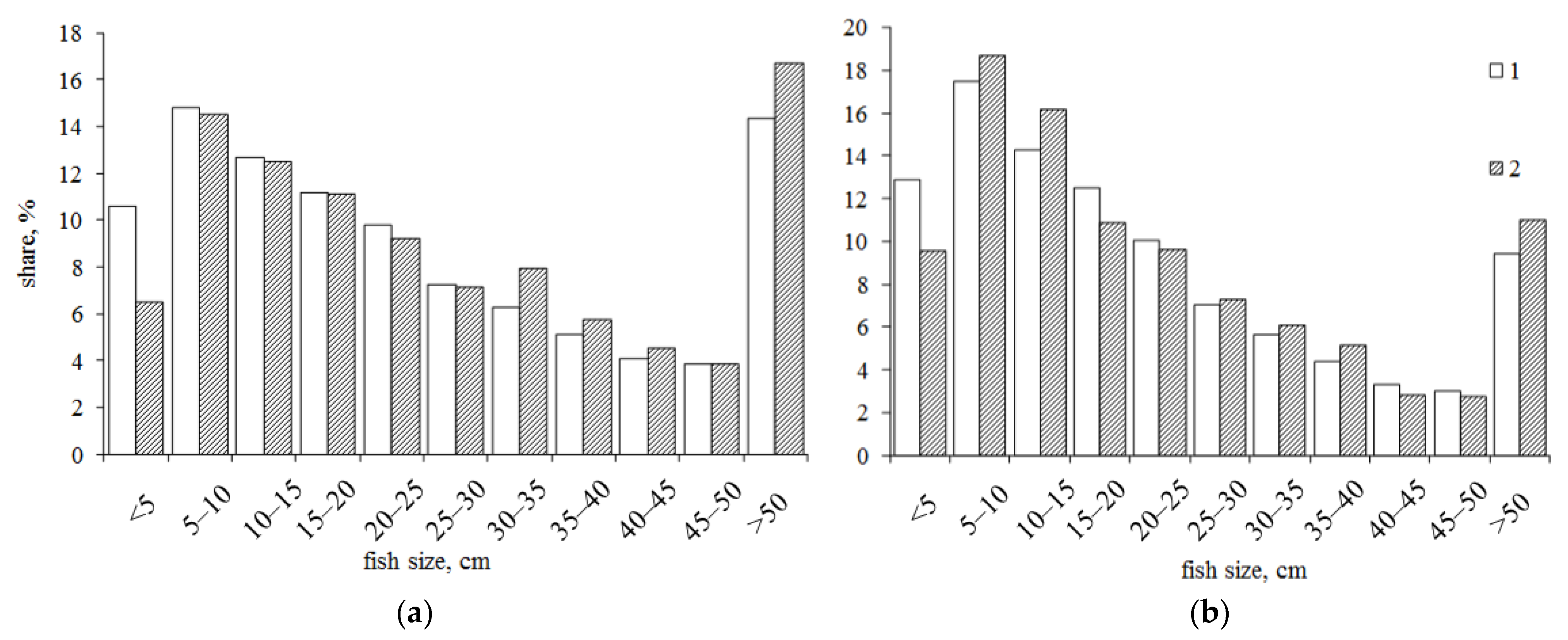
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Górski, K.; Winter, H.V.; De Leeuw, J.J.; Minin, A.E.; Nagelkerke, L.A.J. Fish spawning in a large temperate floodplain: The role of flooding and temperature. Freshw. Biol. 2010, 55, 1509–1519. [Google Scholar] [CrossRef]
- Sommer, T.R.; Harrell, W.C.; Feyrer, F. Large-bodied fish migration and residency in a flood basin of the Sacramento River, California, USA. Ecol. Freshw. Fish 2013, 23, 414–423. [Google Scholar] [CrossRef]
- Górski, K.; Buijse, A.D.; Winter, H.V.; De Leeuw, J.J.; Compton, T.J.; Vekhov, D.A.; Zolotarev, D.V.; Verreth, J.A.; Nagelkerke, L.A. Geomorphology and flooding shape fish distribution in a large-scale temperate floodplain. River Res. Appl. 2012, 29, 1226–1236. [Google Scholar] [CrossRef]
- Górski, K.; DE Leeuw, J.J.; Winter, H.V.; Vekhov, D.A.; Minin, A.E.; Buijse, A.D.; Nagelkerke, L.A.J. Fish recruitment in a large, temperate floodplain: The importance of annual flooding, temperature and habitat complexity. Freshw. Biol. 2011, 56, 2210–2225. [Google Scholar] [CrossRef]
- Bice, C.M.; Gehrig, S.L.; Zampatti, B.P.; Nicol, J.M.; Wilson, P.; Leigh, S.L.; Marsland, K. Flow-induced alterations to fish assemblages, habitat and fish–habitat associations in a regulated lowland river. Hydrobiologia 2013, 722, 205–222. [Google Scholar] [CrossRef]
- Espírito-Santo, H.M.V.; Rodríguez, M.A.; Zuanon, J. Strategies to avoid the trap: Stream fish use fine-scale hydrological cues to move between the stream channel and temporary pools. Hydrobiologia 2016, 792, 183–194. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, Z.; An, R.; Cai, L.; Chen, X.; Zhao, X.; Zou, X. Water flow and substrate preferences of Schizothorax wangchiachii (Fang, 1936). Ecol. Eng. 2019, 138, 1–7. [Google Scholar] [CrossRef]
- Bauer, S.; Hoye, B.J. Migratory Animals Couple Biodiversity and Ecosystem Functioning Worldwide. Science 2014, 344, 1242552. [Google Scholar] [CrossRef] [PubMed]
- Nunn, A.D.; Copp, G.H.; Vilizzi, L.; Carter, M.G. Seasonal and diel patterns in the migrations of fishes between a river and a floodplain tributary. Ecol. Freshw. Fish 2010, 19, 153–162. [Google Scholar] [CrossRef]
- Dutterer, A.C.; Mesing, C.; Cailteux, R.; Allen, M.S.; Pine, W.E.; Strickland, P.A. Fish recruitment is influenced by river flows and floodplain inundation at Apalachicola River, Florida. River Res. Appl. 2012, 29, 1110–1118. [Google Scholar] [CrossRef]
