Human–Shark Interactions: Citizen Science Potential in Boosting Shark Research on Madeira Island
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Data Acquisition
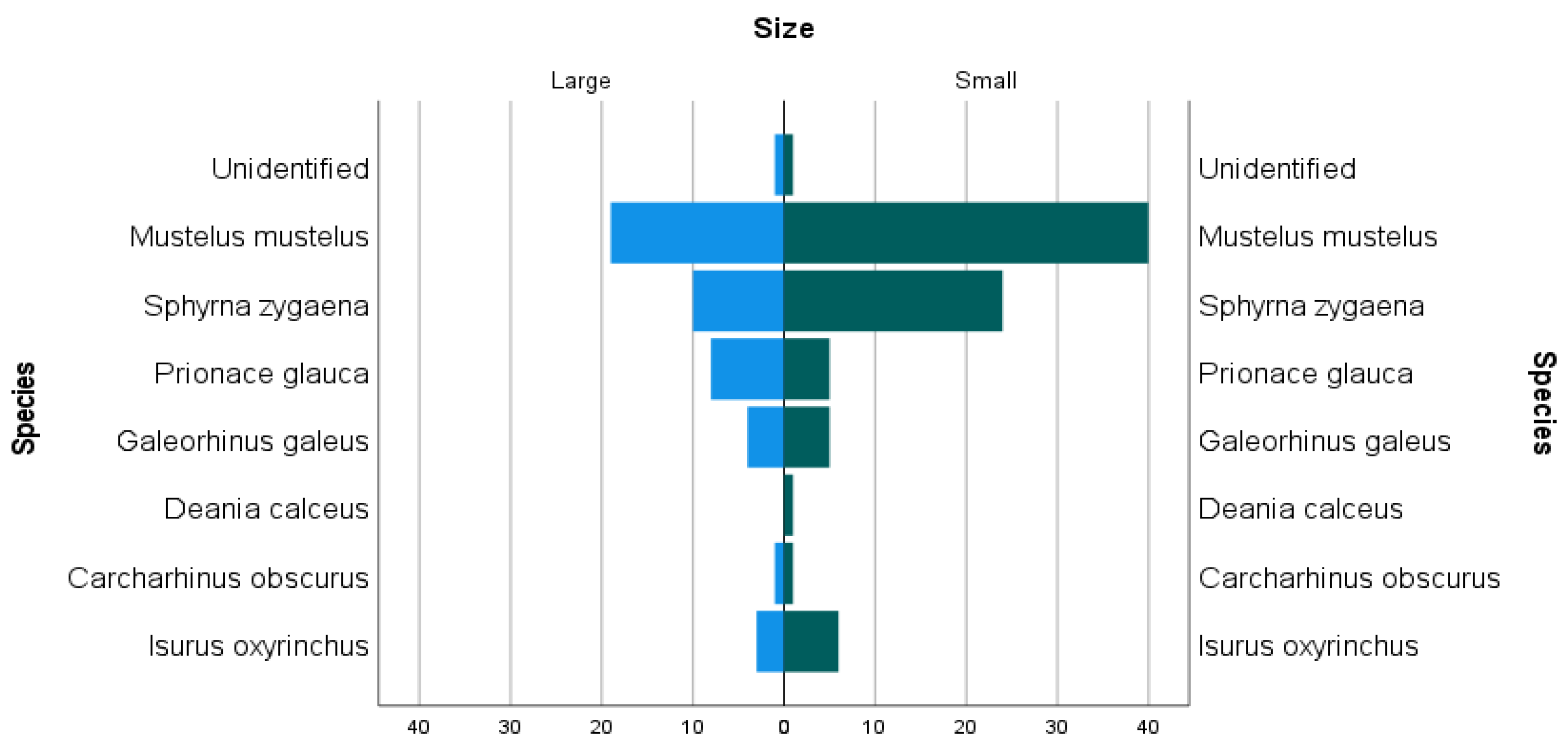
2.3. Species Selection and Size
2.4. Maximum Number of Sharks per Sighting, Sighting Frequency, and Seasonality
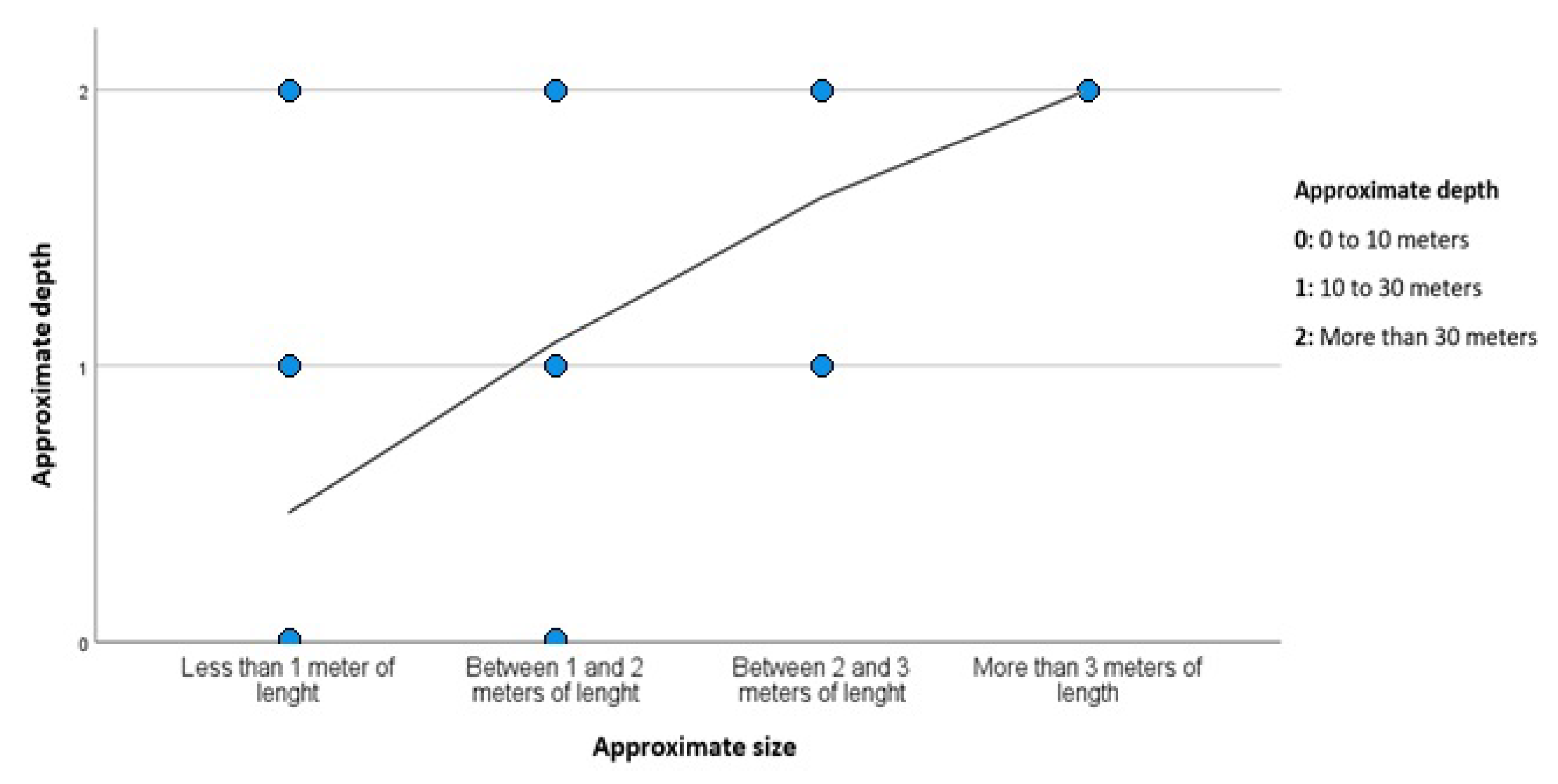
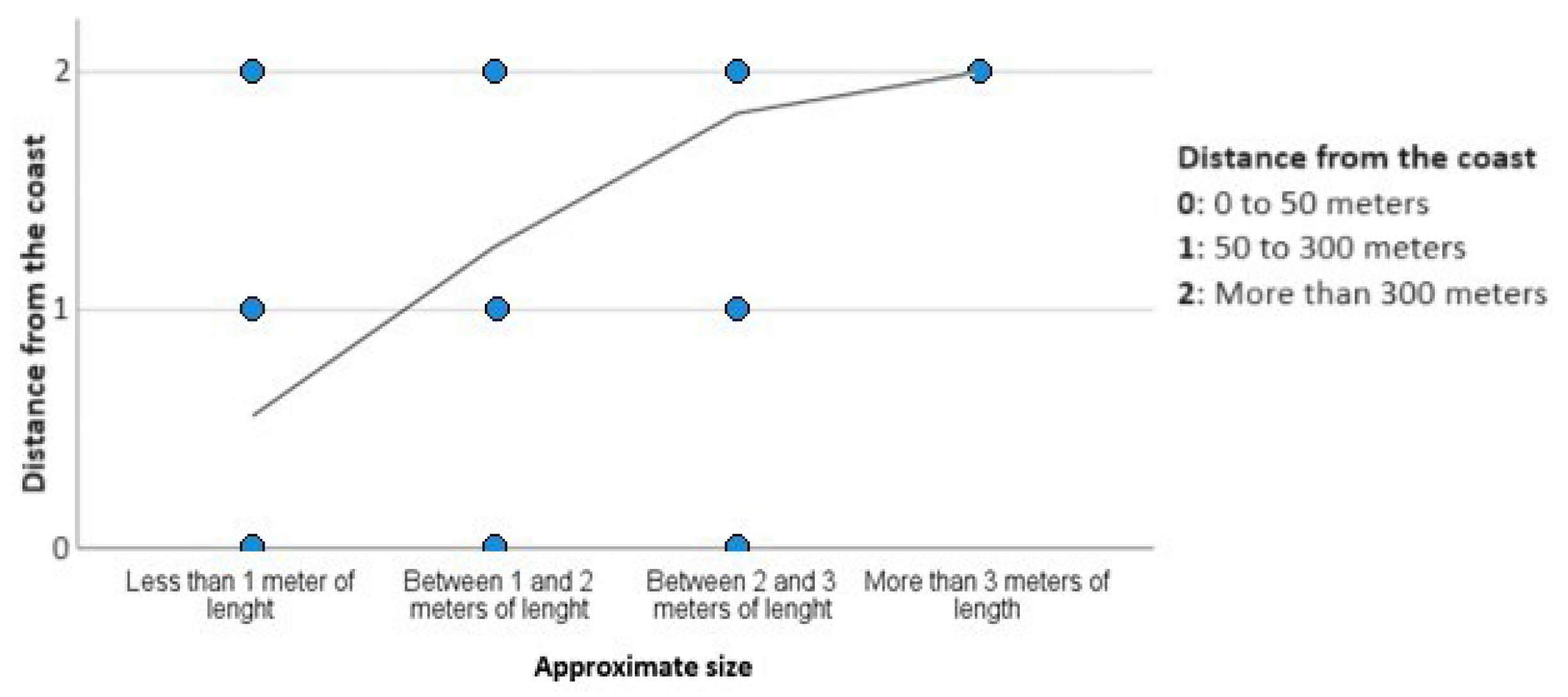
2.5. Depth and Distance from the Coast
