Morphological and Molecular Characterization of the Unarmored Dinoflagellate Gymnodinium trapeziforme (Dinophyceae) from Jiaozhou Bay, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Culture Establishment
2.2. Light Microscopy
2.3. Scanning Electron Microscopy (SEM)
2.4. DNA Extraction, PCR Amplification, and Sequencing
2.5. Phylogenetic Analyses
3. Results
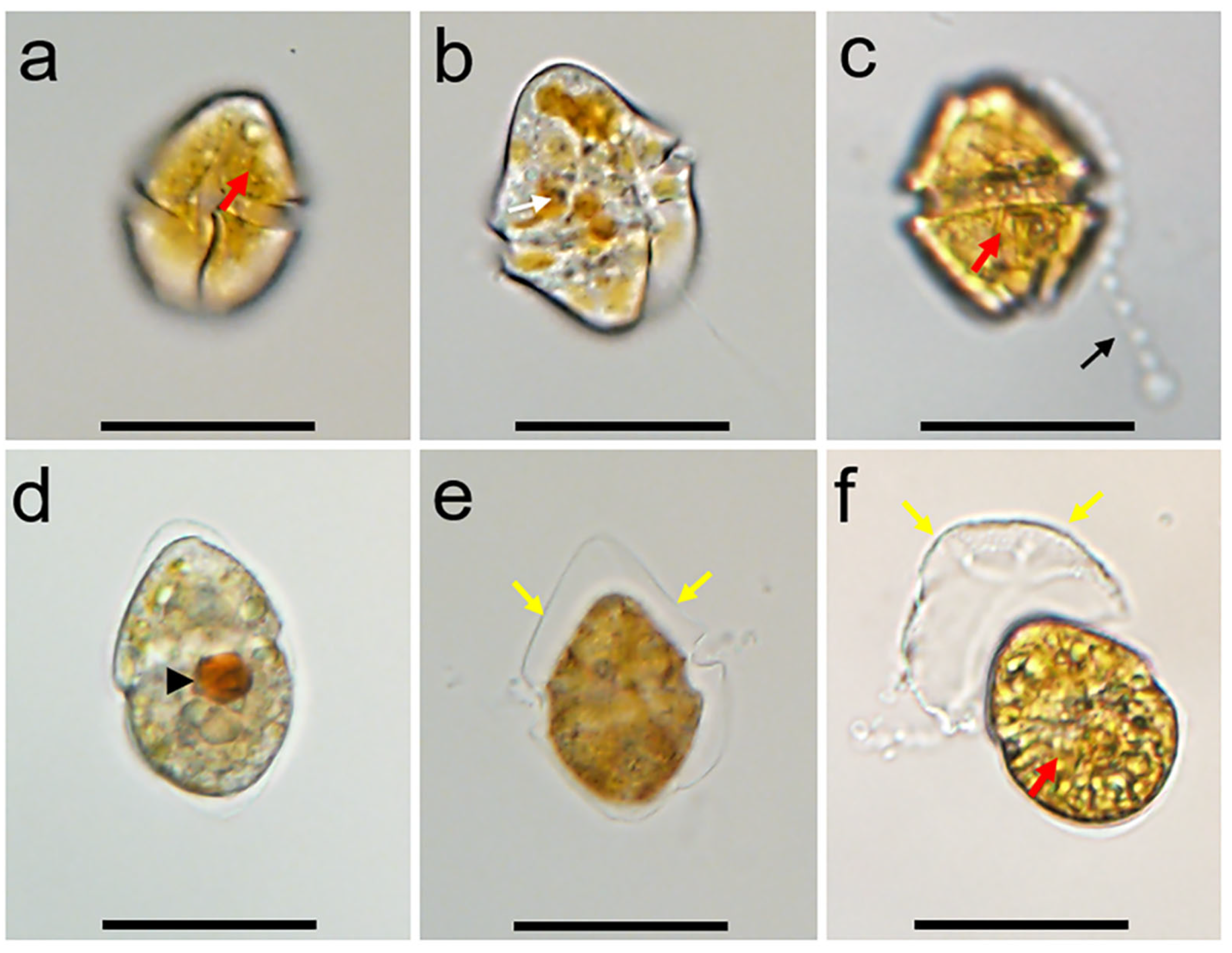
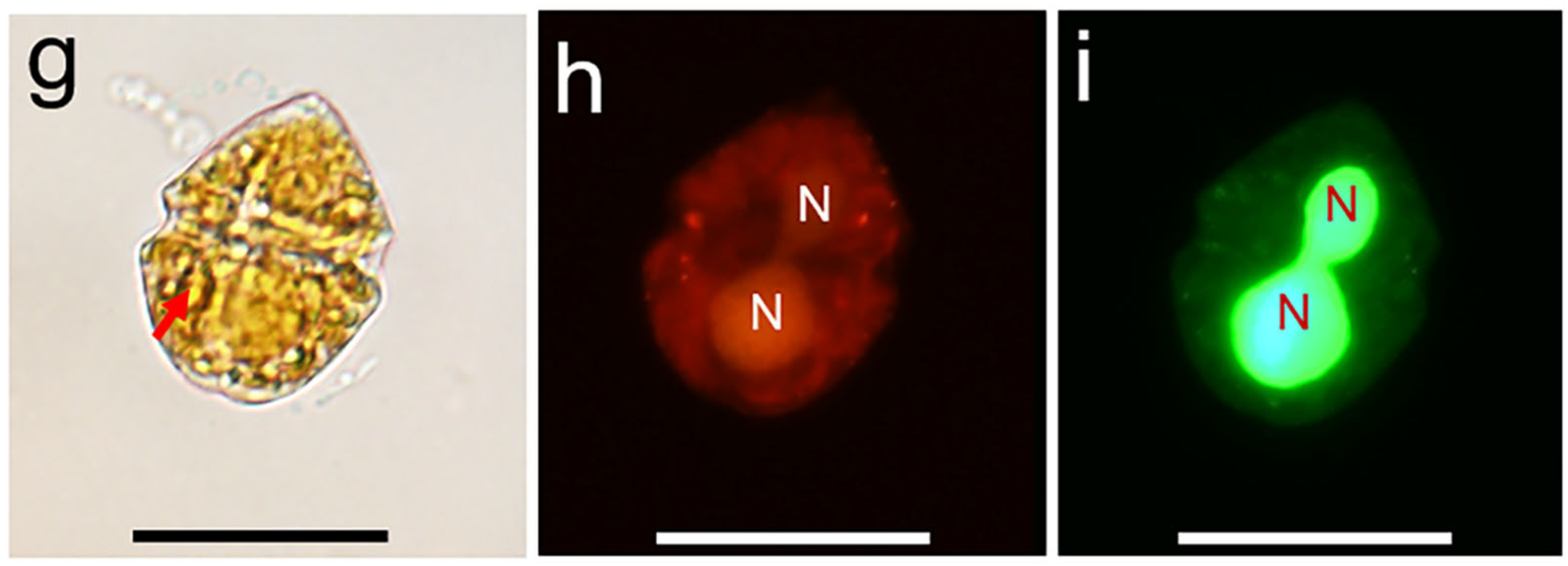
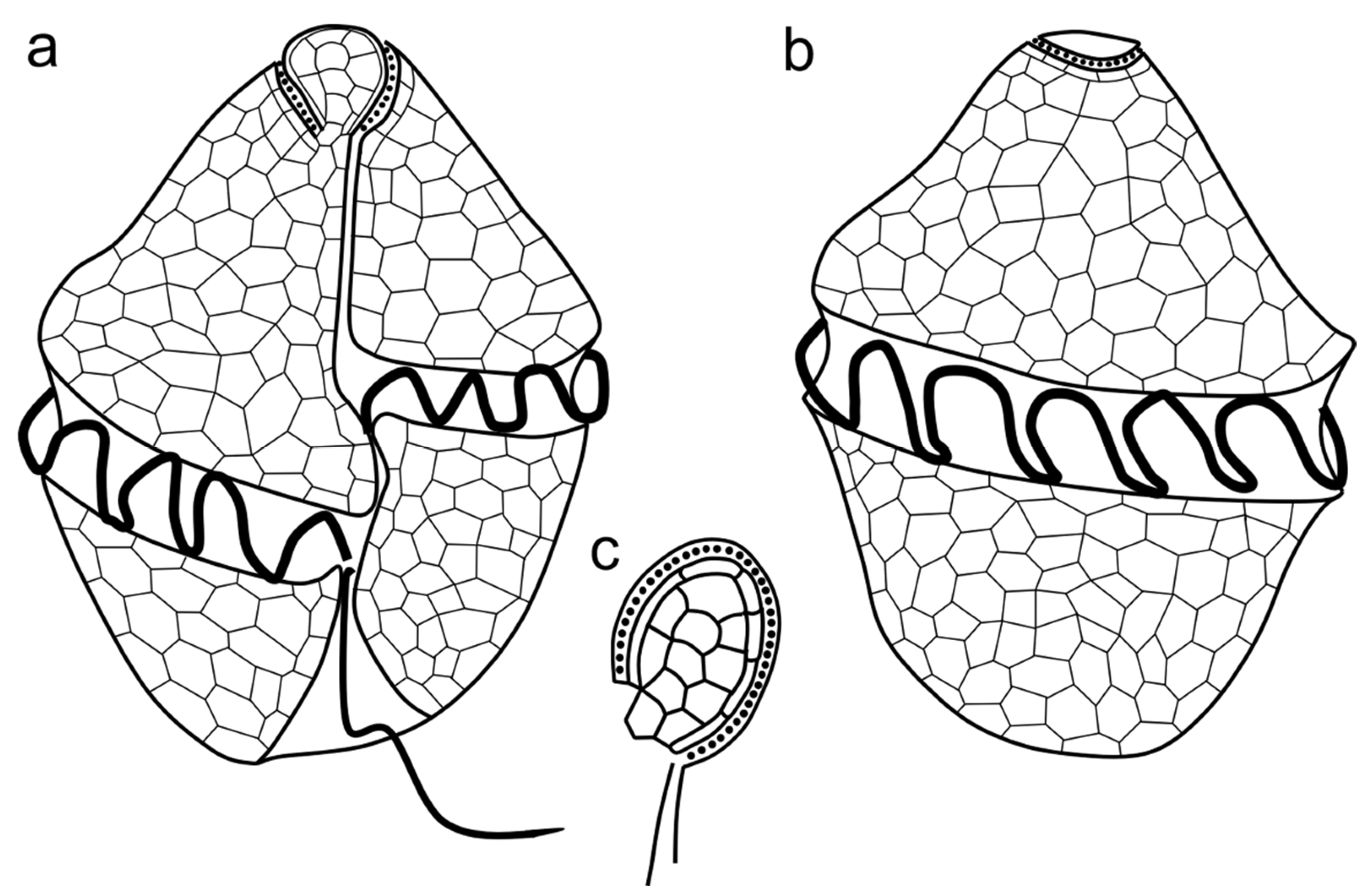
3.1. Morphology
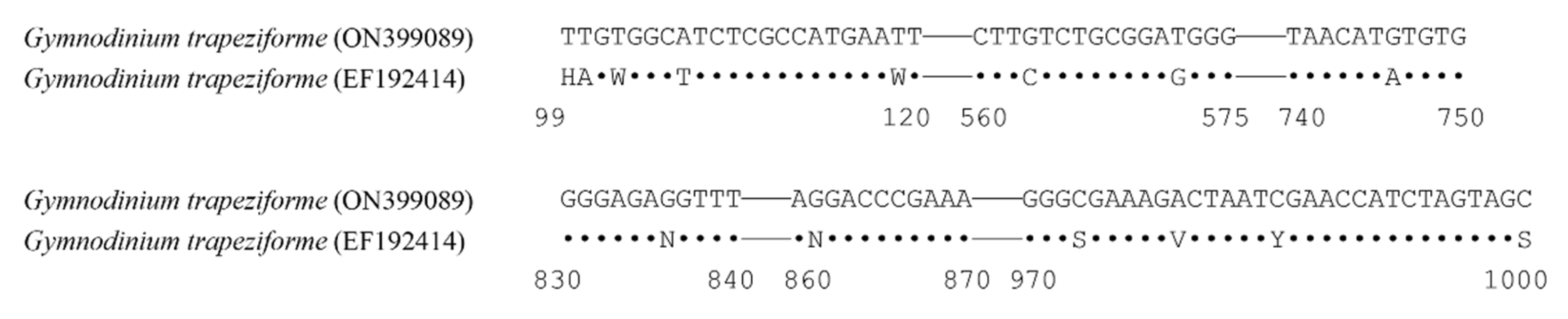
3.2. Phylogeny
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez, F. A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata). CICIMAR Oceánides 2012, 27, 65–140. [Google Scholar] [CrossRef]
- Gómez, F. Diversity and classification of dinoflagellates. In Dinoflagellates: Classification, Evolution, Physiology, and Ecological Significance; Rao, D.V.S., Ed.; Nova Science Publisher: New York, NY, USA, 2020; p. 38. [Google Scholar]
- Tillmann, U.; Gottschling, M.; Nézan, E.; Krock, B.; Bilien, G. Morphological and molecular characterization of three new Azadinium species (Amphidomataceae, Dinophyceae) from the Irminger Sea. Protist 2014, 165, 417–444. [Google Scholar] [CrossRef]
- Tillmann, U.; Gottschling, M.; Wietkamp, S.; Hoppenrath, M. Morphological and phylogenetic characterisation of Prorocentrum spinulentum, sp. nov. (Prorocentrales, Dinophyceae), a small spiny species from the North Atlantic. Microorganisms 2023, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Gómez, F.; López-García, P.; Takayama, H.; Moreira, D. Balechina and the new genus Cucumeridinium gen. nov. (Dinophyceae), unarmored dinoflagellates with thick cell coverings. J. Phycol. 2015, 51, 1088–1105. [Google Scholar] [CrossRef]
- Takahashi, K.; Benico, G.; Lum, W.M.; Iwataki, M. Gertia stigmatica gen. et sp. nov. (Kareniaceae, Dinophyceae), a new marine unarmored dinoflagellate possessing the peridinin-type chloroplast with an eyespot. Protist 2019, 170, 125680. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Moestrup, Ø.; Jordan, R.W.; Iwataki, M. Two new freshwater woloszynskioids Asulcocephalium miricentonis gen. et sp. nov. and Leiocephalium pseudosanguineum gen. et sp. nov. (Suessiaceae, Dinophyceae) Lacking an apical furrow apparatus. Protist 2015, 166, 638–658. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Moestrup, Ø.; Wada, M.; Ishimatsu, A.; Nguyen, V.N.; Fukuyo, Y.; Iwataki, M. Dactylodinium pterobelotum gen. et sp. nov., a new marine woloszynskioid dinoflagellate positioned between the two families Borghiellaceae and Suessiaceae. J. Phycol. 2017, 53, 1223–1240. [Google Scholar] [CrossRef]
- Boutrup, P.V.; Moestrup, Ø.; Tillmann, U.; Daugbjerg, N. Ultrastructure and phylogeny of Kirithra asteri gen. et sp. nov. (Ceratoperidiniaceae, Dinophyceae)—A free-living, thin-walled marine photosynthetic dinoflagellate from Argentina. Protist 2017, 168, 586–611. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, Z.; Tang, Y.; Mertens, K.N.; Leaw, C.P.; Lim, P.T.; Teng, S.T.; Wang, L.; Gu, H. Morphology, ultrastructure, and molecular phylogeny of Wangodinium sinense gen. et sp. nov. (Gymnodiniales, Dinophyceae) and revisiting of Gymnodinium dorsalisulcum and Gymnodinium impudicum. J. Phycol. 2018, 54, 744–761. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, N.; Gu, H.; Chai, Z.; Takahashi, K.; Li, Z.; Deng, Y.; Iwataki, M.; Matsuoka, K.; Tang, Y.Z. Morpho-molecular description of a new HAB species, Pseudocochlodinium profundisulcus gen. et sp. nov., and its LSU rRNA gene based genetic diversity and geographical distribution. Harmful Algae 2021, 108, 102098. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Z.; Deng, Y.; Iwataki, M.; Luo, Z.; Wang, J.; Sun, Y.; Zhao, Z.; Gu, H.; Shin, H.H.; et al. Morphology, ultrastructure, and molecular phylogeny of the unarmoured dinoflagellate Kirithra sigma sp. nov. (Ceratoperidiniaceae, Dinophyceae). Phycologia 2020, 59, 385–396. [Google Scholar] [CrossRef]
- Li, Z.; Mertens, K.N.; Gottschling, M.; Gu, H.; Söhner, S.; Price, A.M.; Marret, F.; Pospelova, V.; Smith, K.F.; Carbonell-Moore, C.; et al. Taxonomy and molecular phylogenetics of Ensiculiferaceae, fam. nov. (Peridiniales, Dinophyceae), with consideration of their life-history. Protist 2020, 171, 125759. [Google Scholar] [CrossRef] [PubMed]