- Jin, B.; Winemiller, K.O.; Shao, B.; Si, J.; Jin, J.; Ge, G. Fish assemblage structure in relation to seasonal environmental variation in sub-lakes of the Poyang Lake floodplain, China. Fish. Manag. Ecol. 2019, 26, 131–140. [Google Scholar] [CrossRef]
- Walton, S.E.; Nunn, A.D.; Probst, W.N.; Bolland, J.D.; Acreman, M.; Cowx, I.G. Do fish go with the flow? The effects of periodic and episodic flow pulses on 0+ fish biomass in a constrained lowland river. Ecohydrology 2016, 10, e1777. [Google Scholar] [CrossRef]
- Espínola, L.A.; Rabuffetti, A.P.; Abrial, E.; Amsler, M.L.; Blettler, M.C.A.; Paira, A.R.; Simões, N.R.; Santos, L.N. Response of fish assemblage structure to changing flood and flow pulses in a large subtropical river. Mar. Freshw. Res. 2016, 68, 319–330. [Google Scholar] [CrossRef]
- Górski, K.; Collier, K.J.; Hamilton, D.P.; Hicks, B.J. Effects of flow on lateral interactions of fish and shrimps with off-channel habitats in a large river-floodplain system. Hydrobiologia 2012, 729, 161–174. [Google Scholar] [CrossRef]
- Naus, C.J.; Adams, S.R. Fish nursery habitat function of the main channel, floodplain tributaries and oxbow lakes of a medium-sized river. Ecol. Freshw. Fish 2016, 27, 4–18. [Google Scholar] [CrossRef]
- Reinhold, A.M.; Bramblett, R.G.; Zale, A.V.; Roberts, D.W.; Poole, G.C. Comparative use of side and main channels by small-bodied fish in a large, unimpounded river. Freshw. Biol. 2016, 61, 1611–1626. [Google Scholar] [CrossRef]
- Schletterer, M.; Shevchenko, A.A.; Yanygina, L.V.; Manakov, Y.A.; Reisenbüchler, M.; Rutschmann, P. Eindrücke vom Oberlauf des Obs in Russland. WasserWirtschaft 2021, 9–10, 77–85. Available online: https://www.springerprofessional.de/eindruecke-vom-oberlauf-des-obs-in-russland/19728084 (accessed on 21 October 2023). [CrossRef]
- Radelyuk, I.; Zhang, L.; Assanov, D.; Maratova, G.; Tussupova, K. A state-of-the-art and future perspectives of transboundary rivers in the cold climate—A systematic review of Irtysh River. J. Hydrol. Reg. Stud. 2022, 42, 101173. [Google Scholar] [CrossRef]
- Pavlov, D.S. (Ed.) Red Book of the Russian Federation, Volume “Animals”. 2021. Available online: https://www.mnr.gov.ru/activity/red_book/krasnaya-kniga-rossiyskoy-federatsii/ (accessed on 21 October 2023).
