Time-Varying Effective Population Sizes of Group-Living Small Mammals
Abstract
:1. Introduction
2. Methods
2.1. Study Site
2.2. Live-Trapping of Daurian Pikas
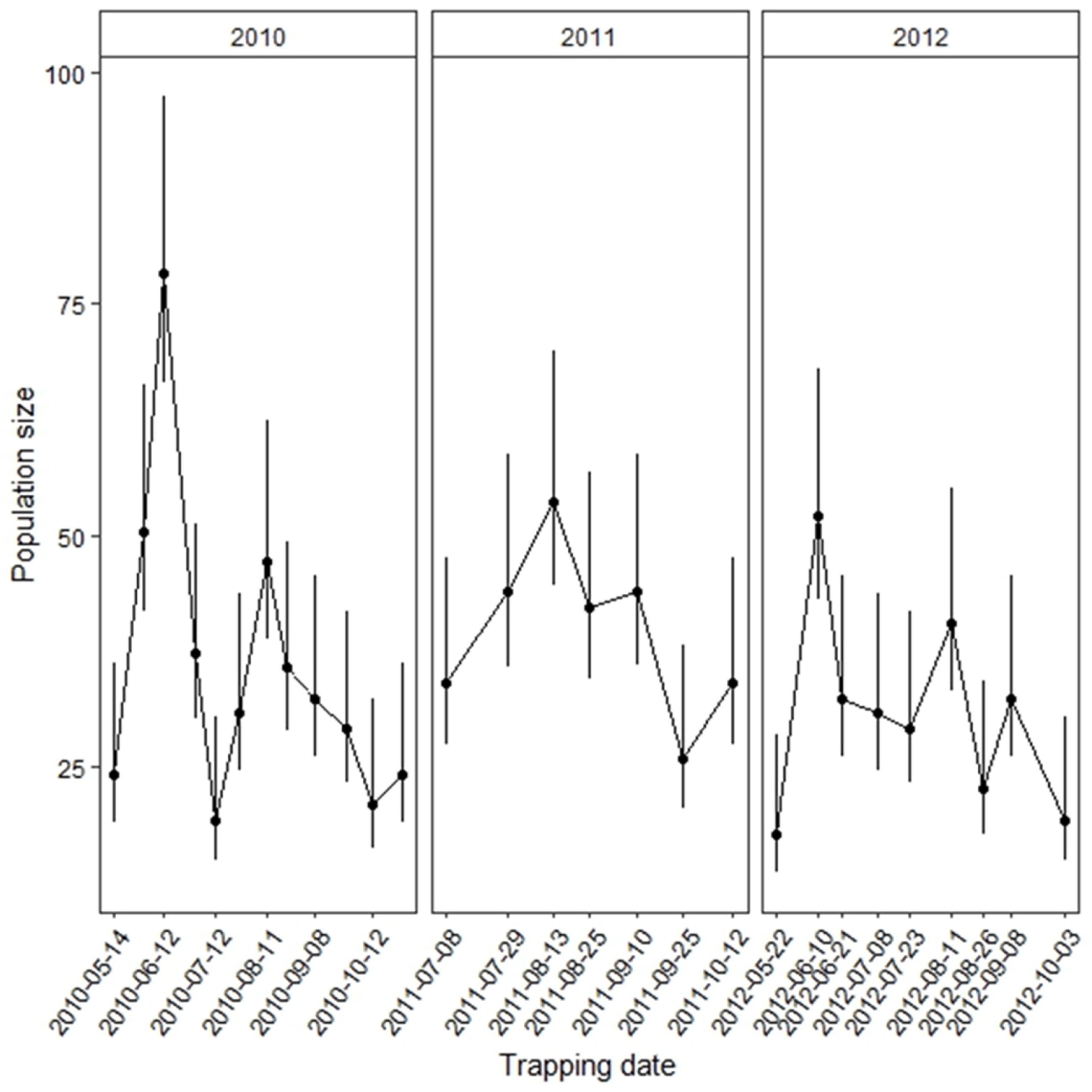
2.3. Estimates of Seasonal Population Sizes
2.4. Microsatellite Analysis
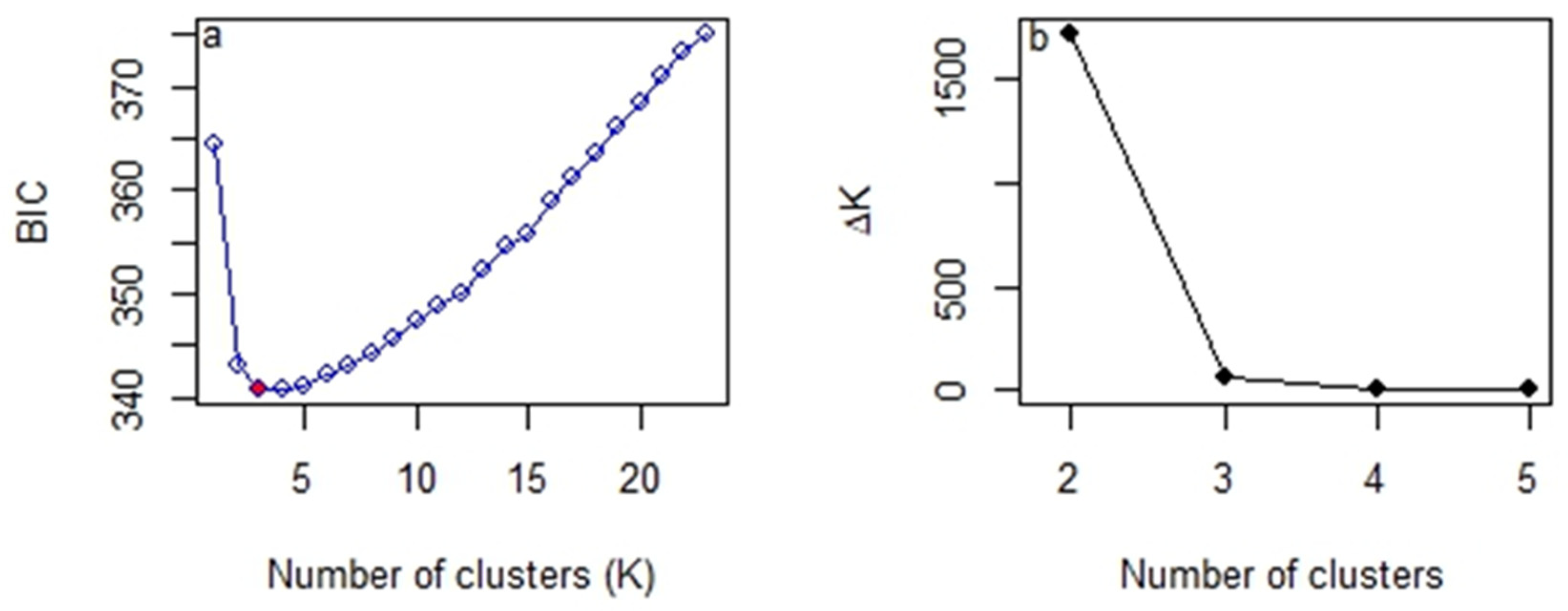
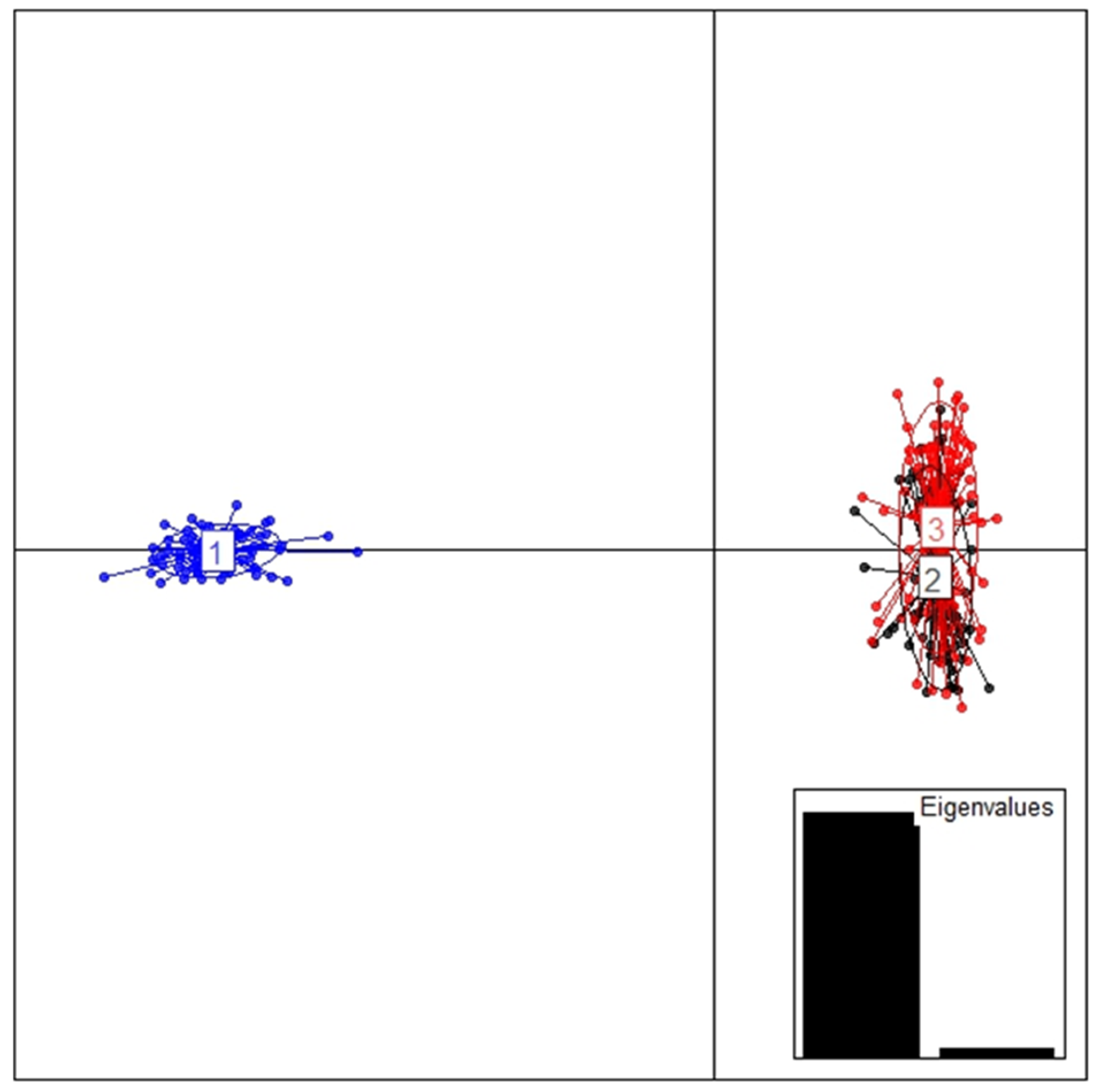
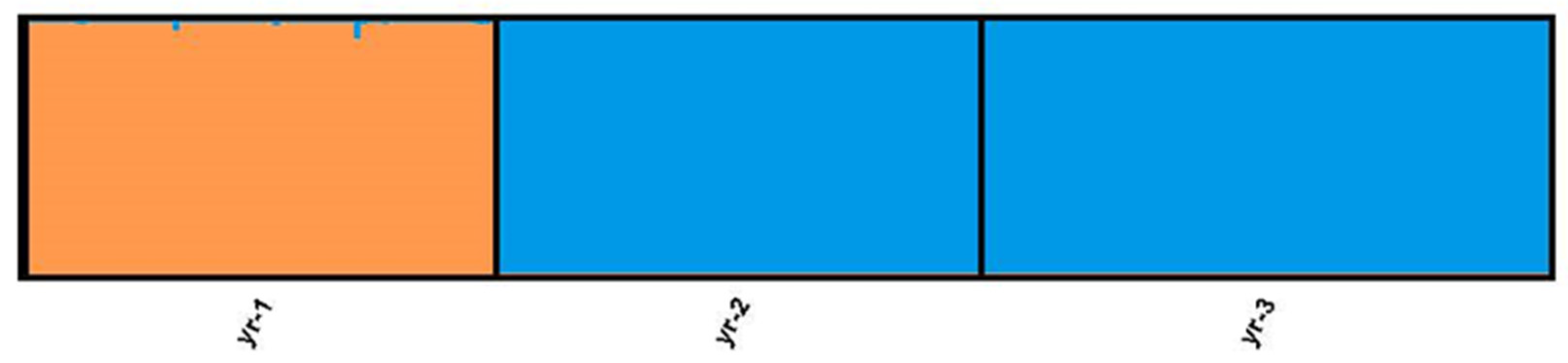
2.5. Between-Year Difference of Daurian Pika Population Genetic Structure
2.6. Estimation of Effective Population Size
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, R.A. The Genetical Theory of Natural Selection; Clarendon Press: London, UK, 1930. [Google Scholar]
- Wright, S. Evolution in Mendelian populations. Genetics 1931, 16, 97–159. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Effective population size/adult population size ratios in wildlife: A review. Genet. Res. 1995, 66, 95–107. [Google Scholar] [CrossRef]
- Hartl, D.L.; Clark, A.G. Principles of Population Genetics; Sinauer Associates: Sunderland, MA, USA, 1997. [Google Scholar]
- Wang, J.; Santiago, E.; Caballero, A. Prediction and estimation of effective population size. Heredity 2016, 117, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Harmon, L.J.; Braude, S. Conservation of small populations: Effective population sizes, inbreeding, and the 50/500 rule. In An Introduction to Methods and Models in Ecology, Evolution, and Conservation Biology; Braude, S., Low, B.S., Eds.; Princeton University Press: Princeton, NJ, USA, 2010; pp. 125–138. [Google Scholar]
- Wright, S. Evolution and the Genetics of Populations: Vol. 2. The Theory of Gene Frequencies; University of Chicago Press: Chicago, IL, USA, 1969. [Google Scholar]