- Ok, J.H.; Jeong, H.J.; Lee, S.Y.; Park, S.A.; Noh, J.H. Shimiella gen. nov. and Shimiella gracilenta sp. nov. (Dinophyceae, Kareniaceae), a kleptoplastidic dinoflagellate from Korean waters and its survival under starvation. J. Phycol. 2021, 57, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Mertens, K.N.; Derrien, A.; Bilien, G.; Li, Z.; Hess, P.; Séchet, V.; Krock, B.; Amorim, A.; Li, Z.; et al. Unraveling the Gonyaulax baltica species complex: Cyst–theca relationship of Impagidinium variaseptum, Spiniferites pseudodelicatus sp. nov. and S. ristingensis (Gonyaulacaceae, Dinophyceae), with descriptions of Gonyaulax bohaiensis sp. nov, G. amoyensis sp. nov. and G. portimonensis sp. nov. J. Phycol. 2022, 58, 465–486. [Google Scholar] [CrossRef]
- Hanifah, A.H.; Teng, S.T.; Law, I.K.; Abdullah, N.; Chiba, S.U.A.; Lum, W.M.; Tillmann, U.; Lim, P.T.; Leaw, C.P. Six marine thecate Heterocapsa (Dinophyceae) from Malaysia, including the description of three novel species and their cytotoxicity potential. Harmful Algae 2022, 120, 102338. [Google Scholar] [CrossRef]
- Abdullah, N.; Teng, S.T.; Hanifah, A.H.; Law, I.K.; Tan, T.H.; Krock, B.; Harris, T.M.; Nagai, S.; Lim, P.T.; Tillmann, U.; et al. Thecal plate morphology, molecular phylogeny, and toxin analyses reveal two novel species of Alexandrium (Dinophyceae) and their potential for toxin production. Harmful Algae 2023, 127, 102475. [Google Scholar] [CrossRef]
- Chomérat, N.; Saburova, M.; Bilien, G.; Zentz, F.; Hoppenrath, M. Morphology and molecular phylogeny of a widely distributed but little-known sand-dwelling phototrophic dinoflagellate, Coutea sabulosa gen. & sp. nov. (Dinophyceae, Alveolata). Phycologia 2023, 62, 244–258. [Google Scholar] [CrossRef]
- Selina, M.S.; Efimova, K.V.; Morozova, T.V.; Hoppenrath, M. Morpho-molecular description of the new sand-dwelling dinoflagellate genus Aliferia gen. nov. (Dinophyceae) from the Sea of Japan, including two new species. Phycologia 2023, 62, 366–382. [Google Scholar] [CrossRef]
- Stein, F. Die Organismus der Flagellaten; Verlag von Wilhelm Engelmann: Leipzig, Germany, 1878; p. 154. [Google Scholar]
- Kofoid, C.A.; Swezy, O. The Free-Living Unarmored Dinoflagellata; University of California Press: Berkeley, CA, USA, 1921; Volume 5, p. 559. [Google Scholar]
- Thessen, A.E.; Patterson, D.J.; Murray, S.A. The taxonomic significance of species that have only been observed once: The genus Gymnodinium (Dinoflagellata) as an example. PLoS ONE 2012, 7, e44015. [Google Scholar] [CrossRef]