- Vodogretsky, V.E. (Ed.) Lower Irtysh and Lower Ob/L. In Surface Water Resources of the USSR. T. Altai and Western Siberia; Gidrometeoizdat: Leningrad, Russia, 1973; p. 35. 424p. [Google Scholar]
- Antonov, A.I. Main causes and consequences of freezing phenomena in the Ob-Irtysh basin. In Proceedings of the Materials of the 2nd National Scientific and Practical Conference “Integration of Science and Practice for the Development of the Agricultural Complex, Bogor, Indonesia, 10–11 October 2019; pp. 26–32. [Google Scholar]
- Chemagin, A.A. Winter refuge for freshwater fish. E3S Web Conf. 2023, 390, 07008. [Google Scholar] [CrossRef]
- Brownscombe, J.W.; Lédée, E.J.I.; Raby, G.D.; Struthers, D.P.; Gutowsky, L.F.G.; Nguyen, V.M.; Young, N.; Stokesbury, M.J.W.; Holbrook, C.M.; Brenden, T.O.; et al. Conducting and interpreting fish telemetry studies: Considerations for researchers and resource managers. Rev. Fish Biol. Fish. 2019, 29, 369–400. [Google Scholar] [CrossRef]
- Matley, J.K.; Klinard, N.V.; Barbosa Martins, A.P.; Aarestrup, K.; Aspillaga, E.; Cooke, S.J.; Cowley, P.D.; Heupel, M.R.; Lowe, C.G.; Lowerre-Barbieri, S.K.; et al. Global trends in aquatic animal tracking with acoustic telemetry. Trends Ecol. Evol. 2022, 37, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Musselman, W.C.; Worthington, T.A.; Mouser, J.; Williams, D.M.; Brewer, S.K. Passive Integrated Transponder Tags: Review of Studies on Warmwater Fishes with Notes on Additional Species. J. Fish Wildl. Manag. 2017, 8, 353–364. [Google Scholar] [CrossRef]
- Vollset, K.W.; Lennox, R.J.; Thorstad, E.B.; Auer, S.; Bär, K.; Larsen, M.H.; Mahlum, S.; Näslund, J.; Stryhn, H.; Dohoo, I. Systematic review and meta-analysis of PIT tagging effects on mortality and growth of juvenile salmonids. Rev. Fish Biol. Fish. 2020, 30, 553–568. [Google Scholar] [CrossRef]
- Maxwell, S. Hydroacoustics: Rivers. In Salmonid Field Protocols Handbook: Techniques for Assessing Status and Trends in Salmon and Trout Populations; Johnson, D.H., Shier, B.M., O’Neal, J.S., Knutzen, J.A., Augerot, X., O’Neill, T.A., Pearsons, T.N., Eds.; American Fisheries Society: Bethesda, MD, USA, 2007; pp. 133–152. [Google Scholar]
- Simmonds, E.J.; MacLennan, D.N. Fisheries Acoustics, 2nd ed.; Blackwell Science: Oxford, UK, 2005. [Google Scholar]
- Ransom, B.H.; Johnston, S.V.; Steig, T.W. Review on monitoring adult salmonid (Oncorhynchus and Salmo spp.) escapement using fixed-location split-beam hydroacoustics. Fish. Res. 1998, 35, 33–42. [Google Scholar] [CrossRef]
- Balk, H.; Lindem, T. Improved fish detection in data from split-beam sonar. Aquat. Living Resour. 2000, 13, 297–303. [Google Scholar] [CrossRef]
- Pavlov, D.S.; Mochek, A.D.; Borisenko, E.S.; Degtev, A.I. Hydroacoustic investigation of taxonomic composition and of vertical distribution of fish in the riverbed depression. J. Ichthyol. 2010, 50, 969–976. [Google Scholar] [CrossRef]
- Borisenko, E.S.; Pavlov, D.S.; Kuzishchin, K.V. Hydroacoustic Studies of the Migration of Anadromous Rainbow Trout Parasalmo mykiss (Salmonidae) in the Kvachina River (Western Kamchatka). J. Ichthyol. 2022, 62, 1149–1159. [Google Scholar] [CrossRef]
- Pavlov, D.S.; Borisenko, E.S.; Mochek, A.D.; Degtev, E.I. Hydroacoustic study of Salmo salar migration in the Shuya River (Onega Lake Basin). J. Ichthyol. 2011, 51, 646–651. [Google Scholar] [CrossRef]
