Abstract
Marine noise is an emerging pollutant inducing a variety of negative impacts on many animal taxa, including fish. Fish population persistence and dynamics rely on the supply of early life stages, which are often very sensitive to disturbance. Impacts of marine noise pollution (MNP) on juvenile fish have rarely been investigated in temperate regions. This is particularly true for the Mediterranean Sea, which is considered as an MNP hotspot due to intensive maritime traffic. In this study, we investigate the relationship between MNP related to boat traffic and (i) assemblage structure and (ii) the density of juvenile fishes (post-settlers at different stages) belonging to the Sparidae family. We quantified MNP produced by boating at four coastal locations in the French Riviera (NW Mediterranean Sea) by linearly combining five variables into a ‘noise index’ (NI): (i) boat visitation, (ii) number of boat passages/hour, (iii) the instantaneous underwater noise levels of passing boats, (iv) continuous boat underwater noise levels and (v) duration of exposure to boat noise. Then, using the NI, we identified an MNP gradient. By using juvenile fish visual censuses (running a total of 1488 counts), we found that (i) the assemblage structure and (ii) the density patterns of three fish species (i.e., Diplodus sargus, D. puntazzo, D. vulgaris) changed along the MNP gradient. Specifically, the density of early D. sargus post-settlers was negatively related to MNP, while late post-settler densities of D. puntazzo and, less evidently, D. vulgaris tended to decrease more rapidly with decreasing MNP. Our findings suggest the following potential impacts of MNP on juvenile sparids related to coastal boat traffic: (i) idiosyncratic effects on density depending on the species and the developmental stage (early vs. late post-settlers); (ii) negative effects on recruitment, due to possible alteration of late post-settlement movement patterns.
1. Introduction
The marine natural soundscape (i.e., acoustic cues changing in time and space in terms of frequency and intensity [1,2]) is composed of both biotic (emitted by marine mammals, fish and invertebrates) and abiotic sounds (e.g., those related to waves, winds and rain [3]). Natural sounds are used by several animals for multiple purposes such as orientation, habitat selection, and intra- and inter-specific relationships [4,5,6,7]. The high acoustic power and the different sound frequencies produced by human activities at sea (e.g., maritime traffic, extraction, digging) can interfere with the natural soundscape and produce a wide array of detrimental effects on marine life. Anthropogenic sound, called noise when it affects the biota (hereinafter indicated as marine noise pollution, MNP), does not only induce physiological responses to stress and behavioral changes because it can impair orientation ability, interfere with intra- and inter-specific relationships and communication, and reduce hearing capabilities, but can also cause internal and external injuries and in some extreme cases even death [8,9,10].
MNP is nowadays listed among the main threats to marine life in European environmental legislation (see the Marine Strategy Framework Directive 56/2008 CE; MSFD; European Union, 2008) and the United Nations Convention on the Law of the Sea (UNCLOS; ICP-19, https://undocs.org/A/73/124 (accessed on 1 January 2023) [11]). EU Member States, more specifically, have developed monitoring programs to track the MNP levels (descriptor 11 within the MSFD), implementing specific measures aimed at keeping MNP at levels that should not harm the marine biota.
The effects of MNP have been primarily investigated on marine mammals, but recent studies have shown that MNP can also affect fishes, invertebrates and seagrasses [10,12,13]. Regarding fishes, most coastal species have a life cycle that includes a pelagic larval stage. Once competent larvae reach suitable coastal habitats, they settle and metamorphose into juveniles (called ‘early post-settlers’ in the beginning, then becoming ‘late post-settlers’ with time) before recruiting to the adult population. Depending on the species and the geographic area, a shift (i.e., movement) in the use of different habitats and/or depth levels between settlement and recruitment can occur, the timing being again species-specific [14,15,16,17]. The coastal soundscape provides cues to fish larvae to identify suitable habitats for settlement [4,18] that MNP can mask, thus disrupting orientation ability and ultimately affecting settlement and recruitment success [4,19,20,21,22,23]. This may have potential consequences for population persistence and dynamics (e.g., in terms of settlement intensity and success, juvenile survival, dispersal and connectivity) for many coastal fishes [24,25,26].
The coastal Mediterranean Sea, like many other coastal regions worldwide, is highly urbanized, with multiple types of coastal infrastructures diffusely present, such as urban structures, resorts, breakwaters, industrial installations, harbors and marinas, inter alia [27,28]. Coastal boating is the most widespread and frequent source of MNP in coastal waters worldwide [13]. Boating activities involve small-scale fishing vessels, recreational boats, yachts of varying sizes, ferries, cargo ships and cruise vessels [29,30]. Coastal boating is particularly intensive during summer at renowned touristic destinations in the Mediterranean, which is the most intensively visited touristic destination worldwide (https://www.medqsr.org/tourism) (accessed on 1 January 2023).