- Takayama, H. Apical grooves of unarmored dinoflagellates. Bull. Plankton Soc. Jpn. 1985, 32, 129–140. [Google Scholar]
- Daugbjerg, N.; Hansen, G.; Larsen, J.; Moestrup, Ø. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia 2000, 39, 302–317. [Google Scholar] [CrossRef]
- de Salas, M.F.; Bolch, C.J.S.; Botes, L.; Nash, G.; Wright, S.W.; Hallegraeff, G.M. Takayama gen. nov. (Gymnodiniales, Dinophyceae), A new genus of unarmored dinoflagellates with sigmoid apical grooves, including the description of two new species. J. Phycol. 2003, 39, 1233–1246. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Hakanen, P.; Hansen, G.; Daugbjerg, N.; Ellegaard, M. On Levanderina fissa gen. & comb. nov. (Dinophyceae) (syn. Gymnodinium fissum, Gyrodinium instriatum, Gyr. uncatenum), a dinoflagellate with a very unusual sulcus. Phycologia 2014, 53, 265–292. [Google Scholar] [CrossRef]
- Reñé, A.; Camp, J.; Garcés, E. Diversity and phylogeny of Gymnodiniales (Dinophyceae) from the NW Mediterranean Sea revealed by a morphological and molecular approach. Protist 2015, 166, 234–263. [Google Scholar] [CrossRef] [PubMed]
- Hoppenrath, M. Dinoflagellate taxonomy—A review and proposal of a revised classification. Mar. Biodivers. 2017, 47, 381–403. [Google Scholar] [CrossRef]
- Kang, N.S.; Jeong, H.J.; Moestrup, Ø.; Shin, W.; Nam, S.W.; Park, J.Y.; De Salas, M.F.; Kim, K.W.; Noh, J.H. Description of a new planktonic mixotrophic dinoflagellate Paragymnodinium shiwhaense n. gen., n. sp. from the coastal waters off western Korea: Morphology, pigments, and ribosomal DNA gene sequence. J. Eukaryot. Microbiol. 2010, 57, 121–144. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, N.; Fraga, S.; Penna, A.; Casabianca, S.; Zapata, M.; Grünewald, C.F.; Riobó, P.; Camp, J. Barrufeta bravensis gen. nov. sp. nov. (Dinophyceae): A new bloom-forming species from the northwest Mediterranean Sea. J. Phycol. 2011, 47, 375–392. [Google Scholar] [CrossRef]
- Kang, N.S.; Jeong, H.J.; Moestrup, Ø.; Park, T.G. Gyrodiniellum shiwhaense n. gen., n. sp., A New planktonic heterotrophic dinoflagellate from the coastal waters of western Korea: Morphology and ribosomal DNA gene sequence. J. Eukaryot. Microbiol. 2011, 58, 284–309. [Google Scholar] [CrossRef]
- Lu, D.; Lin, G.; Gu, H.; Xia, P. Pyrrophyta. In The Living Species in China’s Seas; Huang, Z., Lin, M., Eds.; Ocean Press: Beijing, China, 2012; pp. 94–106. [Google Scholar]
