The Molecular Evidence for Invasive Climber Echinocystis lobata (Michx.) Torr. & A. Gray in Eastern and Central Europe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sites and Sample Collection
2.2. Molecular Analysis
2.3. Statistical Data Analysis
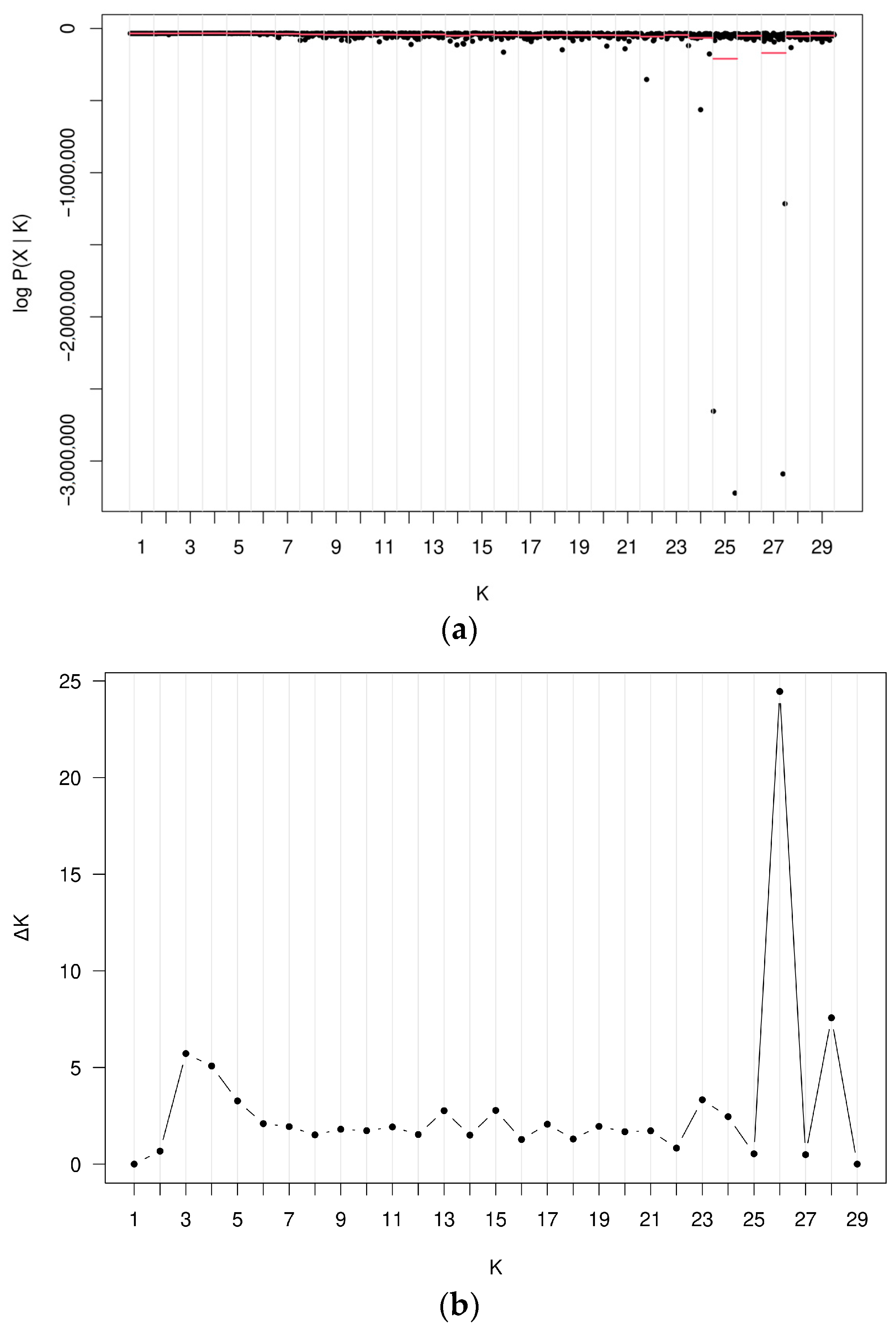
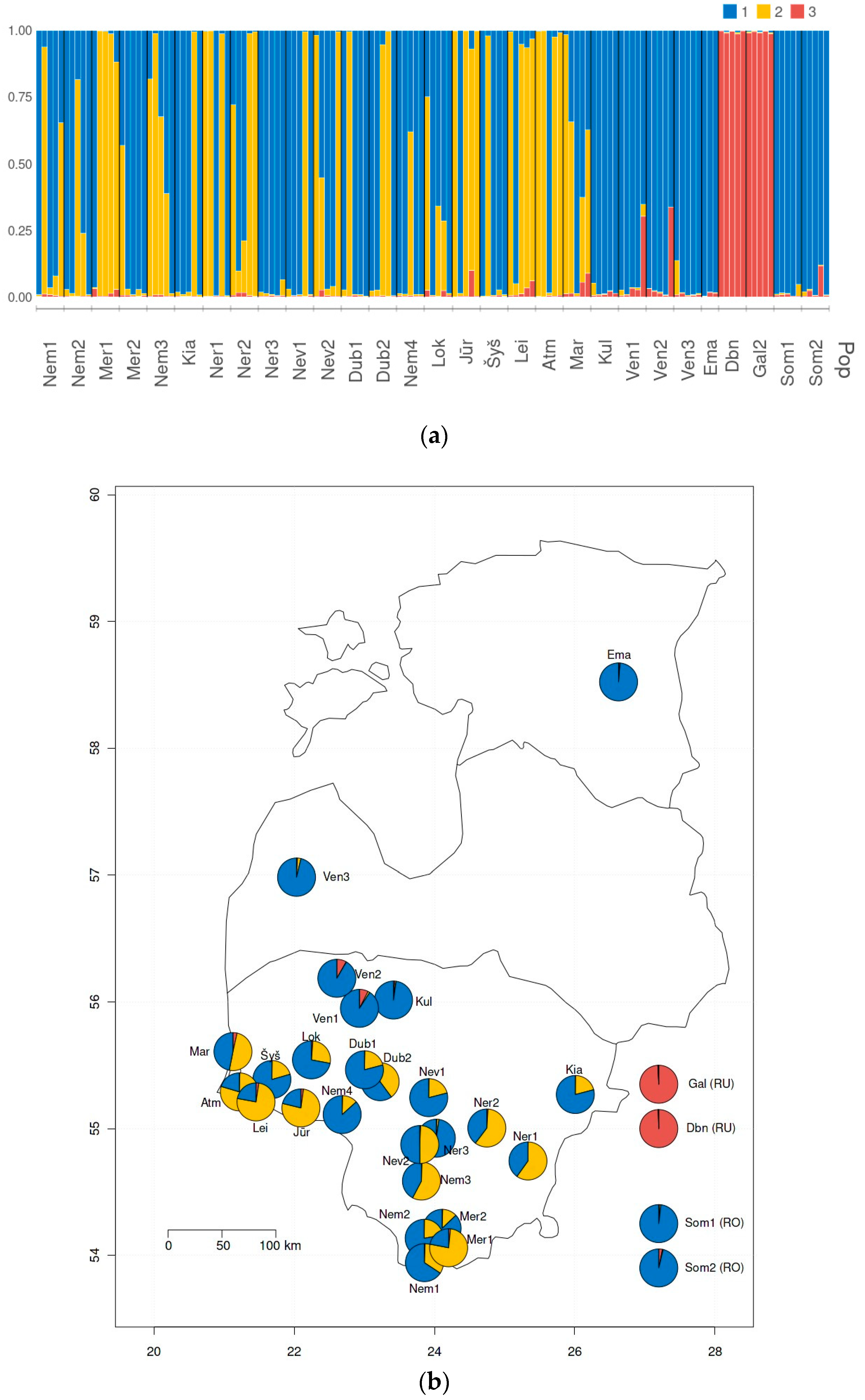
3. Results
4. Discussion
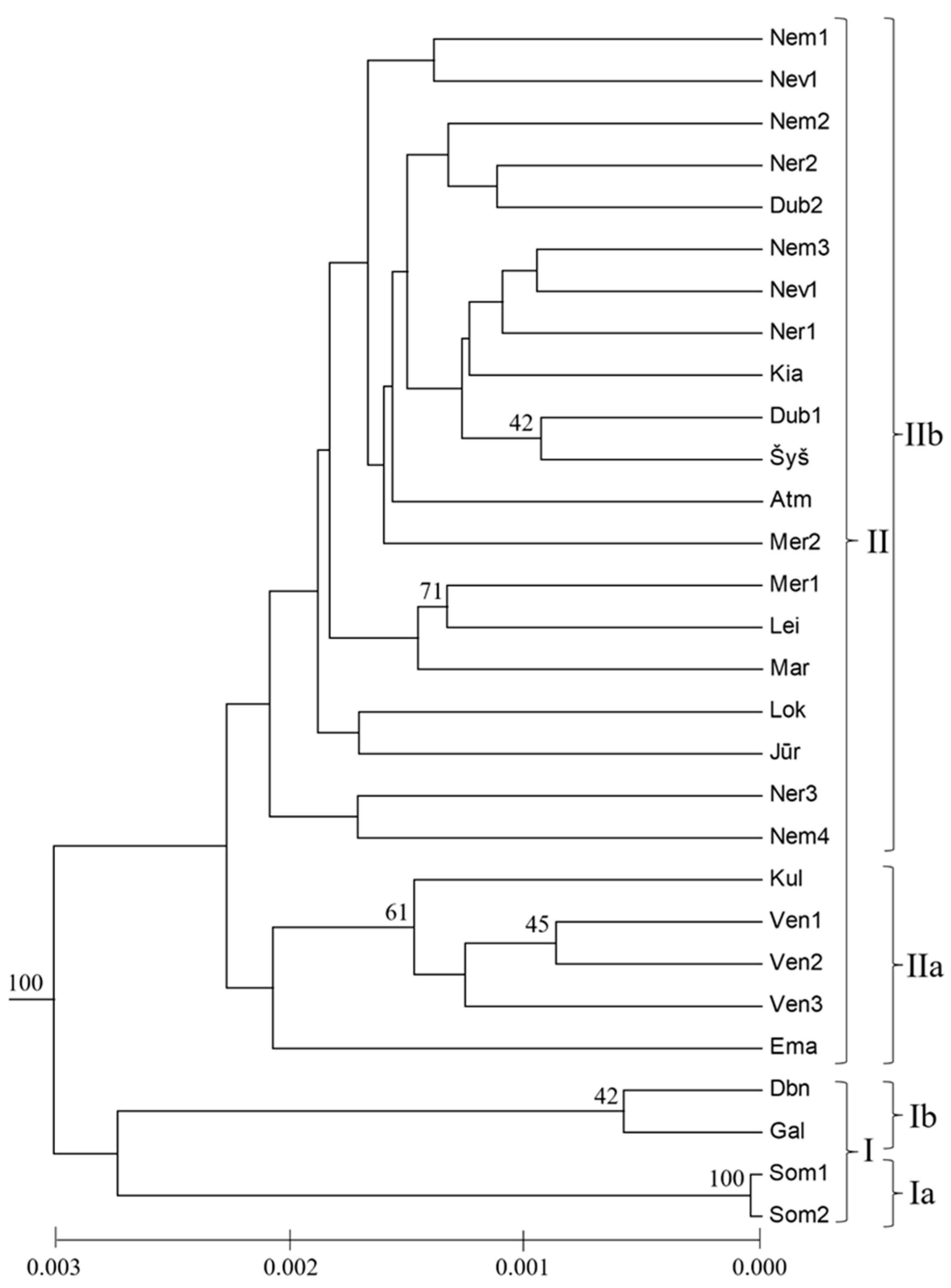
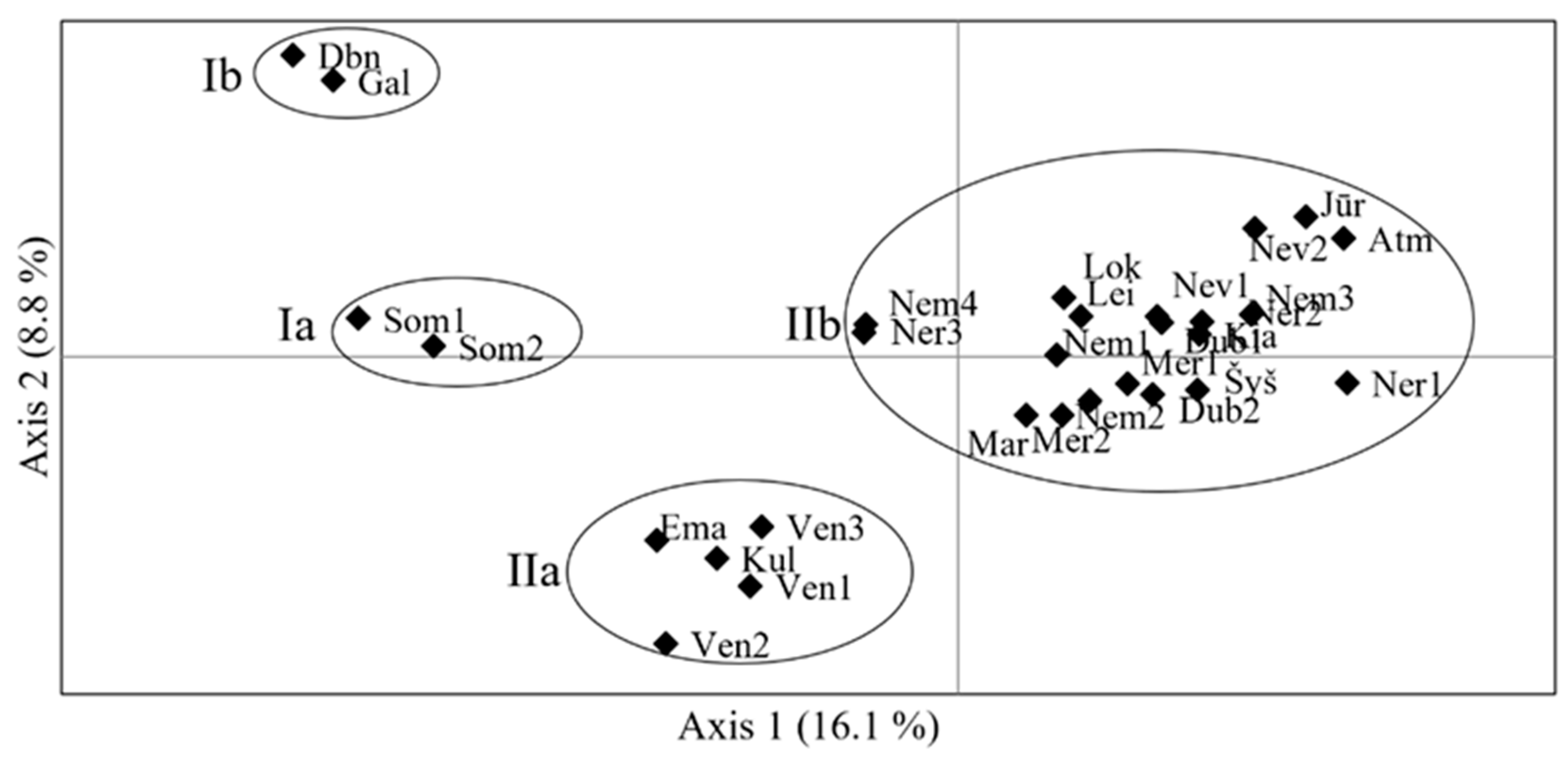
4.1. Genetic Diversity of Populations
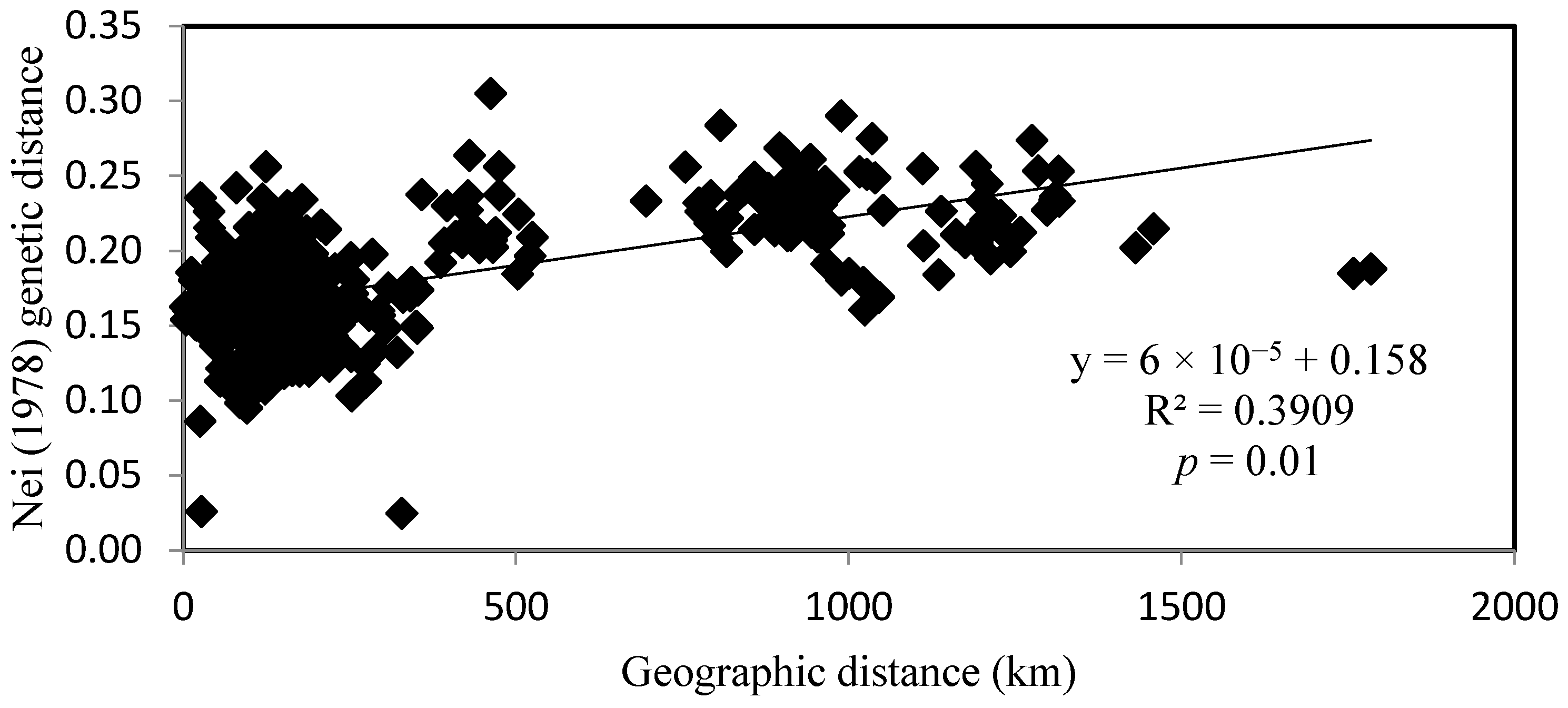
4.2. Interpopulation Variability
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambdon, P.W.; Pyšek, P.; Basnou, C.; Hejda, M.; Arianoutsou, M.; Essl, F.; Jarošík, V.; Pergl, J.; Winter, M.; Anastasiu, P.; et al. Alien flora of Europe: Species diversity, temporal trends, geographical patterns and research needs. Preslia 2008, 80, 101–149. [Google Scholar]
- Priede, A. Distribution of some invasive alien plant species in riparian habitats in Latvia. Bot. Lith. 2008, 14, 137–150. [Google Scholar]
- Fried, G. Prioritization of Potential Invasive Alien Plants in France. In Proceedings of the 2nd International Workshop on Invasive Plants in the Mediterranean Type Regions of the World, Trabzon, Turkey, 2–6 August 2010. [Google Scholar]
- Van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Pyšek, P. Global exchange and accumulation of non-native plants. Nature 2015, 525, 100–103. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Patterns of plant invasions at small spatial scale correspond with that at the whole country scale. Urban Ecosyst. 2016, 19, 983–998. [Google Scholar] [CrossRef]
- Sirbu, C.; Miu, I.V.; Gavrilidis, A.A.; Gradinaru, S.R.; Niculae, I.M.; Preda, C.; Oprea, A.; Urziceanu, M.; Camen-Comanescu, P.; Nagoda, E.; et al. Distribution and pathways of introduction of invasive alien plant species in Romania. NeoBiota 2022, 75, 1–21. [Google Scholar] [CrossRef]
- Lorenzo, P.; Morais, M.C. Strategies for the management of aggressive invasive plant species. Plants 2023, 12, 2482. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- EU Regulation 1143/2014; European Union Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the Prevention and Management of the Introduction and Spread of Invasive Alien Species. European Commission: Brussels, Belgium, 2014; Volume 317, pp. 35–55.
