Diversity of Cyanobacteria and Algae in Biological Soil Crusts of the Northern Ural Mountain Region Assessed through Morphological and Metabarcoding Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. BSCs Sampling Sites
2.2. BSCs Sampling and Algae Cultivation
2.3. Algae and Cyanobacteria Identification
2.4. DNA Extraction
2.5. Amplification, Library Preparation, and Sequencing
2.6. Bioinformatic Pipeline
3. Results
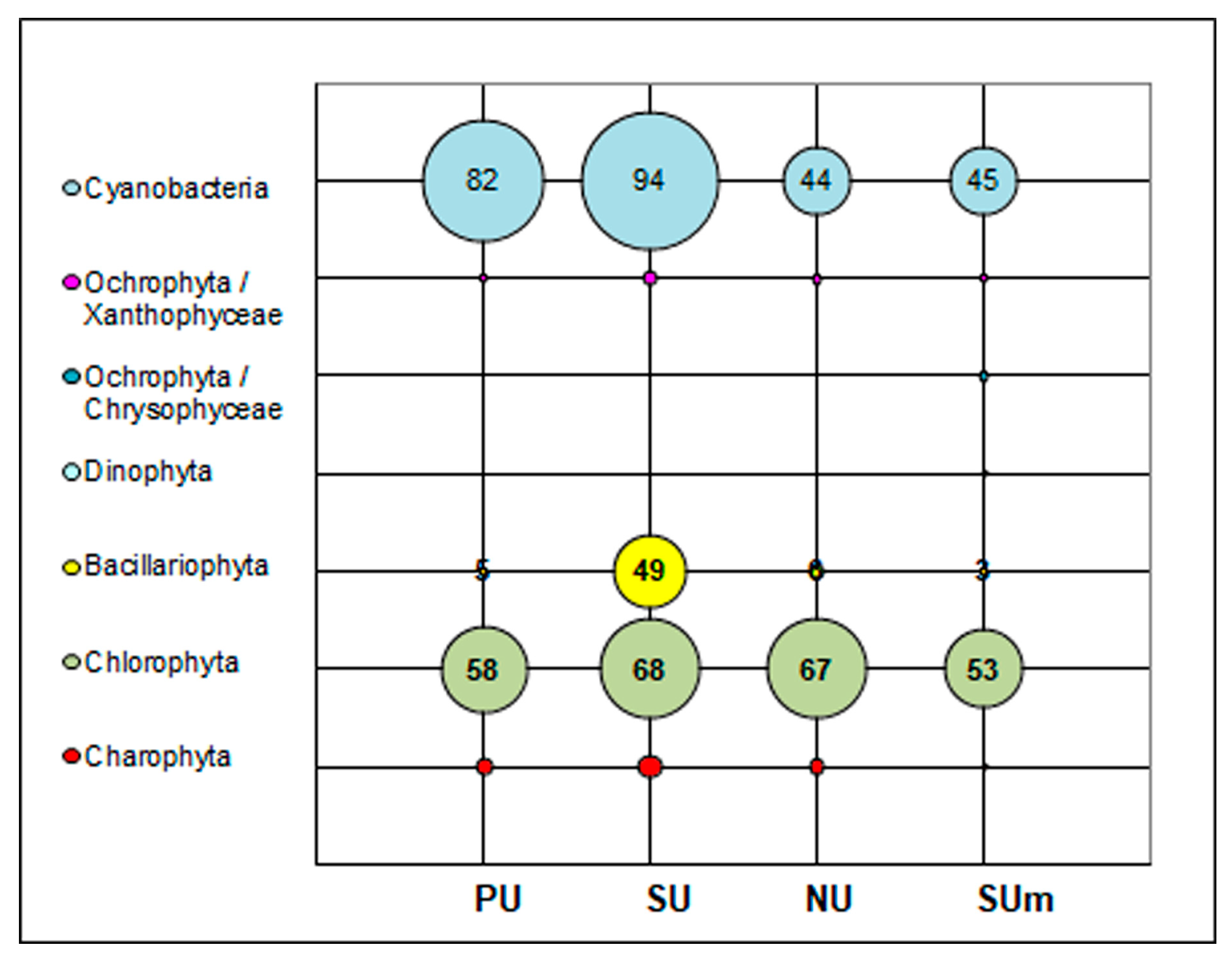
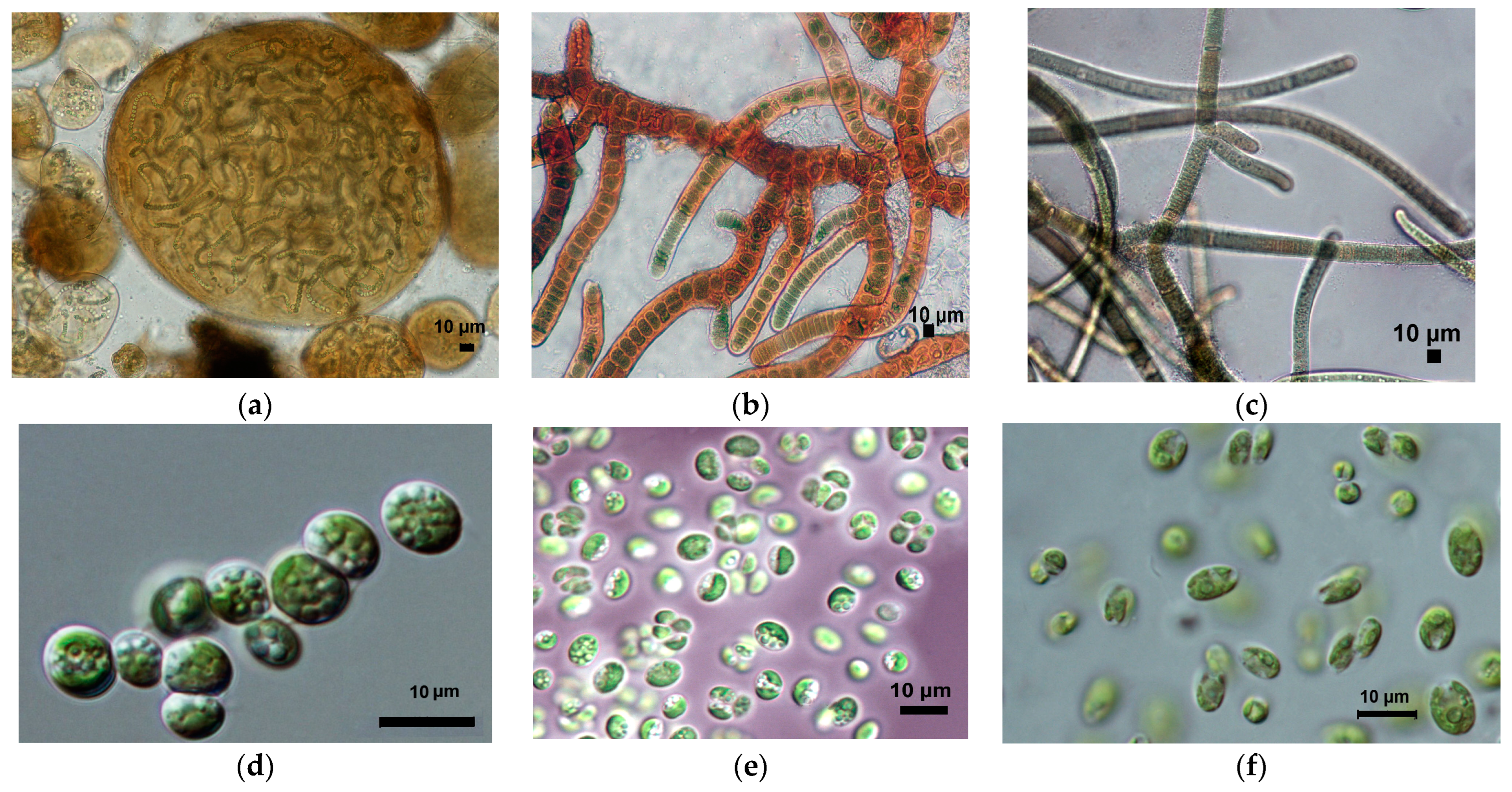
3.1. Diversity of Algae and Cyanobacteria in BSCs Based on Morphological Analysis
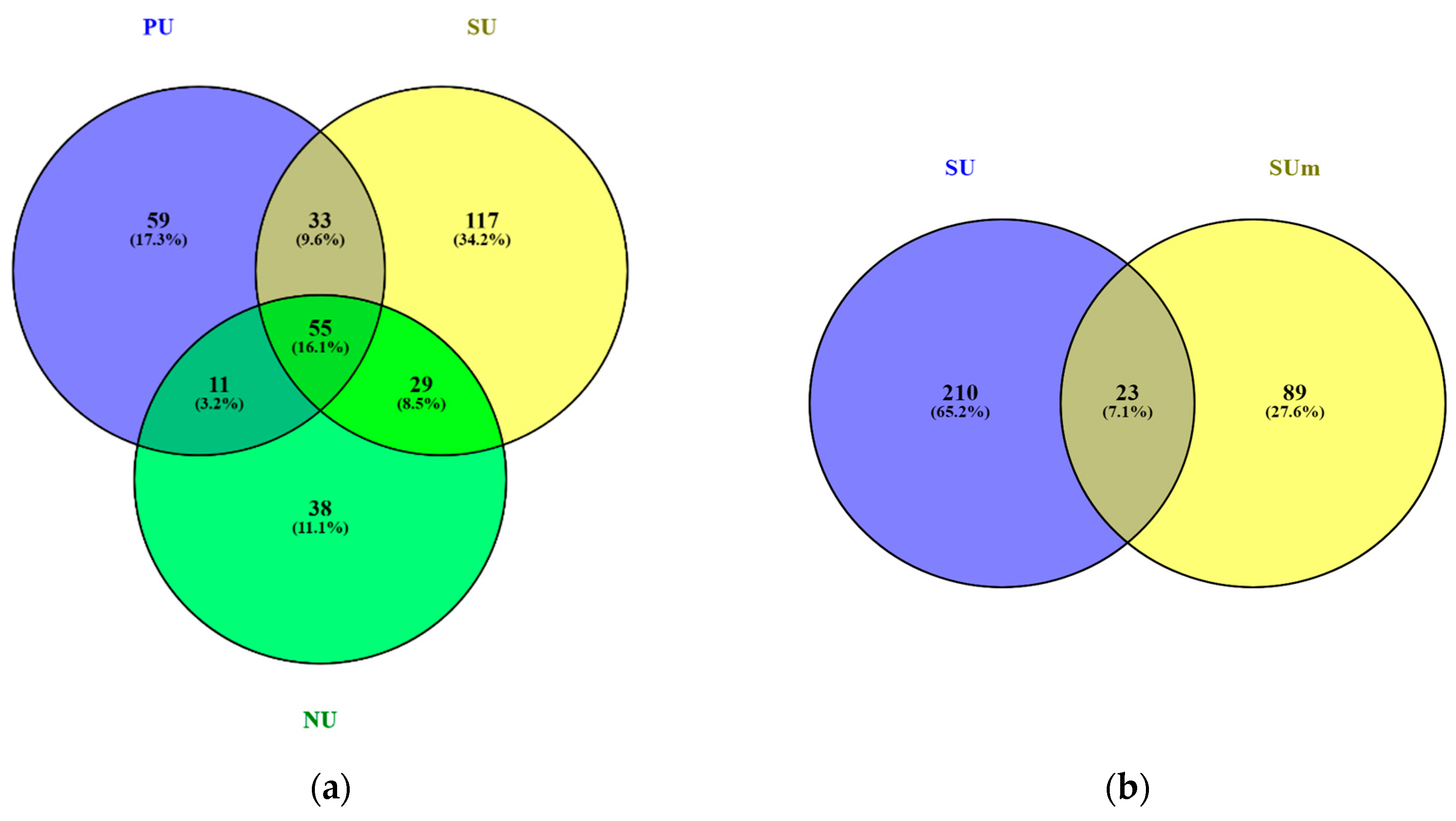
3.2. Diversity of Algae and Cyanobacteria in BSCs Based on Metabarcoding Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Belnap, J.; Lange, O.L. (Eds.) Biological Soil Crusts: Structure, Function, and Management; Springer: Berlin, Germany, 2003; p. 506. [Google Scholar] [CrossRef]
- Řeháková, K.; Chlumská, Z.; Doležal, J. Soil cyanobacterial and microalgal diversity in dry mountains of Ladakh, NW Himalaya, as related to site, altitude, and vegetation. Microb. Ecol. 2011, 62, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Bowker, M.A.; Maetre, F.; Eldridge, D.; Belnap, J.; Castillo-Monroy, A.; Escolar, C.; Soliveres, S. Biological soil crusts (biocrusts) as a model system in community, landscape and ecosystem ecology. Biodivers. Conserv. 2014, 23, 1619–1637. [Google Scholar] [CrossRef]
- Richter, D.; Pietryka, M.; Matuła, J. Relationship of cyanobacterial and algal assemblages with vegetation in the high Arctic tundra (West Spitsbergen, Svalbard Archipelago). Pol. Polar Res. 2015, 36, 239–260. [Google Scholar] [CrossRef]
- Schulz, K.; Mikhailyuk, T.; Dreßler, M.; Leinweber, P.; Karsten, U. Biological soil crusts from coastal dunes at the Baltic Sea: Cyanobacterial and algal biodiversity and related soil properties. Microb. Ecol. 2016, 71, 178–193. [Google Scholar] [CrossRef]
- Glaser, K.; Baumann, K.; Leinweber, P.; Mikhailyuk, T.; Karsten, U. Algal diversity of temperate biological soil crusts depends on land use intensity and affects phosphorus biogeochemical cycling. Biogeosci. Discuss 2017, 1–24. [Google Scholar] [CrossRef]
- Pushkareva, E.; Johansen, J.R.; Elster, J. A review of the ecology, ecophysiology and biodiversity of microalgae in Arctic soil crusts. Polar Biol. 2016, 39, 2227–2240. [Google Scholar] [CrossRef]
- Pushkareva, E.; Kvíderová, J.; Šimek, M.; Elster, J. Nitrogen fixation and diurnal changes of photosynthetic activity in Arctic soil crusts at different development stage. Eur. J. Soil Biol. 2017, 79, 21–30. [Google Scholar] [CrossRef]
- Matuła, J.; Pietryka, M.; Richter, D.; Wojtuń, B. Cyanoprokaryota and algae of Arctic terrestrial ecosystems in the Hornsund area, Spitsbergen. Pol. Polar Res. 2007, 28, 283–315. [Google Scholar]
- Büdel, B.; Dulić, T.; Darienko, T.; Rybalka, N.; Friedl, T. Cyanobacteria and Algae of Biological Soil Crusts. In Photosynthetic Adaptation; Weber, B., Büdel, B., Belnap, J., Eds.; Springer International Publishing: Cham, Germany, 2016; Volume 226, pp. 55–80. [Google Scholar]
- Zielke, M.; Solheim, B.; Spjelkavik, S.; Olsen, R.A. Nitrogen fixation in the high arctic: Role of vegetation and environmental conditions. Arct. Antarct. Alp. Res. 2005, 37, 372–378. [Google Scholar] [CrossRef]
- Yoshitake, S.; Uchida, M.; Koizumi, H.; Kanda, H.; Nakatsubo, T. Production of biological soil crusts in the early stage of primary succession on a High Arctic glacier foreland. New Phytol. 2010, 186, 451–460. [Google Scholar] [CrossRef]
- Komárek, J.; Genuário, D.B.; Fiore, M.F.; Elster, J. Heterocytous cyanobacteria of the ulu peninsula, James Ross island, Antarctica. Polar Biol. 2015, 38, 475–492. [Google Scholar] [CrossRef]
