Prevalence and Genetic Characterization of Morphologically Indistinguishable Sarcocysts of Sarcocystis cruzi in Cattle and Sarcocystis poephagicanis in Yaks
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Prevalence of S. cruzi in Cattle and S. poephagicanis in Yaks
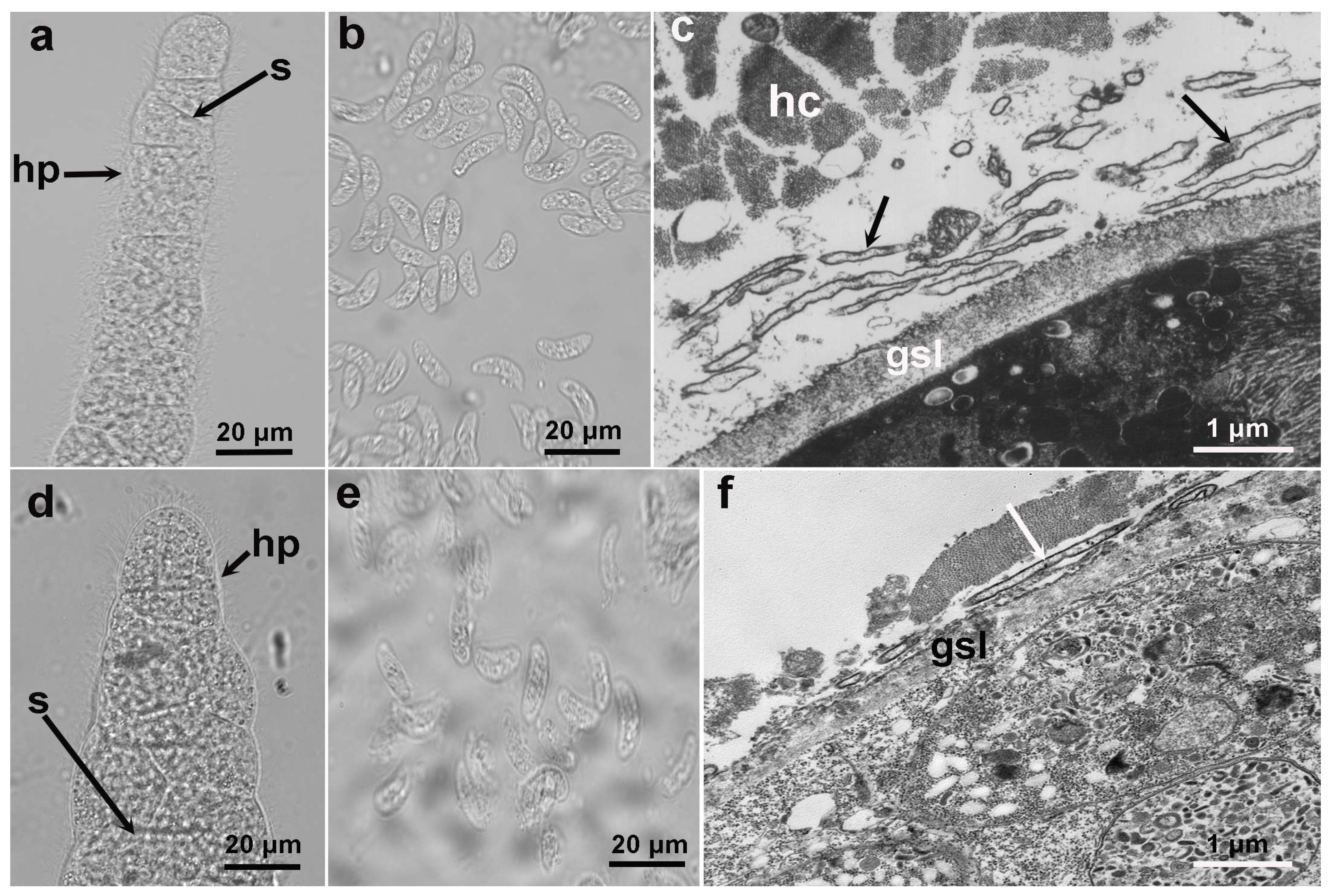
3.2. LM and TEM of Sarcoysts of S. cruzi and S. poephagicanis
3.3. Molecular Characterization of 18S rDNA, 28S rDNA, cox1, and rpl6
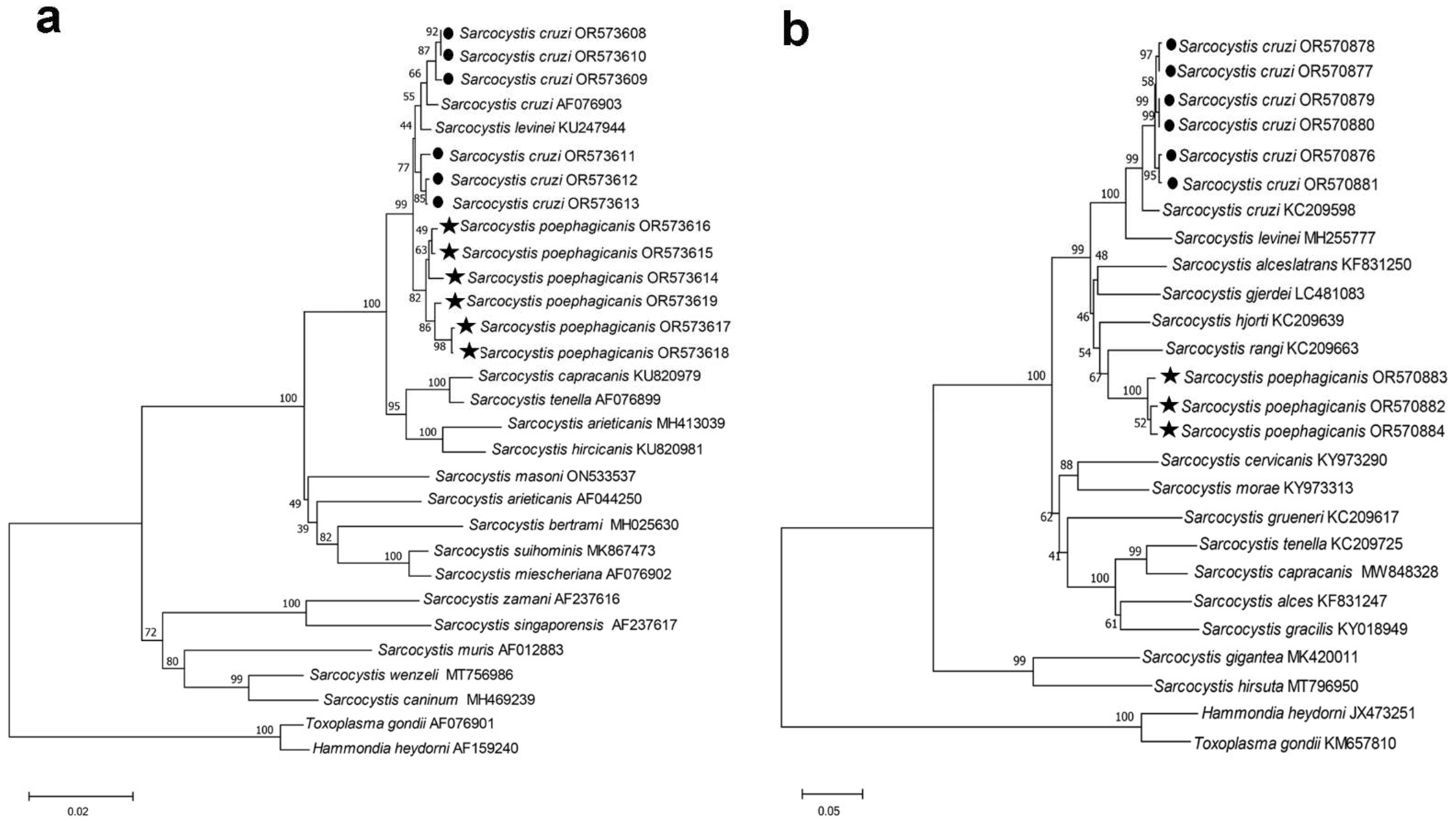
3.4. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 52–59+195–214. [Google Scholar]
- Dubey, J.P.; Rosenthal, B.M. Bovine sarcocystosis: Sarcocystis species, diagnosis, prevalence, economic and public health considerations, and association of Sarcocystis species with eosinophilic myositis in cattle. Inter. J. Parasitol. 2023, 53, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Chang, P.Z.; Dong, M.X.; Wang, X.Y.; Xia, A.Q. Description of two new species of Sarcocystis from the yak (Poephagus grunniens). Sci. Agric. Sin. 1985, 4, 80–85. (In Chinese) [Google Scholar]
- Barsila, S.; Kreuzer, M.; Devkota, N.R.; Ding, L.; Marquardt, S. Adaptation to Himalayan high altitude pasture sites by yaks and different types of hybrids of yaks with cattle. Livest. Sci. 2014, 169, 125–136. [Google Scholar] [CrossRef]
- Geng, T. Prevalence of Sarcocystis spp. from domestic yaks in Xinghai county, Qinghai province, China. Chin. J. Vet. Med. 2009, 25, 49. (In Chinese) [Google Scholar]
- Zhang, G. Prevalence of Sarcocystis spp. from domestic yaks in Wulan county, Qinghai province, China. Chin. J. Vet. Med. 2010, 46, 41–42. (In Chinese) [Google Scholar]
- Chen, L. Prevalence of Sarcocystis spp. from domestic yaks in Chengduo county, Qinghai province, China. Anim. Husb. Vet. Med. 2012, 44, 111–112. (In Chinese) [Google Scholar]
