Predicting Suitable Habitats for China’s Endangered Plant Handeliodendron bodinieri (H. Lév.) Rehder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Establishing Species Occurrence Records
2.2. Selecting Environmental Variables
2.3. MaxEnt Modeling of Species Distribution
3. Results
3.1. Evaluating Model Performance
3.2. Key Environmental Factors and Validating Modeling Results
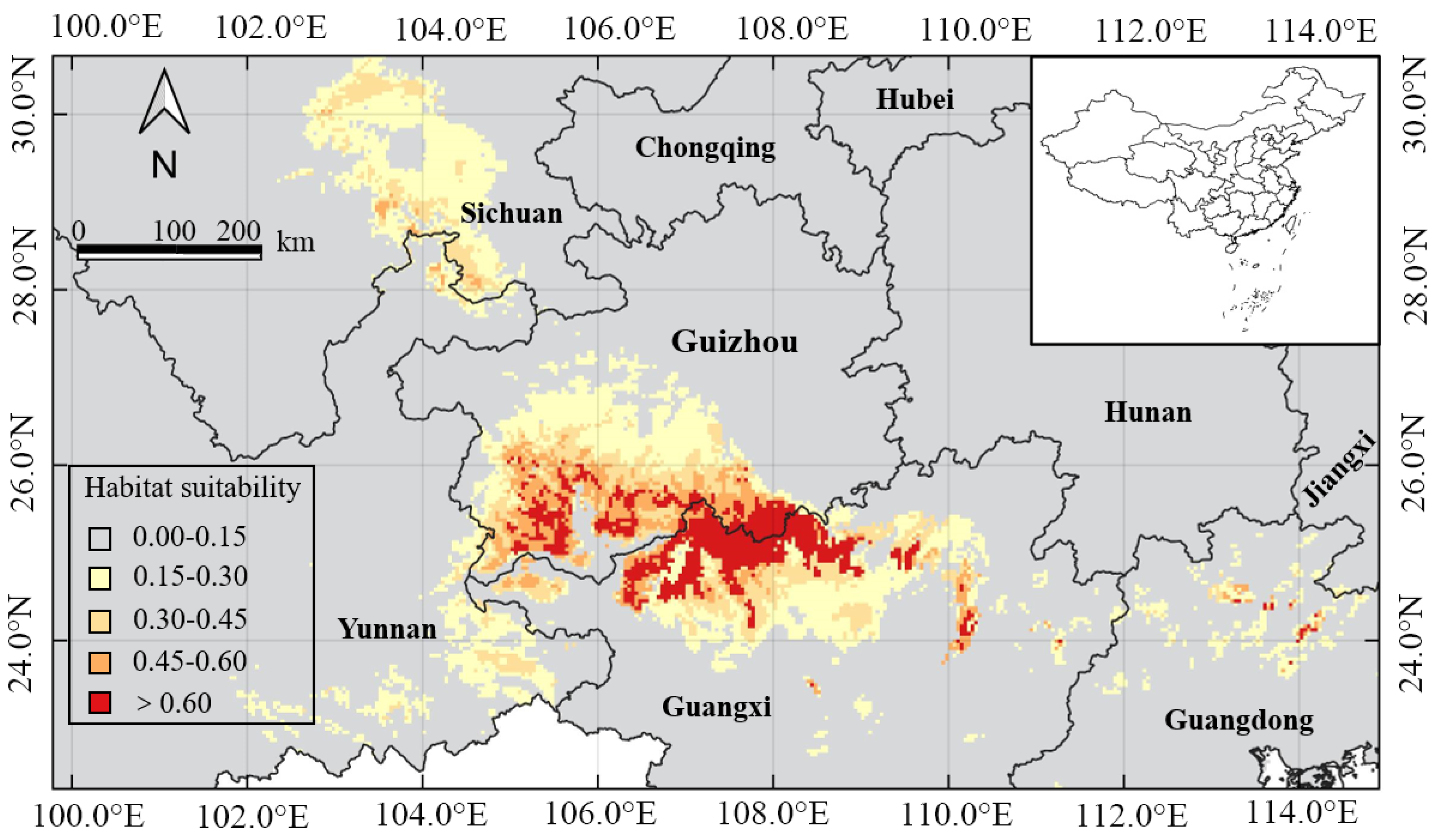
3.3. Predicting the Suitable Habitat of H. Bodinieri in China
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.P.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond predictions: Biodiversity conservation in a changing climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Asch, F.; Brueck, H.; Giese, M.; Dusserre, J.; Ramanantsoanirina, A. Phenological responses of upland rice grown along an altitudinal gradient. Environ. Exp. Bot. 2013, 89, 1–10. [Google Scholar] [CrossRef]
- Graham, E.M.; Reside, A.E.; Atkinson, I.; Baird, D.; Hodgson, L.; James, C.S.; VanDerWal, J.J. Climate change and biodiversity in Australia: A systematic modelling approach to nationwide species distributions. Australas. J. Environ. Manag. 2019, 26, 112–123. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Ibáñez, I.; Blumenthal, D.M.; Molinari, N.A.; Miller, L.P.; Grosholz, E.D.; Diez, J.M.; D’Antonio, C.M.; Olden, J.D.; Jones, S.J.; et al. Poised to prosper? A cross-system comparison of climate change effects on native and non-native species performance. Ecol. Lett. 2013, 16, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Gliottone, I.; Pham, M.P. Current and future predicting habitat suitability map of Cunninghamia konishii Hayata using MaxEnt model under climate change in Northern Vietnam. Eur. J. Ecol. 2021, 7, 1–17. [Google Scholar] [CrossRef]
- Blach-Overgaard, A.; Svenning, J.-C.; Dransfield, J.; Greve, M.; Balslev, H. Determinants of palm species distributions across Africa: The relative roles of climate, non-climatic environmental factors, and spatial constraints. Ecography 2010, 33, 380–391. [Google Scholar] [CrossRef]