- Barger, N.N.; Weber, B.; Garcia-Pichel, F.; Zaady, E.; Belnap, J. Patterns and controls on nitrogen cycling of biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 257–285. [Google Scholar] [CrossRef]
- Borchhardt, N.; Baum, C.; Mikhailyuk, T.; Karsten, U. Biological Soil Crusts of Arctic Svalbard—Water Availability as Potential Controlling Factor for Microalgal Biodiversity. Front. Microbiol. 2017, 8, 1485. [Google Scholar] [CrossRef]
- Davydov, D. Cyanoprokaryotes of the west part of Oscar II Land, West Spitsbergen Island, Spitsbergen archipelago. Czech Polar Rep. 2017, 7, 94–108. [Google Scholar] [CrossRef]
- Malard, L.A.; Pearce, D.A. Microbial diversity and biogeography in Arctic soils. Environ. Microbiol. Rep. 2018, 10, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Rippin, M.; Lange, S.; Sausen, N.; Becker, B. Biodiversity of biological soil crusts from the Polar Regions revealed by metabarcoding. FEMS Microbiol. Ecol. 2018, 94, fiy036. [Google Scholar] [CrossRef]
- Davydov, D. Terrestrial Cyanoprokaryota of the western part of Khibiny Mountains. Bull. MOIP. Otd. Biol. 2012, 117, 72–77. (In Russian) [Google Scholar]
- Patova, E.N.; Novakovskaya, I.V. Soil algae of the Northeastern European Russia. Nov. Sist. Nizshikh Rastenii 2018, 52, 311–353. [Google Scholar] [CrossRef]
- Davydov, D.; Patova, E. The diversity of Cyanoprokaryota from freshwater and terrestrial habitats in the Eurasian Arctic and Hypoarctic. Hydrobiologia 2018, 811, 119–138. [Google Scholar] [CrossRef]
- Patova, E.N.; Novakovskaya, I.V.; Deneva, S.V. Influence of edaphic and orographic factors on the diversity of algae communities of biological soil crusts on medallion spots of the Polar and Subpolar Urals. Eurasian Soil Sci. 2018, 3, 318–330. [Google Scholar] [CrossRef]
- Novakovskaya, I.V.; Patova, E.N.; Kulyugina, E.E. Changes of cyanoprokariota and algae diversity during the overgrowing of bare spots in the mountain tundra communities of the Northern Urals. Bot. Zhurn. 2019, 104, 569–586. (In Russian) [Google Scholar]
- Novakovskaya, I.V.; Dubrovskiy, Y.A.; Patova, E.N.; Novakovskiy, A.B.; Sterlyagova, I.N. Influence of ecological factors on soil algae in different types of mountain tundra and sparse forests in the Northern Urals. Phycologia 2020, 59, 320–329. [Google Scholar] [CrossRef]
- Davydov, D. Cyanobacterial Diversity of the Northern Polar Ural Mountains. Diversity 2021, 13, 607. [Google Scholar] [CrossRef]
- Novakovskaya, I.V.; Patova, E.N.; Dubrovskiy, Y.A.; Novakovskiy, A.B.; Kulyugina, E.E. Distribution of algae and cyanobacteria of biological soil crusts along the elevation gradient in mountain plant communities at the Northern Urals (Russian European Northeast). J. Mt. Sci. 2022, 19, 637–646. [Google Scholar] [CrossRef]
- Patova, E.N.; Novakovskaya, I.V.; Sivkov, M.D. Cyanobacteria and Algae in Biological Soil Crusts of Frost Boils in the Mountainous Tundra of the Nether-Polar Urals. Eurasian Soil Sci. 2023, 56, 184–197. [Google Scholar] [CrossRef]
- Karsten, U.; Holzinger, A. Green algae in Alpine biological soil crust communities: Acclimation strategies against ultraviolet radiation and dehydration. Biodivers Conserv 2014, 23, 1845–1858. [Google Scholar] [CrossRef]
- Semenov, M.V. Metabarcoding and metagenomics in soil ecology research: Achievements, challenges and prospects. Biol. Bull. Rev. 2021, 11, 40–53. [Google Scholar] [CrossRef]
- Stewart, A.; Rioux, D.; Boyer, F.; Gielly, L.; Pompanon, F.; Saillard, A.; Thuiller, W.; Valay, J.-G.; Maréchal, E.; Coissac, E. Altitudinal Zonation of Green Algae Biodiversity in the French Alps. Front. Plant Sci. 2021, 12, 679428. [Google Scholar] [CrossRef]
- Andreyeva, V.M.; Chaplygina, O.J. Terrestrial nonmotile green microalgae (Chlorophyta) of the Polar Urals. Nov. Sist. Nizshikh Rastenii 2007, 41, 15–18. (In Russian) [Google Scholar] [CrossRef]