- Gjerde, B. The resurrection of a species: Sarcocystis bovifelis Heydorn et al., 1975 is distinct from the current Sarcocystis hirsuta in cattle and morphologically indistinguishable from Sarcocystis sinensis in water buffaloes. Parasitol. Res. 2016, 115, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Huang, S.; Wen, T.; Esch, G.W.; Liang, Y.; Li, H.L. Morphology, molecular characteristics, and demonstration of a definitive host for Sarcocystis rommeli from cattle (Bos taurus) in China. J. Parasitol. 2017, 103, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.R.; Martin, D.S.; Liberator, P.A.; Dashkevicz, M.; Anderson, J.W.; Feighner, S.D.; Elbrecht, A.; Perkins-Barrow, A.; Jenkins, M.C.; Danforth, H.D.; et al. Phylogenetic relationships among eight Eimeria species infecting domestic fowl inferred using complete small subunit ribosomal DNA sequences. J. Parasitol. 1997, 83, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Fenger, C.K.; Granstrom, D.E.; Langemeier, J.L.; Stamper, S.; Donahue, J.M.; Patterson, J.S.; Gajadhar, A.A.; Marteniuk, J.V.; Xiaomin, Z.; Dubey, J.P. Identification of opossums (Didelphis virginiana) as the putative definitive host of Sarcocystis neurona. J. Parasitol. 1995, 81, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Mugridge, N.B.; Morrison, D.A.; Johnson, A.M.; Luton, K.; Dubey, J.P.; Votýpka, J.; Tenter, A.M. Phylogenetic relationships of the genus Frenkelia: A review of its history and new knowledge gained from comparison of large subunit ribosomal ribonucleic acid gene sequences. Int. J. Parasitol. 1999, 29, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B. Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int. J. Parasitol. 2013, 43, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B. Sarcocystis species in red deer revisited: With a re-description of two known species as Sarcocystis elongata n. sp. and Sarcocystis truncata n. Sp. based on mitochondrial cox1 sequences. Parasitology 2014, 141, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, P.C.; Dong, M.X.; Wang, X.Y. Host spectrum of two Sarcocystis species from yak (Poephagus grunniens). J. Chin. Trad. Vet. Med. 1990, 5, 8–10+29. (In Chinese) [Google Scholar]