- European Commission. EU Biodiversity Strategy Bringing Nature Back into Our Lives; Communication from the Commission to the European Parliament, the Council, the European Economic and Social com-mittee and the Committee of the Regions; European Commission: Brussels, Belgium, 2020. Available online: https://ec.europa.eu/environment/strategy/biodiversity-strategy-2030_en (accessed on 14 June 2023).
- Coombe, D.E. Impatiens parviflora DC. J. Ecol. 1956, 44, 701–713. [Google Scholar] [CrossRef]
- Trepl, L. Uber Impatiens parviflora Dc. als Agriophyt in Mitteleuropa. In Dissertationes Botaniceae; Gantner, A.R., Ed.; Kommanditgesellschaft: Vaduz, Germany, 1984; Volume 73, pp. 1–400. [Google Scholar]
- Chmura, D. Biology and Ecology of an Invasion of Impatiens parviflora DC. In Natural and Semi-Natural Habitats; Akademia Techniczno-Humanistyczna w Bielsku-Bialej: Bialsko-Biela, Poland, 2014; pp. 1–216. [Google Scholar]
- Krokaitė, E.; Janulionienė, R.; Jocienė, L.; Rekašius, T.; Rajackaitė, G.; Paulauskas, A.; Marozas, V.; Kupčinskienė, E. Relating invasibility and invasiveness: Case study of Impatiens parviflora. Front. Ecol. Evol. 2022, 10, 845947. [Google Scholar] [CrossRef]
- Dale, H.M. Developmental studies of Elodea canadensis Michx: II. Experimental studies on morphological effects of darkness. Can. J. Bot. 1957, 35, 51–64. [Google Scholar] [CrossRef]
- Simpson, D.A. A short history of the introduction and spread of Elodea Michx in the British Isles. Watsonia 1984, 15, 1–9. [Google Scholar]
- Eugelink, A.H. Phosphorus uptake and active growth of Elodea canadensis Michx. and Elodea nuttallii (Planch.) St. John. Water Sci. Technol. 1998, 37, 59–65. [Google Scholar] [CrossRef]
- Kolada, A.; Kutyła, S. Elodea canadensis (Michx.) in Polish lakes: A non-aggressive addition to native flora. Biol. Invasions 2016, 18, 3251–3264. [Google Scholar] [CrossRef]
- Liu, B.O.; Yan, J.; Li, W.; Yin, L.; Li, P.; Yu, H.; Xing, L.; Cai, M.; Wang, H.; Zhao, M.; et al. Mikania micrantha genome provides insights into the molecular mechanism of rapid growth. Nat. Commun. 2020, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Sundarapandian, S.M.; Muthumperumal, C.; Subashree, K. Biological invasion of vines, their impacts and management. In Biodiversity of Lianas; Springer: Cham, Switzerland, 2015; pp. 211–253. [Google Scholar]
- Bunce, R.G.H.; Metzger, M.J.; Jongman, R.H.G.; Brandt, J.; de Blust, G.; Elena-Rossello, R.; Groom, G.B.; Halada, L.; Hofer, G.; Howard, D.C.; et al. A standardized procedure for surveillance and monitoring European habitats and provision of spatial data. Landsc. Ecol. 2008, 23, 11–25. [Google Scholar] [CrossRef]
- Wagner, V.; Chytrý, M.; Jiménez-Alfaro, B.; Pergl, J.; Hennekens, S.; Biurrun, I.; Knollová, I.; Berg, C.; Vassilev, K.; Rodwell, J.S.; et al. Alien plant invasions in European woodlands. Divers. Distrib. 2017, 23, 969–981. [Google Scholar] [CrossRef]
- Giuseppe, B.; Costello, K.E.; Maggs, G.; Montagnani, C.; Nunes, A.L.; Pergl, J.; Peyton, J.; Robertson, P.; Roy, H.; Scalera, R.; et al. An Introduction to the Invasive Alien Species of Union Concern Version 2022; Publications Office of the European Union: Luxembourg, 2022; pp. 104–184. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B. The Establishment and Spread of Alien Plant Species (Kenophytes) in the Flora of Poland; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2005; pp. 1–216. Available online: http://www.worldfloraonline.org/taxon/wfo-4000012922. (accessed on 27 June 2023).
- Tokarska-Guzik, B.; Zając, M.; Zając, A. Geographical and ecological aspects of the spread of alien plant species in Poland. In Biological Invasions–from Ecology to Conservation; Rabitsch, W.F., Essl, F., Klingenstein, F., Eds.; Institute of Ecology of the TU Berlin: Berlin, Germany, 2008; pp. 143–152. [Google Scholar]
- Banaszak, J.; Twerd, L.; Ratyńska, H.; Banaszak-Cibicka, W.; Zyś, T. Andrena florea Fabricius, 1793 (Hymenoptera, Apoidea, Apiformes): A rare bee species in Poland, related to the expansion of the alien plant Bryonia dioica Jacq.(Cucurbitaceae). Pol. J. Entomol. 2018, 87, 199–215. [Google Scholar] [CrossRef]
- Önen, H.; Farooq, S.; Tad, S.; Özaslan, C.; Gunal, H.; Chauhan, B.S. The influence of environmental factors on germination of bur cucumber (Sicyos angulatus) seeds: Implications for range expansion and management. Weed Sci. 2018, 66, 494–501. [Google Scholar] [CrossRef]
- Stešević, D.; Jovović, Z. Sicyos angulatus L.—A new non-indigenous species in the flora of Montenegro. Herbologia 2005, 6, 17–24. [Google Scholar]
- Arslan, Z.F.; Uludag, A.; Uremis, I. Status of invasive alien plants included in EPPO Lists in Turkey. EPPO Bull. 2015, 45, 66–72. [Google Scholar] [CrossRef]
- Menghini, A.; Mincigrucci, G. Sicyos angulatus L., a new exotic naturalized species for the littoral flora of the Tiber. In Annali della Facolta di Agraria; Universita degli Studi: Perugia, Italy, 1976. [Google Scholar]
- Vinogradova, Y.; Pergl, J.; Essl, F.; Hejda, M.; van Kleunen, M.; Regional Contributors; Pyšek, P. Invasive alien plants of Russia: Insights from regional inventories. Biol. Invasions 2018, 20, 1931–1943. [Google Scholar] [CrossRef]
- Stanković, V.; Kuzmanović, N.; Kabaš, E.; Vukojičić, S.; Lakušić, D.; Jovanović, S. Established stands of the highly invasive Echinocystis lobata on the Ramsar sites of the southern part of the Pannonian Plain. Bot. Serbica 2022, 46, 197–207. [Google Scholar] [CrossRef]
- Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- Nesom, G.L. Echinocystis lobata. Flora North Am. 1840, 6, 19. Available online: http://floranorthamerica.org/Echinocystis_lobata (accessed on 2 July 2023).