The aim of this study is to investigate the putative effect of MNP generated by boating activities on the distribution patterns of juvenile sparid fishes of different species and developmental stages. We focused on sparid fishes because (i) they are an important component of Mediterranean coastal fish assemblages (e.g., they account for a significant portion of density and biomass of fish assemblages), (ii) they play a variety of ecological roles in the food web [31] (i.e., they include herbivores, planktivores, omnivores, benthivores and high-level predators) and (iii) they are targeted by recreational and professional fishers (so they are socio-economically relevant as well). Furthermore, juvenile stages of most sparid fishes tend to stay in very shallow waters (0–3 m), display a relatively high site fidelity (at least in the early post-settlement phase) and show a clear ontogenetic shift in habitat use [15,32,33]. Finally, taking into account species-specific settlement timing, different juvenile stages (from early to late post-settlers) of different Mediterranean sparids are potentially exposed to MNP produced by boat traffic in summer, with potential effects on populations and assemblage structure. Juvenile sparids are, from this perspective, suitable indicators to assess the effects of local changes in environmental variables (e.g., inputs of fresh or desalinized waters [34]) or the effects of point-source chemical pollution [35]. Therefore, they can potentially be used to assess the impact of MNP locally generated by boat traffic. The settlement period differs among the different sparid species. Taking into consideration the most abundant and frequent sparid species, Diplodus vulgaris settles from November to February (generally in two pulses) [36], Sparus aurata in February–March, Boops boops from April to June [37], Dentex dentex and Diplodus sargus in May–June [37,38,39], Oblada melanura from July to mid-August, Diplodus puntazzo from October to late November and Sarpa salpa in two peaks, one at the beginning of November and a less intense peak in May [17,38,40]. Sensitivity to MNP impact may change among species, but also intra-specifically between early and late post-settlers, with potential population- and assemblage-wide repercussions.
Here, we have specifically assessed whether the juvenile sparid assemblage structure and species density change across an MNP gradient in a sector (i.e., the eastern French Riviera) of the NW Mediterranean Sea.
2. Materials and Methods
2.1. Study Area
Fish sampling was carried out in the eastern French Riviera (France, NW Mediterranean Sea; Figure 1), during 3 consecutive years (2019–2021) from mid-May to the end of July. This time window corresponds approximately to the period of the year when both (i) the juveniles of many sparid fishes are present in shallow coastal habitats [17,40], and (ii) boat traffic is particularly intensive, due to the historical high tourism concentration in the area [41,42,43]. Worthy to note is that the first sampling was done in 2019 and, then, repeated in the following years (2020 and 2021), when COVID-19 pandemic restrictions occurred.
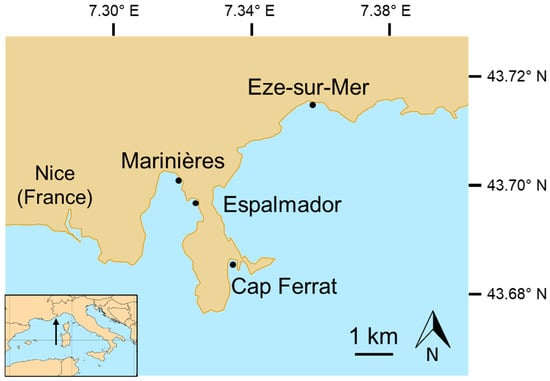
Figure 1.
Study locations from west to east: Marinières (43.7053° N, 7.3188° E), Espalmador (43.7000° N, 7.3243° E), Cap Ferrat (43.6858° N, 7.337° E) and Eze-sur-Mer (43.7220° N, 7.3598° E).
In the study area, the coast is composed of rocky shores separating pocket beaches, often in the vicinity of Posidonia oceanica meadows, which are suitable habitats for settlement for many sparid fishes [17,40,44]. Based on an initial screening of boat traffic frequentation using an Automatic Identification System (International Maritime Organisation (IMO), 2001), four locations (Marinières, Espalmador, Cap Ferrat, Eze-sur-Mer) were selected (Figure 1) to represent an MNP gradient. We did our best to identify places with generally comparable environmental features, even though there could be uncontrolled factors (e.g., current circulation, wind and wave exposure) that could have contributed to the overall variability.
2.2. Noise Measures to Characterize the Sampling Locations
In 2019, to characterize the four study locations in terms of MNP, five variables were measured: (1) boat visitation; (2) instantaneous underwater noise levels generated by passing boats; (3) continuous anthropogenic underwater noise levels emitted by distant boats; (4) number of boat passages per hour; (5) the duration of exposure to boat noise (instantaneous and continuous).
Boat visitation was measured as the number of boats counted by photographic sampling performed in the four study locations between 12 p.m. and 2 p.m., this time window better representing the activity of boating along the coast investigated. In 2019, boat visitation counts were performed on 28 May, 6 June, 12 June, 28 June, 3 July, 12 July, 17 July, 30 July, 7 August and 13 August. In 2020, boats were counted on 9 June, 17 June, 23 June, 1 July, 10 July, 16 July, 31 July and 6 August. In 2021, counts were done on 08 July, 13 July, 21 July, 29 July, 13 August and 17 August. This type of census (data are expressed as ‘number of boats per picture’ per each location) does not distinguish between moving or moored boats, but it was used as a proxy of the boat visitation at each study location. The number of boats counted in 2019 did not significantly change in comparison with 2020 and 2021, in spite of the COVID restrictions (Figure S1).