- Vucetich, J.A.; Waite, T.A.; Nunney, L. Fluctuating population size and the ratio of effective to census population size. Evolution 1997, 51, 2017–2021. [Google Scholar] [CrossRef] [PubMed]
- Nunney, L. Measuring the ratio of effective population size to adult numbers using genetic and ecological data. Evolution 1995, 49, 389–392. [Google Scholar] [CrossRef]
- Mace, G.M.; Lande, R. Assessing extinction threats: Toward a reevaluation of IUCN threatened species categories. Conserv. Biol. 1991, 5, 148–157. [Google Scholar] [CrossRef]
- Wang, J.; Caballero, A. Developments in predicting the effective size of subdivided populations. Heredity 1999, 82, 212–226. [Google Scholar] [CrossRef]
- Chikhi, L.; Sousa, V.C.; Luisi, P.; Goossens, B.; Beaumont, M.A. The confounding effects of population structure, genetic diversity and the sampling scheme on the detection and quantification of population size changes. Genetics 2010, 186, 983–995. [Google Scholar] [CrossRef]
- Chesser, R.K.; Rhodes, O.E., Jr.; Sugg, D.W.; Schnabel, A. Effective sizes for subdivided populations. Genetics 1993, 135, 1221–1232. [Google Scholar] [CrossRef]
- Chesser, R.K.; Sugg, D.W.; Rhodes, O.E., Jr.; Novak, J.M.; Smith, M.H. Evolution of mammalian social structure. Acta Theriol. 1993, 38, 163–174. [Google Scholar] [CrossRef]
- Sugg, D.W.; Chesser, R.K.; Dobson, F.S.; Hoogland, J.L. Population genetics meets behavioral ecology. Trends Ecol. Evol. 1996, 11, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhou, Q.; Zhong, W.; Wang, Z. Spatial overlap between sympatric Microtus brandti (Rodentia, Microtinae) and Ochotona daurica (Lagomorpha, Ochotonidae) in the steppes of inner Mongolia. Mammalia 2003, 67, 349–354. [Google Scholar] [CrossRef]