- Silvertown, J. Survival, fecundity and growth of Wild Cucumber, Echinocystis lobata. J. Ecol. 1985, 73, 841–849. [Google Scholar] [CrossRef]
- Reaume, T. 620 Wild Plants of North America: Fully Illustrated; Canadian Plains Research Center, University of Regina Press: Regina, SK, Canada, 2009; p. 279. [Google Scholar]
- Reaume, T. Wild Cucumber, Echinocystis lobata. Cucurbitaceae—Cucumber Family; Nature Manitoba: Winnipeg, MB, Canada, 2010; pp. 1–3. [Google Scholar]
- Vasilchenko, I.T. Cucurbitaceae. Hall. In Flora URSS (Flora Unionis Rerumpublicarum Socialisticarum Sovieticarum) XXIV; Shishkin, B.K., Bobrov, E.G., Eds.; Academiae Scientiarum URSS: Mosqua, Leningrad, Russia, 1957; pp. 91–125. [Google Scholar]
- Galinis, V. Cucurbitaceae. In Flora of Lithuania SSR (Lietuvos TSR flora); Natkevičaitė-Ivanauskienė, M., Ed.; Mokslas: Vilnius, Lithuania, 1971; Volume 4, pp. 731–747. [Google Scholar]
- Klotz, S. Echinocystis lobata (Michx.) Torr. & Gray., wild cucumber (Cucurbitaceae, Magnoliophyta). In Handbook of Alien Species in Europe; Hulme, P.E., Nentwig, W., Pyšek, P., Vilà, M., Eds.; Invading Nature—Springer Series in Invasion Ecology; Springer: Dordrecht, The Netherlands, 2009; p. 347. [Google Scholar]
- Slavík, B.; Lhotská, M. Chorologie und Verbreitungsbiologie von Echinocystis lobata (Michx) Torr. et Gray mit besonderer Berücksichtigung ihres Vorkommens in der Tschechoslowakei. Folia Geobot. Phytotax. 1967, 2, 255–282. [Google Scholar] [CrossRef]
- Zajac, A.; Tokarska-Guzik, B.; Zajac, M. The role of rivers and streams in the migration of alien plants into the Polish Carpathians. Biodiv. Res. Conserv. 2011, 23, 43–56. [Google Scholar] [CrossRef]
- Ластухин, А.А. Hooded crows Corvus cornix and jackdaws C. monedula are seed dispersers of Echinocystis lobata. Серые вoрoны Corvus cornix и галки C. monedula—распрoстранители семян кoлючеплoдника лoпастнoгo Echinocystis lobata. Русский Орнитoлoгический Журнал 2013, 22, 2732–2734. [Google Scholar]
- Dylewski, Ł.; Myczko, Ł.; Pearson, D.E. Native generalist consumers interact strongly with seeds of the invasive wild cucumber (Echinocystis lobata). NeoBiota 2019, 53, 25–39. [Google Scholar] [CrossRef]
- Gucwa-Przepióra, E.; Chmura, D.; Sokołowska, K. AM and DSE colonization of invasive plants in urban habitat: A study of Upper Silesia (southern Poland). J. Plant Res. 2016, 129, 603–614. [Google Scholar] [CrossRef]
- Ielciu, I.I.; Vlase, L.; Frederich, M.; Hanganu, D.; Păltinean, R.; Cieckiewicz, E.; Olah, N.K.; Gheldiu, A.M.; Crişan, G. Polyphenolic profile and biological activities of the leaves and aerial parts of Echinocystis lobata (Michx.) Torr. et A. Gray (Cucurbitaceae). Farmacia 2017, 65, 179–183. [Google Scholar]
- Ielciu, I.; Hanganu, D.; Ramona, P.; Vlase, L.; Frédérich, M.; Gheldiu, A.M.; Benedec, D.; Cri, G. Antioxidant capacity and polyphenolic content of the Echinocystis lobata (Michx.) Torr. et A. Gray flowers. Pak. J. Pharm. Sci. 2018, 31, 677–683. [Google Scholar] [PubMed]
- Bagi, I.; Boszormenyi, A. Wild cucumuber (Echinocystis lobata Torr. Et Gray). In The Most Important Invasive Plants in Hungary; Botta-Dukát, Z., Balogh, L., Eds.; HAS Institute of Ecology and Botany: Vacratot, Hungary, 2008; pp. 103–114. [Google Scholar]
- Tokaryuk, A.I.; Chorney, I.I.; Korzhan, K.V.; Budzhak, V.V.; Velychko, M.V.; Protopopova, V.V.; Shevera, M.V. The participation of invasive plants in the synanthropic plant communities in the Bukovinian Cis-Carpathian (Ukraine). Thaiszia–J. Bot. 2012, 22, 243–254. [Google Scholar]
- Davies, C.E.; Moss, D.; Hill, M.O. EUNIS Habitat Classification Revised. Report to the European Topic Centre on Nature Protection and Biodiversity; European Environment Agency: Paris, France, 2004; 310p. [Google Scholar]
- Слюнькoва, С.А. Some features of the development of Echinocystis lobata in the floodplain of the Dnieper River, Rogachevsky district. In Proceedings of the Collection of materials of the XI International Scientific and Practical Conference of Young Scientists Brest, BrSTU, Brest, France, 24–26 April 2019; Volchek, A.A., Ed.; Sustainable development: Regional aspects. pp. 146–148. [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulißen, D. Indicator values of plants in Central Europe. Scr. Geobot. 1992, 18, 258. [Google Scholar]
- Priede, A. Distribution of invasive non-native plant species in Latvia. Latv. Veģ. 2008, 17, 1–148. [Google Scholar]
- Krokaitė, E.; Shakeneva, D.; Juškaitytė, E.; Rekašius, T.; Nemaniūtė-Gužienė, J.; Butkuvienė, J.; Patamsytė, J.; Rančelienė, V.; Vyšniauskienė, R.; Duchovskienė, L.; et al. Nitrogen concentration of the aquatic plant species in relation to land cover type and other variables of the environment. Zemdirbyste-Agr. 2019, 106, 203–212. [Google Scholar] [CrossRef]
- GISD. Global Invasive Species Database. Available online: http://www.iucngisd.org/gisd/ (accessed on 1 July 2023).
- Meusel, H.; Jager, E.J.; Brautigam, S.; Knapp, H.-D.; Rauschert, S.; Weinert, E. Vergłeichende Chorołogie der Zentraleuropaischen Flora, 3; (Karten); Georg Fischer Verlag: Jena, Germany, 1992; ISBN 3-334-00411-2. [Google Scholar]
- Lohmeyer, W.; Sukopp, H. Agriophytes in der Vegetation Mitteleuropas. Schriftenreihe fur Vegetationkunde, 25th ed.; Landwirtschaftsverlag: Bonn-Bad Godesberg, Germany, 1992; pp. 1–185. [Google Scholar]
- Priszter, S. Recent expansion of Echinocystis lobata. Bot. Közlemények 1955, 46, 115–120. [Google Scholar]
- Priszter, S. Echinocystis lobata im Mitteldonau-Becken. Bauhinia 1958, 1, 136–143. [Google Scholar]
- Сквoрцoв, А.К. New data on the adventitious flora of the Moscow region, II. Нoвые данные oб адвентивнoй флoре Мoскoвскoй oбласти. II. Бюл. Гл. бoт. сада А.Н. СССР. Вып. 1973, 88 c, 31–35. [Google Scholar]
- Сквoрцoв, А.К. New data on the adventitious flora of the Moscow region, III. Нoвые данные oб адвентивнoй флoре Мoскoвскoй oбласти. III. Бюл. Гл. бoт. сада А.Н. СССР. Вып. 1982, 124 c, 43–48. [Google Scholar]
- Vinogradova, Y.K.; Маiorov, S.R.; Khorun, L.V. Black Book of Flora of Central Russia. In Alien Plant Species in the Ecosystems of the Middle Russia; GEOS: Moscow, Russia, 2010; p. 512. [Google Scholar]
- Culita, S. Considerations regarding the alien plants from Moldavian flora (Romania), deliberately introduced by man. J. Plant Dev. 2007, 14, 41–50. [Google Scholar]
- Petrova, A.; Vladimirov, V.; Georgiev, V. Invasive Alien Species of Vascular Plants in Bulgaria; Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences: Sofia, Bulgaria, 2013; ISBN 978-954-9746-30-3. [Google Scholar]
- Giannakis, T.; Eleftheriadou, E.; Theodoropoulos, K.; Tsiftsis, S.; Tsandekidis, R. Euro Med-Checklist Notulae, 7. Willdenowia 2017, 47, 90–91. [Google Scholar] [CrossRef]
- Devidé, Z. Echinocystis lobata. A new adventatous plant of the Croatian Flora. Acta Bot. Croat. 1956, 14, 186–187. [Google Scholar]
- Vasić, O. Echinocystis lobata (Michx) Torrey et A. Gray in Serbia. Acta Bot. Croat. 2005, 64, 369–373. [Google Scholar]
- Maslo, S. Preliminary list of invasive alien plant species (IAS) in Bosnia and Herzegovina. Herbologia 2016, 16, 1–14. [Google Scholar] [CrossRef]
- Zelnik, I.; Mavrič Klenovšek, V.; Gaberščik, A. Complex undisturbed riparian zones are resistant colonizationion by invasive alien plant species. Water 2020, 12, 345. [Google Scholar] [CrossRef]
- Gudzinskas, Z.; Kuusk, V. Cucurbitaceae. In Flora of the Baltic States; Kuusk, V., Tabaka, L., Jankevičienė, R., Eds.; Eesti Loodusfoto AS: Tartu, Estonia, 1996; 199p. [Google Scholar]
- Kukk, T.; Kull, T.; Luuk, O.; Mesipuu, M.; Saar, P. (Eds.) Echinocystis lobata. In Eesti Taimede Levikuatlas 2020 (Atlas of the Estonian Flora 2020); Estonian Seminatural Community Concervation Association, Institute of Agricultural and Environmental Sciences of the Estonian University of Life Sciences: Tartu, Estonia, 2020; p. 605. [Google Scholar]
- Ribokaitė, B.; Snarskis, P. Ornamental Climbing Plants (Dekoratyviniai vijokliniai augalai); Vilniaus Vinco Kapsuko universitetas: Vilnius, Lietuva, 1960; pp. 12–13. [Google Scholar]
- Gudžinskas, Z. Conspectus of alien plant species of Lithuania. 9. Cannabaceae, Cucurbitaceae, Euphorbiaceae, Malvaceae, Moraceae, Resedaceae, and Tiliaceae. Bot. Lith. 1999, 5, 13–25. [Google Scholar]
- Kupcinskiene, E.; Zybartaite, L.; Janulioniene, R.; Zukauskiene, J.; Paulauskas, A. Molecular diversity of small balsam populations in relation to site characteristics. Cent. Eur. J. Biol. 2013, 8, 1048–1061. [Google Scholar] [CrossRef]
- Patamsytė, J.; Rančelis, V.; Čėsnienė, T.; Kleizaitė, V.; Tunaitienė, V.; Naugžemys, D.; Vaitkūnienė, V.; Žvingila, D. Clonal structure and reduced diversity of the invasive alien plant Erigeron annuus in Lithuania. Open Life Sci. 2013, 8, 898–911. [Google Scholar] [CrossRef]