- Savelyeva, E.A. Atlas of the Komi Republic; Design. Information; Cartography: Moscow, Russia, 2001; p. 551. (In Russian) [Google Scholar]
- Andersen, R.A. Algal Culturing Techniques; Elsevier: New York, NY, USA, 2005; p. 589. [Google Scholar]
- Andreeva, V.M. Soil and Aerophilic Green Algae (Chlorophyta: Tetrasporales Chlorococcales, Chlorosarcinales); Nauka: St. Petersburg, Russia, 1998; p. 351. (In Russian) [Google Scholar]
- Ettl, H.; Gärtner, G. Syllabus der Boden-, Luft-und Flechtenalgen; Auflage 2; Springer: Heidelberg/Berlin, Germany, 2014; p. 773. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Teil: Chroococcales. In Süsswasserflora von Mitteleuropa 19/1; Unaltered, Repr.; Ettl, H., Gärtner, G., Heynig, G., Mollenhauer, D., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2008; p. 548. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil: Oscillatoriales. In Süsswasserflora von Mitteleuropa 19/2; Unaltered, repr., 2 Print; Büdel, B., Gärtner, G., Krienitz, L., Schlager, M., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2008; p. 759. [Google Scholar]
- Komárek, J. Cyanoprokaryota 3. Teil: Heterocytous genera. In Süsswasserflora von Mitteleuropa 19/3; Büdel, B., Gärtner, G., Krienitz, L., Schlager, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; p. 1133. [Google Scholar]
- Škaloud, P.; Rindi, F.; Boedeker, C.; Leliaert, F. Freshwater Flora of Central Europe. Chlorophyta: Ulvophyceae. Bd. 13. In Süβwasserflora von Mitteleuropa; Springer Spektrum: Berlin/Heidelberg, Germany, 2018; p. 289. [Google Scholar] [CrossRef]
- Novakovskaya, I.V.; Patova, E.N.; Boldina, O.N.; Patova, A.D.; Shadrin, D.M. Molecular phylogenetic analyses, ecology and morphological characteristics of Chloromonas reticulata (Goroschankin) Gobi which causes red blooming of snow in the Subpolar Urals. Cryptog. Algol. 2018, 39, 199–213. [Google Scholar] [CrossRef]
- Davydov, D.A.; Patova, E.N.; Shalygin, S.S.; Vilnet, A.A.; Novakovskaya, I.V. The problem of Cyanobacteria cryptic speciation in the Arctic region. Theor. Appl. Ecol. 2020, 1, 110–116. [Google Scholar] [CrossRef]
- Novakovskaya, I.V.; Egorova, I.N.; Kulakova, N.V.; Patova, E.N.; Shadrin, D.M.; Anissimova, O.V. Morphological and phylogenetic relations of members of the genus Coelastrella (Scenedesmaceae, Chlorophyta) from the Ural and Khentii Mountains (Russia, Mongolia). Phytotaxa 2021, 527, 001–020. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. In World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2023. [Google Scholar]
- Boenigk, J.; Wodniok, S.; Bock, C.; Beisser, D.; Hempel, C.; Grossmann, L.; Jensen, M. Geographic distance and mountain ranges structure freshwater protist communities on a European scale. Metabarcoding Metagenomics 2018, 2, e21519. [Google Scholar] [CrossRef]
- Bock, C.; Jensen, M.; Forster, D.; Marks, S.; Nuy, J.; Psenner, R.; Beisser, D.; Boenigk, J. Factors shaping community patterns of protists and bacteria on a European scale. Environ. Microbiol. 2020, 22, 2243–2260. [Google Scholar] [CrossRef] [PubMed]
- Cuzman, O.A.; Ventura, S.; Sili, C.; Mascalchi, C.; Turchetti, T.; D’Acqui, L.P.; Tiano, P. Biodiversity of phototrophic biofilms dwelling on monumental fountains. Microb. Ecol. 2010, 60, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Perkerson, R.B.; Johansen, J.R.; Kovacik, L.; Brand, J.; Kaštovský, J.; Casamatta, D.A. A unique Pseudanabaenalean (Cyanobacteria) genus Nodosilinea gen. nov. based on morphological and molecular data (1). J. Phycol. 2011, 47, 1397–1412. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High throughput Sequence Data. 2015. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 20 July 2023).