- Ferrarini, A.; Dai, J.; Bai, Y.; Alatalo, J.M. Redefining the climate niche of plant species: A novel approach for realistic predictions of species distribution under climate change. Sci. Total Environ. 2019, 671, 1086–1093. [Google Scholar] [CrossRef]
- Liu, L.; Guan, L.; Zhao, H.; Huang, Y.; Mou, Q.; Liu, K.; Chen, T.; Wang, X.; Zhang, Y.; Wei, B.; et al. Modeling habitat suitability of Houttuynia cordata Thunb (Ceercao) using MaxEnt under climate change in China. Ecol. Inform. 2021, 63, 101324. [Google Scholar] [CrossRef]
- Selwood, K.E.; McGeoch, M.A.; Mac Nally, R. The effects of climate change and land-use change on demographic rates and population viability. Biol. Rev. 2015, 90, 837–853. [Google Scholar] [CrossRef]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Wasowicz, P.; Przedpelska-Wasowicz, E.M.; Kristinsson, H. Alien vascular plants in Iceland: Diversity, spatial patterns, temporal trends, and the impact of climate change. Flora Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 648–673. [Google Scholar] [CrossRef]
- Lippmann, R.; Babben, S.; Menger, A.; Delker, C.; Quint, M. Development of wild and cultivated plants under global warming conditions. Curr. Biol. 2019, 29, 1326–1338. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, G.F. Predicting the potential distribution of endangered Parrotia subaequalis in China. Forests 2022, 13, 1595. [Google Scholar] [CrossRef]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2019, 50, 68–75. [Google Scholar] [CrossRef]
- Kamyo, T.; Asanok, L. Modeling habitat suitability of Dipterocarpus alatus (Dipterocarpaceae) using MaxEnt along the Chao Phraya River in Central Thailand. For. Sci. Technol. 2020, 16, 1–7. [Google Scholar] [CrossRef]
- Soilhi, Z.; Sayari, N.; Benalouache, N.; Mekki, M. Predicting current and future distributions of Mentha pulegium L. in Tunisia under climate change conditions, using the MaxEnt model. Ecol. Inform. 2022, 68, 101533. [Google Scholar] [CrossRef]