- Johnson, G.R.; Marcek, B.J. Optimizing data quantity and quality for side-looking echosounder surveys in large rivers. Fish. Res. 2023, 264, 106713. [Google Scholar] [CrossRef]
- Wanzenböck, J.; Mehner, T.; Schulz, M.; Gassner, H.; Winfield, I.J. Quality assurance of hydroacoustic surveys: The repeatability of fish-abundance and biomass estimates in lakes within and between hydroacoustic systems. ICES J. Mar. Sci. 2003, 60, 486–492. [Google Scholar] [CrossRef]
- Mehner, T. Prediction of hydroacoustic target strength of vendace (Coregonus albula) from concurrent trawl catches. Fish. Res. 2006, 79, 162–169. [Google Scholar] [CrossRef]
- Probst, W.N.; Thomas, G.; Eckmann, R. Hydroacoustic observations of surface shoaling behaviour of young-of-the-year perch Perca fluviatilis (Linnaeus, 1758) with a towed upward-facing transducer. Fish. Res. 2008, 96, 133–138. [Google Scholar] [CrossRef][Green Version]
- Wanzenböck, J.; Kubecka, J.; Sajdlova, Z.; Frouzova, J. Hydroacoustic target strength vs. fish length revisited: Data of caged, free-swimming European whitefish (Coregonus lavaretus L.) suggest a bi-phasic linear relationship under a limited range of tilt angles. Fish. Res. 2020, 229, 105620. [Google Scholar] [CrossRef]
- Perivolioti, T.-M.; Frouzova, J.; Tušer, M.; Bobori, D. Assessing the Fish Stock Status in Lake Trichonis: A Hydroacoustic Approach. Water 2020, 12, 1823. [Google Scholar] [CrossRef]
- Goncharov, S.; Popov, S.; Peterfeld, V. The results of hydroacoustic studies of the Baikal omul (Coregonus migratorius) in the fishing waters of Lake Baikal using domestic information developments. Fisheries 2022, 2022, 54–58. [Google Scholar] [CrossRef]
- Ransom, B.H.; Steig, T.W. Using Hydroacoustics to Monitor Fish at Hydropower Dams. Lake Reserv. Manag. 1994, 9, 163–169. [Google Scholar] [CrossRef]
- Schmidt, M.B.; Tuhtan, J.A.; Schletterer, M. Hydroacoustic and Pressure Turbulence Analysis for the Assessment of Fish Presence and Behavior Upstream of a Vertical Trash Rack at a Run-of-River Hydropower Plant. Appl. Sci. 2018, 8, 1723. [Google Scholar] [CrossRef]
- Zhang, P.; Qiao, Y.; Jin, Y.; Lek, S.; Yan, T.; He, Z.; Chang, J.; Cai, L. Upstream migration of fishes downstream of an under-construction hydroelectric dam and implications for the operation of fish passage facilities. Glob. Ecol. Conserv. 2020, 23, e01143. [Google Scholar] [CrossRef]
- Luo, B.; Zhou, X.; Zhang, C.; Bao, J.; Mei, F.; Lian, Y.; Zhang, D.; Hu, S.; Guo, L.; Duan, M. Hydroacoustic survey on fish spatial distribution in the early impoundment stage of Yuwanghe Reservoir in southwest China. Front. Mar. Sci. 2023, 10, 1119411. [Google Scholar] [CrossRef]
- Chemagin, A.A. Distribution of migratory fish in the stream (depth, velocity, body size, predators). Biosyst. Divers. 2019, 27, 221–226. [Google Scholar] [CrossRef]
- Chemagin, A. Distribution of cyprinids in the stream during their spring upstream migration. E3S Web Conf. 2020, 164, 07029. [Google Scholar] [CrossRef]
- Chemagin, A.A. Effects of temperature and water levels on dynamics of density and structure of the fish population of the channel-floodplain complex of a large river in the period of spring floods. Biosyst. Divers. 2019, 27, 291–299. [Google Scholar] [CrossRef]
- Russian Water Register. Available online: https://textual.ru/gvr/ (accessed on 21 October 2023).