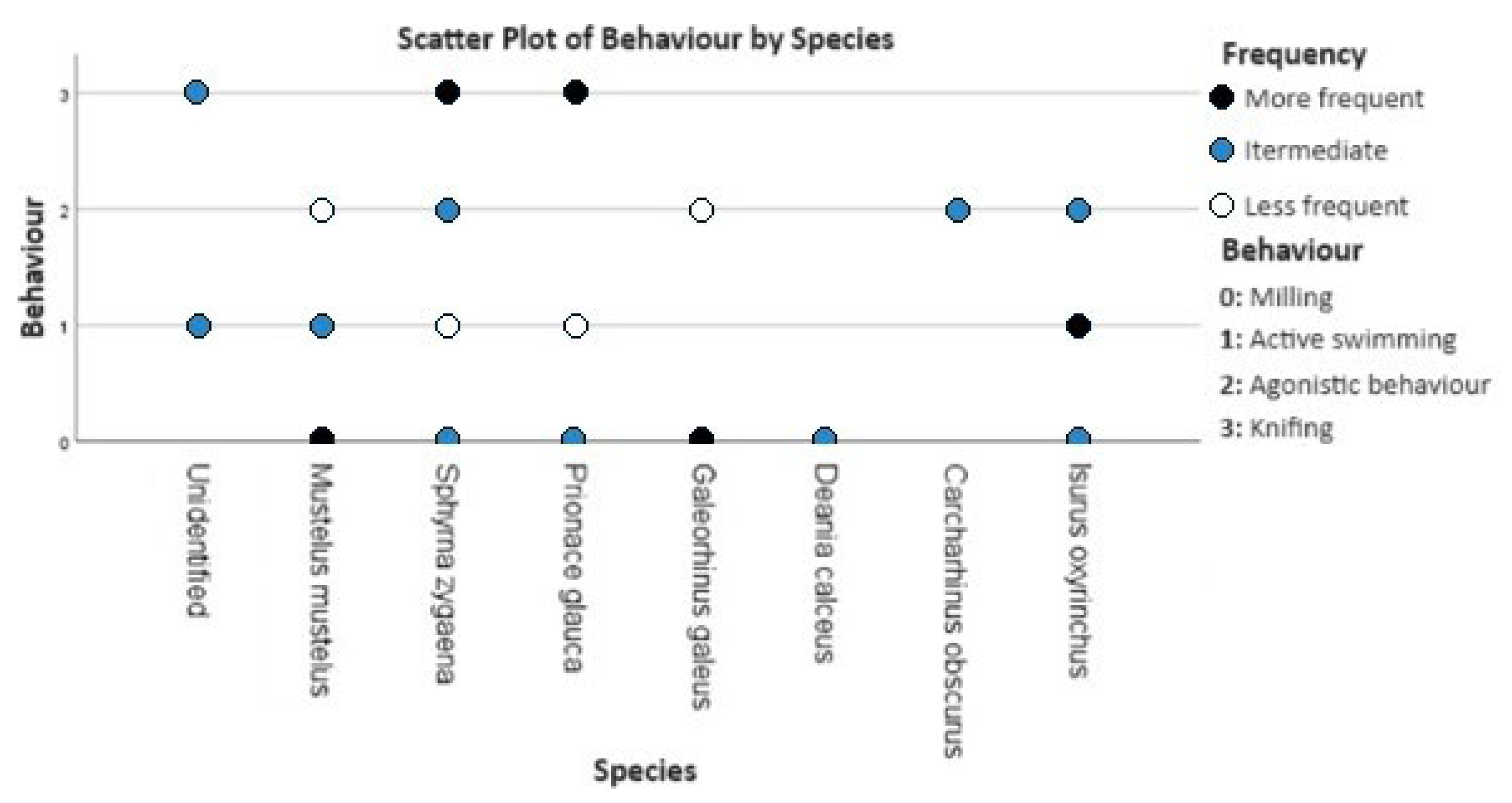
2.6. Behaviour
- Active swimming—The shark swims very actively without significant changes in its direction;
- Agonistic behaviour—The shark is very active, swimming relatively quickly with frequent changes in its direction. Overall, this is a behaviour response to a stressful situation;
- Milling—The shark swims slowly, changing direction frequently;
- Knifing—The shark swims on the surface with its dorsal fin out of water.