- Hu, Z.; Deng, Y.; Li, Y.; Tang, Y.Z. The morphological and phylogenetic characterization for the dinoflagellate Margalefidinium fulvescens (=Cochlodinium fulvescens) isolated from the Jiaozhou Bay, China. Acta Oceanol. Sin. 2018, 37, 11–17. [Google Scholar] [CrossRef]
- Hu, Z.; Deng, Y.; Luo, Z.; Shang, L.; Kong, F.; Gu, H.; Zhao, Z.; Tang, Y.Z. Characterization of the unarmored dinoflagellate Pseliodinium pirum (Ceratoperidiniaceae) from Jiaozhou Bay, China. Phycol. Res. 2020, 68, 3–13. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Gu, H.; Tao, Z.; Deng, Y.; Shang, L.; Chai, Z.; Tang, Y.Z. Biecheleria donggangensis sp. nov. (Suessiaceae, Dinophyceae), a new marine woloszynskioid species germinated from the coastal sediment of Yellow Sea, China. Phycologia 2023, 62, 512–524. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, M.; Liu, S.; Cui, Z. Diversity of HAB species in coastal regions of China. Oceanol. Limnol. Sin. 2023, 54, 599–624. [Google Scholar]
- Gu, H.; Liu, T.; Vale, P.; Luo, Z. Morphology, phylogeny and toxin profiles of Gymnodinium inusitatum sp. nov., Gymnodinium catenatum and Gymnodinium microreticulatum (Dinophyceae) from the Yellow Sea, China. Harmful Algae 2013, 28, 97–107. [Google Scholar] [CrossRef]
- Liu, S.; Gibson, K.; Cui, Z.; Chen, Y.; Sun, X.; Chen, N. Metabarcoding analysis of harmful algal species in Jiaozhou Bay. Harmful Algae 2020, 92, 101772. [Google Scholar] [CrossRef]
- He, L.; Yu, Z.; Xu, X.; Zhu, J.; Yuan, Y.; Cao, X.; Song, X. Metabarcoding analysis identifies high diversity of harmful algal bloom species in the coastal waters of the Beibu Gulf. Ecol. Evol. 2023, 13, e10127. [Google Scholar] [CrossRef]
- Liu, S.; Cui, Z.; Zhao, Y.; Chen, N. Composition and spatial-temporal dynamics of phytoplankton community shaped by environmental selection and interactions in the Jiaozhou Bay. Water Res. 2022, 218, 118488. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Scholin, C.A.; Herzog, M.; Sogin, M.; Anderson, D.M. Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J. Phycol. 1994, 30, 999–1011. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.i.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Posada, D. jModelTest: Phylogenetic Model Averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Fensome, R.A.; Taylor, F.J.R.; Norris, G.; Sargeant, W.A.S.; Wharton, D.I.; Williams, G.L. A Classification of Living and Fossil Dinoflagellates; Sheridan Press: Hanover, PA, USA, 1993; Volume 7, pp. 1–351. [Google Scholar]