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Větrovský, T.; Baldrian, P.; Morais, D. SEED 2: A user-friendly platform for amplicon high-throughput sequencing data analyses. Bioinformatics 2018, 34, 2292–2294. [Google Scholar] [CrossRef]
- Johansen, J.R.; Casamatta, D.A. Recognizing cyanobacterial diversity through adoption of a new species paradigm. Algol. Stud. 2005, 117, 71–93. [Google Scholar] [CrossRef]
- Erwin, P.M.; Thacker, R.W. Cryptic diversity of the symbiotic cyanobacterium Synechococcus spongiarum among sponge hosts. Mol. Ecol. 2008, 17, 2937–2947. [Google Scholar] [CrossRef]
- Osorio-Santos, K.; Pietrasiak, N.; Bohunická, M.; Miscoe, L.H.; Kováčik, L.; Martin, M.P.; Johansen, J.R. Seven new species of Oculatella (Pseudanabaenales, Cyanobacteria): Taxonomically recognizing cryptic diversification. Eur. J. Phycol. 2014, 49, 450–470. [Google Scholar] [CrossRef]
- Bohunická, M.; Pietrasiak, N.; Johansen, J.R.; Gómez, E.B.; Hauer, T.; Gaysina, L.A.; Lukešová, A. Roholtiella, gen. nov. (Nostocales, Cyanobacteria)—A tapering and branching cyanobacteria of the family Nostocaceae. Phytotaxa 2015, 197, 84–103. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef]
- Davydov, D.; Vilnet, A.; Novakovskaya, I.; Patova, E. Terrestrial species of Drouetiella (Cyanobacteria, Oculatellaceae) from the Russian Arctic and Subarctic regions and description of Drouetiella ramosa sp. nov. Diversity 2023, 15, 132. [Google Scholar] [CrossRef]
- Novakovskaya, I.V.; Boldina, O.N.; Shadrin, D.M.; Patova, E.N. Heterochlamydomonas uralensis sp. nov. (Chlorophyta, Chlamydomonadaceae), new species described from the mountain tundra community in the Subpolar Urals (Russia). Diversity 2023, 15, 673. [Google Scholar] [CrossRef]
- Samolov, E.; Baumann, K.; Büdel, B.; Jung, P.; Leinweber, P.; Mikhailyuk, T.; Karsten, U.; Glaser, K. Biodiversity of algae and cyanobacteria in biological soil crusts collected along a climatic gradient in Chile using an integrative approach. Environ. Biol. 2020, 8, 1047. [Google Scholar] [CrossRef]
- González-Resendiz, L.; Johansen, J.R.; León-Tejera, H.; Sanchéz, L.; Segal-Kischinevzky, C.; Escobar-Sánchez, V.; Morales, M. A bridge too far in naming species: A total evidence approach does not support recognition of four species in Desertifilum (Cyanobacteria). J. Phycol. 2019, 55, 898–911. [Google Scholar] [CrossRef]
- Jung, P.; Mikhailyuk, T.; Emrich, D.; Baumann, K.; Dultz, S.; Büdel, B. Shifting boundaries: Ecological and geographical range extension based on three new species in the cyanobacterial genera Cyanocohniella, Oculatella, and, Aliterella. J. Phycol. 2020, 56, 1216–1231. [Google Scholar] [CrossRef]
- Watanabe, S.; Mezaki, N.; Nakada, T. Ultrastructure and phylogeny of Parietochloris toyamaensis sp. nov. and P. bilobata (Trebouxiophyceae). Eur. J. Phycol. 2023, 58, 35–44. [Google Scholar] [CrossRef]
- Darienko, T.; Pröschold, T. Toward a monograph of non-marine Ulvophyceae using an integrative approach (Molecular phylogeny and systematics of terrestrial Ulvophyceae II). Phytotaxa 2017, 324, 1–41. [Google Scholar] [CrossRef]
- Skaloud, P.; Steinova, J.; Rıdka, T.; Vancurova, L. Assembling the challenging puzzle of algal biodiversity: Species delimitation within the genus Asterochloris (Trebouxiophyceae, Chlorophyta). J. Phycol. 2015, 51, 507–527. [Google Scholar] [CrossRef]
- Friedl, T.; Rokitia, C. Species Relationships in the Lichen Alga Trebouxia (Chlorophyta, Trebouxiophyceae): Molecular Phylogenetic Analyses of Nuclear-Encoded Large Subunit rRNA Gene Sequences. Symbiosis 1997, 23, 125–148. [Google Scholar]
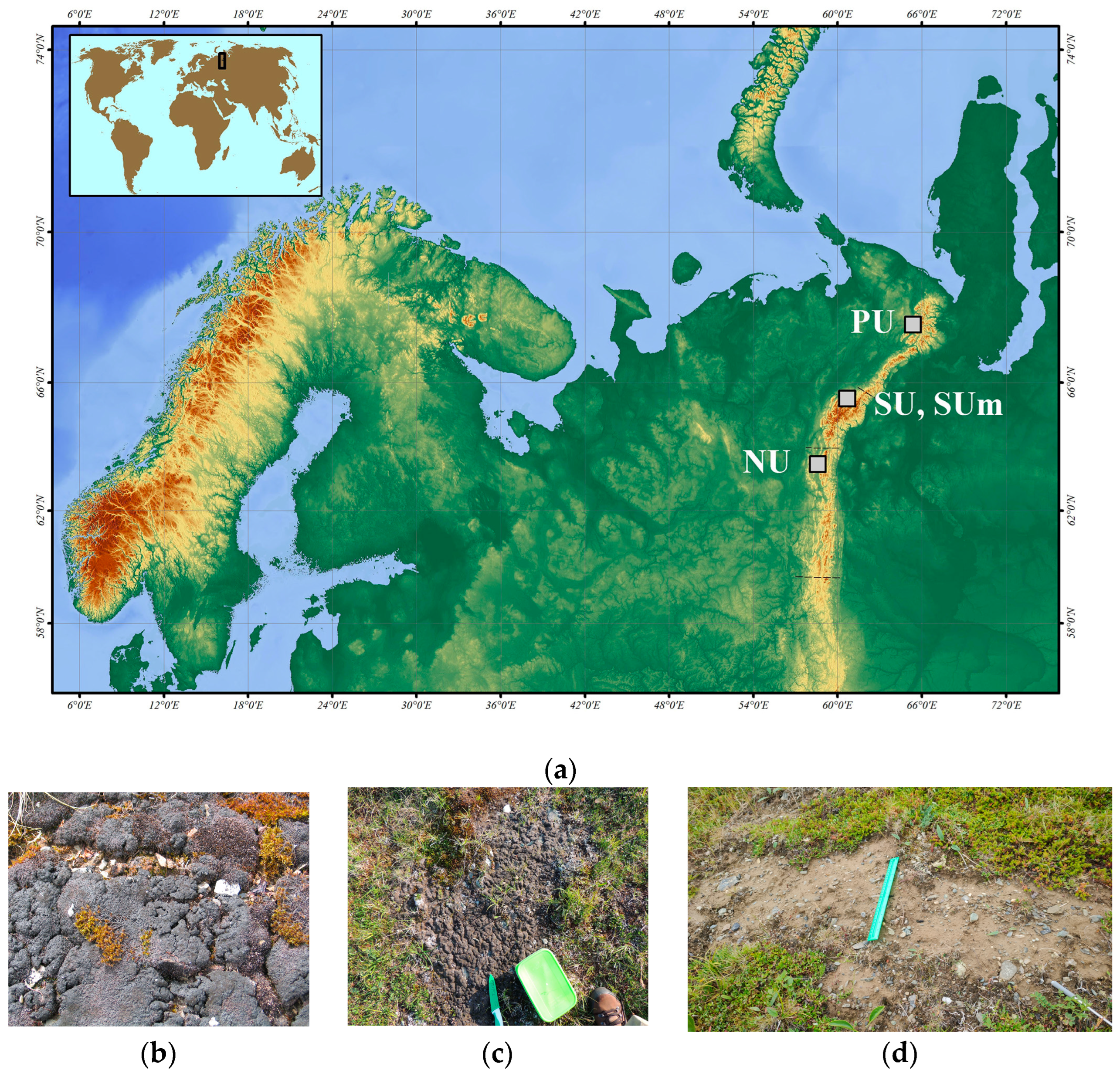

| Region | Collection Date | Sampling Site | Elevation (m) | Coordinates |
|---|---|---|---|---|
| Polar Urals (PU) | August, 2011 | Mount Konstantinov Kamen | 483 | 68.48 N, 66.23 E |
| August, 2011 | Malyi Manisey | 525 | 68.47 N, 66.34 E | |
| July 2019, 2022 | Ochenyrd ridge | 153–1161 | 67.91–68.19 N, 65.21–66.05 E | |
| August 2003, 2013 | Chrebtovy Ridge | 153 | 67.38 N, 64.67 E | |
| Subpolar Urals (SU) and metabarcoding samples (SUm) * | August 2005, 2009; July, 2010, 2012, 2019, 2021 * | Maldynyrd Ridge | 1538 | 65.22–65.31 N, 60.26–60.52 E |
| August, 2005, 2009; July, 2010, 2012, 2019, 2021 * | Barkova Mountain | 1366 | 65.21 N, 60.26 E | |
| July, 2010 | Starik Mountain | 1233 | 65.16 N, 60.29 E | |
| Northern Urals (NU) | June–July, 2018–2019 | Telpos-iz Ridge | 495–1248 | 63.40–63.54 N, 58.54–59.07 E |
| July, 2016, 2018 | Mount Pelener | 585–1067 | 63.38 N, 58.90 E |
| Parameter | PU | SU, SUm | NU |
|---|---|---|---|
| pH (H2O) | 3.3–6.9 | 4.08–6.2 | 4.38–5.48 |
| pH (KCl) | 3.2–6.1 | 2.7–5.3 | 3.40–4.23 |
| C [mg/kg] | 0.42–13.9 | 0.22–3.9 | 0.35–5.8 |
| N [mg/kg] | 0.042–0.97 | 0.026–0.27 | 0.035– 0.51 |
| P2O5 [mg/kg] | 30.90–225.6 | 2.6–1520 | 15–500 |
| Ca2+ [mg/kg] | 0.71–73.71 | 0.26–72 | <0.5–2.79 |
| Mg2+ [mg/kg] | 0.26–2.50 | 0.12–24 | <0.10–0.64 |
| Taxon | PU | SU | NU | SUm |
|---|---|---|---|---|
| Cyanobacteria | ||||
| Albertania spp. | + (N = 2) | |||
| Aliterella sp. | + (N = 1) | |||
| Ammatoidea normanii West et G.S.West | + | |||
| Anabaena cylindrica Lemmerm.1 | + | |||
| Anabaena spp. | + | + | + (N = 1) | |
| Ancylothrix sp. | + (N = 1) | |||
| Aphanocapsa fuscolutea Hansg. | + | |||
| Aphanocapsa muscicola (Menegh.) Wille | + | + | + | |
| Aphanocapsa parietina Nägeli | + | + | ||
| Aphanocapsa rivularis (Carmich.) Rabenh. | + | |||
| Aphanocapsa spp. | + | + | + | |
| Aphanothece castagnei (Kütz.) Rabenh. | + | |||
| Aphanothece microscopica Nägeli | + | |||
| Aphanothece saxicola Nägeli | + | + | + | |
| Aphanothece stagnina (Spreng.) A.Braun | + | + | ||
| Aphanothece pallida (Kütz.) Rabenh. | + | + | ||
| Aphanothece sp. | + | |||
| Calothrix braunii Bornet et Flahault | + | + | ||
| Calothrix clavata G.S.West | + | |||
| Calothrix elenkinii Kossinsk. 1 | + | |||
| Calothrix parietina Thur. ex Bornet et Flahault | + | + | + | |
| Calothrix spp. | + | + | + | |