2.7. Statistical Analyses
3. Results
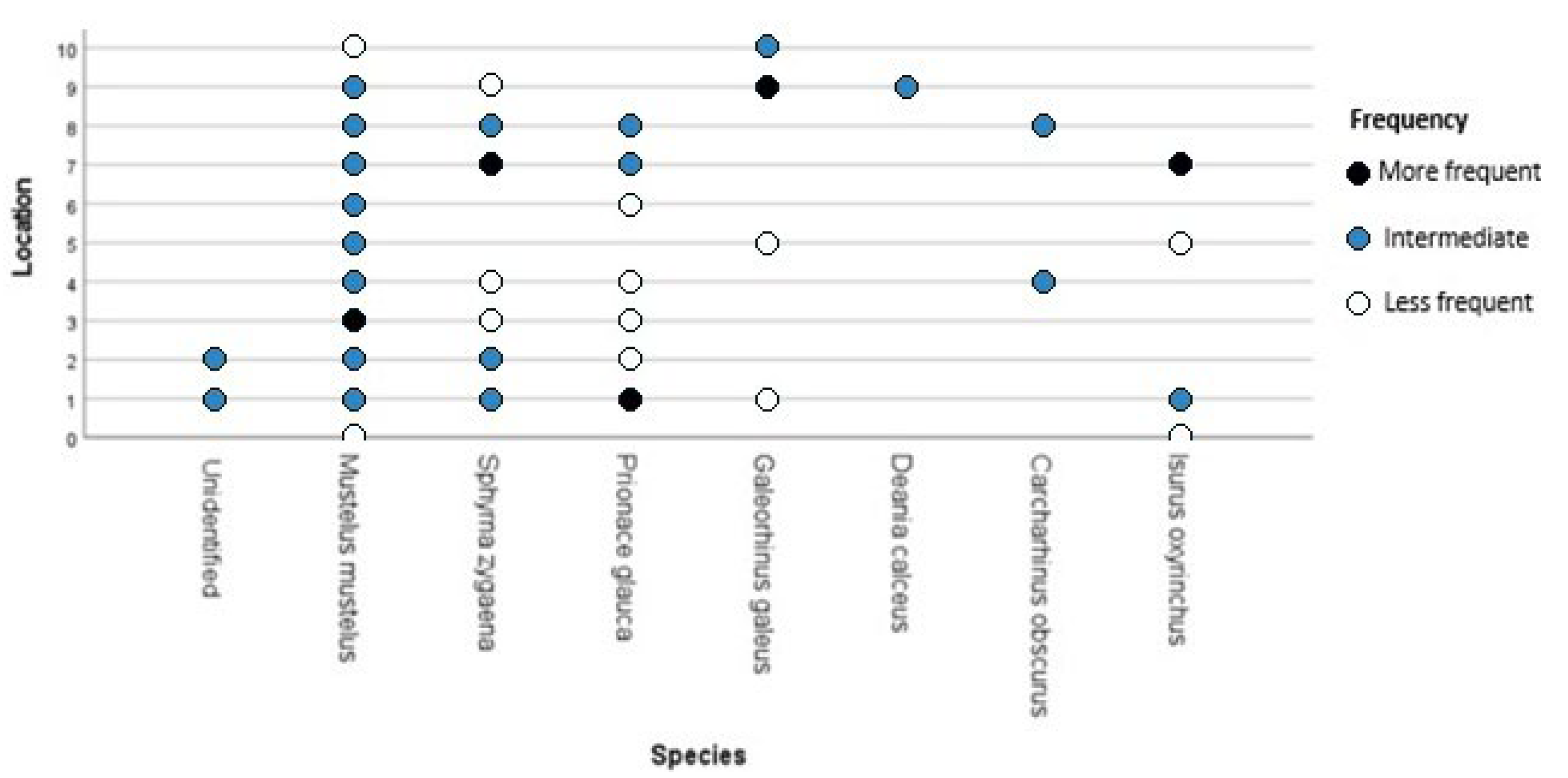
3.1. Species
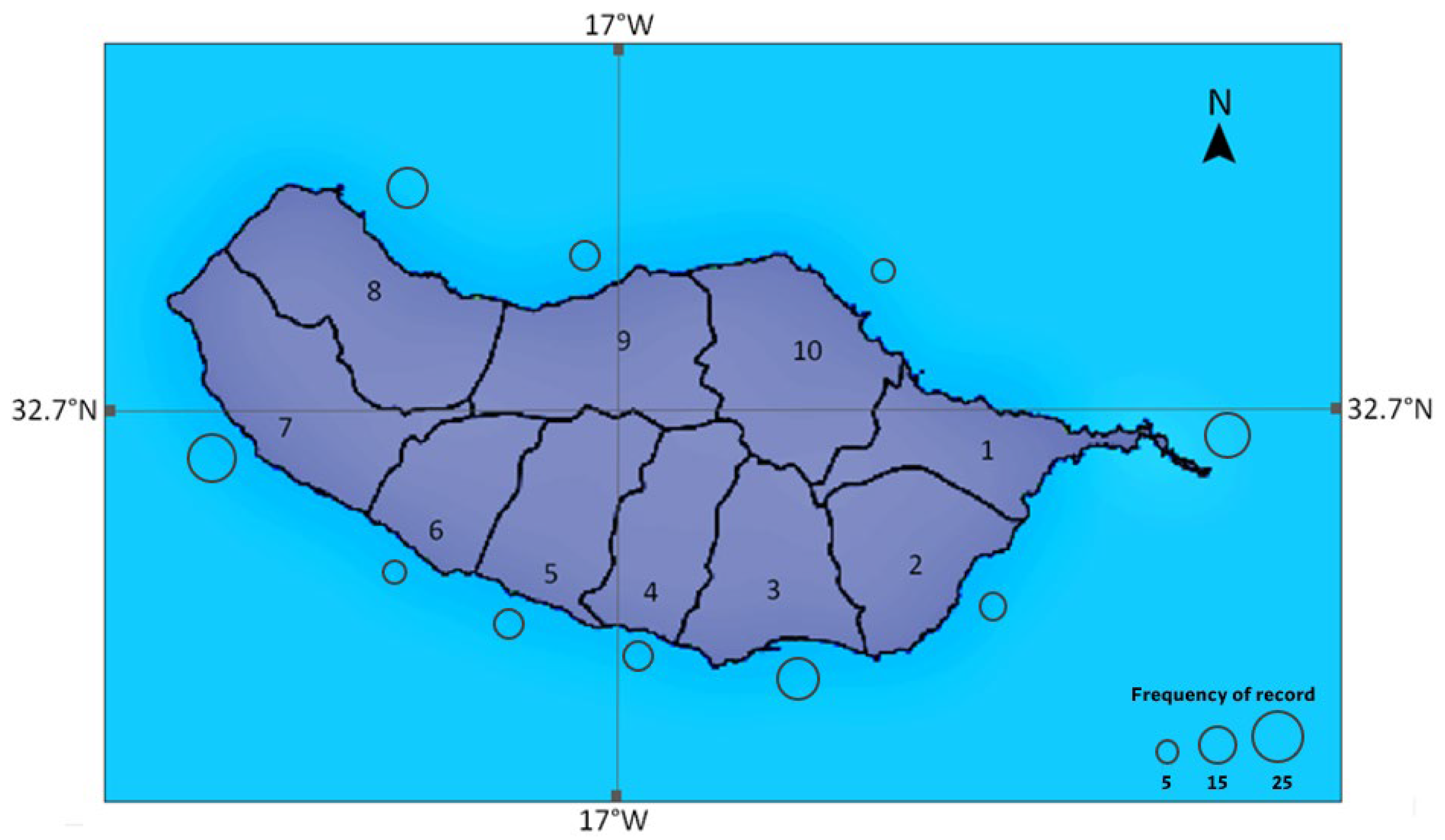
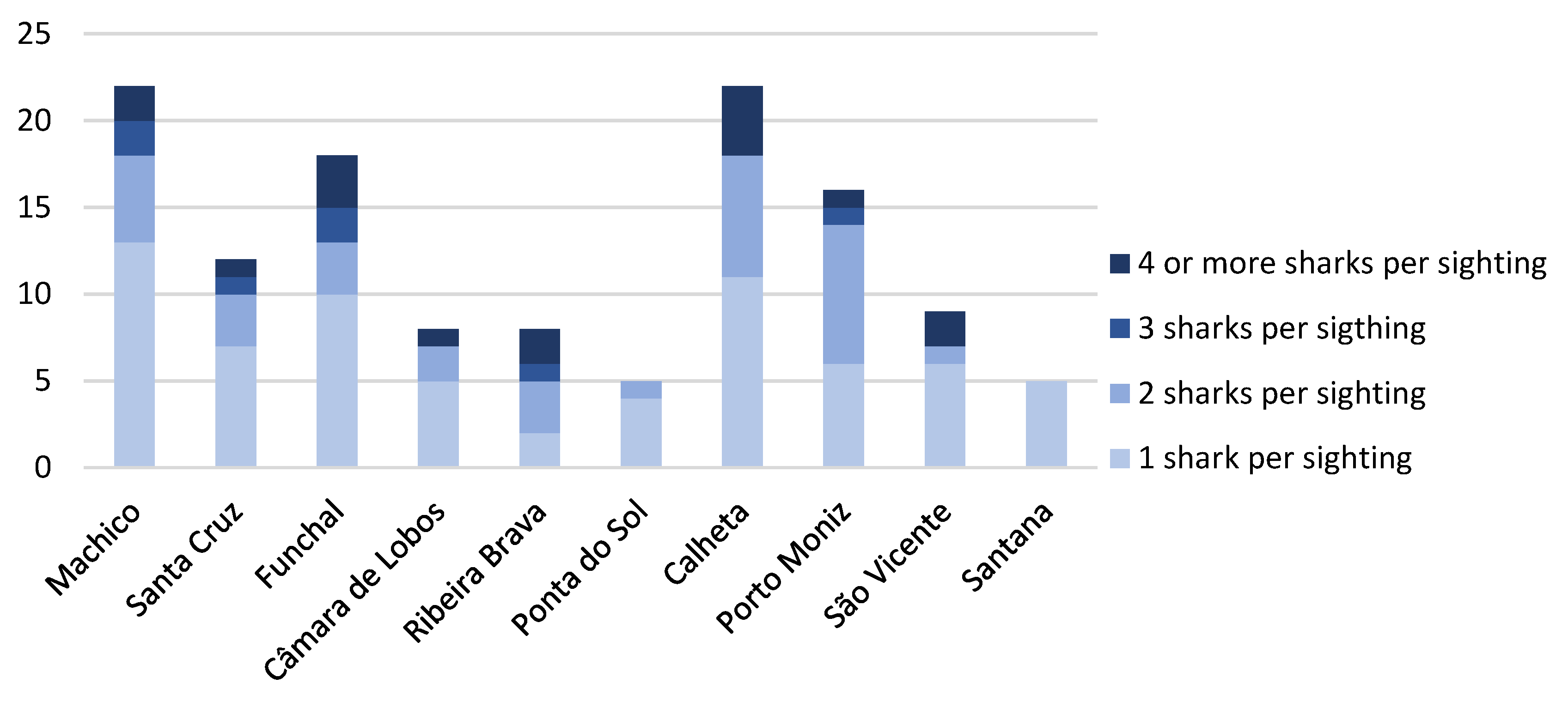
3.2. Spatial and Temporal Distribution
3.3. Behaviour
4. Discussion
4.1. Species
4.2. Spatial and Temporal Distribution
4.3. Behaviour
4.4. Conservation and Awareness
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camhi, M.D.; Pikitch, E.K.; Babcock, E.A. Sharks of the Open Ocean: Biology, Fisheries and Conservation; Blackwell Publishing Ltd.: Oxford, UK, 2008. [Google Scholar]
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The Effects of Fishing on Sharks, Rays, and Chimaeras (Chondrichthyans), and the Implications for Marine Ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Ceccarelli, D.; Ayling, T. Role, Importance and Vulnerability of Top Predators on the Great Barrier Reef—A Review; Great Barrier Reef Marine Park Authority: Townsville City, QLD, Australia, 2010. [Google Scholar]
- Jaiteh, V.F.; Loneragan, N.R.; Warren, C. The End of Shark Finning? Impacts of Declining Catches and Fin Demand on Coastal Community Livelihoods. Mar. Policy 2017, 82, 224–233. [Google Scholar] [CrossRef]
- Seidu, I.; Brobbey, L.K.; Danquah, E.; Oppong, S.K.; Seidu, M.; Dulvy, N.K. Fishing for Survival: Importance of Shark Fisheries for the Livelihoods of Coastal Communities in Western Ghana. Fish. Res. 2022, 246, 106157. [Google Scholar] [CrossRef]
- Huveneers, C.; Meekan, M.G.; Apps, K.; Ferreira, L.C.; Pannell, D.; Vianna, G.M.S. The Economic Value of Shark-Diving Tourism in Australia. Rev. Fish Biol. Fish. 2017, 27, 665–680. [Google Scholar] [CrossRef]
- Zimmerhackel, J.S.; Kragt, M.E.; Rogers, A.A.; Ali, K.; Meekan, M.G. Evidence of Increased Economic Benefits from Shark-Diving Tourism in the Maldives. Mar. Policy 2019, 100, 21–26. [Google Scholar] [CrossRef]
- Torres, P.; Bolhão, N.; Tristão da Cunha, R.; Vieira, J.A.C.; Rodrigues, A.d.S. Dead or Alive: The Growing Importance of Shark Diving in the Mid-Atlantic Region. J. Nat. Conserv. 2017, 36, 20–28. [Google Scholar] [CrossRef]
- Robbins, W.D.; Hisano, M.; Connolly, S.R.; Choat, J.H. Ongoing Collapse of Coral-Reef Shark Populations. Curr. Biol. 2006, 16, 2314–2319. [Google Scholar] [CrossRef]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P.; et al. Half a Century of Global Decline in Oceanic Sharks and Rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef]
- Roff, G.; Brown, C.J.; Priest, M.A.; Mumby, P.J. Decline of Coastal Apex Shark Populations over the Past Half Century. Commun. Biol. 2018, 1, 223. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Simpfendorfer, C.A.; Davidson, L.N.K.; Fordham, S.V.; Bräutigam, A.; Sant, G.; Welch, D.J. Challenges and Priorities in Shark and Ray Conservation. Curr. Biol. 2017, 27, 565–572. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Golbuu, Y.; Caselle, J.E.; Ballesteros, E.; Letessier, T.B.; Meeuwig, J.J.; Gouezo, M.; Olsudong, D.; Turchik, A.; Sala, E. Marine biodiversity and protected areas in Palau. In Scientific Report to the Government of the Republic of Palau; National Geographic Society: Washington, DC, USA, 2014. [Google Scholar]