- Gjerde, B.; Hilali, M.; Abbas, I.E. Molecular differentiation of Sarcocystis buffalonis and Sarcocystis levinei in water buffaloes (Bubalus bubalis) from Sarcocystis hirsuta and Sarcocystis cruzi in cattle (Bos taurus). Parasitol. Res. 2016, 115, 2459–2471. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Primer Name | Primer Sequence (5′–3′) | Reference |
|---|---|---|---|
| 18S rDNA | ERIB1 a | ACCTGGTTGATCCTGCCAG | [10] |
| B b | GATCCTTCTGCAGGTTCACCTAC | [11] | |
| 28S rDNA | KL1 a | TACCCGCTGAACTTAAGC | [12] |
| KL3 b | CCACCAAGATCTGCACTAG | ||
| KL4 a | AGCAGGACGGTGGTC | ||
| KL5 b | CTCAAGCTCAACAGGGTC | ||
| KL6 a | GGATTGGCTCTGAGGG | ||
| KL2 b | ACTTAGAGGCGTTCAGTC | ||
| cox1 | SF1 a | ATGGCGTACAACAATCATAAAGAA | [13] |
| SR9 b | ATATCCATACCRCCATTGCCCAT | [14] | |
| rpl6 | L6F a | CCATGAAACTTAATTTGCACA | This study |
| L6R b | CTTAAAAGTTCTATTATGGGTT |
| Tissues Examined | S. cruzi | S. poephagicanis | ||||
|---|---|---|---|---|---|---|
| No. Sampled | No. Infected | % of Infected | No. Sampled | No. Infected | % of Infected | |
| Esophagus | 301 | 110 | 36.5 | 284 | 227 | 79.9 |
| Tongue | 192 | 23 | 12.0 | 68 | 26 | 38.2 |
| Diaphragm | 947 | 298 | 31.5 | 286 | 232 | 81.1 |
| Heart | 780 | 316 | 40.5 | 279 | 245 | 87.8 |
| Skeletal muscles | 950 | 251 | 26.4 | 320 | 239 | 74.5 |
| Total infected animals | 950 | 405 | 42.6 | 320 | 304 | 95.0 |
| Species | Genetic Markers | Accession Number | Comparison with Sequences Previously Deposited in GenBank | ||
|---|---|---|---|---|---|
| Species | Accession Number | Identity% (Average%) | |||
| S. cruzi | 18S rDNA | OR553288–OR553292 | S. cruzi | #1 | 98.7–99.8 (99.3) |
| S. levinei | KU247914–KU247922 | 99.0–99.5 (99.2) | |||
| S. gjerdei | LC481028–LC481031, LC349475–LC349479 | 98.1–98.6 (98.3). | |||
| 28S rDNA | OR573608–OR573613 | S. cruzi | KT901270–KT901285, AF076903 | 98.6–99.5 (99.1) | |
| S. levinei | KU247937–KU247945, MH793424–MH793426 | 98.1–98.6 (98.4) | |||
| cox1 | OR570876–OR57081 | S. cruzi | #2 | 96.4–99.8 (97.2) | |
| S. levinei | MH255771–MH255781, KU247874–KU247885 | 93.1–94.0 (93.6) | |||