- Seredovskikh, B.A. Hydromorphology of the Konda River: A Retrospective Aspect of the Dynamics of Channel Changes: Monograph; NVGU Publishing House: Nizhnevartovsk, Russia, 2022; 155p. (In Russian) [Google Scholar]
- Borisenko, E.S.; Degtev, A.I.; Mochek, A.D.; Pavlov, D.S. Hydroacoustic characteristics of mass fishes of the Ob-Irtysh Basin. J. Ichthyol. 2006, 46, S227–S234. [Google Scholar] [CrossRef]
- Alimova, G.S.; Dudareva, I.A. Hydrochemistry of the Gornoslinkinskaya wintering pits and floodplain ponds in lower reaches of the river Irtysh. Int. J. Appl. Fundam. Res. 2016, 12, 977–982. [Google Scholar]
- Mochek, A.D.; Borisenko, E.S.; Pavlov, D. Winter Fish Distribution in the Riverbed Depression in the Urtysh [Irtysh] River. J. Ichthyol. 2019, 59, 352–357. [Google Scholar] [CrossRef]
- Chemagin, A.A. Distribution of sturgeon in the River Irtysh. Regul. Mech. Biosyst. 2020, 11, 444–448. [Google Scholar] [CrossRef]
- Neuburg, J.; Friedrich, T. First description of a remnant population of sterlet (Acipenser ruthenus, LINNAEUS 1758) in the eastern Austrian Danube. J. Nat. Conserv. 2023, 75, 126473. [Google Scholar] [CrossRef]
- Buhardinova, M. Distribution and migration cycle of nelma Stenodus leucichthys nelma (Pallas, 1773). Vestnik Astrakhan State Tech. Univ. 2022, 2022, 16–24. [Google Scholar] [CrossRef]
- Bogdanov, V.D.; Agafonov, L.I. Influence of Hydrologic Conditions of the Lower Ob Floodplain on Reproduction in Coregonids. Russ. J. Ecol. 2001, 32, 45–51. [Google Scholar] [CrossRef]
- Semenchenko, S.; Smeshlivaya, N. Spawning behaviour of whitefishes (Coregonidae). Ann. Zool. Fennici 2021, 58, 129–140. [Google Scholar] [CrossRef]
- Hladík, M.; Kubečka, J. Fish migration between a temperate reservoir and its main tributary. Hydrobiologia 2003, 504, 251–266. [Google Scholar] [CrossRef]
- Howell, P.J.; Dunham, J.B.; Sankovich, P.M. Relationships between water temperatures and upstream migration, cold water refuge use, and spawning of adult bull trout from the Lostine River, Oregon, USA. Ecol. Freshw. Fish 2010, 19, 96–106. [Google Scholar] [CrossRef]
- Nagayama, S.; Sueyoshi, M.; Fujii, R.; Harada, M. Basin-scale spatiotemporal distribution of ayu Plecoglossus altivelis and its relationship with water temperature from summer growth to autumn spawning periods. Landsc. Ecol. Eng. 2022, 19, 21–31. [Google Scholar] [CrossRef]
- Bogdanov, V.D.; Mel’nichenko, I.P. Sovremennoye Sostoyaniye Nel’my v Basseyne Reki Severnoy Sos’vy. Vestnik Astra-Khanskogo Gosudarstvennogo Tekhnicheskogo Universiteta. Seriya: Rybnoye Khozyaystvo. 2013, Volume 3, pp. 20–24. Available online: https://vestnik.astu.org/ru/nauka/article/31930/view/ (accessed on 21 May 2022).