- Gallagher, A.J.; Vianna, G.M.S.; Papastamatiou, Y.P.; Macdonald, C.; Guttridge, T.L.; Hammerschlag, N. Biological Effects, Conservation Potential, and Research Priorities of Shark Diving Tourism. Biol. Conser. 2015, 184, 365–379. [Google Scholar] [CrossRef]
- Mieras, P.; Harvey-Clark, C.; Bear, M.; Hodgin, G. The Economy of Shark Conservation in the Northeast Pacific: The Role of Ecotourism and Citizen Science. Adv. Mar. Biol. 2017, 78, 121–153. [Google Scholar] [PubMed]
- Silvertown, J. A New Dawn for Citizen Science. Trends Ecol. Evol. 2009, 24, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Follett, R.; Strezov, V. An Analysis of Citizen Science Based Research: Usage and Publication Patterns. PLoS ONE 2015, 10, e0143687. [Google Scholar] [CrossRef]
- De Sherbinin, A.; Bowser, A.; Chuang, T.R.; Cooper, C.; Danielsen, F.; Edmunds, R.; Elias, P.; Faustman, E.; Hultquist, C.; Mondardini, R.; et al. The Critical Importance of Citizen Science Data. Front. Clim. 2021, 3, 650760. [Google Scholar] [CrossRef]
- Hussey, N.E.; Stroh, N.; Klaus, R.; Chekchak, T.; Kessel, S.T. SCUBA Diver Observations and Placard Tags to Monitor Grey Reef Sharks, Carcharhinus amblyrhynchos, at Sha’ab Rumi, the Sudan: Assessment and Future Directions. J. Mar. Biol. Assoc. U.K. 2013, 93, 299–308. [Google Scholar] [CrossRef]
- Vianna, G.M.S.; Meekan, M.G.; Bornovski, T.H.; Meeuwig, J.J. Acoustic Telemetry Validates a Citizen Science Approach for Monitoring Sharks on Coral Reefs. PLoS ONE 2014, 9, e95565. [Google Scholar] [CrossRef]
- Araujo, G.; Ismail, A.R.; McCann, C.; McCann, D.; Legaspi, C.G.; Snow, S.; Labaja, J.; Manjaji-Matsumoto, M.; Ponzo, A. Getting the Most out of Citizen Science for Endangered Species Such as Whale Shark. J. Fish. Biol. 2020, 96, 864–867. [Google Scholar] [CrossRef]
- Pagel, C.D.; Orams, M.B.; LÜck, M. #Biteme: Considering the Potential Influence of Social Media On In-Water Encounters With Marine Wildlife. Tou. Mar. Environ. 2020, 15, 249–258. [Google Scholar] [CrossRef]
- Malara, D.; Battaglia, P.; Consoli, P.; Arcadi, E.; Longo, F.; Stipa, M.G.; Pagano, L.; Greco, S.; Andaloro, F.; Romeo, T. When Opportunistic Predators Interact with Swordfish Harpoon Fishing Activities: Shark Depredation over Catches in the Strait of Messina (Central Mediterranean Sea). Eur. Zool. J. 2021, 88, 226–236. [Google Scholar] [CrossRef]
- Dickinson, J.L.; Zuckerberg, B.; Bonter, D.N. Citizen Science as an Ecological Research Tool: Challenges and Benefits. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 149–172. [Google Scholar] [CrossRef]