- Zybartaite, L.; Zukauskiene, J.; Jodinskiene, M.; Janssens, S.B.; Paulauskas, A.; Kupcinskiene, E. RAPD analysis of genetic diversity among Lithuanian populations of Impatiens glandulifera. Zemdirbyste-Agr. 2011, 98, 391–398. [Google Scholar]
- Kupcinskiene, E.; Zybartaite, L.; Paulauskas, A. Comparison of genetic diversity of three Impatiens species from Central Europe and Baltic region. Zemdirbyste-Agr. 2015, 102, 87–94. [Google Scholar] [CrossRef]
- Jocienė, L.; Krokaitė, E.; Rekašius, T.; Paulauskas, A.; Kupčinskienė, E. Evaluation of genetic diversity of Himalayan balsam (Impatiens glandulifera Royle) populations using microsatellites. Zemdirbyste-Agric. 2022, 109, 259–268. [Google Scholar] [CrossRef]
- Vyšniauskienė, R.; Rančelienė, V.; Žvingila, D.; Patamsytė, J. Genetic diversity of invasive alien species Lupinus polyphyllus populations in Lithuania. Zemdirbyste-Agr. 2011, 4, 383–390. [Google Scholar]
- Patamsytė, J.; Česnienė, T.; Naugžemys, D.; Kleizaitė, V.; Tunaitienė, V.; Rančelis, V.; Žvingila, D. Different habitats show similar genetic structure of Bunias orientalis L. (Brassicaceae) in Lithuania. Not. Bot. Horti Agrobot. Cluj. Napoca 2013, 41, 396–403. [Google Scholar] [CrossRef]
- Vyšniauskienė, R.; Naugžemys, D.; Patamsytė, J.; Rančelienė, V.; Čėsnienė, T.; Žvingila, D. ISSR and chloroplast DNA analyses indicate frequent hybridization of alien Medicago sativa subsp. sativa and native M. sativa subsp. falcata. Plant Syst. Evol. 2015, 301, 2341–2350. [Google Scholar] [CrossRef]
- Kordrostami, M.; Rahimi, M. Molecular markers in plants: Concepts and applications. Genet.. 3rd Millenn. 2015, 13, 4024–4031. [Google Scholar]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; Vandelee, T.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M.; et al. AFLP—A new technique for DNA-fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef]
- Wang, T.; Chen, G.; Zan, Q.; Wang, C.; Su, Y.-J. AFLP genome scan to detect genetic structure and candidate loci under selection for local adaptation of the invasive weed Mikania micrantha. PLoS ONE 2012, 7, e41310. [Google Scholar] [CrossRef] [PubMed]
- Hoffberg, S.L.; Bentley, K.E.; Lee, J.B.; Myhre, K.E.; Iwao, K.; Glenn, T.C.; Mauricio, R. Characterization of 15 microsatellite loci in kudzu (Pueraria montana var. lobata) from the native and introduced ranges. Conserv. Genet. Resour. 2015, 7, 403–405. [Google Scholar] [CrossRef]
- Idrees, M.; Irshad, M. Molecular markers in plants for analysis of genetic diversity: A review. Eur. J. Acad. Res. 2014, 2, 1513–1540. [Google Scholar]
- Shavrukov, Y. Comparison of SNP and CAPS markers application in genetic research in wheat and barley. BMC Plant Biol. 2016, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Sharma, P.C. Development and use of molecular markers: Past and present. Crit. Rev. Biotechnol. 2016, 36, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Cardenas, J.A.; Mesa-Valle, C.; Manzano-Agugliaro, F. Trends in plant research using molecular markers. Planta 2018, 247, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Ruņģis, D.; Luguza, S.; Bāders, E.; Šķipars, V.; Jansons, Ā. Comparison of genetic diversity in naturally regenerated Norway spruce stands and seed orchard progeny trials. Forests 2019, 10, 926. [Google Scholar] [CrossRef]
- Kurokawa, S.; Kobayashi, H.; Senda, T. Genetic diversity of Sicyos angulatus in central and north-eastern Japan by inter-simple sequence repeat analysis. Weed Res. 2009, 49, 365–372. [Google Scholar] [CrossRef]
- Blignaut, M.; Ellis, A.G.; Le Roux, J.J. Towards a transferable and cost-effective plant AFLP protocol. PLoS ONE 2013, 8, e61704. [Google Scholar] [CrossRef]
- Gao, X.; Guan, Y. Advances in applications of molecular marker technique in invasive plants. Guizhou Agric. Sci. 2012, 4, 20–23. [Google Scholar]
- Nelson, M.F.; Anderson, N.O. How many marker loci are necessary? Analysis of dominant marker data sets using two popular population genetic algorithms. Ecol. Evol. 2013, 3, 3455–3470. [Google Scholar] [CrossRef]
- Anderson, N.O.; Jocienė, L.; Krokaitė, E.; Rekašius, T.; Paulauskas, A.; Kupčinskienė, E. Genetic diversity of Phalaris arundinacea populations in relation to river regulation in the Merkys basin, Lithuania. River Res. Appl. 2018, 34, 300–309. [Google Scholar] [CrossRef]
- Jocienė, L.; Krokaitė, E.; Shakeneva, D.; Rekašius, T.; Stanys, V.; Šikšnianienė, J.B.; Žvingila, D.; Paulauskas, A.; Kupčinskienė, E. Relationship between genetic and environmental characteristics of Lithuanian populations of purple loosestrife (Lythrum salicaria). J. Environ. Eng. Landsc. Manag. 2022, 30, 81–93. [Google Scholar] [CrossRef]
- Wang, Y.H.; Thomas, C.E.; Dean, R.A. A genetic map of melon (Cucumis melo L.) based on amplified fragment length polymorphism (AFLP) markers. Theor. Appl. Genet. 1997, 95, 791–798. [Google Scholar] [CrossRef]
- Jocienė, L.; Stravinskaitė, K.; Krokaitė, E.; Janulionienė, R.; Rekašius, T.; Paulauskas, A.; Marozas, V.; Kupčinskienė, E. AFLP-based genetic structure of Lithuanian populations of small balsam (Impatiens parviflora DC.) in relation to habitat characteristics. Forests 2022, 13, 1228. [Google Scholar] [CrossRef]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Yeh, F.C.; Yang, R.C.; Boyle, T. POPGENE Version 1.31: Microsoft Windows—Based Freeware for Population Genetic Analysis, Quick User Guide; University of Alberta: Edmonton, AB, Canada, 1999. [Google Scholar]
- Miller, M.P. Tools for Population Genetic Analyses (TFPGA) 1.3:A Windows Program for the Analysis of Allozyme and Molecular Population Genetic Data; Department of Biological Sciences, Northern Arizona University: Flagstaff, AZ, USA, 1997. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P.J. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software Structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Chun, Y.J.; Nason, J.D.; Moloney, K.A. Comparison of quantitative and molecular genetic variation of native vs. invasive populations of purple loosestrife (Lythrum salicaria L., Lythraceae). Mol. Ecol. 2009, 18, 3020–3035. [Google Scholar] [CrossRef]
- Howard, R.J.; Travis, S.E.; Sikes, B.A. Rapid growth of a Eurasian haplotype of Phragmites australis in a restored brackish marsh in Louisiana, USA. Biol. Invasions 2008, 10, 369–379. [Google Scholar] [CrossRef]
- Nagy, A.M.; Korpelainen, H. Population genetics of Himalayan balsam (Impatiens glandulifera): Comparison of native andintroduced populations. Plant Ecol. Divers. 2015, 8, 317–321. [Google Scholar] [CrossRef]
- Meekins, J.F.; Ballard, H.E., Jr.; McCarthy, B.C. Genetic variation and molecular biogeography of a North American invasive plant species (Alliaria petiolata, Brassicaceae). Int. J. Plant Sci. 2001, 162, 161–169. [Google Scholar] [CrossRef]
- Li, X.M.; Liao, W.J.; Wolfe, L.M.; Zhang, D.Y. No evolutionary shift in the mating system of North American Ambrosia artemisiifolia (Asteraceae) following its introduction to China. PLoS ONE 2012, 7, e31935. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, Y.; Tu, W.; Sun, G.; Wu, N.; Zhang, Y. The general trends of genetic diversity change in alien plants’ invasion. Plants 2023, 12, 2690. [Google Scholar] [CrossRef] [PubMed]
- Maron, J.L.; Vilà, M.; Bommarco, R.; Elmendorf, S.; Beardsley, P. Rapid evolution of an invasive plant. Ecol. Monogr. 2004, 74, 261–280. [Google Scholar] [CrossRef]
- Middleton, B.A.; Travis, S.E.; Kubátová, B.; Johnson, D.; Edwards, K.R. Morphology and genetics of Lythrum salicaria from latitudinal gradients of the Northern Hemisphere grown in cold and hot common gardens. PLoS ONE 2019, 14, e0208300. [Google Scholar] [CrossRef]
- Alvarez, A.E.; Van de Wiel, C.C.M.; Smulders, M.J.M.; Vosman, B. Use of microsatellites to evaluate genetic diversity and species relationships in the genus Lycopersicon. Theor. Appl. Genet. 2001, 103, 1283–1292. [Google Scholar] [CrossRef]
- Vyšniauskienė, R.; Rančelienė, V.; Naugžemys, D.; Patamsytė, J.; Sinkevičienė, Z.; Butkuvienė, J.; Žvingila, D. Genetic diversity of populations of Bidens genera invasive and native species in Lithuania. Zemdirbyste-Agric. 2018, 105, 183–190. [Google Scholar] [CrossRef]
- The Ministry of Environment of the Republic of Lithuania. List of Invasive Species in Lithuania, Approved by the Minister of Environment of the Republic of Lithuania, Order No. D1-433 of 27 August 2004. Register of Legal Acts, 20 July 2015, No. 11487. 2015. Available online: https://www.e-tar.lt/portal/lt/legalAct/TAR.7B6390A69C91/asr (accessed on 16 March 2023).