- Ellegaard, M.; Moestrup, Ø. Fine structure of the flagellar apparatus and morphological details of Gymnodinium nolleri sp. nov. (Dinophyceae), an unarmored dinoflagellate producing a microreticulate cyst. Phycologia 1999, 38, 289–300. [Google Scholar] [CrossRef]
- Hansen, G. Ultrastructure of Gymnodinium aureolum (Dinophyceae): Toward a further redefinition of Gymnodinium sensu stricto. J. Phycol. 2001, 37, 612–624. [Google Scholar] [CrossRef]
- Murray, S.; De Salas, M.; Luong-Van, J.; Hallegraeff, G. Phylogenetic study of Gymnodinium dorsalisulcum comb. nov. from tropical Australian coastal waters (Dinophyceae). Phycol. Res. 2007, 55, 176–184. [Google Scholar] [CrossRef]
- Reñé, A.; Satta, C.T.; Garcés, E.; Massana, R.; Zapata, M.; Anglès, S.; Camp, J. Gymnodinium litoralis sp. nov. (Dinophyceae), a newly identified bloom-forming dinoflagellate from the NW Mediterranean Sea. Harmful Algae 2011, 12, 11–25. [Google Scholar] [CrossRef]
- Attaran-Fariman, G.; de Salas, M.F.; Negri, A.P.; Bolch, C.J.S. Morphology and phylogeny of Gymnodinium trapeziforme sp. nov. (Dinophyceae): A new dinoflagellate from the southeast coast of Iran that forms microreticulate resting cysts. Phycologia 2007, 46, 644–656. [Google Scholar] [CrossRef]
- Bolch, C.J.S.; Negri, A.P.; Hallegraeff, G.M. Gymnodinium microreticulatum sp. nov. (Dinophyceae): A naked, microreticulate cyst-producing dinoflagellate, distinct from Gymnodinium catenatum and Gymnodinium nolleri. Phycologia 1999, 38, 301–313. [Google Scholar] [CrossRef]
- Hansen, G.; Daugbjerg, N.; Henriksen, P. Comparative study of Gymnodinium mikimotoi and Gymnodinium aureolum, comb. nov. (=Gyrodinium aureolum) based on morphology, pigment composition, and molecular data. J. Phycol. 2000, 36, 394–410. [Google Scholar] [CrossRef]
- Liu, M.; Gu, H.; Krock, B.; Luo, Z.; Zhang, Y. Toxic dinoflagellate blooms of Gymnodinium catenatum and their cysts in Taiwan Strait and their relationship to global populations. Harmful Algae 2020, 97, 101868. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.H.; Li, Z.; Matsuoka, K. Reclassification of Gyrodinium flavescens Kofoid & Swezy as Torquentidium flavescens comb. nov. (Ceratoperidiniaceae, Dinophyceae), based on morphology and phylogeny. Phycologia 2020, 59, 133–139. [Google Scholar] [CrossRef]