- Andrzejaczek, S.; Meeuwig, J.; Rowat, D.; Pierce, S.; Davies, T.; Fisher, R.; Meekan, M. The Ecological Connectivity of Whale Shark Aggregations in the Indian Ocean: A Photo-Identification Approach. R. Soc. Open. Sci. 2016, 3, 160455. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.P.; Jaidah, M.Y.; Jabado, R.W.; Lee-Brooks, K.; Nour El-Din, N.M.; Malki, A.A.A.; Elmeer, K.; McCormick, P.A.; Henderson, A.C.; Pierce, S.J.; et al. Whale Sharks, Rhincodon typus, Aggregate around Offshore Platforms in Qatari Waters of the Arabian Gulf to Feed on Fish Spawn. PLoS ONE 2013, 8, e58255. [Google Scholar] [CrossRef]
- Norman, B.M.; Holmberg, J.A.; Arzoumanian, Z.; Reynolds, S.D.; Wilson, R.P.; Rob, D.; Pierce, S.J.; Gleiss, A.C.; De La Parra, R.; Galvan, B.; et al. Undersea Constellations: The Global Biology of an Endangered Marine Megavertebrate Further Informed through Citizen Science. BioScience 2017, 67, 1029–1043. [Google Scholar] [CrossRef]
- Araujo, G.; Snow, S.; So, C.L.; Labaja, J.; Murray, R.; Colucci, A.; Ponzo, A. Population Structure, Residency Patterns and Movements of Whale Sharks in Southern Leyte, Philippines: Results from Dedicated Photo-ID and Citizen Science. Aquat. Conserv. 2017, 27, 237–252. [Google Scholar] [CrossRef]
- Sims, D.W.; Witt, M.J.; Richardson, A.J.; Southall, E.J.; Metcalfe, J.D. Encounter success of free-ranging marine predator movements across a dynamic prey landscape. Proc. R. Soc. B 2006, 273, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.J.; Streich, M.K.; Topping, T.S.; Stunz, G.W. Utility of Citizen Science Data: A Case Study in Land-Based Shark Fishing. PLoS ONE 2019, 14, e0226782. [Google Scholar] [CrossRef] [PubMed]
- Bargnesi, F.; Lucrezi, S.; Ferretti, F. Opportunities from Citizen Science for Shark Conservation, with a Focus on the Mediterranean Sea. Eur. Zool. J. 2020, 87, 20–34. [Google Scholar] [CrossRef]
- Barreiros, J.P.; Bismarck, O.; Gadig, F. Catálogo Ilustrado dos Tubarões e Raias dos Açores/Sharks and Rays from the Azores—An Illustrated Catalogue; Instituto Açoriano de Cultura: Angra do Heroísmo, Portugal, 2011. [Google Scholar] [CrossRef]
- Das, D.; Afonso, P. Review of the Diversity, Ecology, and Conservation of Elasmobranchs in the Azores Region, Mid-North Atlantic. Front. Mar. Sci. 2017, 4, 354. [Google Scholar] [CrossRef]
- Santos, M.N.; Coelho, R. A general overview of the portuguese pelagic sharks research program in the Atlantic Ocean. Collect. Vol. Sci. Pap. ICCAT 2015, 71, 2551–2556. [Google Scholar]
- Figueiredo, I.; Machado, P.B.; Gordo, L.S. Deep-Water Sharks Fisheries off the Portuguese Continental Coast. J Northwest Atl. Fish. Sci. 2005, 35, 291–298. [Google Scholar] [CrossRef]
- Wirtz, P.; Fricke, R.; Biscoito, M.J. The Coastal Fishes of Madeira Island—New Records and an Annotated Checklist. Zootaxa 2008, 1715, 1–26. [Google Scholar] [CrossRef]
