A Hotspot of Subterranean Biodiversity on the Brink: Mo So Cave and the Hon Chong Karst of Vietnam
Abstract
:1. Introduction
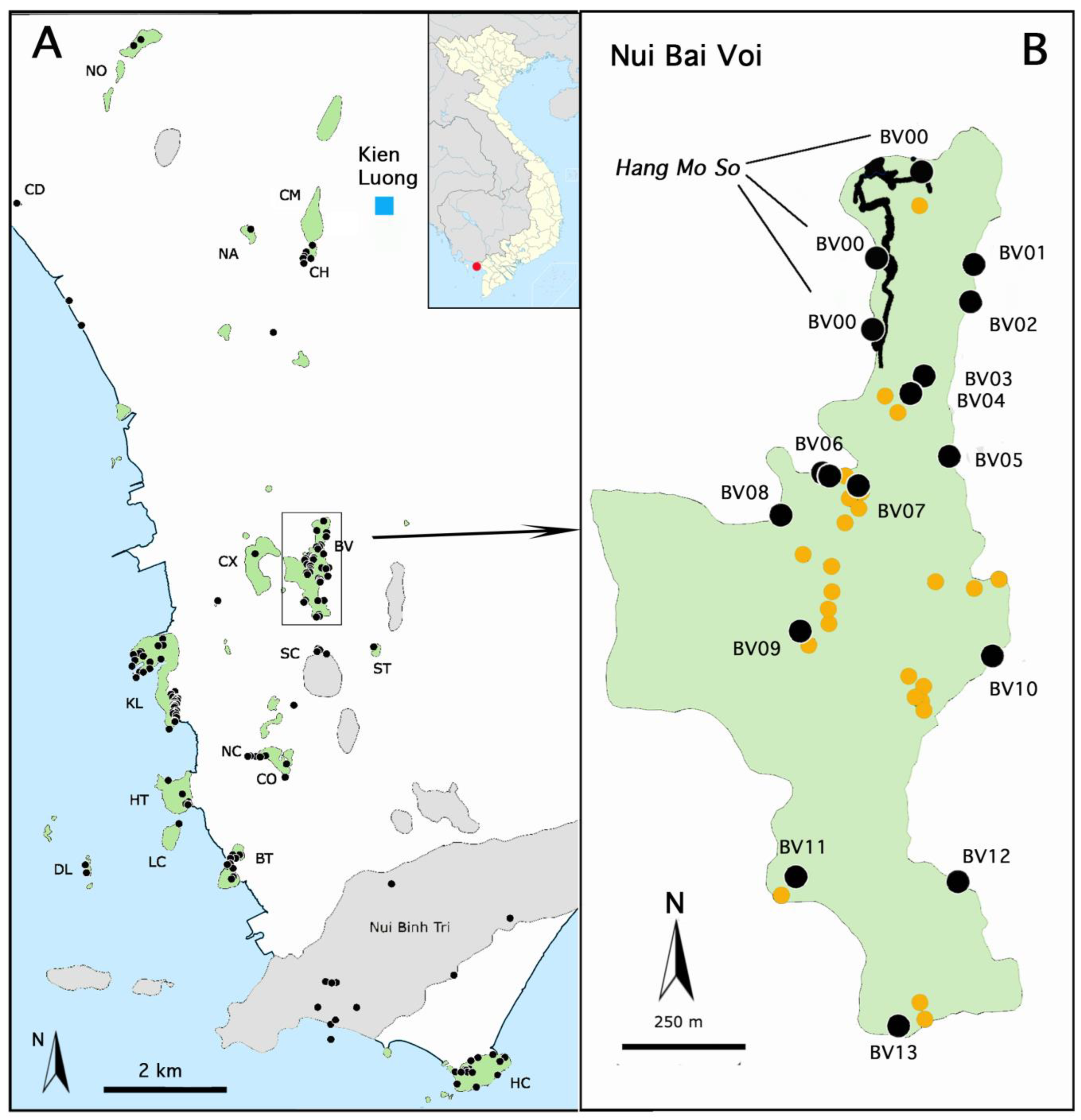
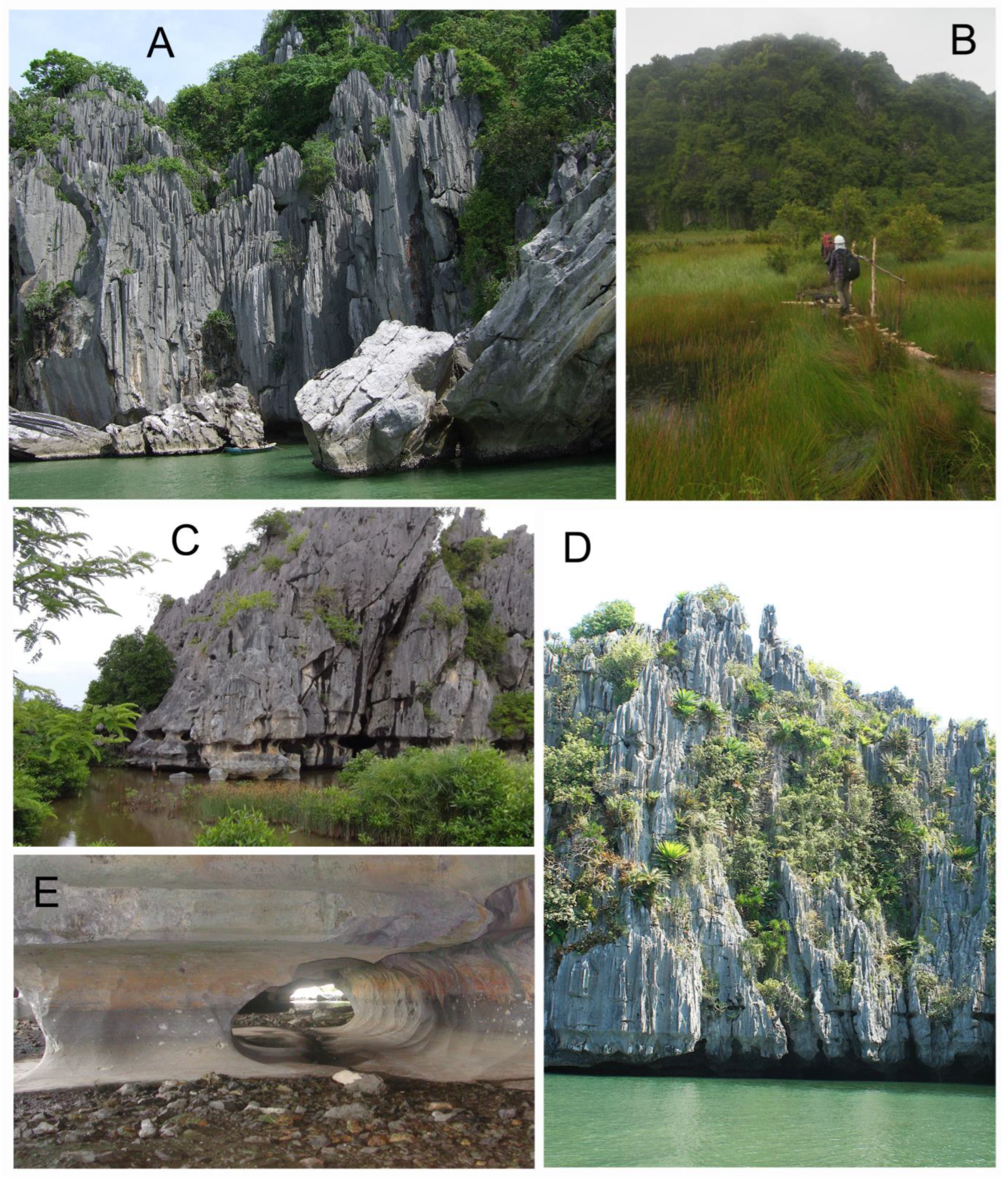
1.1. Karst and Caves of MDL
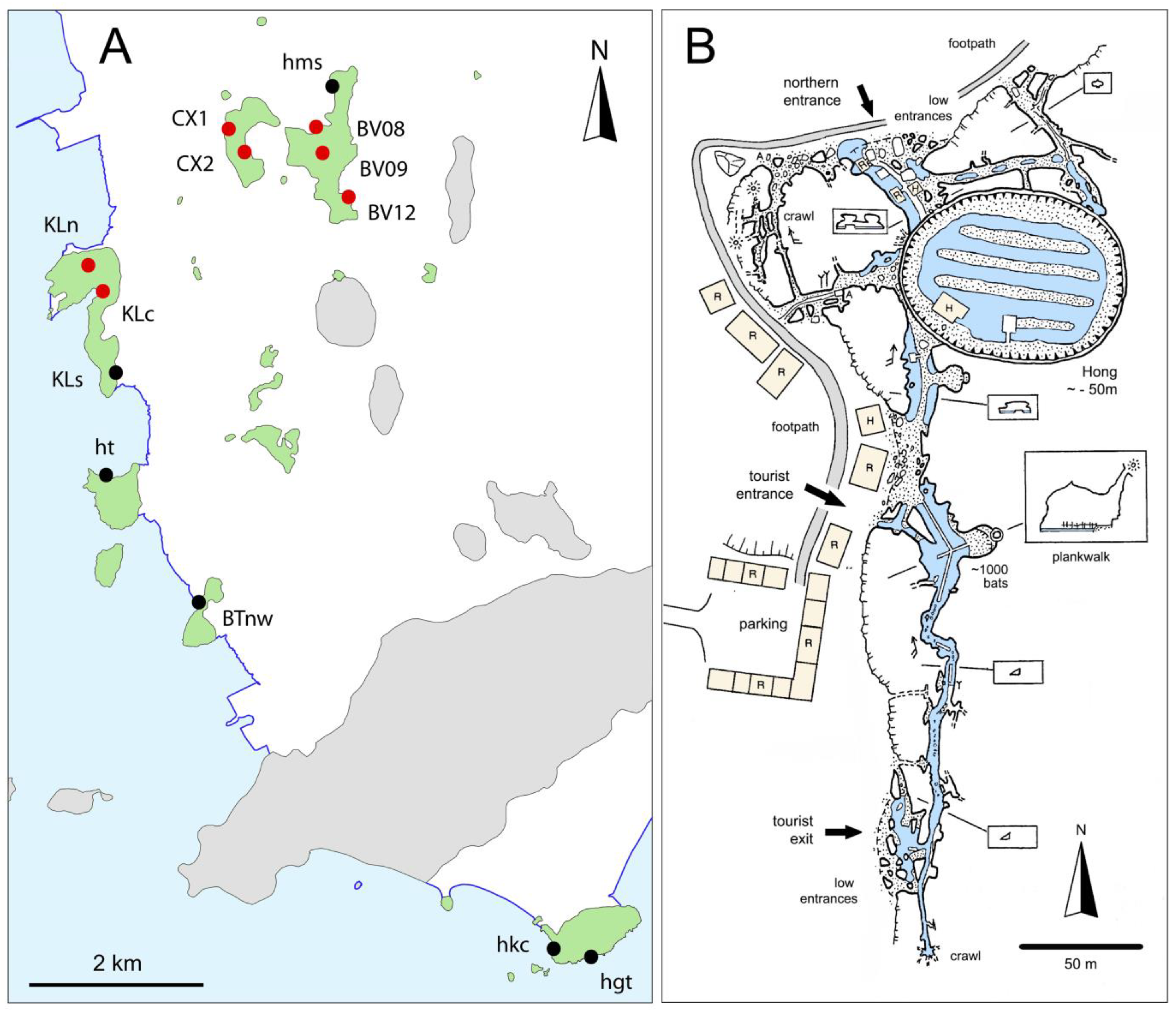
1.2. Description of Hang Mo So
1.3. History of Biological Studies
- (1)
- Cremnophyte and xerophilous shrub formations, dominated by Cycas clivicola subsp. lutea, Euphorbia antiquorum, and Dracaena cambodiana, which develop on patchy soil accumulated between exposed rocks and cliffs.
- (2)
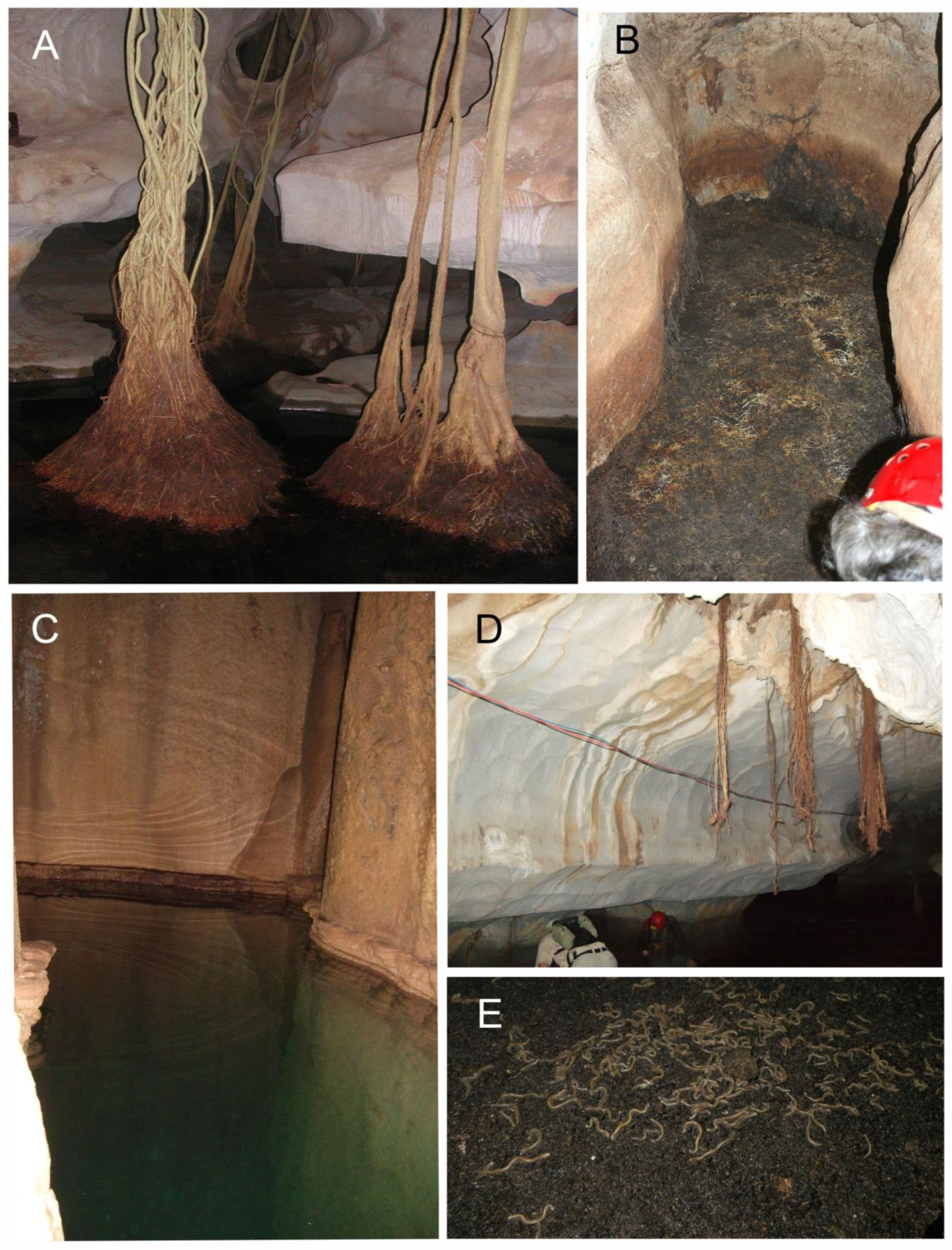
- Mesophilic tree formations, found in small patches on shallow soil, near the base of the hills and on low slopes. These may develop into a semi-deciduous forest dominated by Tetrameles nudiflora, Diospyros crumenata, Sterculia foetida, and Ficus spp. The most significant example was on Nui Com near Kien Luong, but the hill was totally erased by quarrying shortly after the publication of Le Cong Kiet’s study. Roots of Ficus spp. host rich subterranean invertebrate communities in Hang Mo So and in several other caves of MDL-HC.
1.4. Threats and Focus
2. Materials and Methods
2.1. Assessment of the Ecological Status of Species
- (1)
- Morphological inference, based on the presence or absence of troglomorphic traits. This approach is straightforward but raises two caveats. Firstly, depigmentation and eye reduction are frequently considered troglomorphic, but they actually occur in most deep-soil species as well. Thus, a cave arthropod that has these two characteristics can only be qualified as troglomorphic if additional traits are present that have been established as cave-dependent [29], such as appendage elongation or larger body size. It is these additional traits that make the difference between troglomorphic species, which are almost always linked to subterranean life, and euedaphomorphic species, which dominate in the deep soil but are also present in caves. Secondly, troglomorphy is clearly linked to cave-restricted life, whereas euedaphomorphy can be linked to either cave or soil life. Since cave invertebrates are more often euedaphomorphic than troglomorphic, especially in lowland caves of the humid tropics like MDL-HC, morphological inference alone will not work for them.
- (2)
- Parallel-sampling inference, based on the occurrence of species outside subterranean habitats. Many species found in caves are described as troglobionts in the literature, even though they do not exhibit typical troglomorphic traits, or only show euedaphomorphic traits similar to those of many deep-soil species. The absence of a species outside caves can be a good indicator in such cases. This information is often available in the literature for well-investigated regions, but not for the tropics, where it is thus necessary to gather data both inside and outside caves in comparable microhabitats. Extensive parallel sampling in the MDL-HC karst, with 270 cave samples and 674 non-cave samples (including 322 in mineral soil), allows a reasonably reliable ecological status assessment. Because the strength of such inference depends on sampling effort and species frequency, it will be less reliable for species with low population densities or patchy distributions.