| Chamaesiphon polonicus (Rostaf.) Hansg. | + | |||
| Chlorogloea purpurea Geitler | + | |||
| Chroococcidiopsis sp. | + (N = 1) | |||
| Chroococcus cohaerens (Bréb.) Nägeli | + | + | ||
| Chroococcus giganteus W.West | + | |||
| Chroococcus minor (Kütz.) Nägeli | + | |||
| Chroococcus minutus (Kütz.) Nägeli | + | + | ||
| Chroococcus spelaeus Erceg. | + | |||
| Chroococcus tenax (Kirchn.) Hieronymus | + | |||
| Chroococcus turgidus (Kütz.) Nägeli | + | |||
| Chroococcus varius A.Braun | + | |||
| Chroococcus spp. | + | + (N = 2) | ||
| Cyanobacterium cedrorum (Sauv.) Komárek, Kopecky et Cepák | + | |||
| Cyanosarcina chroococcoides (Gaitler) Kováčik | + | |||
| Cyanosarcina sp. | + | |||
| Cyanothece aeruginosa (Nägeli) Komärek | + | |||
| Cyanothece spp. | + (N = 2) | |||
| Cylindrospermum muscicola Kutz. ex Bornet et Flahault | + | |||
| Cylindrospermum spp. | + | + (N = 2) | ||
| Dactylothamnos antarcticus Fiore, Genuario, Komárek et al. | + (N = 1) | |||
| Dasygloea cf. lamyi (Gomont ex Gomont) Senna et Komárek 1 | + | |||
| Dasygloea sp. | + | |||
| Desmonostoc muscorum (Bornet et Flahault) Hrouzek et Ventura 1 | + | + | + | |
| Desmonostoc spp. | + (N = 5) | |||
| Dichothrix gypsophila Bornet et Flahault | + | + | ||
| Dolichospermum spp. | + (N = 3) | |||
| Drouetiella lurida (Gomont) Mai, J.R.Johansen et Pietrasiak 1,2 [41] | + | |||
| Drouetiella ramosa Davydov, Vilnet, Patova et Novakovskaya 1,2 [58] | + | |||
| Drouetiella spp. | + (N = 4) | |||
| Eucapsis minor (Skuja) Elenkin | + | |||
| Fischerella ambigua (Kütz. ex Bornet et Flahault) Gomont f. majuscula (Woron.) Elenkin | + | |||
| Fischerella major Gomont | + | |||
| Fischerella muscicola Gomont 1 | + | + | + | |
| Fischerella spp. | + | + | ||
| Geitlerinema sp. | + (N = 1) | |||
| Gloeocapsa alpina Nägeli | + | + | ||
| Gloeocapsa compacta Kütz. | + | + | ||
| Gloeocapsa kuetzingiana Nägeli ex Kütz. | + | |||
| Gloeocapsa punctata Nägeli | + | |||
| Gloeocapsa ralfsii (Harvey) Lemmerm. | + | |||
| Gloeocapsa rupestris Kütz. | + | + | ||
| Gloeocapsa sanguinea (C.Agardh) Kütz. | + | |||
| Gloeocapsa violacea Kütz. | + | + | ||
| Gloeocapsopsis dvorakii (Novácek) Komárek et Anagn. ex Komárek | + | + | ||
| Gloeocapsopsis magma (Bréb.) Komárek et Anagn. ex Komárek | + | + | + | |
| Gloeocapsopsis sp. | + | |||
| Gloeothece confluens Nägeli | + | + | ||
| Gloeothece rupestris (Lyngb.) Bornet | + | + | ||
| Gloeothece tepidariorum (A.Braun) Lagerh. | + | |||
| Haloleptolyngbya sp. | + (N = 1) | |||
| Hapalosiphon pumilus Kirchner ex Bornet et Flahault | + | |||
| Heteroscytonema crispum (Bornet ex De Toni) G.B.McGregor et Sendall | + | |||
| Jaaginema pseudogeminatum (G.Schmid) Anagn. et Komárek | + | |||
| Kamptonema sp. | + (N = 1) | |||
| Kovacikia sp. | + (N = 1) | |||
| Leptolyngbya angustissima (W.West et G.S.West) Anagn. et Komárek | + | |||
| Leptolyngbya boryana (Gomont) Anagn. et Komärek | + | |||
| Leptolyngbya foveolarum (Gomont) Anagn. et Komárek | + | + | + | |
| Leptolyngbya gracillima (Hansg.) Anagn. et Komärek | + | |||
| Leptolyngbya nostocorum (Bornet ex Gomont) Anagn. et Komárek | + | |||
| Leptolyngbya notata (Schmidle) Anagn. et Komárek | + | + | ||
| Leptolyngbya sieminskae D.Richter et Matula | + | |||
| Leptolyngbya subtilissima (Hansg.) Komárek | + | |||
| Leptolyngbya tenuis (Gomont) Anagn. et Komárek | + | |||
| Leptolyngbya spp. 1 | + | + | + | + (N = 6) |
| Lyngbya sp. | + | |||
| Microchaete tenera Thur. ex Bornet et Flahault | + | |||
| Microcoleus autumnalis (Gomont) Strunecky, Komarek et J.R.Johans. 1 | + | + | + | |
| Microcoleus cf. lacustris Farlow ex Gomont | + | |||
| Microcoleus fonticola (Kirchner ex Hansg.) Strunecky, Komárek et J.R.Johans. | + | |||
| Microcoleus paludosus Gomont | + | + | + | |
| Microcoleus vaginatus Gomont | + | + | + (N = 1) | |
| Microcoleus spp. | + | + | ||
| Microcystis sp. | + | |||
| Nodosilinea sp. | + (N = 1) | |||
| Nostoc commune Vaucher ex Bornet et Flahault f. ulvaceum Elenkin 1,2 | + | + | ||
| Nostoc commune Vaucher ex Bornet et Flahault 1,2 | + | + | + (N = 1) | |
| Nostoc edaphicum N.V.Kondrateva | + | + | + | + (N = 1) |
| Nostoc linckia (Roth) Bornet et Flahault 1,2 | + | |||
| Nostoc microscopicum Carmich. ex Bornet et Flahault | + | |||
| Nostoc paludosum Kütz. ex Bornet et Flahault | + | + | ||
| Nostoc punctiforme Har. 1,2 | + | + | + | |
| Nostoc spp. | + | + | + (N = 30) | |
| Nostoc sp. (photobiont of liver mosses) 1 | + | |||
| Oculatella crustae-formantes P.Jung, Briegel-Williams, Mikhailyuk et Büdel | + (N = 1) | |||
| Oculatella spp. | + (N = 3) | |||
| Oscillatoria tenuis C.Agardh ex Gomont | + | |||
| Petalonema densum (Bornet ex Bornet et Flahault) Migula | + | |||
| Petalonema incrustans Komárek | + | |||
| Phormidesmis mollis (Gomont) Turicchia, Ventura, Komárková et Komárek 1 | + | |||
| Phormidesmis nigrescens (Komarek) Raabová, L.Kovacik, Elster et Strunecký | + (N = 1) | |||
| Phormidesmis sp. | + | |||
| Phormidium ambiguum Gomont 1 | + | + | ||
| Phormidium corium Gomont 1 | + | + | + | |
| Phormidium interruptum Kütz. ex Forti | + | |||
| Phormidium kuetzingianum (Kirchn. ex Hansg.) Anagn. et Komárek | + | + | ||
| Phormidium puteale (Montagne ex Gomont) Anagn. et Komárek 1 | + | + | ||
| Phormidium spp. | + | + | + | + (N = 2) |
| Pleurocapsa aurantiaca Geitler | + | |||
| Porphyrosiphon fuscus Gomont ex Frémy 1 | + | |||
| Porphyrosiphon lomniczensis (Kol) Anagn. et Komárek 1 | + | |||
| Porphyrosiphon sp. | + (N = 1) | |||
| Potamolinea aerugineocaerulea (Gomont) M.D.Martins et Branco 1 | + | + | + | |
| Pseudanabaena spp. | + | + (N = 2) | ||
| Pseudophormidium hollerbachianum (Elenkin) Anagn. 1 | + | |||
| Pycnacronema spp. | + (N = 11) | |||
| Schizothrix calcicola Gomont | + | |||
| Schizothrix lardacea Gomont | + | |||
| Schizothrix fuscescens Kutz. ex Gomont | + | |||
| Schizothrix spp. | + | + | + | |
| Scytonema hoffmannii C.Agardh ex Bornet et Flahault 1 | + | + | + | |