- Biscoito, M.; Ribeiro, C.; Freitas, M. Annotated Checklist of the Fishes of the Archipelago of Madeira (NE Atlantic): I-Chondrichthyes. Zootaxa 2018, 4429, 459–494. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Ferreira, S.; Chada, T.; Delgado, J.; Carvalho, D. First Approach to the Biology of the Deep-Water Shark Deania profundorum (Chondrichthyes: Centrophoridae). Mar. Biodivers. Rec. 2009, 2, e44. [Google Scholar] [CrossRef]
- Severino, R.; Afonso-Dias, I.; Delgado, J.; Afonso-Dias, M. Aspects of the Biology of the Leaf-Scale Gulper Shark Centrophorus squamosus (Bonnaterre, 1788) off Madeira Archipelago. Arquipel. Life Mar. Sci. 2009, 26, 57–61. [Google Scholar]
- Costa, G.; Chada, T.; Melo-Moreira, E.; Cavallero, S.; D’Amelio, S. Endohelminth Parasites of the Leafscale Gulper Shark, Centrophorus squamosus (Bonnaterre, 1788) (Squaliformes:Centrophoridae) off Madeira Archipelago. Acta Parasitol. 2014, 59, 316–322. [Google Scholar] [CrossRef]
- Silvano, R.A.M.; MacCord, P.F.L.; Lima, R.V.; Begossi, A. When Does This Fish Spawn? Fishermen’s Local Knowledge of Migration and Reproduction of Brazilian Coastal Fishes. Environ. Biol. Fishes 2006, 76, 371–386. [Google Scholar] [CrossRef]
- Silvano, R.A.M.; Begossi, A. Local Knowledge on a Cosmopolitan Fish: Ethnoecology of Pomatomus saltatrix (Pomatomidae) in Brazil and Australia. Fish. Res. 2005, 71, 43–59. [Google Scholar] [CrossRef]
- Zukowski, S.; Curtis, A.; Watts, R.J. Using Fisher Local Ecological Knowledge to Improve Management: The Murray Crayfish in Australia. Fish. Res. 2011, 110, 120–127. [Google Scholar] [CrossRef]
- Almojil, D. Local ecological knowledge of fisheries charts decline of sharks in data-poor regions. Mar. Policy 2021, 132, 104638. [Google Scholar] [CrossRef]
- Caldeira, R.M.A.; Groom, S.; Miller, P.; Pilgrim, D.; Nezlin, N.P. Sea-Surface Signatures of the Island Mass Effect Phenomena around Madeira Island, Northeast Atlantic. Remote Sens. Environ. 2002, 80, 336–360. [Google Scholar] [CrossRef]
- Quintal, R. Levadas e Veredas Da Madeira; Francisco Ribeiro: Funchal, Portugal, 2004. [Google Scholar]
- Torres, C.; Andrade, C. Spatial Decision Analysis Process for Selection Marine Aquaculture Suitable: The Exemple of Madeira Island. J. Integr. Coast. Zone Manag. 2010, 10, 321–330. [Google Scholar]
- Hidrográfico, I. Dinâmica Sedimentar da Costa Sul da Ilha da Madeira; REL.TF.GM.02/03; Instituto Hidrográfico: Lisbon, Portugal, 2003. [Google Scholar]
- Morato, T. Description of Environmental Issues, Fish Stocks and Fisheries in the EEZs around the Azores and Madeira. Available online: https://stecf.jrc.ec.europa.eu/documents/43805/465474/Item+6.2+Report+Morato_Azores_Madeira.pdf (accessed on 26 September 2023).
- Hermida, M.; Delgado, J. High Trophic Level and Low Diversity: Would Madeira Benefit from Fishing Down? Mar. Policy 2016, 73, 130–137. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 26 September 2023).