- (3)
- Taxonomic inference, based on the ecological status of related taxa. Certain groups are particularly prone to diversify in subterranean habitats, even though the underlying biological mechanisms are not well understood [30,31,32]. This may cast suspicion on the putative troglobiont status of a species when it belongs to a group that is otherwise not known for having cave-obligate species.
- (4)
- Barcoding inference, based on levels of genetic divergence between populations. Cryptic diversity poses problems for the recognition of species using morphological characters alone. Molecular barcoding often reveals lineages with identical morphologies that show divergence levels as high as those encountered between traditional morphospecies, as has been demonstrated for several Collembola [33]. Moreover, such cryptic lineages may differ in their degree of dependence on cave habitats [34]. Molecular sequencing can, therefore, provide greater accuracy in the delimitation and ecological characterization of species. Another advantage is that it can allow otherwise unidentifiable larval forms to be correlated with their adult stages, especially in insects.
2.2. Sampling
2.2.1. Sampled Habitats
2.2.2. Parallel Sampling and Techniques
- (1)
- Collection by sight (timed or not timed) using a fine brush or pooter in all visited caves
- (2)
- Bulk extraction of arthropods on Berlese funnels from litter and soil cores of standardized volumes, associated with larger unstandardized samples for rare species
- (3)
- Sieving litter and debris for arthropods and gastropods
- (4)
- Baiting and pitfall trapping for arthropods active on the ground—this being the only technique that produces significant numbers of invertebrates in oligotrophic cave habitats
- (5)
- Beating of hanging roots in caves
- (6)
- Mineral soil washing to collect deep-soil arthropods by flotation
- (7)
- Flotation of litter and debris for snails
3. Results
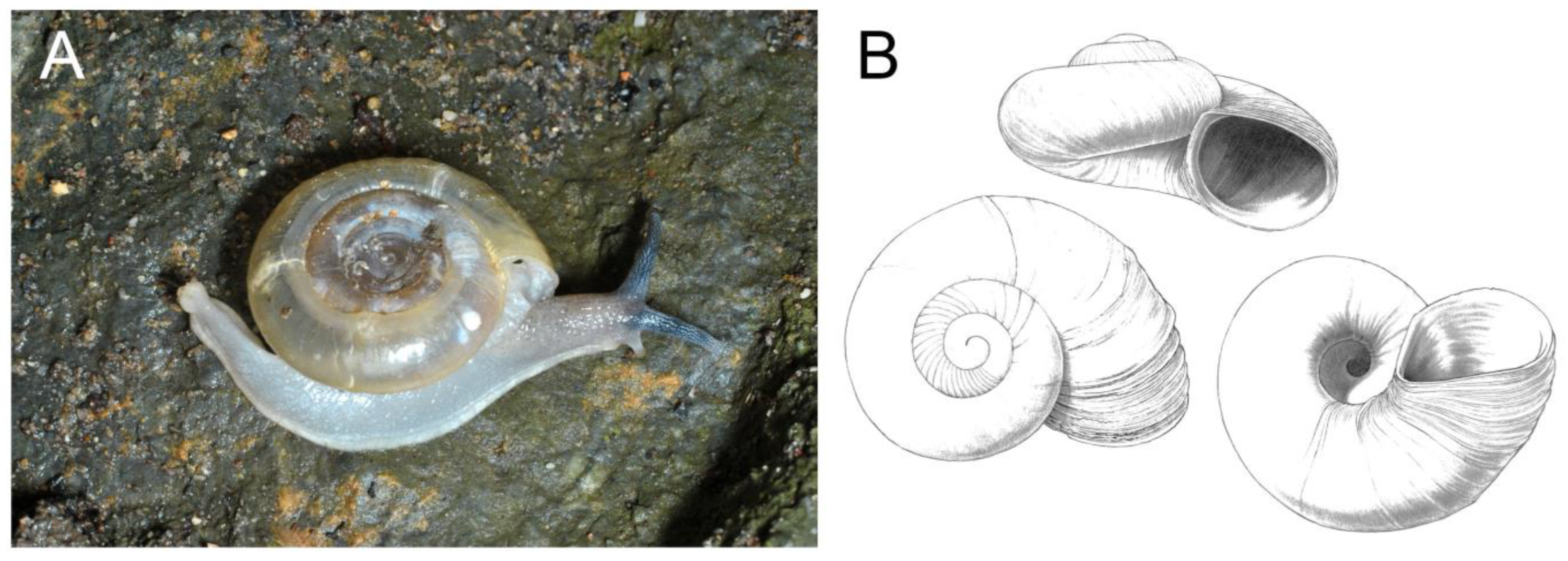
3.1. Gastropoda (Snails)
3.2. Arachnida
- Anactinotrichida (parasitiform mites)
- Actinotrichida (acariform mites)
- Amblypygi (whip spiders)
- Araneae (spiders)
- Opiliones (harvestmen)
- Palpigradi
- Pseudoscorpiones
- Schizomida
- Scorpiones
3.3. Diplopoda (Millipedes)
3.4. Malacostracea: Isopoda
- Oniscidea (woodlice) (Figure 7C–E)
- Asellota (Figure 7F)
3.5. Collembola (Springtails)
- Poduromorpha
- Entomobryomorpha (Figure 8A,B)
- Symphypleona
- Neelipleona (Figure 8C)
3.6. Diplura
3.7. Zygentoma
3.8. Pterygota
- Blattodea (cockroaches)
- Orthoptera (crickets)
- Heteroptera (Figure 8H)
- Homoptera (Figure 8D,E)
- Coleoptera (beetles) (Figure 8F,G)
- Lepidoptera (moths)
4. Discussion
4.1. Species Richness
4.2. Endemism
4.3. Gaps
4.4. Causes of Species Richness
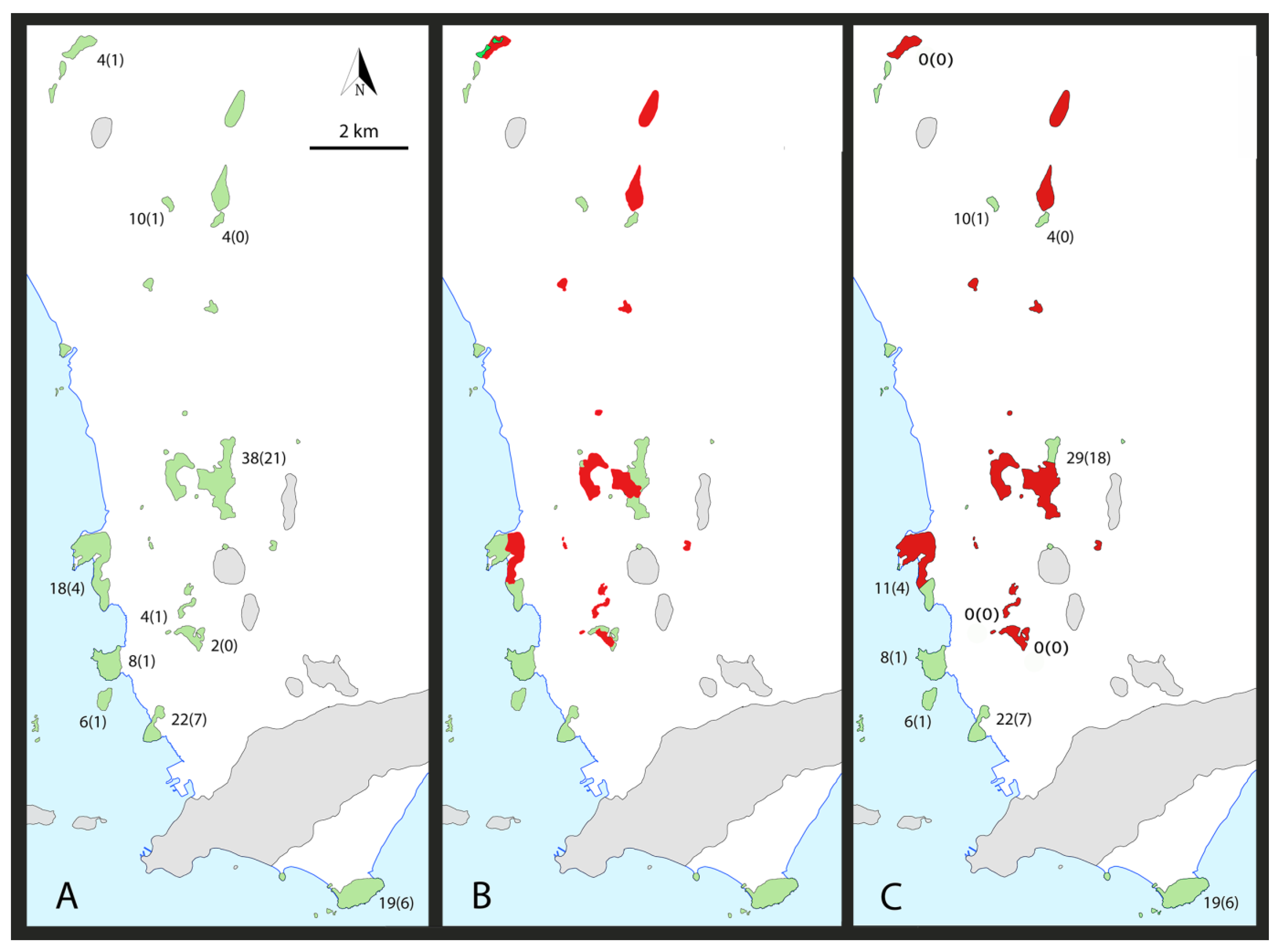
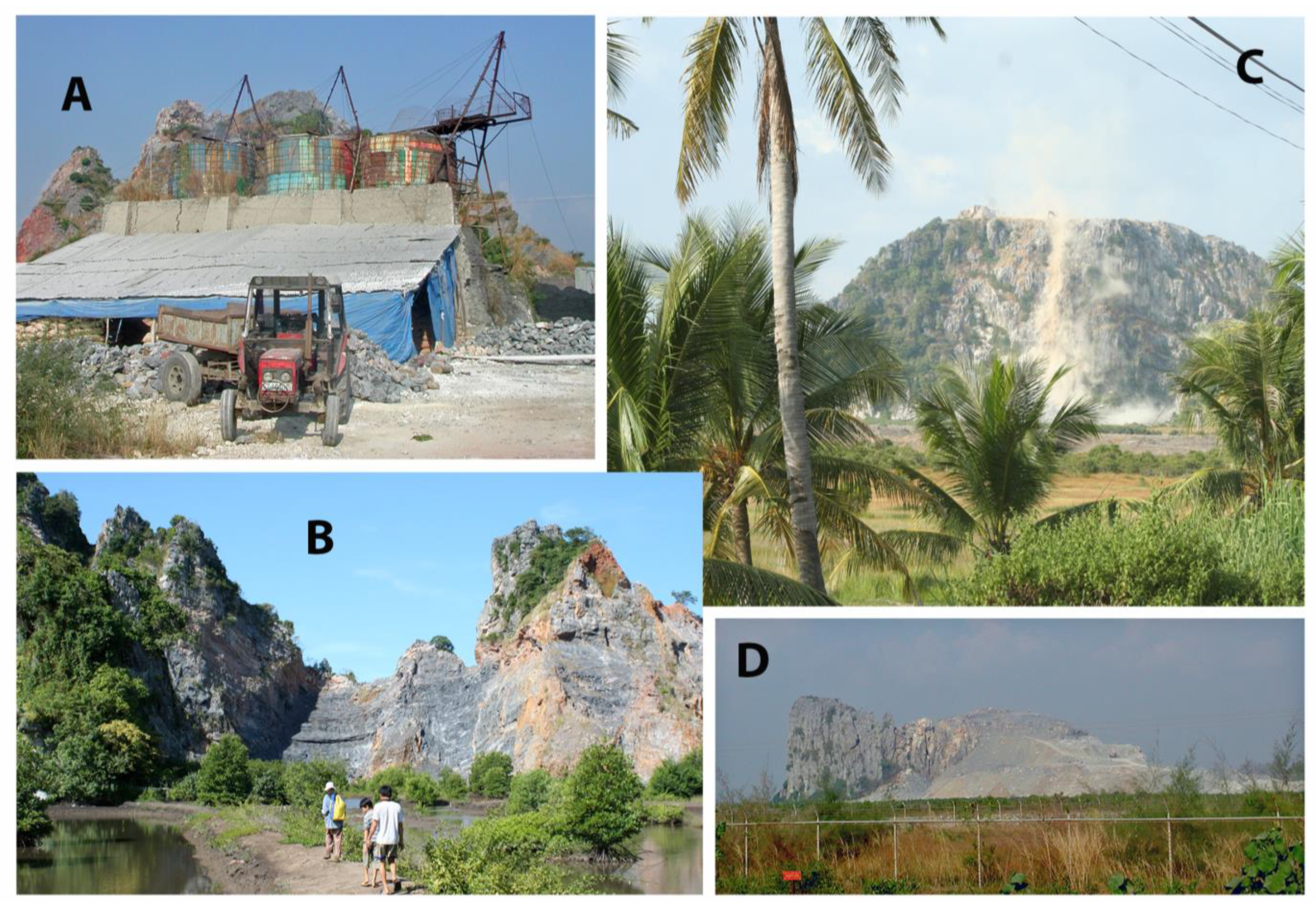
5. Conservation Issues
5.1. How Did We Get Here?
5.2. Species at Risk
5.3. Conservation Actions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Culver, D.C.; Deharveng, L.; Pipan, T.; Bedos, A. An Overview of Subterranean Biodiversity Hotspots. Diversity 2021, 13, 487. [Google Scholar] [CrossRef]
- Niemiller, M.L.; Helf, K.; Toomey, R.S. Mammoth Cave: A Hotspot of Subterranean Biodiversity in the United States. Diversity 2021, 13, 373. [Google Scholar] [CrossRef]
- Zagmajster, M.; Polak, S.; Fišer, C. Postojna-Planina Cave System in Slovenia, a Hotspot of Subterranean Biodiversity and a Cradle of Speleobiology. Diversity 2021, 13, 271. [Google Scholar] [CrossRef]
- Camacho, A.I.; Puch, C. Ojo Guareña: A Hotspot of Subterranean Biodiversity in Spain. Diversity 2021, 13, 199. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, M.; Luo, X.; Bedos, A.; Wang, Y.; Chocat, M.; Tian, M.; Liu, W. Feihu Dong, a New Hotspot Cave of Subterranean Biodiversity from China. Diversity 2023, 15, 902. [Google Scholar] [CrossRef]
- Deharveng, L.; Rahmadi, C.; Suhardjono, Y.R.; Bedos, A. The Towakkalak System, a Hotspot of Subterranean Biodiversity in Sulawesi, Indonesia. Diversity 2021, 13, 392. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Berbert-Born, M.; Souza-Silva, M. The Água Clara Cave System in Northeastern Brazil: The Richest Hotspot of Subterranean Biodiversity in South America. Diversity 2023, 15, 761. [Google Scholar] [CrossRef]
- Laumanns, M. Karsts and Caves of South Vietnam, Part 1: Provinces of Kien Giang, An Giang and Da Nang. Berl. Höhlenkundliche Berichte 2011, 43, 1–73. [Google Scholar]
- Denneborg, M.; Laumanns, M.; Schnadwinkel, M.; Vogt, S. German Speleological Campaign, Cambodia 95/96. Berl. Höhlenkundliche Berichte 2002, 6, 1–92. [Google Scholar]
- Kiernan, K. Human impacts on geodiversity and associated natural values of bedrock hills in the Mekong Delta. Geoheritage 2010, 2, 101–122. [Google Scholar] [CrossRef]
- Pirazzoli, P.A. Marine notches. In Sea-Level Research: A Manual for the Collection and Evaluation of Data; van de Plassche, O., Ed.; Geo Books: Norwich, UK, 1986; pp. 361–400. [Google Scholar]
- Salomon, J. Les influences climatiques sur la géomorphologie karstique: Exemple des milieux tropicaux et arides. Quaternaire 1997, 8, 107–117. [Google Scholar] [CrossRef]
- Deharveng, L.; Truong, Q.T.; Duong, T.D. Explorations au centre et au sud du Vietnam. Spelunca 1995, 59, 8–10. [Google Scholar]
- Le, C.K. La végétation des collines calcaires de la région de Kien-Luong—Ha Tien. Nien-San 1970, 3, 121–200. [Google Scholar]
- Le, C.K. La végétation des collines calcaires de la région de Kien-Luong—Ha Tien (suite). Nien-San 1974, 4, 11–90. [Google Scholar]