| rpl6 | OR590796–OR590798 | T. gondii | NC001799 | 72.7 | |
| S. poephagicanis | 18S rDNA | OR573620–OR573623 | S. cruzi | #1 | 97.9–98.8 (98.7) |
| S. gjerdei | LC481028–LC481031, LC349475–LC349479 | 98.2–98.7 (98.5) | |||
| S. levinei | KU247914–KU247922 | 98.5 | |||
| 28S rDNA | OR573614–OR573619 | S. levinei | KU247937–KU247945, MH793424–MH793426 | 97.2–98.1 (97.5) | |
| S. cruzi | KT901270–KT901285, AF076903 | 95.2–98.1 (97.2) | |||
| cox1 | OR570882–OR570884 | S. rangi | KC209662–KC209668 | 91.6–91.9 (91.8) | |
| S. cruzi | #2 | 89.4–90.5 (90.0) | |||
| S. levinei | MH255771–MH255781, KU247874–KU247885 | 88.8–89.3 (89.1) | |||
| rpl6 | OR590799–OR590800 | T. gondii | NC001799 | 72.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, K.; Lamu, D.; Qin, T.; Liao, Z.; Zhang, M.; Wu, Z.; Deng, S.; Tao, J.; Hu, J. Prevalence and Genetic Characterization of Morphologically Indistinguishable Sarcocysts of Sarcocystis cruzi in Cattle and Sarcocystis poephagicanis in Yaks. Diversity 2023, 15, 1136. https://doi.org/10.3390/d15111136
Tang K, Lamu D, Qin T, Liao Z, Zhang M, Wu Z, Deng S, Tao J, Hu J. Prevalence and Genetic Characterization of Morphologically Indistinguishable Sarcocysts of Sarcocystis cruzi in Cattle and Sarcocystis poephagicanis in Yaks. Diversity. 2023; 15(11):1136. https://doi.org/10.3390/d15111136
Chicago/Turabian StyleTang, Kui, Danqu Lamu, Tao Qin, Zhe Liao, Mingzhu Zhang, Zhipeng Wu, Shuangsheng Deng, Jianping Tao, and Junjie Hu. 2023. "Prevalence and Genetic Characterization of Morphologically Indistinguishable Sarcocysts of Sarcocystis cruzi in Cattle and Sarcocystis poephagicanis in Yaks" Diversity 15, no. 11: 1136. https://doi.org/10.3390/d15111136
APA StyleTang, K., Lamu, D., Qin, T., Liao, Z., Zhang, M., Wu, Z., Deng, S., Tao, J., & Hu, J. (2023). Prevalence and Genetic Characterization of Morphologically Indistinguishable Sarcocysts of Sarcocystis cruzi in Cattle and Sarcocystis poephagicanis in Yaks. Diversity, 15(11), 1136. https://doi.org/10.3390/d15111136