- Wang, G.; Zhong, W. Mongolian gerbils and Daurian pikas responded differently to changes in precipitation in the Inner Mongolian grasslands. J. Arid. Environ. 2006, 66, 648–656. [Google Scholar] [CrossRef]
- Wang, G.; Wan, X.; Liu, W.; Shan, X. Genetic diversity and relatedness of group-living small mammals on the Mongolian Plateau. Diversity 2022, 14, 732. [Google Scholar] [CrossRef]
- Smith, A.T. Patterns of pikas (Genus Ochotona) life history variation. In Evolution of Life Histories of Mammals; Boyce, M.S., Ed.; Yale University Press: New Haven, CT, USA, 1988; pp. 233–256. [Google Scholar]
- Formozov, A.N. Adaptive modifications of behavior in mammals of the Eurasian steppes. J. Mammal. 1966, 47, 208–223. [Google Scholar] [CrossRef]
- Chen, L.; Wang, G.; Wan, X.; Liu, W. Complex and nonlinear effects of weather and density on the demography of small herbivorous mammals. Basic Appl. Ecol. 2015, 16, 172–179. [Google Scholar] [CrossRef]
- White, G.C.; Burnham, K.P. Program MARK: Survival estimation from populations of marked animals. Bird Study 1999, 46, 120–139. [Google Scholar] [CrossRef]
- Cormack, R.M. Estimates of survival from sighting of marked animals. Biometrika 1964, 51, 429–438. [Google Scholar] [CrossRef]
- Jolly, G.M. Explicit estimates from capture-recapture data with both death and immigration-stochastic model. Biometrika 1965, 52, 225–247. [Google Scholar] [CrossRef]
- Seber, G.A.F. A Note on Multiple-Recapture Census. Biometrika 1965, 52, 249–259. [Google Scholar] [CrossRef]