The other four variables (passages of boats per hour, instantaneous noise generated by passing boats, continuous anthropogenic noise and duration of exposure to boat noise) were measured using hydrophones only in 2019, due to the COVID restrictions in the following years. Data were acquired using HTI-96 (High Tech Inc., Long Beach, MS 39560, USA) hydrophones with a sensitivity of −164 dB re 1 V/μPa and a flat frequency response from 2 Hz to 30 kHz connected to an EA-SDA14 compact autonomous recorder (RTSys®, Caudan, France). The devices, which recorded sounds at a sampling rate of 78 kHz and a resolution of 24 bits, were moored to the bottom (8 to 12 m water depth) with the hydrophones positioned 1 m above the seafloor. The loggers recorded data over two weeks in spring (from 23 May to 6 June 2019) and in summer (from 16 July to 1 August 2019). The recording cycle was set to 20 min of recording followed by a 50-min break in spring and a 20-min recording followed by a 40-min break in summer. Due to many hydrodynamic noises covering frequencies and durations similar to boat noises, passing boats were not fully detected automatically. The audio files were split into 5-min bins, which were converted to 5-min spectrograms (FFT 1024 size, Kaiser window with 80% overlap) using a MATLAB® interface (version R2014b) developed at the Chorus institute, enabling the identification of (i) the instantaneous noise emitted by boats in transit, and (ii) the continuous noise of distant boats in the background. The 5-min bins with passing boats and continuous noise were automatically moved to separate folders. This procedure enabled us to count boat passages in each area and quantify the different noise types. Since the sampling regimes were not continuous and identical in spring and summer, boat passages were weighted to get hourly estimates and thus avoid any bias.
The noise generated by passing boats was calculated as the sound pressure level (SPL) of the root mean square (RMS) between 100 Hz and 1 kHz sounds, measured in dB re 1 µPa using the timestamps of the detections within the selected 5-min bins. Continuous noise levels were calculated in third-octave bands, centered at 63 Hz, 125 Hz, 250 Hz, 500 Hz and 1000 Hz measured in dB re 1 µPa, which correspond to the audible bands of fish post-larvae for which audiograms are available [45,46,47]. The recording sequences identified as containing continuous noise only were used for this noise quantification. Since most of the continuous noise was in the 250 Hz octave level band (224 Hz, 282 Hz), the levels in this band were used for subsequent analyses. Finally, the duration of exposure to boat noise was measured as the proportion of time in which the noise from both instantaneous boat passages and distant boats was present throughout the overall recording periods.
2.3. Assessment of Juvenile Fish Assemblages
At each of the four study locations, three sites (each of approximately 150 m of coastline), tens to hundreds of meters apart from each other, were randomly selected and sampled on a weekly basis from mid-May to the end of July. Multiple randomly selected sampling dates were selected within each of the three sampling years: 9 sampling dates in 2019 (from 24 May to 26 July), 11 in 2020 (from 22 May to 30 July) and 11 in 2021 (from 20 May to 29 July).
At each site, juvenile fish (both for early and late post-settlers; for the sake of simplicity, sometimes generically referred to hereinafter as ‘juveniles’) density was recorded by means of an underwater visual census using strip transects 25 m long and 2 m wide [17,40]. Strip transects were conducted by snorkeling in the settlement habitat, parallel to the coast, close to the shore (<5 m away from the shoreline) and to a depth <2 m.
Four strip transects (replicates) were carried out for each combination of sampling site and sampling date, for a total of 1488 fish counts.
2.4. Statistical Data Analyses
The average values of each of the five noise variables per location (sampled in 2019) were linearly combined using principal components analysis (PCA, vegan package in R, version 2.6-2 [48]) and the eigenvalues on the first axis used as a ‘noise index’ (NI; see details in Results). To do that, we used data on boat visitation only for the dates when audio recordings were also available (i.e., 28 May, 6 June, 17 and 30 July).
Analyses of the density distribution of the juvenile sparid assemblage were run separately for each year because of the non-negligible interannual natural variability in the sparid settlement and because measurements of noise were obtained only in 2019 [32]. The 2019 data were fitted to a generalized linear model (GLM) with NI, site (nested in NI) and sampling date as factors. NI and sampling date were continuous variables. The density distribution data from the following years (2020 and 2021) were fitted to a GLM model with location (fixed, 4 levels), site (as nested in location) and sampling date. We used the ‘manyglm’ function from the ‘mvabund’ R package, version 4.2.1 [49] to run the model and the ‘anova’ function implemented in the same package to test for the significant effects of the factors in explaining the patterns. Before running the analyses, we checked dispersion of the residual using the PERMDISP [50]. Multivariate results were visualized using non-metric multidimensional scaling ordinations (nMDS).
The density distribution of the most abundant species was further investigated using Generalized Linear Mixed Models (GLMMs): the ‘glmmTMB’ function from the ‘glmmTMB’ package [51]. The other multivariate and univariate models were run using a negative binomial distribution and they were checked for normality and homoscedasticity by visually inspecting model residuals. The significance of the terms of the model was then assessed using the ‘Anova’ function from the ‘car’ package [52].
Data analyses were performed using R version 3.4.3 software [53]. PERMDISP analysis and nMDS plots were performed using PRIMER v6 with the PERMANOVA+ Add On package [50].
3. Results
3.1. Noise Measures
The four study locations displayed differences in the five noise variables (averaged across the sampling window, i.e., spring and summer 2019, for each study location) considered in this study (Table 1). Boat visitation was highest at Marinières, followed by Espalmador, and far lower at Cap Ferrat and Eze-sur-Mer. Values of boat passages and duration of boat exposure were higher at Espalmador, followed by Marinières and lower, again, at Eze-sur-Mer and Cap Ferrat. The other two variables tended to be higher at Marinières, followed by Espalmador, while the lowest values were found at Cap Ferrat (Table 1).

Table 1.
Mean values of the five MNP-related variables measured at the four study locations.