- Truong, Q.T.; Kiew, R.; Vermeulen, J.J. Begonia bataiensis Kiew, a new species in section Leprosae (Begoniaceae) from Vietnam. Gard. Bull. Singap. 2005, 57, 119–123. [Google Scholar]
- Middleton, D.J.; Ly, N.S. A new species of Ornithoboea (Gesneriaceae) from Vietnam. Edinb. J. Bot. 2008, 65, 353–357. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Luu, H.T.; Nguyen, Q.D.; Hetterscheid, W.L.A. Amorphophallus kienluongensis (Araceae), a new species from the Mekong Delta, Southern Vietnam. Blumea 2016, 61, 1–3. [Google Scholar] [CrossRef]
- Deharveng, L.; Le, C.K.; Bedos, A. Vietnam. In Encyclopaedia Biospeologica Tome III; Juberthie, C., Decu, V., Eds.; Société Internationale de Biospéologie: Moulis, France; Bucarest, Romania, 2001; pp. 2027–2037. [Google Scholar]
- Boutin, C. Cambodge. In Encyclopaedia Biospeologica Tome III; Juberthie, C., Decu, V., Eds.; Société Internationale de Biospéologie: Moulis, France; Bucarest, Romania, 2001; pp. 1755–1762. [Google Scholar]
- Deharveng, L.; Bedos, A.; Le, C.K.; Le, C.M.; Truong, Q.T. Endemic arthropods of the Hon Chong hills (Kien Giang), an unrivaled biodiversity heritage in Southeast Asia. In Beleaguered Hills: Managing the Biodiversity of the Remaining Karst Hills of Kien Giang, Vietnam; Le, C.K., Truong, Q.T., Ly, N.S., Eds.; Nha Xuat Ban Nong Nghiep: Ho Chi Minh, Vietnam, 2009; pp. 31–57. [Google Scholar]
- Steiner, H. Karsts and Caves of South Vietnam, Part 1: Provinces of Kien Giang, An Giang and Da Nang. Biospeleology of southern Vietnam. Berl. Höhlenkundliche Berichte 2011, 43, 53–73. [Google Scholar]
- Deharveng, L.; Le, C.K.; Le, C.M.; Bedos, A. Hot issues in karst conservation: The biodiversity of Hon Chong hills (southern Vietnam), with emphasis on invertebrate endemism. In Trans-KARST 2004, Proceedings of the International Transdisciplinary Conference on Development and Conservation of Karst Regions, Hanoi, Vietnam, 13–18 September 2004; Batelaan, O., Dusar, M., Masschelein, J., Vu, T.T., Tran, T.V., Nguyen, X.K., Eds.; Research Institute of Geology and Mineral Resources: Hanoi, Vietnam, 2005; p. 40. [Google Scholar]
- Humphreys, W.F. Community extinction: The groundwater (stygo-) fauna of Curaçao, Netherlands Antilles. Hydrobiologia 2022, 849, 4605–4611. [Google Scholar] [CrossRef]
- Ng, P.K.L.; Kottelat, M.; Deharveng, L. Tony Whitten and biodiversity: Personal recollections. Raffles Bull. Zool. 2020, 35, 11–13. [Google Scholar]
- Ives, M. The Big Story. Threatened Vietnam Cave Bugs Draw Little Sympathy; Associated Press: New York, NY, USA, 2012. [Google Scholar]
- Santos, A.J.; Ferreira, R.L.; Buzatto, B.A. Two New Cave-Dwelling Species of the Short-Tailed Whipscorpion Genus Rowlandius (Arachnida: Schizomida: Hubbardiidae) from Northeastern Brazil, with Comments on Male Dimorphism. PLoS ONE 2013, 8, e63616. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, S.M.; Howarth, F.G. Undara lava cave fauna in tropical Queensland with an annotated list of Australian subterranean biodiversity hotspots. Diversity 2021, 13, 326. [Google Scholar] [CrossRef]
- Deharveng, L.; Bedos, A. Diversity of Terrestrial Invertebrates in Subterranean Habitats. In Cave Ecology, Ecological Studies 235; Moldovan, O.T., Kovác, L., Halse, S., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 107–172. [Google Scholar]
- Faille, A. Beetles. In Encyclopedia of Caves; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press: New York, NY, USA, 2019; pp. 102–108. [Google Scholar]
- Lukić, M. Collembola. In Encyclopedia of Caves; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press: New York, NY, USA, 2019; pp. 308–319. [Google Scholar]
- Fišer, C. Niphargus—A model system for evolution and ecology. In Encyclopedia of Caves; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press: New York, NY, USA, 2019; pp. 746–755. [Google Scholar]
- Porco, D.; Bedos, A.; Greenslade, P.; Janion, C.; Skarzynski, D.; Stevens, M.I.; Jansen van Vuuren, B.; Deharveng, L. Challenging species delimitation in Collembola: Cryptic diversity among common springtails unveiled by DNA barcoding. Invertebr. Syst. 2012, 26, 470–477. [Google Scholar] [CrossRef]
- Lukić, M.; Delic, T.; Pavlek, M.; Deharveng, L.; Zagmajster, M. Distribution pattern and radiation of the European subterranean genus Verhoeffiella (Collembola, Entomobryidae). Zool. Scr. 2020, 49, 86–100. [Google Scholar] [CrossRef]
- Jasinska, E.J.; Knott, B.; McComb, A.J. Root mats in ground water: A fauna-rich cave habitat. J. N. Am. Benthol. Soc. 1996, 15, 508–519. [Google Scholar] [CrossRef]
- Howarth, F.G. Ecology of cave Arthropods. Annu. Rev. Entomol. 1983, 28, 365–389. [Google Scholar] [CrossRef]
- Novak, T.; Perc, M.; Lipovšek, S.; Janžekovič, F. Duality of terrestrial subterranean fauna. Int. J. Speleol. 2012, 41, 181–188. [Google Scholar] [CrossRef]
- Oromí, P.; Socorro, S. Biodiversity in the Cueva del Viento lava tube system (Tenerife, Canary Islands). Diversity 2021, 13, 226. [Google Scholar] [CrossRef]
- Vandel, A. Biospéologie. La Biologie des Animaux Cavernicoles; Gauthier-Villars: Paris, France, 1964; pp. 1–619. [Google Scholar]
- Wynne, J.J.; Howarth, F.G.; Sommer, S.; Dickson, B.G. Fifty years of cave arthropod sampling: Techniques and best practices. Int. J. Speleol. 2019, 48, 33–48. [Google Scholar] [CrossRef]
- Vermeulen, J.J.; Phung, L.C.; Truong, Q.T. New species of terrestrial molluscs (Caenogastropoda, Pupinidae & Pulmonata, Vertiginidae) of the Hon Chong-Ha Tien limestone hills, Southern Vietnam. Basteria 2007, 71, 81–92. [Google Scholar]