- Kendall, W.L. The robust design for capture-recapture studies: Analysis using program MARK. In Wildlife, Land, and People: Priorities for the 21st Century; Field, R., Warren, R.J., Okarma, H., Sievert, P.R., Eds.; The Wildlife Society: Bethesda, MD, USA, 2001; pp. 361–367. [Google Scholar]
- Pollock, K.H. A capture-recapture design robust to unequal probability of capture. J. Wildl. Manag. 1982, 46, 752–757. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Bailey, L.L.; Simons, T.R.; Pollock, K.H. Estimating detection probability parameters for plethodon salamanders using the robust capture-recapture design. J. Wildl. Manag. 2004, 68, 1–13. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring, NY, USA, 2001. [Google Scholar]
- Peacock, M.M.; Kirchoff, V.S.; Merideth, S.J. Identification and characterization of nine polymorphic microsatellite loci in the North American pika, Ochotona princeps. Mol. Ecol. Notes 2002, 2, 360–362. [Google Scholar] [CrossRef]
- Zgurski, J.M.; Davis, C.S.; Hik, D.S. Isolation and characterization of microsatellite loci for the collared pika (Ochotona collaris) and their cross-amplification in five other Ochotona species. Mol. Ecol. Resour. 2009, 9, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Li, K.X.; Geng, J.N.; Yang, J.; Zhang, Y.M.; Hu, S.N. Isolation and characterization of 13 microsatellite loci in the plateau pika (Ochotona curzoniae). Conserv. Genet. 2009, 10, 785–787. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Wang, J. MLNe: Simulating and estimating effective size and migration rate from temporal changes in allele frequencies. J. Hered. 2022, 113, 563–567. [Google Scholar] [CrossRef]