The PCA (Figure 2) showed a clear separation of the four locations on the first axis that explains 79.4% of the total variance, whereas the second axis only explains 15.5%. The first axis was best related to the ‘boat passage noise level’ and ‘continuous anthropogenic noise level’, while ‘duration of noise exposure’, ‘number of boat passages’ and ‘boat visitation’ better explained part of the residual variance along the second axis. Cap Ferrat was best separated along the first axis, followed by Eze-sur-Mer. Marinières and Espalmador were better differentiated along the second axis, with Marinières characterized by the highest boat visitation (see also Figure S1). Due to the high variability explained by the first axis, the eigenvalues of each location along this axis were used to attribute an MNP index to each location, hereafter referred to as ‘noise index’ (NI), which was defined as the inverse of the eigenvalues so that lower values of NI corresponded to lower MNP (Figure 2).
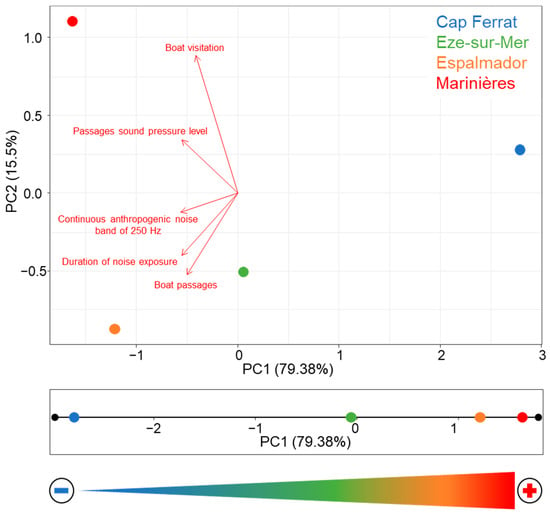
Figure 2.
(Top): principal component analysis computed on the five variables related to boat MNP estimated in this study. (Bottom): projection of the coordinates of the locations on the first PCA axis and inversion of the axis, showing the MNP gradient, from the lowest (Cap Ferrat) to the highest (Marinières) NI value. The arrow represents the gradient, from the lowest (−) to the highest (+) MNP produced by boat traffic.
3.2. Juvenile Sparid Assemblage Structure
The juvenile fish assemblage was chiefly characterized by five sparid species: the sharpsnout sea bream Diplodus puntazzo, the white sea bream D. sargus, the common two-banded sea bream D. vulgaris, the saddled sea bream Oblada melanura and the salema porgy Sarpa salpa. Other species were occasionally found in the transects (e.g., two juveniles of Sparus aurata in one transect of 2021), but they were extremely uncommon and thus excluded from statistical analyses. Regarding the reproductive timing of each sparid species and our sampling windows (see Introduction for details), D. sargus and O. melanura were mostly early post-settlers, while D. puntazzo, D. vulgaris and to some extent S. salpa were mostly late post-settlers.
In 2019, the assemblage structure varied according to the interaction between the NI and the sampling date (Table S1). In the subsequent years, assemblages differed among locations and, in 2020, these differences varied through sampling dates (significant interaction ‘Location × Sampling Date’ in 2020; Table S1). The nMDS plots showed that, in 2019, the two locations with less impact of boat noise (i.e., Cap Ferrat and Eze-sur-Mer) separated from the others, especially starting from the third sampling date (Figure 3A), whereas in 2020 and 2021 only Cap Ferrat separated from the other locations, for a few sampling dates in 2020 (Figure 3B,C). Post-hoc analyses showed that the assemblage structure differed significantly across all locations except for the assemblage of Espalmador, which was not significantly different from the one of Eze-sur-Mer (Table S2).
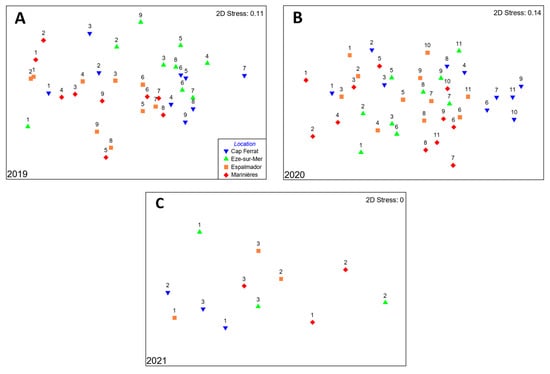
Figure 3.
Non-metric multidimensional scaling ordinations (nMDSs) of juvenile sparid assemblages (based on density data) for each study year. In the 2019 (A) and 2020 (B) nMDS plots, each point corresponds to the centroid of three sites and four transects sampled on each sampling date (labelled with a number) at each location. In the 2021 (C) nMDS plot, each point corresponds to a location. The centroids of sites and sampling dates are shown here since the interaction ‘Location × Sampling Date’ was non-significant. Colors and symbols indicate the NI values per location as in the legend.
3.3. Single Species Density Patterns
D. puntazzo, D. sargus and D. vulgaris were the species that contributed the most to the differences among juvenile sparid assemblages in relation to NI values or to the interaction ‘NI × Sampling Date’ in 2019, and to the differences among locations in the two subsequent years (Table S3). In 2020, the contribution of S. salpa was also important (Table S3). Worthy to note, juveniles of these four species (the three Diplodus and S. salpa) differed in size (and therefore likely in age) during the sampling windows in each of the three sampling years: censused D. sargus were early post-settlers (they were smaller in size than the other two, Diplodus and S. sarpa), while D. puntazzo, D. vulgaris and S. salpa were late post-settlers (see Figure S2, showing the size–frequency distributions of post-settler sparids). Based on otolith aging (data not reported), early D. sargus post-settlers were censused approx. between 7 and 60 days after settlement, while late post-settlers of D. puntazzo and D. vulgaris were censused approx. 200–260 days after settlement. These three species were analyzed separately for each year.