- Vermeulen, J.J.; Luu, H.T.; Theary, K.; Anker, K. New species of land snails (Mollusca: Gastropoda: Caenogastropoda and Pulmonata) of the Mekong Delta limestone hills (Cambodia, Vietnam). Folia Malacol. 2019, 27, 7–41. [Google Scholar] [CrossRef]
- Vermeulen, J.; Whitten, T. Biodiversity and Cultural Property in the Management of Limestone Resources. Lessons from East Asia; Directions in Development Series; The World Bank: Washington, DC, UAS, 1999; pp. 1–120. [Google Scholar]
- Leclerc, P. Considérations paléogéographiques à propos de la découverte en Thaïlande d’Opilioacariens nouveaux (Acari-Notostigmata). C. R. Soc. Biogéogr. 1989, 65, 162–174. [Google Scholar]
- Beron, P. Comparative study of the invertebrate cave faunas of Southeast Asia and New Guinea. Hist. Nat. Bulg. 2015, 21, 169–210. [Google Scholar]
- Fage, L. Scorpions et pédipalpes de l’Indochine française. Ann. Soc. Entomol. Fr. 1946, 113, 71–81. [Google Scholar]
- Miranda, G.S.; Giupponi, A.P.; Prendini, L.; Scharff, N. Systematic revision of the pantropical whip spider family Charinidae Quintero, 1986 (Arachnida, Amblypygi). Eur. J. Taxon. 2021, 772, 1–409. [Google Scholar] [CrossRef]
- Nguyen, T.T.A.; Phung, T.H.L. A new species of the whip spider genus Weygoldtia (Arachnida: Amblypygi: Charinidae) from Con Dao National Park, Vietnam. Acad. J. Biol. 2022, 44, 67–76. [Google Scholar]
- Zhu, X.Y.; Wu, S.Y.; Liu, Y.J.; Reardon, C.R.; Roman-Palacios, C.; Li, Z.; He, Z.Q. A new species of whip spider, Weygoldtia hainanensis sp. nov., from Hainan, China (Arachnida: Amblypygi: Charinidae). Zootaxa 2021, 5082, 65–76. [Google Scholar] [CrossRef]
- Mammola, S.; Cardoso, P.; Ribera, C.; Pavlek, M.; Isaia, M. A synthesis on cave-dwelling spiders in Europe. J. Zool. Syst. Evol. Res. 2018, 56, 301–316. [Google Scholar] [CrossRef]
- Brignoli, P.M. New or interesting Anapidae (Arachnida, Araneae). Rev. Suisse Zool. 1981, 88, 109–134. [Google Scholar] [CrossRef]
- Decae, A.E. Three new species in the genus Latouchia Pocock, 1901 (Araneae, Mygalomorphae, Halonoproctidae) from Vietnam. Rev. Suisse Zool. 2019, 126, 275–289. [Google Scholar]
- Tong, Y.; Li, F.; Song, Y.; Chen, H.; Li, S. Thirty-two new species of the genus Speocera Berland, 1914 (Araneae: Ochyroceratidae) from China, Madagascar and Southeast Asia. Zool. Syst. 2019, 44, 1–75. [Google Scholar]
- Lehtinen, P.T. Spiders of the Oriental-Australian region III. Tetrablemmidae, with a world revision. Acta Zool. Fenn. 1981, 162, 1–151. [Google Scholar]
- Lin, Y.; Li, S. New cave-dwelling armored spiders (Araneae, Tetrablemmidae) from Southwest China. Zookeys 2014, 388, 35–67. [Google Scholar]
- Schwendinger, P.; Martens, J. A taxonomic revision of the family Oncopodidae V. Gnomulus from Vietnam and China, with the description of five new species (Opiliones, Laniatores). Rev. Suisse Zool. 2006, 113, 595–615. [Google Scholar] [CrossRef]
- Condé, B. Nouveaux palpigrades de Trieste, de Slovénie, de Malte, du Paraguay, de Thaïlande et de Bornéo. Rev. Suisse Zool. 1988, 95, 723–750. [Google Scholar] [CrossRef]
- Condé, B. Palpigrades cavernicoles et endogés de Thaïlande et des Célèbes (première note). Rev. Suisse Zool. 1992, 99, 655–672. [Google Scholar] [CrossRef]
- Condé, B. Palpigrades cavernicoles et endogés de Thaïlande et de Célèbes (2e note). Rev. Suisse Zool. 1994, 101, 233–263. [Google Scholar] [CrossRef]
- Judson, M.L.I. A new and endangered species of the pseudoscorpion genus Lagynochthonius from a cave in Vietnam, with notes on chelal morphology and the composition of the Tyrannochthoniini (Arachnida, Chelonethi, Chthoniidae). Zootaxa 2007, 1627, 53–68. [Google Scholar] [CrossRef]
- Judson, M.L.I. A new subfamily of Feaellidae (Arachnida, Chelonethi, Feaelloidea) from Southeast Asia. Zootaxa 2017, 4258, 1–33. [Google Scholar] [CrossRef]
- Harvey, M.S. Two new species of the pseudoscorpion genus Cybella (Pseudoscorpiones: Feaellidae) from Malaysian caves. Zool. Anz. 2018, 273, 124–132. [Google Scholar] [CrossRef]
- Harvey, M.S.; Berry, O.; Edward, K.L.; Humphreys, G. Molecular and morphological systematics of hypogean schizomids (Schizomida: Hubbardiidae) in semiarid Australia. Invertebr. Syst. 2008, 22, 167–194. [Google Scholar] [CrossRef]
- Lourenço, W.R.; Duhem, B. Buthid scorpions found in caves; a new species of Isometrus Ehrenberg, 1828 (Scorpiones, Buthidae) from southern Vietnam. C. R. Biol. 2010, 333, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, W.R.; Leguin, E.A. A new species of Isometrus Ehrenberg 1828 (Scorpiones: Buthidae) from Laos. Acta Arachnol. 2012, 61, 71–76. [Google Scholar]
- Golovatch, S.I. Cave Diplopoda of southern China with reference to millipede diversity in Southeast Asia. Zookeys 2015, 510, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Golovatch, S.I.; Liu, W.X. Diversity, distribution patterns, and fauno-genesis of the millipedes (Diplopoda) of mainland China. Zookeys 2020, 930, 153–198. [Google Scholar] [CrossRef]
- Likhitrakarn, N.; Golovatch, S.I.; Thach, P.; Chhuoy, S.; Ngor, P.B.; Srisonchai, R.; Sutcharit, C.; Panha, S. Two new species of the millipede genus Plusioglyphiulus Silvestri, 1923 from Cambodia (Diplopoda, Spirostreptida). Zookeys 2020, 938, 137–151. [Google Scholar] [CrossRef]
- Golovatch, S.I.; Geoffroy, J.J.; Mauriès, J.P.; VandenSpiegel, D. New or poorly-known species of the millipede genus Trachyjulus Peters, 1864 (Diplopoda: Spirostreptida: Cambalopsidae). Arthropoda Sel. 2012, 21, 103–129. [Google Scholar] [CrossRef]