- Attaran-Fariman, G.; Khodami, S.; Bolch, C.J.S. First observation of dinoflagellate resting cysts from recent sediments of the southeast coast of Iran. Algol. Stud. 2012, 140, 51–80. [Google Scholar] [CrossRef]
- Mertens, K.N.; Gu, H.; Gurdebeke, P.R.; Takano, Y.; Clarke, D.; Aydin, H.; Li, Z.; Pospelova, V.; Shin, H.H.; Li, Z.; et al. A review of rare, poorly known, and morphologically problematic extant marine organic-walled dinoflagellate cyst taxa of the orders Gymnodiniales and Peridiniales from the Northern Hemisphere. Mar. Micropaleontol. 2020, 159, 101773. [Google Scholar] [CrossRef]
- Gray, D.D.; Zonneveld, K.A.F.; Versteegh, G.J.M. Species-specific sensitivity of dinoflagellate cysts to aerobic degradation: A five-year natural exposure experiment. Rev. Palaeobot. Palynol. 2017, 247, 175–187. [Google Scholar] [CrossRef]
- Rigel, C.Q.; Javier, H.; Ernesto, G.M.; Rafael, R.M. Assemblages of dinoflagellate resistance cysts and copepod eggs in superficial sediments at the upper Gulf of California. Cont. Shelf Res. 2022, 235, 104648. [Google Scholar] [CrossRef]
- Chen, N.; Cui, Z.; Xu, Q. Advances in the study of biodiversity of phytoplankton and red tide species in China (IV): The Changjiang Estuary. Oceanol. Limnol. Sin. 2021, 52, 402–417. [Google Scholar]
- Chen, N.; Chen, Y. Advances in the study of biodiversity of phytoplankton and red tide species in China (II): The East China Sea. Oceanol. Limnol. Sin. 2021, 52, 363–384. [Google Scholar]
- Chen, N.; Huang, H. Advances in the study of biodiversity of phytoplankton and red tide species in China (I): The Bohai Sea. Oceanol. Limnol. Sin. 2021, 52, 346–362. [Google Scholar]
- Chen, N.; Zhang, M. Advances in the study of biodiversity of phytoplankton and red tide species in China (III): The South China Sea. Oceanol. Limnol. Sin. 2021, 52, 385–401. [Google Scholar]


| Species | GenBank Accession Number | Identity | Origin |
|---|---|---|---|
| G. trapeziforme | EF192414 | 98.7% (926/938) | Pasabander, South coast of Iran |
| G. fuscum (Ehrenberg) F. Stein | AF200676 | 82.9% (819/988) | La Trobe, Australia |