- Smith, K.; Scarr, M.; Scarpaci, C. Grey Nurse Shark (Carcharias taurus) Diving Tourism: Tourist Compliance and Shark Behaviour at Fish Rock, Australia. Environ. Manag. 2010, 46, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.K.; Bennison, A.; Jessopp, M.; Haberlin, D.; Harman, L.A.A. Dawn Peak in the Occurrence of “knifing Behaviour” in Blue Sharks. Anim. Biotelemetry 2015, 3, 46. [Google Scholar] [CrossRef][Green Version]
- Francis, M.P. Distribution, habitat and movement of juvenile smooth hammerhead sharks (Sphyrna zygaena) in northern New Zealand. N. Z. J. Mar. Freshw. Res. 2016, 50, 506–525. [Google Scholar] [CrossRef]
- Martínez-Escauriaza, R.; Vieira, C.; Gouveia, L.; Gouveia, N.; Hermida, M.; Trenkel, V. Characterization and Evolution of Spearfishing in Madeira Archipelago, Eastern Atlantic. Aquat. Living Resour. 2020, 33, 15. [Google Scholar] [CrossRef]
- Mendonça, A. Diet of the Blue Shark, Prionace glauca, in the Northeast Atlantic. Master’s Thesis, Faculdade de Ciências da Universidade do Porto, Porto, Portugal, 2009. [Google Scholar]
- Saïdi, B.; Enajjar, S.; Bradaï, M.N.; Bouaïn, A. Diet Composition of Smooth-Hound Shark, Mustelus mustelus (Linnaeus, 1758), in the Gulf of Gabès, Southern Tunisia. J. Appl. Ichthyol. 2009, 25, 113–118. [Google Scholar] [CrossRef]
- Lippmann, J. Fatal Shark Attacks on Divers in Australia, 1960-2017. Diving Hyperb. Med. 2018, 48, 224–228. [Google Scholar] [CrossRef]
- Lopes, J.T.; Bicudo, P. Surfing Tourism Plan: Madeira Island Case Study. Eur. J. Tour. Res. 2017, 16, 45–56. [Google Scholar] [CrossRef]
- Nakano, H.; Seki, M.P. Synopsis of Biological Data on the Blue Shark, Prionace glauca Linnaeus. Bull. Fish. Res. Agency Jpn. 2003, 6, 18–55. [Google Scholar]
- Abascal, F.J.; Quintans, M.; Ramos-Cartelle, A.; Mejuto, J. Movements and Environmental Preferences of the Shortfin Mako, Isurus oxyrinchus, in the Southeastern Pacific Ocean. Mar. Biol. 2011, 158, 1175–1184. [Google Scholar] [CrossRef]
- Barreto, R.R.; De Farias, W.K.T.; Andrade, H.; Santana, F.M.; Lessa, R. Age, Growth and Spatial Distribution of the Life Stages of the Shortfin Mako, Isurus oxyrinchus (Rafinesque, 1810) Caught in the Western and Central Atlantic. PLoS ONE 2016, 11, e0153062. [Google Scholar] [CrossRef]
- Francis, M.P.; Shivji, M.S.; Duffy, C.A.J.; Rogers, P.J.; Byrne, M.E.; Wetherbee, B.M.; Tindale, S.C.; Lyon, W.S.; Meyers, M.M. Oceanic Nomad or Coastal Resident? Behavioural Switching in the Shortfin Mako Shark (Isurus oxyrinchus). Mar. Biol. 2019, 166, 5. [Google Scholar] [CrossRef]
- Nosal, A.P.; Cartamil, D.P.; Ammann, A.J.; Bellquist, L.F.; Ben-Aderet, N.J.; Blincow, K.M.; Burns, E.S.; Chapman, E.D.; Freedman, R.M.; Klimley, A.P.; et al. Triennial Migration and Philopatry in the Critically Endangered Soupfin Shark Galeorhinus galeus. J. Appl. Ecol. 2021, 58, 1570–1582. [Google Scholar] [CrossRef]
- Walker, T.I.; Rigby, C.L.; Pacoureau, N.; Ellis, J.; Kulka, D.W.; Chiaramonte, G.E.; Herman, K. Galeorhinus galeus. IUCN Red List Threat. Species 2020, 12, 1–21. [Google Scholar] [CrossRef]
- Maul, G. Lista Sistemática dos Peixes Assinalados nos Mares da Madeira e Índice Alfabético. In Vertebrados da Madeira; Noronha, A., Sarmento AA, Eds.; Junta Geral do Distrito Autónomo do Funchal: Madeira, Portugal, 1949; Volume 2, pp. 135–159. [Google Scholar]
- Clarke, M.; Bracken, J.J.; Institute, M. Aspects of the Biology of Three Exploited Deepwater Sharks Centrophorus squamosus, Centroscymnus coelolepis and Deania calceus (Elasmobranchii: Squalidae) from the Continental Slopes of the Rockall Trough and Porcupine Bank. Ph.D. Thesis, National University of Ireland, Galway, Ireland, 2000. [Google Scholar]
- Heithaus, M. Nursery Areas as Essential Shark Habitats: A Theoretical Perspective. In Shark Nursery Grounds of the Gulf of Mexico and the East Coast Waters of the United States; McCandless, C.T., Kohler, N.E., Pratt, H.L., Jr., Eds.; American Fisheries Society: Bethesda, MD, USA, 2007; Volume 50, pp. 3–13. [Google Scholar]
- Driggers, W.B.; Ingram, G.W.; Grace, M.A.; Gledhill, C.T.; Henwood, T.A.; Horton, C.N.; Jones, C.M. Pupping Areas and Mortality Rates of Young Tiger Sharks Galeocerdo cuvier in the Western North Atlantic Ocean. Aquat. Biol. 2008, 2, 161–170. [Google Scholar] [CrossRef]
- Heupel, M.R.; Carlson, J.K.; Simpfendorfer, C.A. Shark Nursery Areas: Concepts, Definition, Characterization and Assumptions. Mar. Ecol. Prog. Ser. 2007, 337, 287–297. [Google Scholar] [CrossRef]
- Power, M.E. Depth Distributions of Armored Catfish: Predator-induced Resource Avoidance? Ecology 1984, 65, 523–528. [Google Scholar] [CrossRef]
- Simpfendorfer, C.; Heupel, M. Assessing Habitat Use and Movement. In Biology of Sharks and Their Relatives; CRC Press: Boca Raton, FL, USA, 2012; pp. 553–572. [Google Scholar]
- Oddone, C.; Paesch, L.; Norbis, W. Reproductive Biology and Seasonal Distribution of Mustelus schmitti (Elasmobranchii: Triakidae) in the Rio de La Plata Oceanic Front, South-Western Atlantic. J. Mar. Biol. Assoc. UK 2005, 85, 1193–1198. [Google Scholar] [CrossRef]
- Ward-Paige, C.A.; Britten, G.L.; Bethea, D.M.; Carlson, J.K. Characterizing and Predicting Essential Habitat Features for Juvenile Coastal Sharks. Mar. Ecol. 2015, 36, 419–431. [Google Scholar] [CrossRef]
- Hacohen-Domené, A.; Martínez-Rincón, R.O.; Galván-Magaña, F.; Cárdenas-Palomo, N.; de la Parra-Venegas, R.; Galván-Pastoriza, B.; Dove, A.D.M. Habitat Suitability and Environmental Factors Affecting Whale Shark (Rhincodon typus) Aggregations in the Mexican Caribbean. Environ. Biol. Fish. 2015, 98, 1953–1964. [Google Scholar] [CrossRef]
- Sabarros, P.S.; Dominique, D.; Bach, P.; Selles, J.; Romanov, E.; Dagorne, D.; Le Foulgoc, L. Characterisation of Blue Shark (Prionace glauca) Hotspots in the South-West Indian Ocean; FAO, Reference IOTC–2014–WPEB10–23. Available online: https://www.iotc.org/documents/characterisation-blue-shark-prionace-glauca-hotspots-south-west-indian-ocean (accessed on 26 September 2023).