- Mauriès, J.P.; Golovatch, S.I. Stemmiulus deharvengi sp.n., le premier Stemmiulida signalé en Indonésie (Diplopoda: Stemmiulida). Arthropoda Sel. 2006, 15, 91–98. [Google Scholar]
- Srisonchai, R.; Likhitrakarn, N.; Sutcharit, C.; Jeratthitikul, E.; Siriwut, W.; Thrach, P.; Chhuoy, S.; Ngor, P.B.; Panha, S. A new micropolydesmoid millipede of the genus Eutrichodesmus Silvestri, 1910 from Cambodia, with a key to species in mainland Southeast Asia (Diplopoda, Polydesmida, Haplodesmidae). Zookeys 2020, 996, 59–91. [Google Scholar] [CrossRef]
- Golovatch, S.I.; Geoffroy, J.J.; VandenSpiegel, D. Review of the millipede family Trichopolydesmidae in the Oriental realm (Diplopoda, Polydesmida), with descriptions of new genera and species. Zookeys 2014, 414, 19–65. [Google Scholar] [CrossRef]
- Schmalfuss, H. World catalog of terrestrial isopods (Isopoda: Oniscidea). Stuttg. Beitr. Naturk. 2003, (A) 654, 1–341. [Google Scholar]
- Deharveng, L.; Bedos, A. Rambutsinella, a new genus of Entomobryidae (Insecta: Collembola) from Southeast Asia. Raffles Bull. Zool. 1996, 44, 279–285. [Google Scholar]
- Deharveng, L. Fauna of Thai caves. II. New Entomobryoidea Collembola from Chiang Dao Cave, Thailand. Bish. Mus. Occas. Pap. 1990, 30, 279–287. [Google Scholar]
- Deharveng, L.; Bedos, A. The cave fauna of southeast Asia. Origin, evolution and ecology. In Ecosystems of the World 30. Subterranean Ecosystems; Wilkens, H., Culver, D.C., Humphreys, W.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 603–632. [Google Scholar]
- Deharveng, L.; Suhardjono, Y.R. Pseudosinella maros sp. n., a troglobitic Entomobryidae (Collembola) from Sulawesi Selatan, Indonesia. Rev. Suisse Zool. 2004, 111, 979–984. [Google Scholar] [CrossRef]
- Yoshii, R. Finding of Lepidosinella armata Handschin from East Java. In Proceedings of the 3rd International Seminar on Apterygota, Siena, Italy, 21–26 August 1989; Dallai, R., Ed.; 1989; pp. 89–91. [Google Scholar]
- Deharveng, L.; Bedos, A. Lepidonella lecongkieti n.sp., premier Collembole cavernicole du Vietnam (Collembola, Insecta). Bull. Soc. Entomol. Fr. 1995, 100, 21–24. [Google Scholar] [CrossRef]
- Deharveng, L.; Jantarit, S.; Bedos, A. Revisiting Lepidonella Yosii (Collembola: Paronellidae): Character overview, checklist of world species and reassessment of Pseudoparonella doveri Carpenter. Ann. Soc. Entomol. Fr. 2018, 54, 381–400. [Google Scholar] [CrossRef]
- Jantarit, S.; Satasook, C.; Deharveng, L. Cyphoderus (Cyphoderidae) as a major component of collembolan cave fauna in Thailand, with description of two new species. Zookeys 2014, 368, 1–21. [Google Scholar]
- Papáč, V.; Palacios-Vargas, J.G. A new genus of Neelidae (Collembola) from Mexican caves. Zookeys 2016, 569, 37–51. [Google Scholar]
- Schneider, C.; Deharveng, L. First record of the genus Spinaethorax Papác & Palacios Vargas, 2016 (Collembola, Neelipleona, Neelidae) in Asia, with a new species from a Vietnamese cave. Eur. J. Taxon. 2017, 363, 1–20. [Google Scholar]
- Schneider, C. Morphological review of the order Neelipleona (Collembola) through the redescription of the type species of Acanthoneelidus, Neelides and Neelus. Zootaxa 2017, 4308, 1–94. [Google Scholar] [CrossRef]
- Lucañas, C.C.; Bláha, M.; Rahmadi, C.; Patoka, J. The first Nocticola Bolivar 1892 (Blattodea: Nocticolidae) from New Guinea. Zootaxa 2021, 5082, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Moulds, T.; Anderson, J.; Anderson, R.; Nykiel, P. Preliminary Survey of Cave Fauna in the Gunung Mulu World Heritage Area, Sarawak, Malaysia. 2013, unpublished. [Google Scholar]
- Hoch, H.; Howarth, F.G. Multiple cave invasions by species of the planthopper genus Oliarus in Hawaii (Homoptera: Fulgoroidea: Cixiidae). Zool. J. Linn. Soc. 1999, 127, 453–475. [Google Scholar] [CrossRef]
- Hoch, H.; Mühlethaler, R.; Wachmann, E.; Stelbrink, B.; Wessel, A. Celebenna thomarosa gen. n., sp. n. (Hemiptera, Fulgoromorpha, Cixiidae, Bennini) from Indonesia: Sulawesi with notes on its ecology and behaviour. Dtsch. Entomol. Z. 2011, 58, 241–250. [Google Scholar] [CrossRef]
- Casale, A.; Taglianti, A.V.; Juberthie, C.; Decu, V. Coleoptera Carabidae. In Encyclopaedia Biospeologica Tome II; Juberthie, C., Decu, V., Eds.; Société Internationale de Biospéologie: Moulis, France; Bucarest, Romania, 1998; pp. 1047–1081. [Google Scholar]
- Deuve, T. Description d’un Coléoptère troglobie du genre Eustra, découvert dans un karst du Vietnam méridional. Rev. Fr. Entomol. 1996, 18, 23–26. [Google Scholar]
- Ferrer, J. Description d’un nouveau genre de Stenosini troglobie du Vietnam (Coleoptera, Tenebrionidae). Nouv. Rev. Entomol. 2004, 20, 367–371. [Google Scholar]
- Kaszab, Z. Eine neue höhlenbewohnende Dichillus-Art aus Thailand (Coleoptera: Tenebrionidae). Ann. Nat. Hist. Mus. Wien 1980, 83, 585–588. [Google Scholar]
- Robinson, G.S. Cave-dwelling Tineid moths: A taxonomic review of the world species (Lepidoptera: Tineidae). Trans. Br. Cave Res. Assoc. 1980, 7, 83–120. [Google Scholar]
- Howarth, F.G.; James, S.A.; Preston, D.J.; Imada, C.T. Identification of roots in lava tube caves using molecular techniques: Implications for conservation of cave arthropod faunas. J. Insect Conserv. 2007, 11, 251–261. [Google Scholar] [CrossRef]
- Trajano, E.; Bichuette, M.E. Diversity of Brazilian subterranean invertebrates, with a list of troglomorphic taxa. Subterr. Biol. 2010, 7, 1–16. [Google Scholar]