| Scytonema hyalinum N.L.Gardner | + (N = 1) | |||
| Scytonema ocellatum Lyngbye ex Bornet et Flahault | + | + | ||
| Scytonema spp. | + | + | + | |
| Scytonematopsis crustacea (Thur. ex Bornet et Flahault) Koválik et Komárek 1,2 | + | |||
| Shackletoniella spp. | + (N = 2) | |||
| Snowella sp. | + (N = 1) | |||
| Stenomitos frigidus (F.E.Fritsch) Miscoe et J.R.Johans. | + | + | + | |
| Stenomitos hiloensis Johansen, Gargas et Shalygin | + (N = 1) | |||
| Stenomitos kolaensis Shalygin, Shalygina et Johansen | + (N = 1) | |||
| Stenomitos spp. 1,2 [41] | + | + (N = 10) | ||
| Stigonema hormoides Bornet et Flahault | + | + | ||
| Stigonema informe Kütz. ex Bornet et Flahault 1,2 | + | + | ||
| Stigonema mamillosum C.Agardh ex Bornet et Flahault | + | + | + | |
| Stigonema minutum Hassall ex Bornet et Flahault | + | + | + | |
| Stigonema ocellatum Thur. ex Bornet et Flahault 1,2 | + | + | + | |
| Stigonema spp. | + | + | + | + (N = 10) |
| Symplocastrum friesii (Gomont) Kirchn. 1 | + | + | + | |
| Synechococcus elongatus (Nägeli) Nägeli | + | + | ||
| Synechocystis crassa Woron. | + | |||
| Synechocystis fuscopigmentosa Kovácik | + (N = 1) | |||
| Synechocystis spp. | + (N = 2) | |||
| Tenebriella curviceps (C.Agardh ex Gomont) Hauerová, Hauer et Kaštovský | + (N = 1) | |||
| Tildeniella alaskaensis Strunecky, Raabova, Bernardova, A.P.Ivanova, Semanova, Crossley et Kaftan | + (N = 1) | |||
| Tildeniella sp. | + (N = 1) | |||
| Timaviella spp. | + (N = 8) | |||
| Tolypothrix distorta Kütz. ex Bornet et Flahault | + | + | + (N = 1) | |
| Tolypothrix lanata Wartm. ex Bornet et Flahault | + | |||
| Tolypothrix saviczii Kossinsk. | + | |||
| Tolypothrix tenuis Kütz. ex Bornet et Flahault 1 | + | + | + | |
| Tolypothrix fasciculata Gomont 1 | + | |||
| Tolypothrix spp. | + | + | + (N = 3) | |
| Trichormus variabilis (Kütz. ex Bornet et Flahault) Komárek et Anagn. | + | |||
| Wilmottia sp. | + (N = 1) | |||
| Total | 83 | 94 | 44 | 221 OTUs Identified—135 Not identified— 86 |
| Dinophyta | ||||
| Nusuttodinium sp. | + (N = 1) | |||
| Peridinium sp. | + (N = 1) | |||
| Total | 0 | 0 | 0 | 8 OTUs Identified—2 Not identified—6 |
| Euglenophyta | ||||
| Astasia sp. | + | |||
| Total | 0 | 0 | 1 | 3 OTUs Identified—0 Not identified—3 |
| Cryptophyta | ||||
| Total | 3 OTUs Identified—0 Not identified—3 | |||
| Ochrophyta/Chrysophyceae | ||||
| Chromulina chionophilia Stein | + (N = 1) | |||
| Chromulina spp. | + (N = 3) | |||
| Chrysocapsa vernalis Starmach | + (N = 1) | |||
| Kremastochrysopsis austriaca Remias, Procházková et R.A.Andersen | + (N = 1) | |||
| Spumella spp. | + (N = 4) | |||
| Total | 0 | 0 | 0 | 19 OTUs Identified—10 Not identified—9 |
| Ochrophyta/Xanthophyceae | ||||
| Botrydiopsis eriensis J.W.Snow 1 | + | + | ||
| Bumilleria sicula Borzi 1 | + | |||
| cf. Chlorobotrys simplex Pascher | + | |||
| Chlorobotrys sp. | + (N = 1) | |||
| Characiopsiella minima (Pascher) R.Amaral, K.P.Fawley, Nemcová, T.Sevcíková, Lukesová, M.W.Fawley, L.M.A.Santos et M.Eliás | + | |||
| Characiopsis minutissima Pascher 1 | + | |||
| Monodopsis subterranea (J.B.Petersen) D.J.Hibberd | + (N = 1) | |||
| Pleurochloris pyrenoidosa Pascher | + | |||
| Tribonema vulgare Pascher 1 | + | |||
| Tribonema sp. | + | |||
| Vischeria helvetica (Vischer et Pascher) D.J.Hibberd 1 | + | + | ||
| Vischeria magna (Petersen) Kryvenda, Rybalka, Wolf et Friedl 1,2 | + | + | + | + (N = 1) |
| Vischeria stellata (Chodat) Pascher | + (N = 1) | |||
| Vischeria sp. | + | |||
| Xanthonema bristolianum (Pascher) P.C.Silva 1 | + | |||
| Total | 3 | 8 | 5 | 4 OTUs Identified—4 Not identified—0 |
| Bacillariophyta | ||||
| Achnanthidium lineare W.Smith | + | |||
| Achnanthidium minutissimum (Kütz.) Czarnecki | + | |||
| Aulacoseira italica (Ehrenb.) Simonsen | + | |||
| Caloneis aerophila W.Bock | + | |||
| Cavinula cf. lapidosa (Krasske) Lange-Bert. | + | |||
| Chamaepinnularia begeri (Krasske) Lange-Bert. | + | |||
| Chamaepinnularia soehrensis (Krasske) Lange-Bert. et Krammer | + | |||
| Diatoma tenuis C.Agardh | + | |||
| Discostella sp. | + (N = 1) | |||
| Encyonema gracile Rabenh. | + | |||
| Encyonema minutum (Hilse) D.G.Mann | + | |||
| Eunotia bilunaris (Ehrenb.) Schaarschm. | + | |||
| Eunotia diodon Ehrenb. | + | |||
| Eunotia fallax A.Cleve | + | |||
| Eunotia incisa W.Smith ex W.Gregory | + | |||
| Eunotia intermedia (Krasske ex Hustedt) Nörpel et Lange-Bert. | + | |||
| Eunotia microcephala Krasske | + | |||
| Eunotia paludosa Grunow | + | |||
| Eunotia praerupta Ehrenb. | + | |||
| Eunotia septentrionalis Østrup | + | |||
| Eunotia sp. | + | |||
| Fragilaria radians (Kütz.) D.M.Williams et Round | + | |||
| Fragilaria vaucheriae (Kütz.) J.B.Petersen | + | |||
| Fragilaria sp. | + | |||
| Gomphonema angustatum (Kütz.) Rabenh. | + | |||
| Gomphonema brebissonii Kütz. | + | |||
| Hannaea arcus (Ehrenb.) R.M.Patrick | + | |||
| Hantzschia amphioxys (Ehrenb.) Grunow | + | + | + | |
| Hantzschia amphioxys (Ehrenb.) Grunow f. capitata O.Müll. | + | |||
| Microcostatus krasskei (Hustedt) J.R.Johans. et Sray | + | |||
| Navicula spp. | + | + | + | |
| Neidium alpinum Hustedt | + | |||
| Neidium bisulcatum (Lagerst.) Cleve | + | |||
| Nitzschia frustulum (Kütz.) Grunow | + | |||
| Nitzschia palea (Kütz.) W.Smith | + | |||
| Nitzschia perminuta Grunow | + | |||
| Pinnularia appendiculata (C.Agardh) Schaarschm. | + | |||
| Pinnularia borealis Ehrenb. | + | + | ||
| Pinnularia cf. bullacostae Krammer et Lange-Bert. | + | |||
| Pinnularia cf. microstauron (Ehrenb.) Cleve var. rostrata Krammer | + | |||
| Pinnularia streptoraphe Cleve | + | |||
| Pinnularia subcapitata W.Gregory | + | |||
| Pinnularia subrostrata (A.Cleve) A.Cleve | + | |||
| Pinnularia spp. | + | + | + | |
| Psammothidium helveticum (Hust.) Bukht. et Round | + | |||
| Psammothidium kryophilum (J.B.Petersen) E.Reichardt | + | |||
| Sellaphora spp. | + (N = 3) | |||
| Stauroneis agrestis Petersen | + | + | ||
| Stauroneis anceps Ehrenb. | + | |||