The density of D. sargus juveniles varied significantly across NI levels consistently across sampling dates in 2019 (χ2 1 = 11.2043; p = 0.001), with the lowest densities associated with the highest NI values (Figure 4A). In 2020, there was a significant interaction ‘location × sampling date’, and density increased across sampling dates in the Cap-Ferrat location (Table S4; Figure 4B). In 2021, densities were considerably lower than in the previous years. The analyses evidenced differences among locations and the largest densities in Eze-sur-Mer (Table S4; Figure 4C).
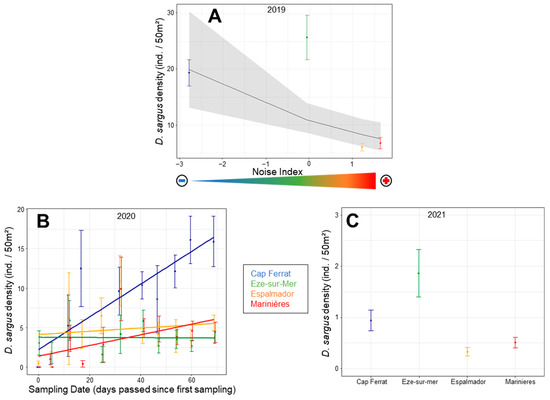
Figure 4.
Mean (±SE) density values of D. sargus juveniles for the NI levels in 2019 (A), and the sampling days for each location in 2020 (B) and for each location in 2021 (C), according to the results of the glmm models. In (A), the line and the grey area are the fitted values and the 95% confidence interval of the regression model. The arrow represents the MNP gradient as in Figure 2. In (A,C), the mean and SE are averaged across sampling days, transects and sites. In (B), they are averaged across sites.
The density of D. puntazzo juveniles in 2019 showed a significant interaction ‘NI × sampling date’ (χ2 3 = 6.1026; p = 0.013). In general, the density decreased through sampling dates for all NI values and no specimens were sampled during the last dates of sampling, especially for the Cap-Ferrat location (Table S6; Figure 5A). Moreover, the density decrease was steeper for the locations with low NI than for the other two (Figure 5A). In 2020, the density showed a similar pattern of decrease in time among locations (Table S6; Figure 5B), whereas in 2021, there was a significant effect of location, consistently through sampling dates (χ2 3 = 11.4456; p = 0.010), with the highest densities in Marinières and Espalmador (Figure 5C, Table S7). There was also a significant change through sampling dates (Table S6).
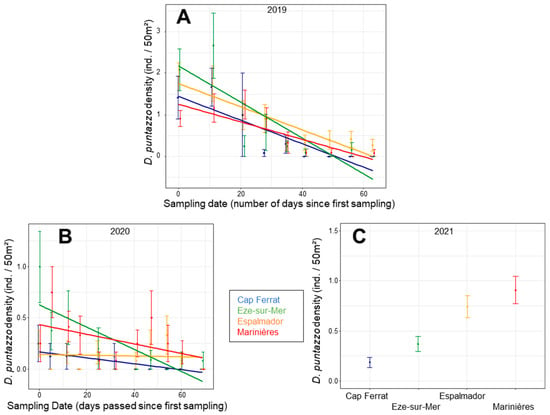
Figure 5.
Mean (±SE) density values of D. puntazzo juveniles for the sampling days per each location in 2019 (A) and 2020 (B) and for each location in 2021 (C), according to the results of the glmm models. In (A,B), mean and SE are averaged across sampling days, transects and sites. In (C), they are averaged across sites.
Density patterns of D. vulgaris juveniles in 2019 showed a significant interaction ‘NI × Sampling Date’ (χ2 3 = 7.6929; p = 0.005) (Table S8). Densities were the highest in Marinières and decreased over time, except for Espalmador (Figure 6A). As for the other species, there was a significant interaction ‘Location × sampling date’ in 2020 and a difference among locations in 2021 (Table S8). As for 2019, densities decreased over time in 2020 and were the highest in Marinières, except for the location Espalmador (Figure 6B). In 2021, densities were the highest in Marinières (Figure 6C, Table S9).
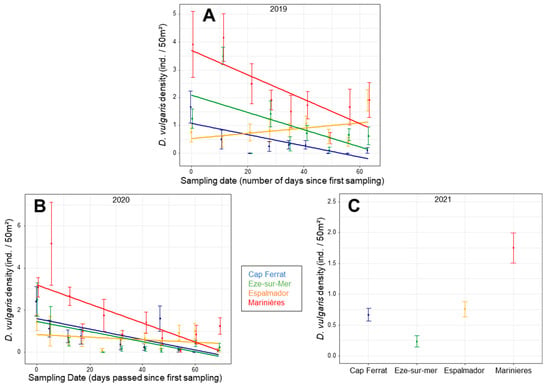
Figure 6.
Mean (±SE) density values of D. vulgaris juveniles for the sampling days per each location in 2019 (A) and 2020 (B) and for each location in 2021 (C), according to the results of the glmm models. In (A,B), mean and SE are averaged across sampling days, transects and sites. In (C), they are averaged across sites.