- Tran, V.D.; Vu, T.T.; Tran, Q.B.; Nguyen, T.H.; Ta, T.N.; Ha, T.M.; Nguyen, H.V. Predicting suitable distribution for an endemic, rare and threatened species (Grey-shanked douc langur, Pygathrix cinerea Nadler, 1997) using MaxEnt model. Appl. Ecol. Environ. Res. 2018, 16, 1275–1291. [Google Scholar] [CrossRef]
- Bentlage, B.; Peterson, A.T.; Barve, N.; Cartwright, P. Plumbing the depths: Extending ecological niche modelling and species distribution modelling in three dimensions. Glob. Ecol. Biogeogr. 2013, 22, 952–961. [Google Scholar] [CrossRef]
- Mousazade, M.; Ghanbarian, G.; Pourghasemi, H.R.; Safaeian, R.; Cerdà, A. Maxent Data Mining Technique and its comparison with a bivariate statistical model for predicting the potential distribution of Astragalus Fasciculifolius Boiss. in Fars, Iran. Sustainability 2019, 11, 3452. [Google Scholar] [CrossRef]
- Çoban, H.O.; Örücü, Ö.K.; Arslan, E.S. MaxEnt modeling for predicting the current and future potential geographical distribution of Quercus libani Olivier. Sustainability 2020, 12, 2671. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. ORIGINAL ARTICLE: Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Anand, V.; Oinam, B.; Singh, I.H. Predicting the current and future potential spatial distribution of endangered Rucervus eldii eldii (Sangai) using MaxEnt model. Environ. Monit. Assess. 2021, 193, 147. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Cotrina-Sánchez, A.; Rojas Briceño, N.B.; Bandopadhyay, S.; Ghosh, S.; Torres Guzmán, C.; Oliva, M.; Guzman, B.K.; Salas López, R. Biogeographic distribution of Cedrela spp. genus in Peru using MaxEnt modeling: A conservation and restoration approach. Diversity 2021, 13, 261. [Google Scholar] [CrossRef]
- Dad, J.M.; Rashid, I. Differential responses of Kashmir Himalayan threatened medicinal plants to anticipated climate change. Environ. Conserv. 2022, 49, 33–41. [Google Scholar] [CrossRef]