- Walsh, C.T.; Reinfelds, I.V.; Gray, C.A.; West, R.J.; van der Meulen, D.E.; Craig, J.R. Seasonal residency and movement patterns of two co-occurring catadromous percichthyids within a south-eastern Australian river. Ecol. Freshw. Fish 2011, 21, 145–159. [Google Scholar] [CrossRef]
- Jones, M.J.; Stuart, I.G. Lateral movement of common carp (Cyprinus carpio L.) in a large lowland river and floodplain. Ecol. Freshw. Fish 2009, 18, 72–82. [Google Scholar] [CrossRef]
- Hohausová, E.; Copp, G.H.; Jankovský, P. Movement of fish between a river and its backwater: Diel activity and relation to environmental gradients. Ecol. Freshw. Fish 2003, 12, 107–117. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Q.; Liu, C.; Xian, W. Seasonal and Spatial Variations in Fish Assemblage in the Yangtze Estuary and Adjacent Waters and Their Relationship with Environmental Factors. J. Mar. Sci. Eng. 2022, 10, 1679. [Google Scholar] [CrossRef]
- Garner, P. Swimming ability and differential use of velocity patches by 0+ cyprinids. Ecol. Freshw. Fish 1999, 8, 55–58. [Google Scholar] [CrossRef]
- Clark, S.P.; Toews, J.S.; Tkach, R. Beyond average velocity: Modelling velocity distributions in partially filled culverts to support fish passage guidelines. Int. J. River Basin Manag. 2014, 12, 101–110. [Google Scholar] [CrossRef]
- Dockery, D.R.; McMahon, T.E.; Kappenman, K.M.; Blank, M. Evaluation of swimming performance for fish passage of longnose dace Rhinichthys cataractae using an experimental flume. J. Fish Biol. 2016, 90, 980–1000. [Google Scholar] [CrossRef]
- Crisp, D.T.; Hurley, M.A. Stream channel experiments on downstream movement of recently emerged trout, Salmo trutta L. and salmon, S. salar L.—I. Effect of four different water velocity treatments upon dispersal rate. J. Fish Biol. 1991, 39, 347–361. [Google Scholar] [CrossRef]
- Tudorache, C.; Viaene, P.; Blust, R.; Vereecken, H.; De Boeck, G. A comparison of swimming capacity and energy use in seven European freshwater fish species. Ecol. Freshw. Fish 2007, 17, 284–291. [Google Scholar] [CrossRef]
- Kopf, S.M.; Humphries, P.; Watts, R.J. Ontogeny of critical and prolonged swimming performance for the larvae of six Australian freshwater fish species. J. Fish Biol. 2014, 84, 1820–1841. [Google Scholar] [CrossRef]
- Heggenes, J.; Traaen, T. Downstream migration and critical water velocities in stream channels for fry of four salmonid species. J. Fish Biol. 1988, 32, 717–727. [Google Scholar] [CrossRef]
- Heggenes, J.; Baglinière, J.L.; Cunjak, R.A. Spatial niche variability for young Atlantic salmon (Salmo salar) and brown trout (S. trutta) in heterogeneous streams. Ecol. Freshw. Fish 1999, 8, 1–21. [Google Scholar] [CrossRef]