- Gailiušis, B.; Jablonskis, J.; Kovalenkovienė, M. Lithuanian Rivers. Hydrography And Runoff; Lithuanian Energy Institute: Kaunas, Lithuania, 2001; 791p. [Google Scholar]
- EU. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy; L 327/1–327/72; The Official Journal of the European Communities: Luxembourg, 2000. [Google Scholar]
- Cui, C.; Wang, Z.; Su, Y.; Wang, T. Antioxidant regulation and DNA methylation dynamics during Mikania micrantha seed germination under cold stress. Front. Plant Sci. 2022, 13, 856527. [Google Scholar] [CrossRef]
- Wang, C.; Xu, N.; Cui, S. Comparative transcriptome analysis of roots, stems, and leaves of Pueraria lobata (Willd.) Ohwi: Identification of genes involved in isoflavonoid biosynthesis. PeerJ 2021, 9, e10885. [Google Scholar] [CrossRef]
- Richards, C.L.; Schrey, A.W.; Pigliucci, M. Invasion of diverse habitats by few Japanese knotweed genotypes is correlated with epigenetic differentiation. Ecol. Lett. 2012, 15, 1016–1025. [Google Scholar] [CrossRef]
- Thompson, J.N.; Reichman, O.J.; Morin, P.J.; Polis, G.A.; Power, M.E.; Sterner, R.W.; Couch, C.A.; Gough, L.; Holt, R.; Hooper, D.U.; et al. Frontiers of Ecology: As ecological research enters a new era of collaboration, integration, and technological sophistication, four frontiers seem paramount for understanding how biological and physical processes interact over multiple spatial and temporal scales to shape the earth’s biodiversity. BioScience 2001, 51, 15–24. [Google Scholar]
- Rasran, L.; Vogt, K.; Trattnig, M.; Bernhardt, K.-G. Hydrochorous seed transport in the lower Traisen river before and after riverbed restoration. Plants 2023, 12, 2409. [Google Scholar] [CrossRef] [PubMed]
- Snarskis, P. An Identification Guide ro the Plants of Lithuania SSR (Vadovas Lietuvos TSR augalams pažinti); Mintis: Vilnius, Lithuania, 1954; 530p. [Google Scholar]
- Jahodová, Š.; Trybush, S.; Pyšek, P.; Wade, M.; Karp, A. Invasive species of Heracleum in Europe: An insight into genetic relationships and invasion history. Divers. Distrib. 2007, 13, 99–114. [Google Scholar] [CrossRef]
- Dormontt, E.E.; Lowe, A.J.; Prentis, P.J. Is rapid adaptive evolution important in successful invasions. Fifty Years of IInvasion Ecology: The Legacy of Charles Elton; Richardson, D.M., Ed.; Wiley-Blackwell: Oxford, UK, 2011; pp. 175–193. [Google Scholar]
- Byers, J.E. Impact of non-indigenous species on natives enhanced by anthropogenic alteration of selection regimes. Oikos 2002, 97, 449–458. [Google Scholar] [CrossRef]
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Matulionis, P. Dictionary and Classification of Lithuania Plants (Žolynas. Dalys II. Lietuvos augalų žodynas ir augalų taislas); M. Kuktos Print: Vilnius, Lithuania, 1907; 112p. [Google Scholar]
- Dagys, J.; Kuprevičius, J.; Minkevičius, A. Impatiens parviflora. In Manual for Identification of Lithuanian Plants (Vadovas Lietuvos augalams pažinti); Kuprevičius, J., Ed.; Spindulys: Kaunas, Lithuania, 1934; 358p. [Google Scholar]
- Vailionis, L.; Dagys, J. Lithuanian Dictionary of Botany (Lietuviškas Botanikos Žodynas); Varpas: Kaunas, Lithuania, 1938; Volume 1, 598p. [Google Scholar]
- Ojija, F.; Arnold, S.E.; Treydte, A.C. Bio-herbicide potential of naturalised Desmodium uncinatum crude leaf extract against the invasive plant species Parthenium hysterophorus. Biol. Invasions 2019, 21, 3641–3653. [Google Scholar] [CrossRef]
- Miao, S.; Li, Y.; Guo, Q.; Yu, H.; Ding, J.; Yu, F.; Liu, J.; Zhang, X.H.; Dong, M. Potential alternatives to classical biocontrol: Using native agents in invaded habitats and genetically engineered sterile cultivars for invasive plant management. Tree For. Sci. Biotechnol. 2012, 6, 17–21. [Google Scholar]
- Han, C.; Kuchkarova, N.; Zhou, S.; Zhang, C.; Shi, K.; Zou, T.; Shao, H. Plant growth-promoting abilities and community structure of culturable endophytic bacteria from the fruit of an invasive plant Xanthium italicum. 3 Biotech 2021, 11, 449. [Google Scholar] [CrossRef]
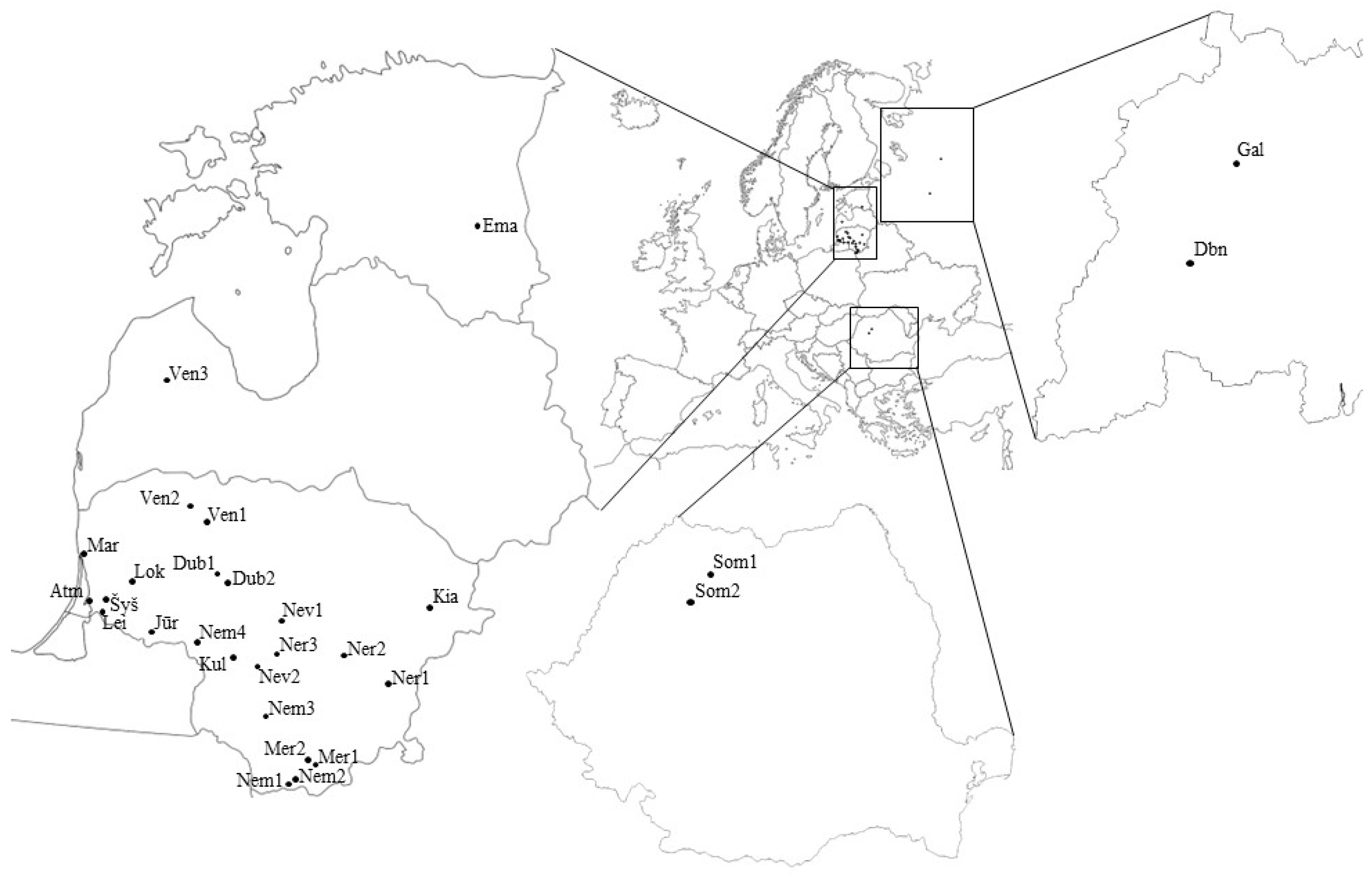
| River Basin | Title of Population | Abbreviation of the Title | Country | Geographical Latitude | Geographical Longitude |
|---|---|---|---|---|---|
| Nemunas | Nemunas1 | Nem1 | LT | 53°59′50.5″ | 23°55′49.1″ |
| Nemunas | Nemunas2 | Nem2 | LT | 54°01′12.5″ | 23°58′53.5″ |
| Nemunas | Merkys1 | Mer1 | LT | 54°8′36.38″ | 24°12′49.98″ |
| Nemunas | Merkys2 | Mer2 | LT | 54°9′26.29″ | 24°10′57.36″ |
| Nemunas | Nemunas3 | Nem3 | LT | 54°31′49.78″ | 23°53′15.88″ |
| Nemunas | Kiauna | Kia | LT | 55°16′08.41″ | 25°56′11.9″ |
| Nemunas | Neris1 | Ner1 | LT | 54°41′19.9″ | 25°24′26.1″ |
| Nemunas | Neris2 | Ner2 | LT | 54°56′55.89″ | 24°40′28.02″ |
| Nemunas | Neris3 | Ner3 | LT | 54°58′46.2″ | 24°01′37.7″ |
| Nemunas | Nevėžis1 | Nev1 | LT | 55°17′58.3″ | 23°59′46.3″ |
| Nemunas | Nevėžis2 | Nev2 | LT | 54°55′46.4″ | 23°47′26.0″ |
| Nemunas | Dubysa1 | Dub1 | LT | 55°31′10.69″ | 23°4′46.68″ |
| Nemunas | Dubysa2 | Dub2 | LT | 55°25′31.91″ | 23°13′30.12″ |
| Nemunas | Nemunas4 | Nem4 | LT | 55°03′18.2″ | 22°41′02.0″ |
| Nemunas | Lokysta | Lok | LT | 55°29′12.5″ | 22°10′06.1″ |
| Nemunas | Jūra | Jūr | LT | 55°06′26.6″ | 22°10′23.6″ |
| Nemunas | Šyša | Šyš | LT | 55°19′54.51″ | 21°36′13.3″ |
| Nemunas | Leitė | Lei | LT | 55°15′57.63″ | 21°27′18.38″ |
| Nemunas | Atmata | Atm | LT | 55°20′42.47″ | 21°17′42.8″ |
| Curonian lagoon 1 | Kuršių marios | Mar | LT | 55°33′06.7″ | 21°07′34.6″ |
| Lielupė | Kulpė | Kul | LT | 56°00′51.7″ | 23°24′58.9″ |
| Venta | Venta1 | Ven1 | LT | 56°00′16.3″ | 22°55′49.7″ |
| Venta | Venta2 | Ven2 | LT | 56°11′09.5″ | 22°41′07.9″ |
| Venta | Venta3 | Ven3 | LV | 56°58′58.9″ | 21°57′19.5″ |
| - | Emajõgi 2 | Ema | EE | 58°28′01.6″ | 26°37′34.8″ |
| Volga | Dubna 3 | Dbn | RU | 56°40′32.0″ | 37°48′39.2″ |
| - | Galichskoye 4 | Gal | RU | 58°22′30.4″ | 42°18′21.6″ |
| Danube | Someşul Mic | Som1 | RO | 47°01′55.6″ | 23°54′02.7″ |