- Fernandez, N.; Theron, P.; Rollard, C.; Castillo, E.R. Oribatid mites from deep soils of Hòn Chông limestone hills, Vietnam: The family Lohmanniidae (Acari: Oribatida), with the descriptions of Bedoslohmannia anneae n. gen., n. sp., and Paulianacarus vietnamese n. sp. Zoosystema 2014, 36, 771–787. [Google Scholar] [CrossRef]
- Meregalli, M.; Osella, G. Studies on Oriental Molytinae. IV. Anonyxmolytes lilliput new genus and new species from Vietnam (Coleoptera, Curculionidae). Ital. J. Zool. 2007, 74, 381–388. [Google Scholar] [CrossRef]
- Jaloszynski, P. Subterranean Scydmaeninae of Southeast Asia (Coleoptera: Staphylinidae). Ann. Zool. 2017, 67, 713–723. [Google Scholar] [CrossRef]
- Mauriès, J.P.; Golovatch, S.I.; Geoffroy, J.J. Un nouveau genre et une nouvelle espèce de l’ordre Stemmiulida du Viet-Nam (Diplopoda). Arthropoda Sel. 2010, 19, 73–80. [Google Scholar] [CrossRef]
- Deharveng, L.; Bedos, A. Chapter 18—Diversity Patterns in the Tropics. In Encyclopedia of Caves; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 146–162. [Google Scholar]
- Moseley, M.; Lim, T.W.; Lim, T.T. Fauna reported from Batu caves, Selangor, Malaysia: Annotated checklist and bibliography. Cave Karst Sci. 2012, 39, 77–92. [Google Scholar]
- Chapman, P. Invertebrates in Niah Great Cave, Batu Niah National Park, Sarawak. Cave Sci. 1984, 11, 89–91. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Isaac, N.J.B.; Pocock, M.J.O. Bias and information in biological records. Biol. J. Linn. Soc. 2015, 115, 522–531. [Google Scholar] [CrossRef]
- Li, F.; Li, S. Paleocene–Eocene and Plio–Pleistocene sea-level changes as “species T pumps” in Southeast Asia: Evidence from Althepus spiders. Mol. Phylogenet. Evol. 2018, 127, 545–555. [Google Scholar] [CrossRef]
- Voris, H.K. Maps of Pleistocene sea levels in Southeast Asia: Shorelines, river systems and time durations. J. Biogeogr. 2001, 27, 1153–1167. [Google Scholar] [CrossRef]
- Tamura, T.; Saito, Y.; Sieng, S.; Ben, B.; Kong, M.; Sim, I.; Choup, S.; Akiba, F. Initiation of the Mekong River delta at 8 ka: Evidence from the sedimentary succession in the Cambodian lowland. Quat. Sci. Rev. 2009, 28, 327–344. [Google Scholar] [CrossRef]
- Liu, J.P.; DeMaster, D.J.; Nguyen, T.T.; Saito, Y.; Nguyen, V.L.; Ta, T.K.O.; Li, X. Stratigraphic formation of the Mekong River Delta and its recent shoreline changes. Oceanography 2017, 30, 72–83. [Google Scholar] [CrossRef]
- Mylroie, J.; Tronvig, K. Karst Waters Institute creates Top Ten List of endangered karst ecosystem. NSS News 1998, 56, 41–43. [Google Scholar]
- IFC. Cement Manufacturing in a Critical Ecosystem. A Guide to Biodiversity for the Private Sector; Holcim Vietnam Ltd.: Ho Chi Minh City, Vietnam, 2005; pp. 1–3. [Google Scholar]
- Sinclair Knight Merz (Australia). Limestone Biodiversity Study, Hon Chong (EIA Report); Sinclair Knight Merz (Australia): St. Leonards, Australia, 2002; (Unpublished report). [Google Scholar]
- Le, C.K.; Truong, Q.T.; Ly, N.S. (Eds.) Beleaguered Hills: Managing the Biodiversity of the Remaining Karst Hills of Kien Giang, Vietnam, Proceedings of the International Workshop on Biodiversity of Kien Giang Karst, Rach Gia, Vietnam, 5–6 June 2008; Nha Xuat Ban Nong Nghiep: Ho Chi Minh, Vietnam, 2009; pp. 1–199. [Google Scholar]
- Lang, C. Vietnam: Unique biodiversity threatened by World Bank-funded cement plant. World Rainfor. Mov. Bull. 2003, 71, 1–3. [Google Scholar]




| Taxon | Species | Ecol | RL | End | HMS | NBV | MDL-HC |
|---|---|---|---|---|---|---|---|
| Gastropoda: Pomatiopsidae | |||||||
| Pseudoiglica sp. | STB | x | 0 | HC (2) | |||
| Actinotrichida: Leeuwenhoekiidae | |||||||
| gen. sp. | TB | 1 | bv00 (1), bv10 (1), bv12 (2) | BV (4), HT (1), KL (1) | |||
| Anactinotrichida: Opilioacaridae | |||||||
| Siamacarus sp. | TB | 0 | bv12 (1) | BV (1), HC (2), KL (2), LC (1), NA (1) | |||
| Amblypygi: Charinidae | |||||||
| Weygoldtia sp. | TB? | 3 | bv00 (3), bv12 (2), bv13 (1) | BT (1), BV (6), HC (8), KL (1), LC (1), NA (1) | |||
| Araneae: Ctenidae | |||||||
| gen.sp. 1 | TB | x | 0 | KL (1) | |||
| gen. sp. 2 | TB | 5 | bv00 (5), bv08 (1), bv11 (1), bv12 (1), bv13 (1) | BT (1), BV (9), CH (1), HC (1), HT (3), KL (3), NA (1), NO (1) | |||
| Araneae: Halonoproctidae | |||||||
| Latouchia schwendingeri Decae, 2019 | TB | + | 0 | HT (1) | |||
| Araneae: Ochyroceratidae | |||||||
| gen. sp. 1 | TB? | x | 0 | BT (1) | |||
| gen. sp. 2 | TB | 0 | BT (1), HC (3) | ||||
| gen. sp. 3 | TB? | 0 | bv12 (2) | BT (2), BV (2), KL (2), NO (1) | |||
| Araneae: Oonopidae | |||||||
| gen. sp. 1 | TB? | x | 0 | bv12 (1) | BV (1) | ||
| gen. sp. 2 | TB | 3 | bv00 (3), bv08 (1) | BV (4), KL (1) | |||
| gen. sp. 3 | TB? | 0 | bv13 (1) | BT (1), BV (1) | |||
| Araneae: Pholcidae | |||||||
| gen. sp. 1 | TB? | x | 0 | NA (1) | |||
| gen. sp. 2 | TB | 0 | bv13 (1) | BT (2), BV (1), HC (1), LC (1), NA (1) | |||
| gen. sp. 3 | TB? | 0 | HC (1), NA (1) | ||||
| gen. sp. 4 | TB? | 1 | bv00 (1), bv08 (1) | BV (2), HC (1), KL (1) | |||
| Araneae: Telemidae | |||||||
| gen. sp. 1 | TB? | x | 0 | HC (1) | |||
| gen. sp. 2 | TB? | x | 1 | bv00 (1) | BV (1) | ||
| gen. sp. 3 | TB? | x | 0 | bv13 (1) | BV (1) | ||
| gen. sp. 4 | TB | x | 0 | KL (2) | |||
| gen. sp. 5 | TB? | x | 0 | bv13 (1) | BV (1) | ||
| Araneae: Tetrablemmidae | |||||||
| gen. sp. | TB? | x | 1 | bv00 (1) | BV (1) | ||
| Opiliones: Epedanidae | |||||||
| gen. sp. 1 | TB | 4 | bv00 (4), bv09 (1) | BT (1), BV (5) | |||
| gen. sp. 2 | TB | x | 0 | HC (1) | |||
| Opiliones: family undet. | |||||||
| gen. sp. | TB? | 0 | BT (1) | ||||