Finally, for S. salpa in 2020, only a significant variability across sampling dates was detected (Table S10; Figure S3).
4. Discussion and Conclusions
This study sheds new light on the possible effects of marine noise pollution (MNP) produced by boat traffic on the assemblage of the juvenile stages of some commercially and ecologically important Mediterranean sparid fishes that spend part of their life cycle in shallow coastal waters. During the first sampling year, we observed relationships between MNP (measured by the noise index, NI, at multiple sampling locations) and: (i) juvenile sparid assemblage structure; (ii) spatio-temporal distribution density patterns of juveniles belonging to some species (i.e., Diplodus sargus, D. vulgaris and D. puntazzo), but not to other species (i.e., Sarpa salpa and Oblada melanura). These relationships were sometimes variable across sampling dates.
One point to stress to properly interpret our data concerns the non-negligible temporal variability of juvenile density values, i.e., among sampling dates (see statistical outputs) and years (from the visual inspection of data and graphs). The 2020 and 2021 years coincided with the global pandemic restrictions due to COVID-19. The 2020 lockdown caused a likely reduction in boat traffic (see for examples in other geographical areas [54,55,56,57]). Unfortunately, we could not collect noise data due to the impossibility of obtaining the necessary authorization. Data on boat frequentation were fairly similar across years, but photo-sampling cannot allow us to distinguish between mooring and moving boats, with only the latter producing noise. The PCA done on the 2019 data allowed us to detect a fairly clear MNP gradient among the four study locations. The analysis of differences in assemblages among locations in data from 2020 and 2021 also showed variations among sampling dates and among years, which are likely to be related to the environmental variability of a number of here-untested factors, possibly changing between locations and playing a role in affecting fish settlement, such as changes in water currents that influenced the arrival of larvae, changes in water temperature affecting noise diffusion or the specific structure of each sampling embayment, possibly inducing a more or less important ‘funneling’ of settling larvae [58].
Literature data show that fish are widely used as indicator taxa, due to their sensitivity (depending on the species and/or life stage) to a wide array of disturbances, such as chemical pollution [59,60], fishing [61,62], protection within MPAs [63,64] and habitat alteration [65,66], among other factors, including in the coastal Mediterranean Sea [67,68]. The responses may change in terms of direction (positive or negative) or intensity, depending on the specific tolerance each species or life stage may have towards specific sources of disturbance which can change in terms of intensity and/or frequency [69,70,71]. Specifically, considering the disturbance associated with MNP due to boat traffic, other studies assessed its impact on the density of coastal fishes. For instance, Simpson et al. (2016) [19] recorded a lower settlement intensity of the Ambon damselfish (Pomacentrus amboinensis) on coral patches subject to experimentally played boat noise in the Australian Great Barrier Reef. Becker et al. (2013) [72] found a decrease in mid-sized fish in an estuarine environment in South Africa following the passage of boats. Engås et al. (1996) [73] and Paxton et al. (2017) [74] showed a significant decrease in multiple fish species, while Slotte et al. (2004) [75] observed that pelagic fish tend to swim deeper during seismic surveys. Recent studies carried out at the Tagus estuary (Portugal) using the Lusitanian toadfish (Halobatrachus didactylus) as a model species show that males exposed to boat noise depressed their metabolism and their activity (such as parental care and mate attraction) to cope with an acoustic stressor, consistent with a freezing defensive response/behavior, which implies that boat noise may have a severe impact on reproductive fitness [76]. In the same study area and using the same model species, Faria et al. (2022) [77] found that boat noise induced a detrimental effect on embryos and larvae stress response and on larvae development, providing evidence of detrimental effects of boat noise exposure on fish development in the field and on stress biomarker responses. Jain-Schlaepfer et al. (2018) [78] also investigated the impact of motorboat noise on embryos directly in the field in the Great Barrier Reef and revealed a significant increase in the embryos’ heart rate during motorboat exposure with the magnitude of the impact depending on the engine type. Recently, by associating field manipulation on the Great Barrier Reef with laboratory experiments, it has been demonstrated that limiting motorboat activity on reefs leads to the survival of more fish offspring compared to reefs experiencing busy motorboat traffic, showing that the enhanced reproductive success on protected reefs is likely due to improvements in parental care and offspring length [79].
The fact that juveniles of two sparid species (i.e., S. salpa and O. melanura) did not show any significant relationship with MNP could be explained by several not mutually exclusive processes: (1) the species-specific tolerance/sensitivity/auditive capabilities of juvenile stages to MNP or to specific noise levels [80,81]; (2) the capability for rapid habituation to MNP [82,83]; (3) the possible effects of other uncontrolled sources of variance (e.g., local levels of human visitation, chemical pollution, even though the study locations are less than 10 km apart and are as similar as possible in terms of substrate, habitat types and depth; Figure S4) [35,38,84,85]; and (4) the intrinsically high variability in settlement intensity [14,38], which could mask the MNP effects, as well as the high variance around the mean density values estimated which could be particularly important for gregarious species like O. melanura.