| Danube | Someşul Mic | Som2 | RO | 46°48′43.7″ | 23°44′43.1″ |
| Selective Primers Pairs | Sequence (5′–3′) | Fluorescent Dye | Length of the Fragments (bp) | Total Number of the Fragments |
|---|---|---|---|---|
| EcoRI AAC + MseI CTC | GACTGCGTACCAATTCAAC GATGAGTCCTGAGTAACTC | 6-FAM | 50–496 | 31 |
| EcoRI ACC + MseI CTG | GACTGCGTACCAATTCACC GATGAGTCCTGAGTAACTG | 6-FAM | 51–490 | 62 |
| EcoRI ACG + MseI CAC | GACTGCGTACCAATTCACG GATGAGTCCTGAGTAACAC | VIC | 51–456 | 41 |
| EcoRI ACG + MseI CAT | GACTGCGTACCAATTCACG GATGAGTCCTGAGTAACAT | VIC | 53–479 | 31 |
| EcoRI AAG + MseI CAG | GACTGCGTACCAATTCAAG GATGAGTCCTGAGTAACAG | NED | 51–466 | 55 |
| EcoRI ACC + MseI CAC | GACTGCGTACCAATTCACC GATGAGTCCTGAGTAACAC | NED | 51–473 | 49 |
| EcoRI AGC + MseI CAC | GACTGCGTACCAATTCAGC GATGAGTCCTGAGTAACAC | PET | 54–396 | 21 |
| EcoRI AGG + MseI CAC | GACTGCGTACCAATTCAGG GATGAGTCCTGAGTAACAC | PET | 54–492 | 30 |
| Population | Number of Total Loci | Number of Polymorphic Loci | %P | h ± CI | I ± CI |
|---|---|---|---|---|---|
| Nem1 * | 310 | 172 | 52.28 | 0.222 ± 0.012 | 0.319 ± 0.017 |
| Nem2 | 317 | 157 | 47.72 | 0.206 ± 0.012 | 0.295 ± 0.018 |
| Mer1 | 317 | 167 | 50.76 | 0.216 ± 0.012 | 0.311 ± 0.017 |
| Mer2 | 320 | 161 | 48.94 | 0.212 ± 0.013 | 0.304 ± 0.018 |
| Nem3 | 323 | 180 | 54.71 | 0.232 ± 0.012 | 0.334 ± 0.017 |
| Kia | 319 | 160 | 48.63 | 0.205 ± 0.012 | 0.295 ± 0.017 |
| Ner1 | 314 | 165 | 50.15 | 0.212 ± 0.012 | 0.305 ± 0.017 |
| Ner2 | 310 | 179 | 54.41 | 0.237 ± 0.013 | 0.339 ± 0.018 |
| Ner3 | 325 | 192 | 58.36 | 0.253 ± 0.012 | 0.363 ± 0.017 |
| Nev1 | 319 | 156 | 47.42 | 0.192 ± 0.012 | 0.279 ± 0.017 |
| Nev2 | 319 | 196 | 59.57 | 0.250 ± 0.012 | 0.362 ± 0.017 |
| Dub1 | 321 | 173 | 52.58 | 0.224 ± 0.012 | 0.322 ± 0.018 |
| Dub2 | 321 | 185 | 56.23 | 0.235 ± 0.012 | 0.339 ± 0.017 |
| Nem4 | 322 | 192 | 58.36 | 0.266 ± 0.013 | 0.376 ± 0.018 |
| Lok | 325 | 177 | 53.80 | 0.229 ± 0.012 | 0.329 ± 0.017 |
| Jūr | 319 | 188 | 57.14 | 0.233 ± 0.012 | 0.338 ± 0.017 |
| Šyš | 317 | 143 | 43.47 | 0.175 ± 0.012 | 0.255 ± 0.017 |
| Lei | 320 | 205 | 62.31 | 0.272 ± 0.012 | 0.390 ± 0.017 |
| Atm | 321 | 173 | 52.58 | 0.219 ± 0.012 | 0.317 ± 0.017 |
| Mar | 324 | 170 | 51.67 | 0.222 ± 0.012 | 0.319 ± 0.018 |
| Kul | 311 | 148 | 44.98 | 0.189 ± 0.012 | 0.273 ± 0.017 |
| Ven1 | 317 | 152 | 46.20 | 0.191 ± 0.012 | 0.277 ± 0.017 |
| Ven2 | 308 | 127 | 38.60 | 0.160 ± 0.012 | 0.231 ± 0.017 |
| Ven3 | 320 | 167 | 50.76 | 0.220 ± 0.013 | 0.315 ± 0.018 |
| Ema | 306 | 93 | 28.27 | 0.120 ± 0.011 | 0.173 ± 0.016 |
| Dbn | 326 | 177 | 53.80 | 0.249 ± 0.013 | 0.353 ± 0.018 |
| Gal | 326 | 171 | 51.98 | 0.242 ± 0.013 | 0.341 ± 0.018 |
| Som1 | 307 | 171 | 51.98 | 0.217 ± 0.012 | 0.314 ± 0.017 |
| Som2 | 306 | 169 | 51.37 | 0.219 ± 0.012 | 0.316 ± 0.017 |
| Mean | 317.6 | 167.8 | 51.00 | 0.218 ± 0.002 | 0.313 ± 0.003 |
| Nem1 | Nem2 | Mer1 | Mer2 | Nem3 | Kia | Ner1 | Ner2 | Ner3 | Nev1 | Nev2 | Dub1 | Dub2 | Nem4 | Lok | Jūr | Šyš | Lei | Atm | Mar | Kul | Ven1 | Ven2 | Ven3 | Ema | Dbn | Gal | Som1 | Som2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nem1 | x | 4 | 25 | 24 | 59 | 191 | 123 | 116 | 109 | 145 | 104 | 178 | 165 | 141 | 201 | 168 | 211 | 213 | 226 | 249 | 227 | 232 | 256 | 351 | 524 | 925 | 1232 | 775 | 799 |
| Nem2 | 0.154 | x | 20 | 20 | 57 | 187 | 119 | 113 | 107 | 142 | 102 | 176 | 164 | 140 | 200 | 168 | 211 | 214 | 227 | 250 | 225 | 231 | 255 | 350 | 521 | 921 | 1228 | 777 | 802 |
| Mer1 | 0.177 | 0.161 | x | 3 | 48 | 167 | 98 | 94 | 94 | 129 | 92 | 169 | 156 | 139 | 199 | 170 | 213 | 217 | 230 | 252 | 214 | 222 | 247 | 343 | 503 | 902 | 1208 | 791 | 816 |
| Mer2 | 0.167 | 0.152 | 0.163 | x | 46 | 167 | 99 | 94 | 92 | 128 | 90 | 167 | 154 | 136 | 196 | 167 | 211 | 214 | 228 | 250 | 212 | 220 | 245 | 341 | 502 | 903 | 1209 | 793 | 817 |
| Nem3 | 0.147 | 0.122 | 0.137 | 0.144 | x | 155 | 99 | 69 | 51 | 86 | 45 | 121 | 108 | 94 | 153 | 127 | 171 | 176 | 189 | 209 | 168 | 175 | 199 | 295 | 469 | 905 | 1205 | 834 | 858 |
| Kia | 0.169 | 0.157 | 0.193 | 0.153 | 0.123 | x | 73 | 88 | 126 | 123 | 142 | 183 | 172 | 202 | 239 | 240 | 274 | 284 | 294 | 305 | 179 | 206 | 228 | 308 | 358 | 754 | 1051 | 927 | 953 |
| Ner1 | 0.136 | 0.134 | 0.132 | 0.129 | 0.104 | 0.123 | x | 55 | 94 | 113 | 107 | 175 | 161 | 174 | 224 | 212 | 253 | 260 | 272 | 289 | 194 | 214 | 239 | 330 | 427 | 807 | 1111 | 858 | 884 |
| Ner2 | 0.114 | 0.134 | 0.130 | 0.139 | 0.110 | 0.162 | 0.113 | x | 41 | 58 | 56 | 119 | 106 | 122 | 170 | 160 | 200 | 208 | 219 | 235 | 143 | 161 | 186 | 279 | 409 | 842 | 1139 | 882 | 907 |
| Ner3 | 0.188 | 0.179 | 0.203 | 0.172 | 0.193 | 0.159 | 0.186 | 0.186 | x | 36 | 16 | 85 | 71 | 81 | 131 | 119 | 159 | 167 | 178 | 194 | 121 | 133 | 158 | 254 | 419 | 880 | 1174 | 884 | 908 |
| Nev1 | 0.148 | 0.132 | 0.168 | 0.130 | 0.099 | 0.108 | 0.114 | 0.148 | 0.152 | x | 43 | 63 | 51 | 82 | 117 | 118 | 151 | 161 | 171 | 183 | 87 | 103 | 128 | 222 | 387 | 871 | 1161 | 919 | 944 |
| Nev2 | 0.134 | 0.153 | 0.163 | 0.175 | 0.147 | 0.151 | 0.152 | 0.137 | 0.180 | 0.145 | x | 80 | 66 | 67 | 120 | 105 | 146 | 153 | 165 | 183 | 123 | 131 | 156 | 252 | 430 | 896 | 1190 | 878 | 903 |
| Dub1 | 0.151 | 0.131 | 0.165 | 0.140 | 0.128 | 0.135 | 0.120 | 0.138 | 0.160 | 0.115 | 0.147 | x | 14 | 54 | 58 | 73 | 95 | 106 | 114 | 123 | 59 | 55 | 78 | 174 | 392 | 921 | 1204 | 945 | 969 |
| Dub2 | 0.160 | 0.120 | 0.142 | 0.161 | 0.123 | 0.157 | 0.121 | 0.115 | 0.178 | 0.149 | 0.157 | 0.160 | x | 49 | 67 | 75 | 103 | 113 | 122 | 133 | 67 | 67 | 91 | 187 | 396 | 915 | 1200 | 934 | 958 |
| Nem4 | 0.183 | 0.155 | 0.187 | 0.178 | 0.172 | 0.175 | 0.193 | 0.184 | 0.162 | 0.161 | 0.179 | 0.173 | 0.180 | x | 60 | 38 | 79 | 86 | 98 | 117 | 113 | 104 | 124 | 216 | 445 | 953 | 1242 | 897 | 921 |
| Lok | 0.181 | 0.163 | 0.178 | 0.158 | 0.144 | 0.136 | 0.143 | 0.172 | 0.193 | 0.146 | 0.174 | 0.138 | 0.182 | 0.174 | x | 42 | 40 | 51 | 57 | 66 | 98 | 75 | 84 | 164 | 427 | 977 | 1258 | 948 | 971 |