| Pseudoscorpiones: Chthoniidae | |||||||
| Lagynochthonius fragilis Judson, 2007 | TB | x | 5 | bv00 (5) | BV (5) | ||
| Diplopoda: Haplodesmidae | |||||||
| gen. sp. | TB? | x | 1 | bv00 (1) | BV (1) | ||
| Diplopoda: Pyrgodesmidae | |||||||
| gen. sp. | TB | 0 | HC (1), HT (1) | ||||
| Diplopoda: Trichopolydesmidae | |||||||
| gen. sp. | TB? | 0 | HT (1) | ||||
| Diplopoda: Siphonophoridae | x | ||||||
| gen. sp. | TB? | x | 1 | bv00 (1) | BV (1) | ||
| Diplopoda: Cambalopsidae | |||||||
| Glyphiulus sp. | TB? | 0 | BT (3), NC (1) | ||||
| Trachyjulus singularis Attems, 1938 | TB? | 14 | bv00 (14), bv01 (3), bv05 (2), bv08 (3), bv09 (1), bv10 (1), bv11 (1), bv12 (3), bv13 (1) | BV (29), CH (2), HT (4), KL (6), LC (3), NA (3) | |||
| Diplopoda: Stemmiulidae | |||||||
| Eostemmiulus caecus Mauriès, Golovatch & Geoffroy, 2010 | TB | CR | x | 1 | bv00 (1) | BV (1) | |
| Amphipoda: Bogidiellidae | |||||||
| gen. sp. | STB | x | 0 | BT (1) | |||
| Isopoda Oniscidea: family undet. | |||||||
| gen. sp. | TB? | x | 0 | BT (1) | |||
| Isopoda Oniscidea: Armadillidae | |||||||
| Sumatrillo sp. | TB | VU | 0 | HC (1), HT (2) | |||
| Isopoda Oniscidea: Philosciidae | |||||||
| Burmoniscus sp. | TB | EN | x | 3 | bv00 (3), bv12 (2) | BV (5) | |
| gen. sp. | TB? | x | 0 | LC (1) | |||
| Isopoda Asellota: Stenasellidae | |||||||
| Stenasellus sp. | STB | x | 0 | HC (1) | |||
| Collembola: Hypogastruridae | |||||||
| Acherontiella sp. | TB? | x | 0 | NO (1) | |||
| Ceratophysella sp. | TB | CR | x | 0 | HC (1) | ||
| Collembola: Tullbergiidae | |||||||
| gen. sp. | TB | 0 | BT (1), HT (1), LC (1), NC (1) | ||||
| Collembola: Entomobryidae | |||||||
| Acrocyrtus (cf.) sp. | TB | VU | x | 0 | KL (1) | ||
| Ascocyrtus sp. | TB? | x | 1 | bv00 (1) | BV (1) | ||
| Coecobrya sp. | TB | x | 1 | bv00 (1), bv11 (1) | BV (2) | ||
| Lepidocyrtinae gen. sp. | TB | 0 | BT (1), LC (1) | ||||
| Lepidosinella sp. | TB | 2 | bv00 (2) | BV (2), KL (1) | |||
| Collembola: Isotomidae | |||||||
| Folsomides anops Deharveng, Bedos & Lukić, 2020 | TB | VU | x | 0 | BT (2) | ||
| Folsomides whitteni Deharveng, Bedos & Lukić, 2020 | TB | x | 2 | bv00 (2), bv08 (3) | BV (5) | ||
| Collembola: Paronellidae | |||||||
| Lepidonella lecongkieti Deharveng & & Bedos, 1995 | TB | NT | 7 | bv00 (7), bv01 (2), bv08 (3), bv12 (2), bv13 (3) | BT (3), BV (17), CH (1), HC (6), HT (8), KL (9), NA (1), NO (2) | ||
| Lepidonella sp. | TB? | x | 1 | bv00 (1) | BV (1) | ||
| Collembola: Neelidae | |||||||
| Spinaethorax adamantis Schneider & Deharveng 2017 | TB | x | 0 | HC (4) | |||
| Spinaethorax sp. 1 | TB | x | 0 | bv01 (1) | BV (1) | ||
| Spinaethorax sp. 2 | TB? | x | 0 | NC (1) | |||
| Diplura: Japygidae | |||||||
| gen. sp. | TB? | x | 0 | bv01 (1), bv12 (1) | BV (2) | ||
| Zygentoma: Family ind. | |||||||
| gen. sp. | TB | 0 | bv13 (1) | BV (1), HC (1) | |||
| Zygentoma: Nicoletiidae | |||||||
| gen. sp. 1 | TB? | x | 0 | BT (1) | |||
| gen. sp. 2 | TB | x | 1 | bv00 (1) | BV (1) | ||
| Blattodea: Nocticolidae | |||||||
| Spelaeoblatta sp. | TB | 4 | bv00 (4), bv01 (1), bv08 (1), bv12 (1), bv13 (1) | BV (8), CH (1), KL (3), NA (1) | |||
| Coleoptera: Carabidae | |||||||
| Eustra honchongensis Deuve, 1996 | TB | EN | x | 4 | bv00 (4), bv01 (1), bv12 (1), bv13 (1) | BV (7) | |
| Coleoptera: Curculionidae | |||||||
| gen. sp. | TB | 0 | BT (1), KL (1) | ||||
| Coleoptera: Tenebrionidae | |||||||
| Harvengia vietnamita Ferrer, 2004 | TB | EN | 0 | BT (2), CO (1), HC (5), KL (1) | |||
| Pseudochillus honchongensis Schawaller & Faille, 2023 | TB | x | 1 | bv00 (1) | BV (1) | ||
| Heteroptera: Reduviidae: Harpactorinae | |||||||
| gen. sp. | TB? | 0 | bv12 (1) | BV (1), HC (1), NA (1), NC (1) | |||
| Heteroptera: Schizopteridae | |||||||
| gen. sp. | TB? | x | 0 | BT (1) | |||
| Homoptera: Cixiidae | |||||||
| gen. sp. 1 | TB | 2 | bv00 (2), bv07 (1), bv08 (1), bv11 (1), bv12 (1), bv13 (2) | BT (1), BV (8), CO (1), KL (2) | |||
| gen. sp. 2 | TB | x | 0 | KL (2) | |||
| Homoptera: Delphacidae | |||||||
| gen. sp. | TB | x | 1 | bv00 (1), bv08 (2) | BV (3) | ||
| Homoptera: Kinnaridae: Kinnarini | |||||||
| gen. sp. | TB? | x | 1 | bv00 (1) | BV (1) | ||
| Total | 43 | 27 | 38 | 70 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deharveng, L.; Le, C.K.; Bedos, A.; Judson, M.L.I.; Le, C.M.; Lukić, M.; Luu, H.T.; Ly, N.S.; Nguyen, T.Q.T.; Truong, Q.T.; et al. A Hotspot of Subterranean Biodiversity on the Brink: Mo So Cave and the Hon Chong Karst of Vietnam. Diversity 2023, 15, 1058. https://doi.org/10.3390/d15101058
Deharveng L, Le CK, Bedos A, Judson MLI, Le CM, Lukić M, Luu HT, Ly NS, Nguyen TQT, Truong QT, et al. A Hotspot of Subterranean Biodiversity on the Brink: Mo So Cave and the Hon Chong Karst of Vietnam. Diversity. 2023; 15(10):1058. https://doi.org/10.3390/d15101058
Chicago/Turabian StyleDeharveng, Louis, Cong Kiet Le, Anne Bedos, Mark L. I. Judson, Cong Man Le, Marko Lukić, Hong Truong Luu, Ngoc Sam Ly, Tran Quoc Trung Nguyen, Quang Tam Truong, and et al. 2023. "A Hotspot of Subterranean Biodiversity on the Brink: Mo So Cave and the Hon Chong Karst of Vietnam" Diversity 15, no. 10: 1058. https://doi.org/10.3390/d15101058
APA StyleDeharveng, L., Le, C. K., Bedos, A., Judson, M. L. I., Le, C. M., Lukić, M., Luu, H. T., Ly, N. S., Nguyen, T. Q. T., Truong, Q. T., & Vermeulen, J. (2023). A Hotspot of Subterranean Biodiversity on the Brink: Mo So Cave and the Hon Chong Karst of Vietnam. Diversity, 15(10), 1058. https://doi.org/10.3390/d15101058