- Deslauriers, D.; Kieffer, J.D. The influence of flume length and group size on swimming performance in shortnose sturgeon Acipenser brevirostrum. J. Fish Biol. 2011, 79, 1146–1155. [Google Scholar] [CrossRef]
- Cattanéo, F.; Grimardias, D.; Carayon, M.; Persat, H.; Bardonnet, A. A multidimensional typology of riverbank habitats explains the distribution of European grayling (Thymallus thymallus L.) fry in a temperate river. Ecol. Freshw. Fish 2013, 23, 527–543. [Google Scholar] [CrossRef]
- Deslauriers, D.; Heironimus, L.B.; Rapp, T.; Graeb, B.D.S.; Klumb, R.A.; Chipps, S.R. Growth potential and habitat requirements of endangered age-0 pallid sturgeon (Scaphirhynchus albus) in the Missouri River, USA, determined using a individual-based model framework. Ecol. Freshw. Fish 2017, 27, 198–208. [Google Scholar] [CrossRef]
- Helfman, G.S. Fish Behaviour by Day, Night and Twilight. In The Behaviour of Teleost Fishes; Pitcher, T.J., Ed.; Springer: Boston, MA, USA, 1986. [Google Scholar] [CrossRef]
- Perkin, E.K.; Hölker, F.; Richardson, J.S.; Sadler, J.P.; Wolter, C.; Tockner, K. The influence of artificial light on stream and riparian ecosystems: Questions, challenges, and perspectives. Ecosphere 2011, 2, 1–16. [Google Scholar] [CrossRef]
- Brüning, A.; Hölker, F.; Franke, S.; Preuer, T.; Kloas, W. Spotlight on fish: Light pollution affects circadian rhythms of European perch but does not cause stress. Sci. Total. Environ. 2015, 511, 516–522. [Google Scholar] [CrossRef]
- Bassi, A.; Love, O.P.; Cooke, S.J.; Warriner, T.R.; Harris, C.M.; Madliger, C.L. Effects of artificial light at night on fishes: A synthesis with future research priorities. Fish Fish. 2021, 23, 631–647. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Reebs, S.G. Circadian Rhythms in Fish. Behav. Physiol. Fish 2005, 24, 197–238. [Google Scholar] [CrossRef]
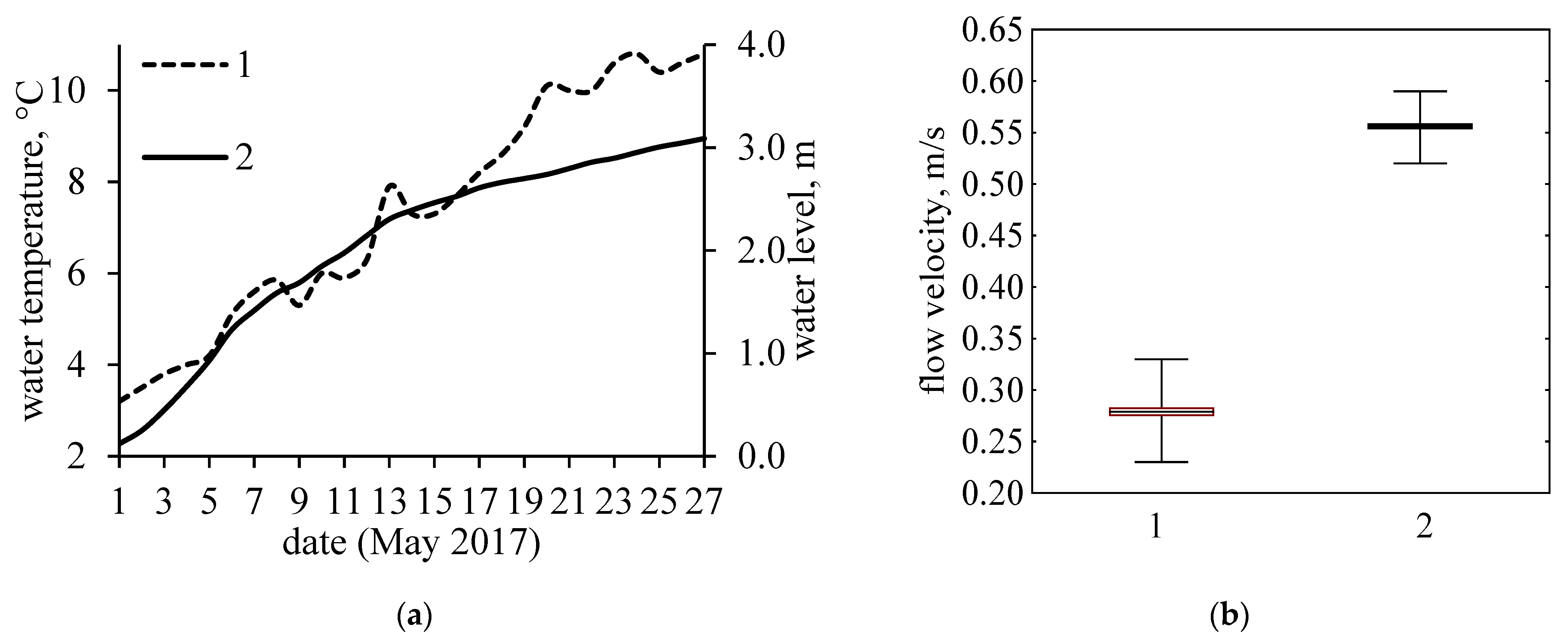


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chemagin, A.A.; Schletterer, M. Insights into Daily Dynamics of Fish Migration during Spring in the Konda River. Diversity 2023, 15, 1211. https://doi.org/10.3390/d15121211
Chemagin AA, Schletterer M. Insights into Daily Dynamics of Fish Migration during Spring in the Konda River. Diversity. 2023; 15(12):1211. https://doi.org/10.3390/d15121211
Chicago/Turabian StyleChemagin, Andrey A., and Martin Schletterer. 2023. "Insights into Daily Dynamics of Fish Migration during Spring in the Konda River" Diversity 15, no. 12: 1211. https://doi.org/10.3390/d15121211
APA StyleChemagin, A. A., & Schletterer, M. (2023). Insights into Daily Dynamics of Fish Migration during Spring in the Konda River. Diversity, 15(12), 1211. https://doi.org/10.3390/d15121211