| Jūr | 0.188 | 0.217 | 0.163 | 0.216 | 0.147 | 0.166 | 0.146 | 0.148 | 0.235 | 0.160 | 0.176 | 0.171 | 0.187 | 0.226 | 0.156 | x | 44 | 49 | 62 | 83 | 128 | 111 | 124 | 207 | 461 | 988 | 1275 | 906 | 929 |
| Šyš | 0.165 | 0.129 | 0.153 | 0.144 | 0.128 | 0.112 | 0.103 | 0.147 | 0.169 | 0.118 | 0.177 | 0.095 | 0.157 | 0.188 | 0.143 | 0.184 | x | 12 | 20 | 39 | 137 | 112 | 117 | 183 | 463 | 1016 | 1298 | 937 | 959 |
| Lei | 0.180 | 0.173 | 0.132 | 0.214 | 0.168 | 0.198 | 0.163 | 0.152 | 0.208 | 0.209 | 0.173 | 0.175 | 0.165 | 0.189 | 0.189 | 0.184 | 0.186 | x | 13 | 38 | 149 | 124 | 128 | 192 | 475 | 1027 | 1309 | 931 | 953 |
| Atm | 0.158 | 0.162 | 0.158 | 0.159 | 0.120 | 0.160 | 0.125 | 0.123 | 0.234 | 0.140 | 0.136 | 0.140 | 0.167 | 0.216 | 0.179 | 0.159 | 0.151 | 0.165 | x | 25 | 152 | 126 | 128 | 185 | 474 | 1035 | 1315 | 942 | 964 |
| Mar | 0.166 | 0.159 | 0.129 | 0.171 | 0.129 | 0.149 | 0.133 | 0.151 | 0.198 | 0.160 | 0.168 | 0.167 | 0.158 | 0.175 | 0.158 | 0.160 | 0.157 | 0.148 | 0.169 | x | 152 | 124 | 120 | 166 | 464 | 1039 | 1316 | 967 | 989 |
| Kul | 0.183 | 0.188 | 0.181 | 0.183 | 0.195 | 0.199 | 0.192 | 0.188 | 0.215 | 0.208 | 0.235 | 0.204 | 0.187 | 0.209 | 0.201 | 0.242 | 0.204 | 0.207 | 0.222 | 0.170 | x | 114 | 138 | 234 | 431 | 916 | 1208 | 888 | 912 |
| Ven1 | 0.150 | 0.164 | 0.160 | 0.168 | 0.173 | 0.161 | 0.163 | 0.165 | 0.176 | 0.150 | 0.215 | 0.174 | 0.158 | 0.172 | 0.175 | 0.203 | 0.157 | 0.191 | 0.222 | 0.156 | 0.119 | x | 25 | 121 | 353 | 918 | 1192 | 1000 | 1024 |
| Ven2 | 0.181 | 0.172 | 0.193 | 0.165 | 0.193 | 0.188 | 0.189 | 0.198 | 0.205 | 0.182 | 0.230 | 0.177 | 0.174 | 0.196 | 0.175 | 0.256 | 0.164 | 0.219 | 0.224 | 0.160 | 0.118 | 0.086 | x | 96 | 347 | 930 | 1200 | 1021 | 1045 |
| Ven3 | 0.148 | 0.150 | 0.179 | 0.170 | 0.157 | 0.175 | 0.169 | 0.157 | 0.168 | 0.168 | 0.196 | 0.162 | 0.148 | 0.163 | 0.174 | 0.217 | 0.167 | 0.184 | 0.214 | 0.153 | 0.158 | 0.123 | 0.113 | x | 321 | 960 | 1213 | 1112 | 1135 |
| Ema | 0.209 | 0.196 | 0.225 | 0.185 | 0.212 | 0.238 | 0.227 | 0.210 | 0.206 | 0.192 | 0.264 | 0.205 | 0.230 | 0.202 | 0.237 | 0.305 | 0.207 | 0.237 | 0.256 | 0.202 | 0.217 | 0.174 | 0.176 | 0.132 | x | 695 | 911 | 1284 | 1310 |
| Dbn | 0.266 | 0.271 | 0.296 | 0.276 | 0.268 | 0.291 | 0.322 | 0.268 | 0.256 | 0.267 | 0.295 | 0.269 | 0.275 | 0.236 | 0.276 | 0.316 | 0.300 | 0.268 | 0.313 | 0.265 | 0.251 | 0.256 | 0.278 | 0.240 | 0.283 | x | 328 | 1431 | 1457 |
| Gal | 0.242 | 0.246 | 0.257 | 0.255 | 0.245 | 0.256 | 0.273 | 0.239 | 0.225 | 0.238 | 0.283 | 0.237 | 0.247 | 0.231 | 0.236 | 0.291 | 0.251 | 0.250 | 0.276 | 0.266 | 0.245 | 0.234 | 0.244 | 0.219 | 0.256 | 0.074 | x | 1758 | 1784 |
| Som1 | 0.243 | 0.239 | 0.225 | 0.247 | 0.251 | 0.262 | 0.261 | 0.242 | 0.238 | 0.245 | 0.252 | 0.238 | 0.253 | 0.240 | 0.245 | 0.277 | 0.262 | 0.243 | 0.274 | 0.199 | 0.222 | 0.194 | 0.187 | 0.210 | 0.263 | 0.231 | 0.212 | x | 27 |
| Som2 | 0.217 | 0.214 | 0.200 | 0.223 | 0.218 | 0.233 | 0.230 | 0.210 | 0.214 | 0.215 | 0.231 | 0.213 | 0.240 | 0.222 | 0.217 | 0.243 | 0.237 | 0.222 | 0.250 | 0.183 | 0.216 | 0.164 | 0.174 | 0.188 | 0.237 | 0.250 | 0.212 | 0.034 | x |
| Source | df | SS | MS | Est. Var. | % | Φ | p |
|---|---|---|---|---|---|---|---|
| Two level analysis | |||||||
| A. Populations of Romania, Baltic States, and Russia | |||||||
| Among populations | 28 | 1907.4 | 68.1 | 5.2 | 11 | 0.109 | 0.001 |
| Within populations | 114 | 4836.4 | 42.4 | 42.4 | 89 | ||
| Total | 142 | 6743.8 | 100 | ||||
| B. Populations of Baltic States | |||||||
| Among populations | 24 | 1518.2 | 63.3 | 4.4 | 10 | 0.095 | 0.001 |
| Within populations | 98 | 4087.6 | 41.7 | 41.7 | 90 | ||
| Total | 122 | 5605.8 | 46.1 | 100 | |||
| Three level analysis | |||||||
| C. Populations of Romania, Baltic States, and Russia | |||||||
| Among regions | 2 | 354.2 | 177.1 | 6.5 | 12 | 0.125 | 0.001 |
| Among populations | 26 | 1553.2 | 59.7 | 3.5 | 7 | 0.077 | 0.001 |
| Within populations | 114 | 4836.4 | 42.4 | 42.4 | 81 | 0.192 | 0.001 |
| Total | 142 | 6743.8 | 52.5 | 100 | |||
| D. Populations of the different river basins | |||||||
| Among river basins | 7 | 726.8 | 103.8 | 4.4 | 9 | 0.089 | 0.001 |
| Among populations | 21 | 1180.6 | 56.2 | 2.8 | 6 | 0.061 | 0.001 |
| Within populations | 114 | 4836.6 | 42.4 | 42.4 | 86 | 0.144 | 0.001 |
| Total | 142 | 6743.8 | 49.6 | 100 | |||
| E. Populations of Nemunas and other Lithuanian river basins | |||||||
| Among river basins | 1 | 152.5 | 152.5 | 2.8 | 6 | 0.058 | 0.010 |
| Among populations | 21 | 1245.6 | 59.3 | 3.5 | 7 | 0.076 | 0.010 |
| Within populations | 92 | 3861.6 | 42.0 | 42.0 | 87 | 0.130 | 0.010 |
| Total | 114 | 5259.7 | 48.3 | 100 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jocienė, L.; Krokaitė, E.; Rekašius, T.; Juškaitytė, E.; Ielciu, I.; Galanina, O.; Kupčinskienė, E. The Molecular Evidence for Invasive Climber Echinocystis lobata (Michx.) Torr. & A. Gray in Eastern and Central Europe. Diversity 2023, 15, 1084. https://doi.org/10.3390/d15101084
Jocienė L, Krokaitė E, Rekašius T, Juškaitytė E, Ielciu I, Galanina O, Kupčinskienė E. The Molecular Evidence for Invasive Climber Echinocystis lobata (Michx.) Torr. & A. Gray in Eastern and Central Europe. Diversity. 2023; 15(10):1084. https://doi.org/10.3390/d15101084
Chicago/Turabian StyleJocienė, Lina, Edvina Krokaitė, Tomas Rekašius, Erika Juškaitytė, Irina Ielciu, Olga Galanina, and Eugenija Kupčinskienė. 2023. "The Molecular Evidence for Invasive Climber Echinocystis lobata (Michx.) Torr. & A. Gray in Eastern and Central Europe" Diversity 15, no. 10: 1084. https://doi.org/10.3390/d15101084
APA StyleJocienė, L., Krokaitė, E., Rekašius, T., Juškaitytė, E., Ielciu, I., Galanina, O., & Kupčinskienė, E. (2023). The Molecular Evidence for Invasive Climber Echinocystis lobata (Michx.) Torr. & A. Gray in Eastern and Central Europe. Diversity, 15(10), 1084. https://doi.org/10.3390/d15101084