| Staurosira subsalina (Hustedt) Lange-Bert. | + | |||
| Staurosirella pinnata (Ehrenb.) D.M.Williams et Round | + | |||
| Tabellaria flocculosa (Roth) Kütz. | + | |||
| Tabellaria sp. | + | |||
| Thalassiosira pseudonana Hasle et Heimdal | + (N = 1) | |||
| Ulnaria ulna (Nitzsch) Compère | + | |||
| Total | 5 | 49 | 6 | 11 OTUs Identified—5 Not identified—6 |
| Chlorophyta | ||||
| Apatococcus lobatus (Chodat) J.B.Petersen | + (N = 1) | |||
| Asterochloris excentrica (Archibald) Skaloud et Peksa 1 | + | + | ||
| Asterochloris italiana (Archibald) Skaloud et Peksa | + (N = 1) | |||
| Asterococcus superbus (Cienk.) Scherff. | + | |||
| Auxenochlorella sp. | + (N = 1) | |||
| Borodinellopsis oleifera Schwarz | + | |||
| Borodinellopsis texensis Dykstra 1 | + | |||
| Botryokoryne simplex Reisigl | + | |||
| Bracteacoccus aggregatus Tereg 1,2 | + | |||
| Bracteacoccus giganteus H.W.Bischoff et H.C.Bold 1,2 | + | + | ||
| Bracteacoccus grandis H.W.Bischoff et H.C.Bold 1,2 | + | |||
| Bracteacoccus minor (Schmidle ex Chodat) Petrová 1,2 | + | + | + | |
| Bracteacoccus pseudominor H.W. Bischoff et H.C. Bold 1,2 | + | + | ||
| Bracteacoccus spp. 1,2 | + | + | + (N = 1) | |
| Carteria sp. | + | |||
| Cecidochloris adnata (Korshikov) H.Ettl | + | |||
| Chlamydocapsa cf. maxima (Mainx) H.Ettl et Gärtner 1 | + | |||
| Chlamydocapsa lobata Broady 1 | + | + | + | |
| Chlamydocapsa spp. | + | + (N = 1) | ||
| Chlamydomonas cf. applanata E.G.Pringsh. | + | |||
| Chlamydomonas cf. asymmetrica Korshikov | + | |||
| Chlamydomonas elliptica Korshikov | + | |||
| Chlamydomonas cf. gloeogama Korshikov | + | |||
| Chlamydomonas hindakii H.Ettl 1 | + | |||
| Chlamydomonas macrostellata J.W.G.Lund 1 | + | |||
| Chlamydomonas cf. noctigama Korshikov | + | + | ||
| Chlamydomonas pseudagloë Pascher | + (N = 1) | |||
| Chlamydomonas radiata T.R.Deason et Bold | + | |||
| Chlamydomonas cf. reinhardtii P.A.Dang. | + | |||
| Chlamydomonas cf. reisiglii H.Ettl 1 | + | + | + | |
| Chlamydomonas cf. thomassonii H.Ettl | + | |||
| Chlamydomonas spp. | + | + | + | + (N = 1) |
| Chlorella chlorelloides (Naumann) C.Bock, L.Krienitz et T.Pröschold | + | |||
| Chlorella vulgaris W.Beij 1,2 | + | + | + | |
| Chlorella vulgaris W.Beij. f. globosa V.M.Andreeva 1,2 | + | + | ||
| Chlorella sp. | + | |||
| Chlorococcum costatozygotum H.Ettl et Gärtner 1 | + | |||
| Chlorococcum infusionum (Schrank) Menegh. | + | |||
| Chlorococcum isabeliense P.A.Archibald et Bold 1 | + | |||
| Chlorococcum lobatum (Korshikov) F.E.Fritsch et R.P.John 1 | + | + | ||
| Chlorococcum spp. | + | + | ||
| Chloroidium lichinum (Chodat) Darienko et Pröschold | + (N = 1) | |||
| Chloroidium spp. | + (N = 2) | |||
| Chloroidium orientalis Gontcharov, Abdullin, A.Nikulin, V.Nikulin et Bagmet | + (N = 1) | |||
| Chloroidium saccharophilum (W.Krüger) Darienko, Gustavs, Mudimu, Menendez, Schumann, Karsten, Friedl et Proschold 1 | + | + (N = 1) | ||
| Chlorolobion lunulatum Hindák | + | + | ||
| Chlorominima sp. | + (N = 1) | |||
| Chloromonas reticulata (Gorozh.) Gobi 1,2 [40] | + | + | ||
| Chloromonas cf. rosae H.Ettl. 1 | + | |||
| Chloromonas spp. | + (N = 2) | |||
| Chloroplana terricola Hollerb. | + | |||
| Chlororustica terrestris (Herndon) Shin Watan., N.Mezaki et Tatsuya Suzuki | + | |||
| Chlorosarcinopsis spp. | + | + | + | |
| Chromochloris zofingiensis (Dönz) Fucíková et L.A.Lewis | + (N = 1) | |||
| Coccomyxa confluens (Kütz.) Fott | + (N = 1) | |||
| Coccomyxa elongata Chodat et Jaag | + (N = 1) | |||
| Coccomyxa polymorpha T.Darienko et T.Pröschold | + (N = 1) | |||
| Coccomyxa subellipsoidea E.Acton | + (N = 1) | |||
| Coccomyxa subglobosa Pascher | + | + | ||
| Coccomyxa viridis Chodat 1 | + | + (N = 1) | ||
| Coccomyxa spp. | + (N = 13) | |||
| Coelastrella aeroterrestrica Tschaikner, Gärtner et Kofler | + (N = 1) | |||
| Coelastrella multistriata (Trenkwalder) Kalina et Punčochárová | + | |||
| Coelastrella oocystiformis (J.W.G.Lund) E.Hegewald et N.Hanagata 1,2 [42] | + | + | + (N = 1) | |
| Coelastrella rubescens (Vinatzer) Kaufnerová et Eliás 1,2 [42] | + | + | + | + (N = 1) |
| Coelastrella terrestris (Reisigl) E.Hegewald et N.Hanagata | + | + | ||
| Coelastrella spp. 1,2 [42] | + | + (N = 1) | ||
| Coenochloris bilobata (Broady) Hindák | + | |||
| Coenochloris signiensis (Broady) Hindák 1 | + | + | + | |
| Coenocystis cf. oleifera (Broady) Hindák var. antarctica (Broady) V.M.Andreeva | + | |||
| Coenocystis spp. | + (N = 2) | |||
| Coleochlamys apoda Korshikov | + | |||
| Deasonia multinucleata (Deason et H.C.Bold) H.Ettl et Komárek 1 | + | + | ||
| Desmodesmus abundans (Kirchner) E.Hegewald | + | |||
| Desmotetra stigmatica (T.R.Deason) T.R.Deason et G.L.Floyd 1 | + | |||
| Dictyochloropsis splendida Geitler | + (N = 1) | |||
| Dictyochloropsis spp. | + (N = 3) | |||
| cf. Dictyococcus varians Gerneck 1 | + | + | ||
| Diplosphaera chodatii Bialosukniá 1 | + | |||
| Diplosphaera chodatii var. mucosa (Broady) Pröschold et Darienko | + | |||
| Elliptochloris bilobata Tscherm.-Woess 1 | + | + | + | |
| Elliptochloris reniformis H.Ettl et G.Gärtner 1 | + | + | ||
| Elliptochloris subsphaerica (Reisigl) H.Ettl et G.Gärtner 1 | + | + | + | + (N = 1) |
| Elliptochloris spp. | + | + | + (N = 6) | |
| Eremochloris sp. | + (N = 1) | |||
| Ettlia minuta (Arce et H.C.Bold) Komárek | + | |||
| Eubrownia aggregata (R.M.Brown et Bold) Shin Watan. et L.A.Lewis | + | + | ||
| Eubrownia dissociata (R.M.Brown et Bold) Shin Watan. et L.A.Lewis | + | + | ||
| Fernandinella semiglobosa (F.E.Fritsch et R.P.John) Škaloud et Leliaert | + | |||
| Gloeococcus sp. | + | |||
| Hariotina compacta Wang, Liu, Hu et Liu | + (N = 1) | |||
| Herndonia botryoides (Hernon) Shin Watan. | + | |||
| Heterochlamydomonas uralensis Novakovskaya, Boldina, Shadrin, Patova 1,2 [59] | + | |||
| cf. Heterotetracystis intermedia Ed.R.Cox et T.R.Deason | + | + | + | |
| Interfilum paradoxum Chodat et Topali | + | |||
| Interfilum terricola (J.B.Petersen) Mikhailyuk, Sluiman, Massalski, Mudimu, Demchenko, Friedl et Kondratyuk | + | + | + | |