- Cao, L.M.; Xia, N.H.; Deng, Y.F. Embryology of Handeliodendron bodinieri (Sapindaceae) and its systematic value: Development of male and female gametophytes. Plant Syst. Evol. 2008, 274, 17–23. [Google Scholar] [CrossRef]
- He, R.; Wang, J.; Huang, H. Long-distance gene dispersal inferred from spatial genetic structure in Handeliodendron bodinieri, an endangered tree from karst forest in southwest China. Biochem. Syst. Ecol. 2012, 44, 295–302. [Google Scholar] [CrossRef]
- Xiong, Z.; Ran, J.; Tan, C.; Yu, P.; Qin, H.; Wei, J. The seed ecological characteristics of endangered Handeliodendron bod inierei. Acta Ecol. Sin. 2003, 23, 820–825. [Google Scholar]
- Huang, S.; Luo, W. Biological behaviors and conservation of Handeliodendron Bodinieri. J. Ecol. Rural Environ. 2001, 17, 21–22. [Google Scholar] [CrossRef]
- Xie, C.; Li, X.; Guo, S.; Jiang, M.; Li, Z. Research trends of the rare and endangered plant Handeliodendron bodinierei. Mol. Plant Breed. 2020, 18, 1725–1730. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Puchałka, R.; Dyderski, M.K.; Vítková, M.; Sádlo, J.; Klisz, M.; Netsvetov, M.; Prokopuk, Y.; Matisons, R.; Mionskowski, M.; Wojda, T.; et al. Black locust (Robinia pseudoacacia L.) range contraction and expansion in Europe under changing climate. Glob. Chang. Biol. 2021, 27, 1587–1600. [Google Scholar] [CrossRef]
- Liu, D.W.; Xie, C.P.; Jim, C.Y.; Liu, Y.J.; Hou, S.L. Predicting the Potential Distribution of the alien invasive alligator gar Atractosteus spatula in China. Sustainability 2023, 15, 6419. [Google Scholar] [CrossRef]
- Xu, D.P.; Zhuo, Z.H.; Wang, R.L.; Ye, M.; Pu, B. Modeling the distribution of Zanthoxylum armatum in China with MaxEnt modeling. Glob. Ecol. Conserv. 2019, 19, e00691. [Google Scholar] [CrossRef]
- Xie, C.P.; Huang, B.Y.; Jim, C.Y.; Han, W.D.; Liu, D.W. Predicting differential habitat suitability of Rhodomyrtus tomentosa under current and future climate scenarios in China. For. Ecol. Manag. 2021, 501, 119696. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for MaxEnt ecological niche models. Methods Ecol. Evol. 2014, 11, 1198–1205. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Sarma, K.; Roy, S.J.; Kalita, B.; Baruah, P.S.; Bawri, A.; Nath, M.J.; Baruah, U.D.; Sahariah, D.; Saikia, A.; Tanti, B. Habitat suitability of Gymnocladus assamicus-A critically endangered plant of Arunachal Pradesh, India using machine learning and statistical modeling. Acta Ecol. Sin. 2022, 42, 398–406. [Google Scholar] [CrossRef]
- Beale, C.M.; Lennon, J.J. Incorporating uncertainty in predictive species distribution modelling. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, L.; Wu, G.; Yuan, L.; Meng, C.; Guo, H.; Zhou, Y.; Ma, C.; Gao, Y.; Xue, Y.; et al. Habitat suitability assessment of Panthera uncia in Qilian Mountains of Qinghai based on MaxEnt modeling. Acta Ecol. Sin. 2023, 43, 2202–2209. [Google Scholar] [CrossRef]
- Gao, B.; Wei, H.; Guo, Y.; Gu, W. Using GIS and MaxEnt to analyze the potential distribution of Abies chensiensis. Chin. J. Ecol. 2015, 34, 843–852. [Google Scholar] [CrossRef]
- Remya, K.; Ramachandran, A.; Jayakumar, S. Predicting the current and future suitable habitat distribution of Myristica dactyloides Gaertn. using MaxEnt model in the Eastern Ghats, India. Ecol. Eng. 2015, 82, 184–188. [Google Scholar] [CrossRef]
- Veloz, S.D.; Williams, J.W.; Blois, J.L.; He, F.; Otto-Bliesner, B.; Liu, Z. No-analog climates and shifting realized niches during the late quaternary: Implications for 21st-century predictions by species distribution models. Glob. Change Biol. 2012, 18, 1698–1713. [Google Scholar] [CrossRef]
- Wang, G.H.; Pan, Y.; Qin, G.L.; Tan, W.N.; Lu, C.H. Population structure and spatial distribution pattern of Kmeria septentrionalis an endangered species in karst habitat. For. Res. 2021, 34, 81–87. [Google Scholar] [CrossRef]
- Gao, J.W.; Xie, G.W.; Lin, Z.G.; He, J.L.; Wang, Y.L.; Qiu, C.M.; Huang, J.J. Population dynamics of rare and endangered plant Handeliodendron bodinieri (Levl.) Rehd in Leye County of Guangxi region. Guangdong Agric. Sci. 2015, 18, 43–48. [Google Scholar] [CrossRef]