| G. fuscum var. rubrum Baumeister ex C.Romeikat, J. Knechtel & M. Gottschling | MK405489 | 85.5% (1260/1473) | Seeon, Traunstein, Germany |
| G. microreticulatum | ON392356 | 94.6% (1427/1508) | Yellow Sea, China |
| G. catenatum | AY036073 | 84.5% (594/703) | Australia |
| G. inusitatum | KF234071 | 84.7% (609/719) | Lianyungang, China |
| G. smaydae | HG005135 | 84.0% (771/918) | Shiwha Bay, Korea |
| G. venator Flo Jørgensen & Shauna Murray | AY455681 | 82.1% (1212/1477) | - |
| G. plasticum | KY688184 | 95.5% (734/769) | Plastic Lake, Canada |
| G. aureolum | AF200670 | 84.5% (843/998) | Pettaquamscutt R., USA |
| G. corollarium A.M. Sundström, Kremp & Daugbjerg | FJ211386 | 88.3% (1217/1378) | Northern Baltic proper, Baltic Sea, Sweden |
| Levanderina fissa | EF192407 | 83.0% (356/429) | - |
| Akashiwo sanguinea | AF260397 | 86.3% (341/395) | - |
| Barrufeta bravensis | FN647674 | 79.9% (591/740) | La Fosca, Cataluña |
| Lepidodinium chlorophorum | AY331681 | 85.5% (882/1032) | - |
| Wangodinium sinense | MH732681 | 85.5% (508/594) | Beihai, South China Sea, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Hu, Z.; Luo, Z.; Deng, Y.; Shang, L.; Sun, Y.; Tang, Y. Morphological and Molecular Characterization of the Unarmored Dinoflagellate Gymnodinium trapeziforme (Dinophyceae) from Jiaozhou Bay, China. Diversity 2023, 15, 1186. https://doi.org/10.3390/d15121186
Gao M, Hu Z, Luo Z, Deng Y, Shang L, Sun Y, Tang Y. Morphological and Molecular Characterization of the Unarmored Dinoflagellate Gymnodinium trapeziforme (Dinophyceae) from Jiaozhou Bay, China. Diversity. 2023; 15(12):1186. https://doi.org/10.3390/d15121186
Chicago/Turabian StyleGao, Menghan, Zhangxi Hu, Zhaohe Luo, Yunyan Deng, Lixia Shang, Yuanyuan Sun, and Yingzhong Tang. 2023. "Morphological and Molecular Characterization of the Unarmored Dinoflagellate Gymnodinium trapeziforme (Dinophyceae) from Jiaozhou Bay, China" Diversity 15, no. 12: 1186. https://doi.org/10.3390/d15121186
APA StyleGao, M., Hu, Z., Luo, Z., Deng, Y., Shang, L., Sun, Y., & Tang, Y. (2023). Morphological and Molecular Characterization of the Unarmored Dinoflagellate Gymnodinium trapeziforme (Dinophyceae) from Jiaozhou Bay, China. Diversity, 15(12), 1186. https://doi.org/10.3390/d15121186