- Wang, J.; Whitlock, M.C. Estimating effective population size and migration rates from genetic samples over space and time. Genetics 2003, 163, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Parreira, B.R.; Chikhi, L. On some genetic consequences of social structure, mating systems, dispersal, and sampling. Proc. Natl. Acad. Sci. USA 2015, 112, E3318–E3326. [Google Scholar] [CrossRef]
- Chesser, R.K. Influence of gene flow and breeding tactics on gene diversity within populations. Genetics 1991, 129, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Chesser, R.K. Gene diversity and female philopatry. Genetics 1991, 127, 437–447. [Google Scholar] [CrossRef]
- Beaumont, M.A. Detecting population expansion and decline using microsatellites. Genetics 1999, 153, 2013–2029. [Google Scholar] [CrossRef]
- Wang, G.M.; Hobbs, N.T.; Slade, N.A.; Merritt, J.F.; Getz, L.L.; Hunter, M.; Vessey, S.H.; Witham, J.; Guillaumet, A. Comparative population dynamics of large and small mammals in the Northern Hemisphere: Deterministic and stochastic forces. Ecography 2013, 36, 439–446. [Google Scholar] [CrossRef]
- Archie, E.A.; Chiyo, P.I. Elephant behaviour and conservation: Social relationships, the effects of poaching, and genetic tools for management. Mol. Ecol. 2012, 21, 765–778. [Google Scholar] [CrossRef]
- Sjodin, P.; Kaj, I.; Krone, S.; Lascoux, M.; Nordborg, M. On the meaning and existence of an effective population size. Genetics 2005, 169, 1061–1070. [Google Scholar] [CrossRef]
- Wakeley, J.; Sargsyan, O. Extensions of the coalescent effective population size. Genetics 2009, 181, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Kingman, J.F.C. The coalescent. Stoch. Process. Appl. 1982, 13, 235–248. [Google Scholar] [CrossRef]