- Lan, X.; Wang, J.; Fu, C.; Li, L.; Yuan, M.; Tan, T.; Du, F. Prediction of suitable distribution area of Magnolia sieboldii in China based on the optimized MaxEnt model. J. Northwest For. Univ. 2022, 37, 100–106. [Google Scholar] [CrossRef]
- Leng, X.H.; Xue, L.; Wang, J.; Li, S.; Yang, Z.L.; Ren, H.D.; Yao, X.H.; Wu, Z.Y.; Li, J.Y. Physiological Responses of Handeliodendron bodinieri(Levl.) Rehd. to Exogenous Calcium Supply under Drought Stress. Forests 2020, 11, 69. [Google Scholar] [CrossRef]
- Khanum, R.; Mumtaz, A.S.; Kumar, S. Predicting impacts of climate change on medicinal asclepiads of Pakistan using Maxent modeling. Acta Oecologica 2013, 49, 23–31. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, W.; Cheng, B.; Zhang, J. Potential Suitable Areas of Crataegus pinnatifida in China based on MaxEnt Modeling. Sci. Silvae Sin. 2022, 58, 43–50. [Google Scholar] [CrossRef]
- Khodorova, N.V.; Boitel-Conti, M. The Role of Temperature in the Growth and Flowering of Geophytes. Plants 2013, 2, 699–711. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Brennan, R.M.; Jones, H.G. Declining chilling and its impact on temperate perennial crops. Environ. Exp. Bot. 2013, 91, 48–62. [Google Scholar] [CrossRef]
- Rupa, D.; Saikat, B. Influence of abiotic stresses on seed production and quality. In Seed Biology Updates; Jimenez-Lopez, J.C., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Zhou, Y.R.; Lu, X.; Zhang, G.F. Potentially differential impacts on niche overlap between Chinese endangered Zelkova schneideriana and its associated tree species under climate change. Front. Ecol. Evol. 2023, 11, 1218149. [Google Scholar] [CrossRef]
- Yang, X.Q.; Kushwaha, S.P.S.; Saran, S.; Xu, J.C. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Z.N.; Li, Z.Y.; Liu, Y.; Fang, S.Z. Predictive modeling of suitable habitats for Cinnamomum camphora (L.) presl using maxent model under climate change in China. Int. J. Environ. Res. Public Health 2019, 16, 3185. [Google Scholar] [CrossRef]