| Keratococcus bicaudatus (A.Braun ex Rabenh.) J.B.Petersen | + | |||
| Leptosira obovata Vischer | + (N = 1) | |||
| Leptosira polychloris Reisigl 1 | + | + | ||
| Leptosira spp. | + | + | + (N = 1) | |
| Lobochlamys culleus (H.Ettl) T.Pröschold, B.Marin, U.W.Schlösser et M.Melkonian | + | + | + | |
| Lobosphaera incisa (Reisigl) Karsten, Friedl, Schumannn, Hoyer et Lembcke 1 | + | + | ||
| Macrochloris dissecta Korshikov 1 | + | + | ||
| Massjukichlorella sp. | + (N = 1) | |||
| Microspora sp. | + | |||
| Mychonastes homosphaera (Skuja) Kalina et Punčoch. 1,2 | + | + | + | |
| Myrmecia bisecta Reisigl 1 | + | + | + | |
| Myrmecia israelensis (S.Chantanachat et H.Bold) T.Friedl | + (N = 1) | |||
| Myrmecia macronucleata (Deason) V.M.Andreeva | + | |||
| Myrmecia pyriformis J.B.Petersen | + (N = 1) | |||
| Myrmecia sp. | + (N = 1) | |||
| cf. Nannochloris sp. | + | |||
| Nautococcus terrestris P.A.Archibald | + | |||
| Neochloris gelatinosa Herndon | + | |||
| Neochloris sp. | + | |||
| Neocystis broadiensis Kostikov, Darienko, Lukesova et L.Hoffm. | + | |||
| Neocystis curvata (Broady) Kostikov, Darienko, Lukesova et L.Hoffm. 1 | + | + | ||
| Neocystis sp. | + | |||
| Oedogonium sp. | + | |||
| Palmellopsis gelatinosa Korshikov | + | |||
| Parietochloris alveolaris (H.C.Bold) Shin Watan. et G.L.Floyd 1 | + | + | + | |
| Parietochloris bilobata (Vinatzer) V.M.Andreeva 1 | + | + | + | + (N = 1) |
| Parietochloris cf. pseudoalveolaris (Deason et H.C.Bold) Shin Watan. et G.L.Floyd 1 | + | |||
| Parietochloris spp. 1 | + | + (N = 1) | ||
| Planophila asymmetrica (Gerneck) Wille | + | |||
| Planophila laetevirens Gerneck | + (N = 1) | |||
| Pleurastrum terricola (Bristol) D.M.John 1 | + | + | + | + (N = 1) |
| Pleurastrum sp. | + (N = 1) | |||
| Pseudendoclonium sp. | + | |||
| Pseudodictyochloris multinucleata (Broady) H.Ettl et G.Gärtner | + | |||
| Pseudosphaerocystis sp. | + | |||
| Pseudotrochiscia areolata Vinatzer | + | |||
| Radiosphaera minuta Herndon 1 | + | + | ||
| Rhexinema sp. | + (N = 2) | |||
| Sanguina nivaloides Procházková, Leya et Nedbalová | + (N = 1) | |||
| Scenedesmus acuminatus (Lagerheim) Chodat | + (N = 1) | |||
| Schizochlamydella minutissima Broady 1 | + | |||
| Scotiellopsis levicostata (Hollerb.) Punčoch. et Kalina | + | + | ||
| Scotinosphaera sp. | + (N = 1) | |||
| Spongiochloris lamellata Deason et H.C.Bold | + | |||
| Sporotetras polydermatica (Kütz.) Kostikov, Darienko, Lukesová et L.Hoffm 1 | + | + | + | |
| Stichococcus bacillaris Nägeli 1 | + | + | ||
| Stichococcus sp. | + | |||
| Stigeoclonium sp. | + (N = 1) | |||
| Symbiochloris symbiontica (Tscherm.-Woess) Skaloud, Friedl, A.Beck& Dal Grande | + (N = 1) | |||
| Tetracystis compacta K.Schwarz 1 | + | |||
| Tetracystis excentrica R.M.Brown et H.C.Bold | + | |||
| Tetracystis pulchra R.M.Brown et H.C.Bold | + | |||
| Tetracystis tetraspora (Arce et H.C.Bold) R.M.Brown et H.C.Bold 1 | + | |||
| Tetracystis cf. vinatzeri H.Ettl et Gärtner. 1 | + | |||
| Tetracystis spp. | + | + | + (N = 1) | |
| Tetradesmus obliquus (Turpin) M.J.Wynne | + | |||
| Tetrasporidium javanicum Möbius | + | |||
| Trebouxia arboricola Puymaly | + | |||
| Trebouxia spp. | + | + | ||
| Ulothrix tenerrima Kutz. | + | + | + | |
| Uvulifera mucosa (Broady et M.Ingerfeld) Molinari | + (N = 1) | |||
| Uvulifera sp. | + (N = 1) | |||
| Valeriella excentrica (R.C.Starr) Darienko et Pröschold | + | |||
| Valeriella incrassata (Chantanachat & H.C.Bold) Darienko et Pröschold | + | |||
| Watanabea reniformis N.Hanagata, I.Karube, M.Chihara et P.C.Silva | + (N = 1) | |||
| Watanabea sichuanensis Shuyin Li et al. | + (N = 1) | |||
| Xerochlorella spp. | + (N = 2) | |||
| Total | 58 | 68 | 67 | 80 OTUs Identified—78 Not identified—2 |
| Charophyta | ||||
| Closterium pusillum Hantzsch 1 | + | + | ||
| Cosmarium anceps P.Lundell | + | |||
| Cosmarium saxicola Kaiser | + | + | ||
| Cosmarium undulatum Corda ex Ralfs | + | |||
| Cosmarium spp. | + | + | ||
| Cylindrocystis brebissonii var. turgida Schmidle | + | + | ||
| Cylindrocystis crassa De Bary | + | + | ||
| Cylindrocystis sp. | + | |||
| Euastrum binale Ehrenb. ex Ralfs | + | + | ||
| Klebsormidium crenulatum (Kütz.) Lokhorst | + | |||
| Klebsormidium dissectum (F.Gay) H.Ettl et G.Gärtner 1 | + | + | + | |
| Klebsormidium flaccidum (Kütz.) P.C.Silva, Mattox et W.H.Blackwel 1 | + | + | ||
| Klebsormidium nitens (Kütz.) Lokhorst 1 | + | + | + | + (N = 1) |
| Mesotaenium caldariorum (Lagerh.) Hansg. | + | + | ||
| Mesotaenium endlicherianum Nägeli | + | + | + | |
| Mesotaenium pyrenoidosum (P.A.Broady) Petlovany 1 | + | + | ||
| Mesotaenium sp. | + | |||
| Total | 9 | 14 | 9 | 2 OTUs Identified—1 Not identified—1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patova, E.; Novakovskaya, I.; Gusev, E.; Martynenko, N. Diversity of Cyanobacteria and Algae in Biological Soil Crusts of the Northern Ural Mountain Region Assessed through Morphological and Metabarcoding Approaches. Diversity 2023, 15, 1080. https://doi.org/10.3390/d15101080
Patova E, Novakovskaya I, Gusev E, Martynenko N. Diversity of Cyanobacteria and Algae in Biological Soil Crusts of the Northern Ural Mountain Region Assessed through Morphological and Metabarcoding Approaches. Diversity. 2023; 15(10):1080. https://doi.org/10.3390/d15101080
Chicago/Turabian StylePatova, Elena, Irina Novakovskaya, Evgeniy Gusev, and Nikita Martynenko. 2023. "Diversity of Cyanobacteria and Algae in Biological Soil Crusts of the Northern Ural Mountain Region Assessed through Morphological and Metabarcoding Approaches" Diversity 15, no. 10: 1080. https://doi.org/10.3390/d15101080
APA StylePatova, E., Novakovskaya, I., Gusev, E., & Martynenko, N. (2023). Diversity of Cyanobacteria and Algae in Biological Soil Crusts of the Northern Ural Mountain Region Assessed through Morphological and Metabarcoding Approaches. Diversity, 15(10), 1080. https://doi.org/10.3390/d15101080