- Papastamatiou, Y.P.; Itano, D.G.; Dale, J.J.; Meyer, C.G.; Holland, K.N. Site Fidelity and Movements of Sharks Associated with Ocean-Farming Cages in Hawaii. Mar. Freshw. Res. 2010, 61, 1366–1375. [Google Scholar] [CrossRef]
- Boyra, A.; Sanchez-Jerez, P.; Tuya, F.; Espino, F.; Haroun, R. Attraction of Wild Coastal Fishes to an Atlantic Subtropical Cage Fish Farms, Gran Canaria, Canary Islands. Environ. Biol. Fish. 2004, 70, 93–401. [Google Scholar] [CrossRef]
- Wosnick, N.; Leite, R.D.; Balanin, S.; Chaves, A.P.; Gastal, E.R.; Hauser-Davis, R.A.; Giareta, E.P. Behavioral and visual stress-induced proxies in elasmobranchs. Rev. Fish. Biol. Fish. 2023, 33, 175–199. [Google Scholar] [CrossRef]
- Giovos, I.; Barash, A.; Barone, M.; Barría, C.; Borme, D.; Brigaudeau, C.; Charitou, A.; Brito, C.; Currie, J.; Dornhege, M.; et al. Understanding the Public Attitude towards Sharks for Improving Their Conservation. Mar. Policy 2021, 134, 104811. [Google Scholar] [CrossRef]
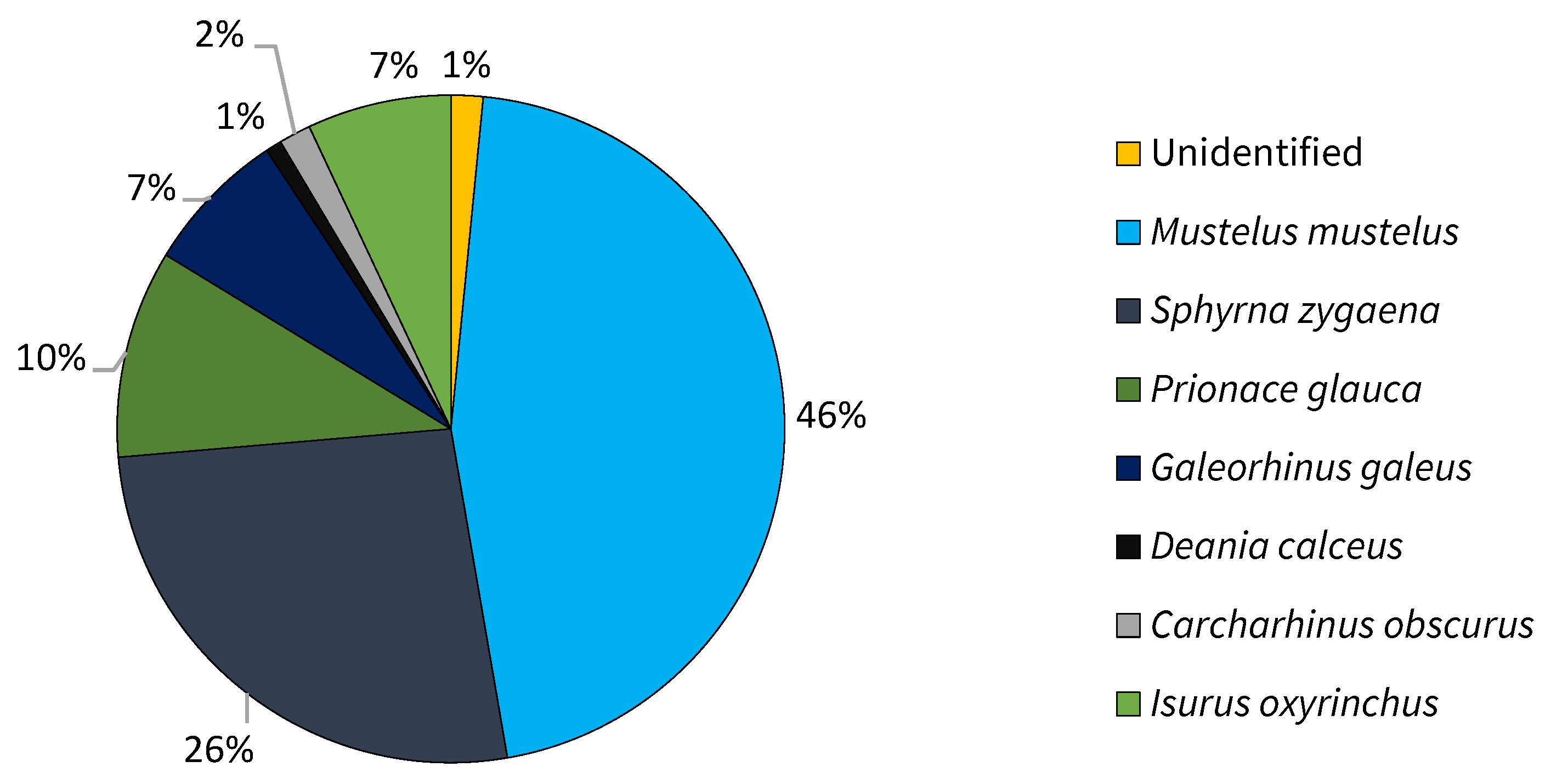
| Species | Small | Large | Maximum Length | Length at First Maturity |
|---|---|---|---|---|
| Mustelus mustelus | <1 m | ≥1 m | 2 m | 0.90 m |
| Sphyrna zygaena | ≤2 m | >2 m | 4 m | 2.65 m |
| Prionace glauca | <2 m | ≥2 m | 4 m | 1.99 m |
| Galeorhinus galeus | ≤1 m | >1 m | 1.95 m | 1.44 m |
| Deania calceus | ≤60 cm | >60 cm | 1.27 m | 0.98 m |
| Carcharhinus obscurus | ≤2 m | >2 m | 4.20 m | 2.5 m |
| Isurus oxyrinchus | ≤2 m | >2 m | 4.45 m | 2.78 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berimbau, L.; Larrea, A.; Costa, A.C.; Torres, P. Human–Shark Interactions: Citizen Science Potential in Boosting Shark Research on Madeira Island. Diversity 2023, 15, 1062. https://doi.org/10.3390/d15101062
Berimbau L, Larrea A, Costa AC, Torres P. Human–Shark Interactions: Citizen Science Potential in Boosting Shark Research on Madeira Island. Diversity. 2023; 15(10):1062. https://doi.org/10.3390/d15101062
Chicago/Turabian StyleBerimbau, Luís, Ander Larrea, Ana Cristina Costa, and Paulo Torres. 2023. "Human–Shark Interactions: Citizen Science Potential in Boosting Shark Research on Madeira Island" Diversity 15, no. 10: 1062. https://doi.org/10.3390/d15101062
APA StyleBerimbau, L., Larrea, A., Costa, A. C., & Torres, P. (2023). Human–Shark Interactions: Citizen Science Potential in Boosting Shark Research on Madeira Island. Diversity, 15(10), 1062. https://doi.org/10.3390/d15101062