One point deserving major attention is that when a significant relation with MNP was detected, densities of juveniles of D. puntazzo and, to a lesser extent, D. vulgaris were positively related with MNP (more individuals associated with higher NI values), especially at the end of the sampling period, while those of D. sargus showed an opposite pattern. This species-specific response could be interpreted by considering the life cycle, the ontogenetic shift in habitat use from settlement to recruitment, and the timing for settlement and recruitment of the different species in relation to the peak of boat traffic. Even though specific experimental studies are needed, we can draw some hypotheses to interpret the patterns we have observed. The settlement of D. sargus takes place in the first half of the tourism season (i.e., the end of May to June), when boat traffic progressively intensifies. The negative relationship we have found between early settlers of D. sargus (as shown by their very small sizes and ages) and MNP (due to the rising MNP during the D. sargus settlement) could be explained in terms of disturbance that could impair the ability of competent (i.e., ready to settle) larvae to locate suitable settling habitats/sites. This could determine a lower settlement intensity and thus a lower density of settlers compared to locations where NI values are lower. Considering that early post-settlers are relatively site-attached [15,32,40], the patterns we have observed could suggest a negative impact of MNP caused by boat traffic on settlement intensity and/or a negative effect on fish survival rates, attributable, e.g., to stress or to an increased predation on juveniles of D. sargus, as observed for other species elsewhere [86]. In contrast, during the peak of boat traffic, juveniles of D. puntazzo and D. vulgaris are late post-settlers (as they settle well before D. sargus, as observed on the basis of their larger sizes and ages than D. sargus) that tend to move much more than early settlers, both horizontally (as observed for other Diplodus species [15,32,33]) and/or towards deeper stands for recruiting to the adult populations some months after settlement [40,87,88]. The positive relationship between late post-settlers of D. puntazzo and, to a lesser extent, D. vulgaris and NI values could be explained in terms of noise disturbance, which could impair the ability of late post-settlers (i.e., ready to recruit or disperse) to move away from their shallow settlement habitats/sites. This could determine a higher late post-settler density compared to the values observed in locations where NI values are lower and where late post-settlers at a given stage and time would naturally tend to swim away in the absence of such a disturbance. Considering that late post-settlers are far less site-attached [33,87,89] than early post-settlers, the patterns we have observed could suggest a negative impact of coastal MNP caused by boat traffic also on recruitment/emigration intensity, something that to the best of our knowledge has never been reported before. Preliminary measures of boat noise taken at very shallow settlement sites (about 1 m depth; data not reported) show that boat noise is much less intense (with a difference of about 20 dB; see Figure S5) than at 10 m depth because of low-frequency cutoff. This would suggest that the negative effects of coastal boat MNP on juvenile sparids could be buffered in very shallow settlement habitats/sites, which could thus act as ‘noise shelters’ for sparid settlers. Some months after settlement (i.e., at the time of recruitment), however, they could become ‘noise traps’, preventing post-settlers from moving away, i.e., towards the deeper recruitment habitats (see Figure 7 for a schematic representation of this hypothesis).
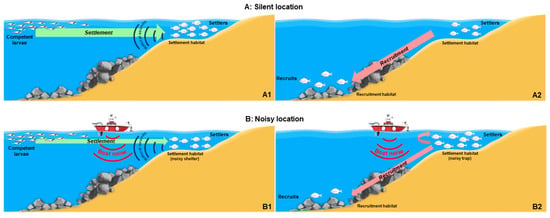
Figure 7.
Schematic representation of the hypothesis proposed in this study. In silent conditions (panel (A1)), competent larvae use the natural sound cues to reach a suitable settlement habitat/site (green arrow), and then (A2) late post-settlers leave the settlement habitat/site (pink arrow) to recruit into the adult population. MNP caused by boat traffic (B1) masks the natural sound cues used by competent larvae to reach the suitable settlement habitat/site, thus reducing settlement intensity, and then (B2) late post-settlers are disturbed by boat noise when they should leave the settlement habitat/site, which could reduce and delay recruitment and cause unnaturally higher late post-settler abundances in settlement habitats/sites.
As discussed before in this section, our data are substantially in line with the available literature on the multiple negative effects caused by boat traffic on juvenile fish. This is especially true in terms of the potential of MNP to mask the sound cues used by fish larvae or juvenile fish to identify suitable settlement and recruitment habitats/sites, which could reduce settlement and recruitment intensity with possible repercussions on population dynamics [19]. Furthermore, MNP is reported (i) to increase juvenile fish mortality by affecting anti-predator behaviors [86,90,91] and (ii) worsen the physiological condition of juvenile fish, factors that negatively affect survival and population density [92,93,94].
Our findings based on mensurative experiments (sensu Underwood, 1997 [95]) do not logically imply a causal link between boat MNP and the effects observed on juvenile fish. Further specific experiments would thus be needed to better understand if and how MNP related to boat traffic may affect the density of early or late post-settler sparid fishes and of any other Mediterranean coastal fish. The patterns that emerged in this study, however, enable us to draw a number of hypotheses on the possible mechanisms (causal processes) regarding the MNP effects, which could help formulate sound hypotheses to be then tested through appropriate manipulative experiments [95].
The use of an NI composed of multiple parameters is likely more representative of the effect of boat traffic on marine organisms than noise levels alone. Moreover, it may allow us to identify which component (noise exposure duration, noise level of passing boat, number of boats, etc.) mostly influences specific species. An NI of this kind may therefore help identify thresholds of species-specific responses to be considered in risk assessment [96].