- Wang, L.; Yilihamu, Y. Prediction of potential suitable areas for a Chinese endemic shrub Sophora davidii using the MaxEnt Model. Chin. J. Ecol. 2021, 40, 3114–3124. [Google Scholar] [CrossRef]
- Aiba, M.; Takafumi, H.; Hiura, T. Interspecific differences in determinants of plant species distribution and the relationships with functional traits. J. Ecol. 2012, 100, 950–957. [Google Scholar] [CrossRef]
- Clark, A.T.; Detto, M.; Muller-Landau, H.C.; Schnitzer, S.A.; Wright, S.J.; Condit, R.; Hubbell, S.P. Functional traits of tropical trees and lianas explain spatial structure across multiple scales. J. Ecol. 2018, 106, 795–806. [Google Scholar] [CrossRef]
- Asanok, L.; Kamyo, T.; Marod, D. Maximum entropy modeling for the conservation of Hopea odorata in riparian forests, central Thailand. Biodiversitas 2020, 21, 4663–4670. [Google Scholar] [CrossRef]
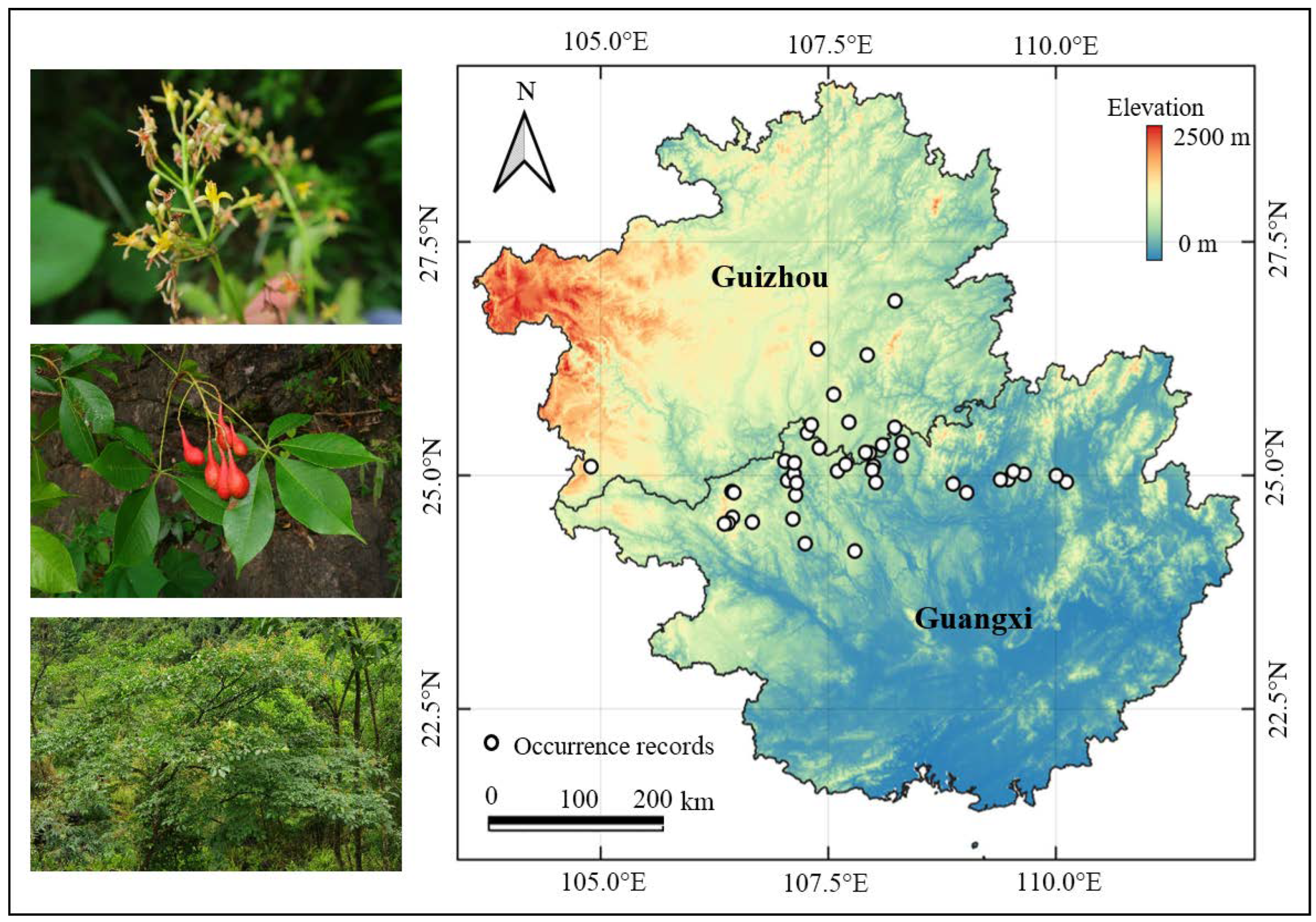
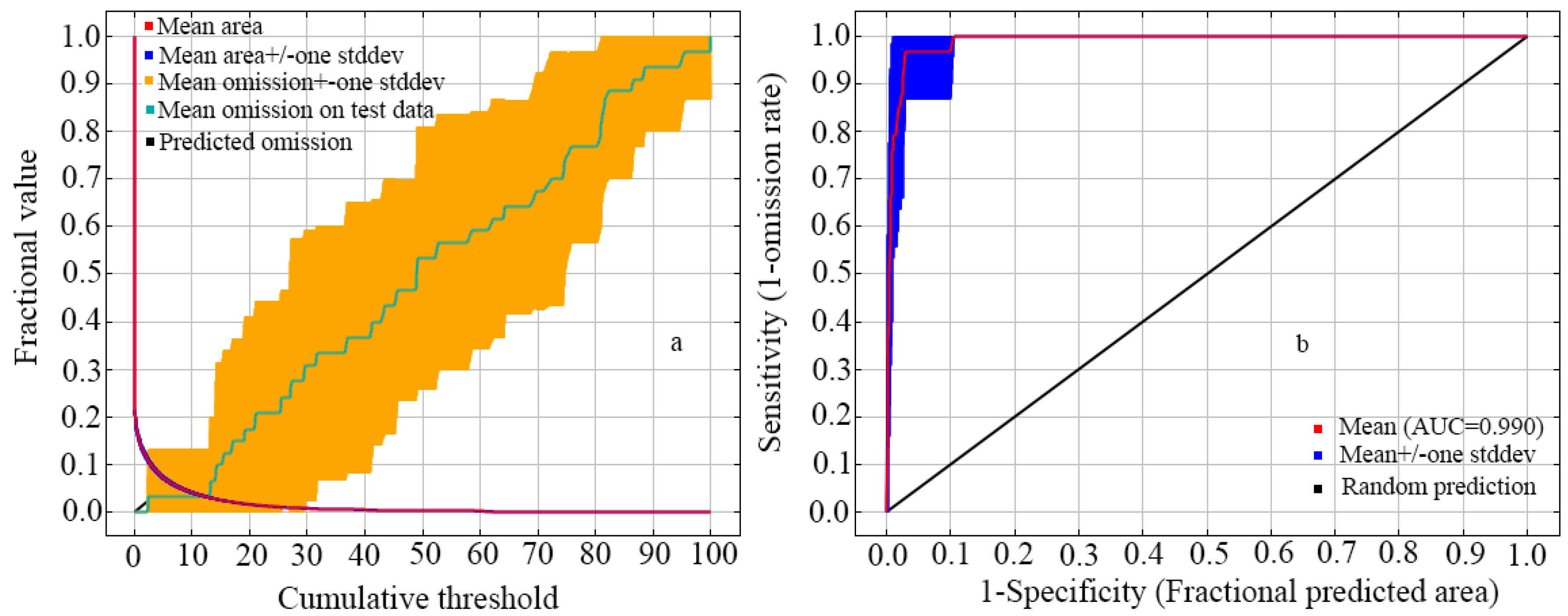
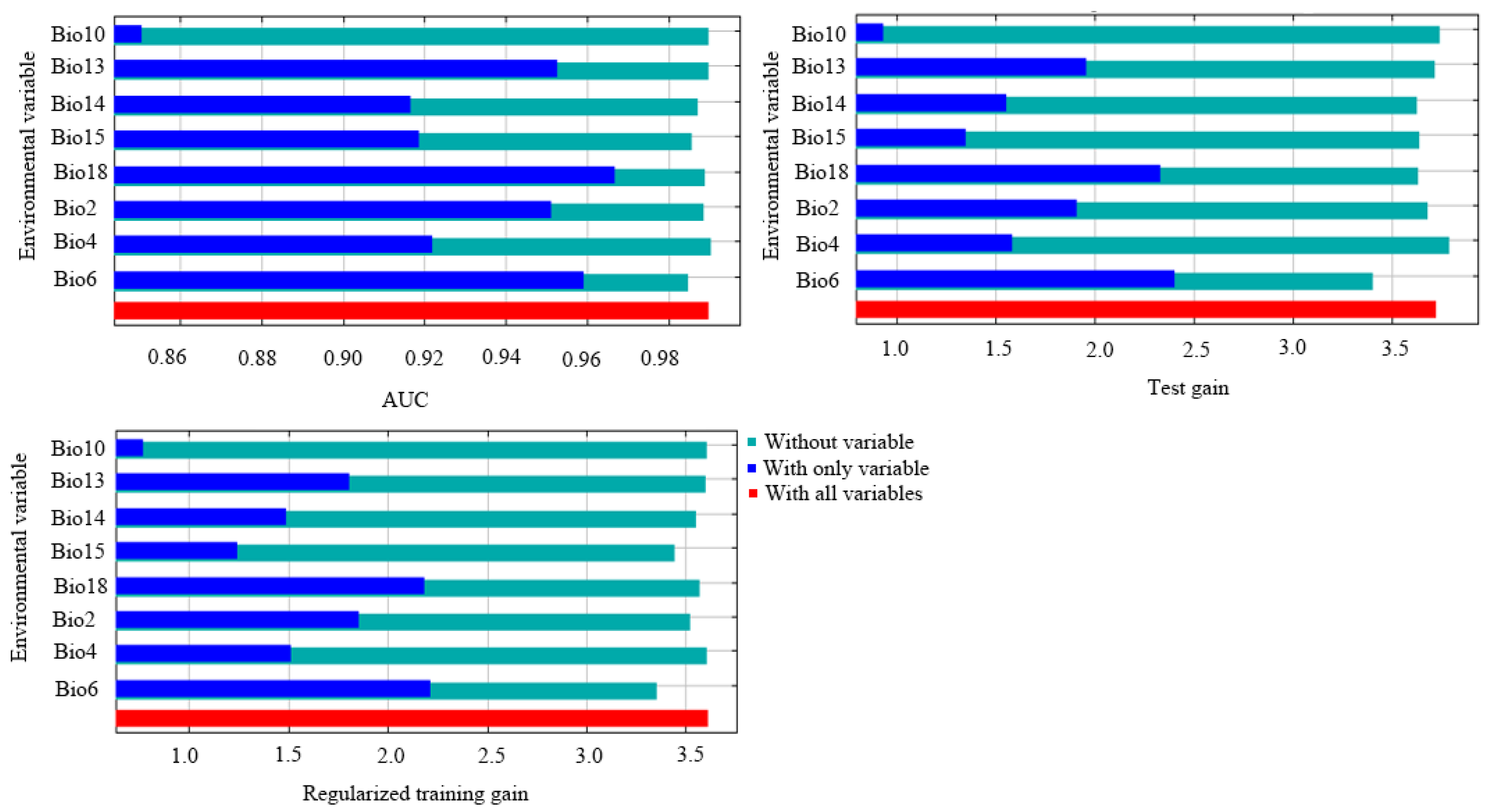
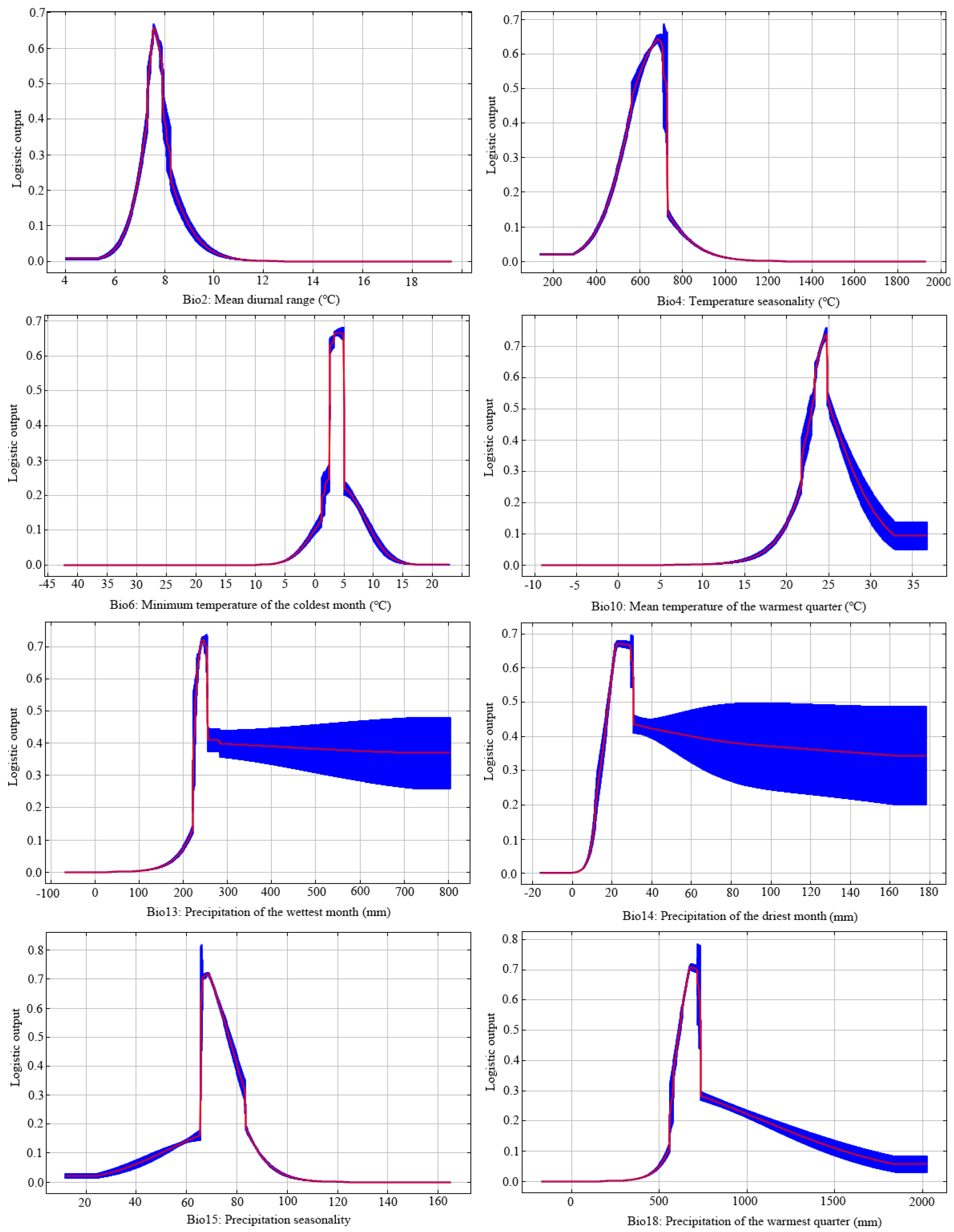
| Code | Environmental Variable | Unit |
|---|---|---|
| Bio1 | Annual mean temperature | °C |
| Bio2 | Mean diurnal range (mean of monthly (maximum temp-minimum temp)) | °C |
| Bio3 | Isothermality (Bio2/Bio7) (×100) | - |
| Bio4 | Temperature seasonality (standard deviation×100) | °C |
| Bio5 | Maximum temperature of the warmest month | °C |
| Bio6 | Minimum temperature of the coldest month | °C |
| Bio7 | Temperature annual range (Bio5-Bio6) | °C |
| Bio8 | Mean temperature of the wettest quarter | °C |
| Bio9 | Mean temperature of the driest quarter | °C |
| Bio10 | Mean temperature of the warmest quarter | °C |
| Bio11 | Mean temperature of the coldest quarter | °C |
| Bio12 | Annual precipitation | mm |
| Bio13 | Precipitation of the wettest month | mm |
| Bio14 | Precipitation of the driest month | mm |
| Bio15 | Precipitation seasonality (coefficient of variation) | - |
| Bio16 | Precipitation of the wettest quarter | mm |
| Bio17 | Precipitation of the driest quarter | mm |
| Bio18 | Precipitation of the warmest quarter | mm |