- Taylor, J.; Véber, A. Coalescent processes in subdivided populations subject to recurrent mass extinctions. Electron. J. Probab. 2009, 14, 242–288. [Google Scholar] [CrossRef]
- Tarantino, R.; Panunzi, G.; Romano, V. Modeling of Hardy-Weinberg Equilibrium Using Dynamic Random Networks in an ABM Framework. In Complex Networks and Their Applications XI; Cherifi, H., Mantegna, R.N., Rocha, L.M., Cherifi, C., Miccichè, S., Eds.; Springer: Cham, Switzerland, 2022; pp. 241–250. [Google Scholar]
- Tellier, A.; Lemaire, C. Coalescence 2.0: A multiple branching of recent theoretical developments and their applications. Mol. Ecol. 2014, 23, 2637–2652. [Google Scholar] [CrossRef] [PubMed]
| Period | LLM | ULM | Actual Size (N) | ||
|---|---|---|---|---|---|
| 2010–2011 | 15.8 | 17.29 | 18.36 | 37.14 | 0.46 |
| 2011–2012 | 32.53 | 39.83 | 53.84 | 34.56 | 1.13 |
| 2010–2012 | 18.07 | 19.41 | 20.7 | 35.16 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Wan, X.; Chen, L.; Shan, X. Time-Varying Effective Population Sizes of Group-Living Small Mammals. Diversity 2023, 15, 1173. https://doi.org/10.3390/d15121173
Wang G, Wan X, Chen L, Shan X. Time-Varying Effective Population Sizes of Group-Living Small Mammals. Diversity. 2023; 15(12):1173. https://doi.org/10.3390/d15121173
Chicago/Turabian StyleWang, Guiming, Xinrong Wan, Lijun Chen, and Xueyan Shan. 2023. "Time-Varying Effective Population Sizes of Group-Living Small Mammals" Diversity 15, no. 12: 1173. https://doi.org/10.3390/d15121173
APA StyleWang, G., Wan, X., Chen, L., & Shan, X. (2023). Time-Varying Effective Population Sizes of Group-Living Small Mammals. Diversity, 15(12), 1173. https://doi.org/10.3390/d15121173