| Bio19 | Precipitation of the coldest quarter | mm |
| Code | Bioclimatic Variable | Percent Contribution | Permutation Importance |
|---|---|---|---|
| Bio18 | Precipitation of the warmest quarter | 32.7 | 6.9 |
| Bio6 | Minimum temperature of the coldest month | 26.3 | 75.7 |
| Bio2 | Mean diurnal range | 20.5 | 13 |
| Bio15 | Precipitation seasonality | 8.4 | 2.1 |
| Bio4 | Temperature seasonality | 7.7 | 0.3 |
| Bio14 | Precipitation of the driest month | 3.1 | 1.3 |
| Bio13 | Precipitation of the wettest month | 0.9 | 0.4 |
| Bio10 | Mean temperature of the warmest quarter | 0.4 | 0.2 |
| Province or Autonomous Region | Predicted Suitable Area Ratio | ||||
|---|---|---|---|---|---|
| Fail | Poor | Fair | Good | Excellent | |
| Guangxi | 15.87 | 2.10 | 1.15 | 0.50 | 1.19 |
| Guizhou | 10.99 | 1.92 | 1.11 | 1.22 | 0.71 |
| Guangdong | 14.72 | 0.64 | 0.06 | 0.05 | 0.03 |
| Sichuan | 42.42 | 2.29 | 0.63 | 0.06 | 0.00 |
| Yunnan | 32.40 | 1.40 | 0.27 | 0.02 | 0.00 |
| Total | 116.4 | 8.35 | 3.22 | 1.85 | 1.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Xie, C.; Wei, L.; Gao, Z.; Yang, H.; Jim, C. Predicting Suitable Habitats for China’s Endangered Plant Handeliodendron bodinieri (H. Lév.) Rehder. Diversity 2023, 15, 1033. https://doi.org/10.3390/d15101033
Wang G, Xie C, Wei L, Gao Z, Yang H, Jim C. Predicting Suitable Habitats for China’s Endangered Plant Handeliodendron bodinieri (H. Lév.) Rehder. Diversity. 2023; 15(10):1033. https://doi.org/10.3390/d15101033
Chicago/Turabian StyleWang, Guohai, Chunping Xie, Lijuan Wei, Zequn Gao, Honglan Yang, and Chiyung Jim. 2023. "Predicting Suitable Habitats for China’s Endangered Plant Handeliodendron bodinieri (H. Lév.) Rehder" Diversity 15, no. 10: 1033. https://doi.org/10.3390/d15101033
APA StyleWang, G., Xie, C., Wei, L., Gao, Z., Yang, H., & Jim, C. (2023). Predicting Suitable Habitats for China’s Endangered Plant Handeliodendron bodinieri (H. Lév.) Rehder. Diversity, 15(10), 1033. https://doi.org/10.3390/d15101033