In conclusion, this study provides evidence of possible effects of boat MNP on juvenile Mediterranean coastal fishes, exploring plausible hypotheses to explain the observed patterns that future studies could test via well-designed manipulative experiments. Although we observed a non-negligible variability, our results should be considered as a promising step towards gathering information on the MNP caused by boat traffic and its impact on juvenile fish that may propagate at population and community levels and potentially affect ecosystem functioning and services, in the light of the various and important ecological and economic roles that coastal fishes (including sparids) play [97,98].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15010092/s1. Figure S1: Boats counted by photo-sampling at the four sampling locations during the sampling period in 2019 (A), 2020 (B) and 2021(C). Colors of the four lines representing the four sampling locations refer the NI gradient, from the lowest level of noise (blue/green lines) to the highest (red line). In spite of a non-negligible variability in time (especially among sampling dates), a consistent pattern across years emerges, with Marinières being the location with the highest NI value, and Eze-sur-Mer and Cap Ferrat being the locations with the lowest NI values. Figure S2: Size-frequency distributions of juveniles of Diplodus sargus, D. puntazzo, D. vulgaris and Sarpa salpa at the two first sampling dates (data were pooled for 2019, 2020 and 2021, the general patterns being similar) showing that, initially, D. sargus were early settlers (smaller in size: modal class = 2 cm, max = 3 cm standard length, SL), while the other species (D. puntazzo, D. vulgaris and Sarpa salpa) were late post-settlers (bigger in size: modal classes = 3–5 cm; max = 7–8 cm SL). Figure S3: Mean (±SE) values of density of juveniles of S. salpa across sampling dates in 2020. Each point represents the mean density for each level of sampling date investigated. The line represents the linear relation between density of juveniles of S. salpa across sampling dates. Figure S4: Bathymetry (from −5 to −40 m; red lines) and biocenoses of the study area. Blue ovals represent the 4 study locations (DONIA: Habitat cartography-Data consulted 10/2021 on the MEDTRIX surveillance platform) (https://plateforme.medtrix.fr) (accessed on 8 January 2023)). Figure S5: Mean values (±SE) of sound pressure level in the third octave band centered around 250 Hz (where most of the anthropogenic continuous noise is produced) recorded within contemporaneous temporal windows by hydrophones located at 10 m (fixed) and 1 m depth (portable; the microhabitat for settlement of most sparid fishes), at the four sampling locations. Table S1: Outputs of the ANOVA computed on the ManyGLM for the three sampling years. Table S2: Outputs of the post-hoc analyses performed the ManyGLM for 2020 and 2021. Table S3. Results of the univariate test from the output of the ANOVA computed on the ManyGLM for the three sampling years. Table S4: Outputs of the ANOVA computed on the D. sargus glmmTMB model for the three sampling years. Table S5: Post-hocs analyses of the ANOVA computed on the D. sargus glmmTMB model for the factor location in 2021. Table S6: Outputs of the ANOVA computed on the D. puntazzo glmmTMB model for the three sampling years. Table S7: Post-hocs analyses of the ANOVA computed on the D. puntazzo glmmTMB model for the factor location in 2021. Table S8: Outputs of the ANOVA computed on the D. vulgaris glmmTMB model for the three sampling years. Table S9: Post-hocs analyses of the ANOVA computed on the D. vulgaris glmmTMB model for the factor location in 2021. Table S10. Output of the ANOVA computed on the S. salpa glmmTMB model for 2020.
Author Contributions
Conceptualization, E.D.F., F.R., L.D.I., P.P. and P.G.; methodology, E.D.F., F.R., L.D.I. and P.G.; field data collection, E.D.F., L.D.I., K.S., A.C., A.D.F., G.S., J.-M.C., B.D., S.B., P.G.; software, validation and formal analysis, E.D.F., F.R., L.D.I. and P.G.; data curation, E.D.F., F.R., L.D.I.; writing, from original draft preparation to review and editing, all co-authors; visualization, E.D.F., F.R., L.D.I.; supervision, P.G., F.R., P.P.; funding acquisition and project administration, P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out within the framework of the Nautilus Project, supported by the Academy of Excellence 3 (Space, Environment, Risk and Resilience), and has been supported by the French government, through the UCA JEDI Investments in the Future project managed by the National Research Agency (ANR) with the reference number ANR-15-IDEX-01.
Institutional Review Board Statement
Our study employed the use of non-destructive nor intrusive methods, so it did not require any ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Mate-data are available on request.
Acknowledgments
We wish to thank Coralie Meinesz and Adrien Lyonnet of the Metropole Nice Côte d’Azur for the data on boat frequentation as well as Alexis Pey and Patricia Ventura of ‘THALASSA Marine research & Environmental awareness’ for their help in setting and retrieving the hydrophones. Finally, we would like to thank Michael Paul for the revision of the English language.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pijanowski, B.C.; Villanueva-Rivera, L.J.; Dumyahn, S.L.; Farina, A.; Krause, B.L.; Napoletano, B.M.; Gage, S.H.; Pieretti, N. Soundscape Ecology: The Science of Sound in the Landscape. BioScience 2011, 61, 203–216. [Google Scholar] [CrossRef]
- Sueur, J.; Farina, A. Ecoacoustics: The Ecological Investigation and Interpretation of Environmental Sound. Biosemiotics 2015, 8, 493–502. [Google Scholar] [CrossRef]
- Mooney, T.A.; Di Iorio, L.; Lammers, M.; Lin, T.-H.; Nedelec, S.L.; Parsons, M.; Radford, C.; Urban, E.; Stanley, J. Listening Forward: Approaching Marine Biodiversity Assessments Using Acoustic Methods. R. Soc. Open Sci. 2020, 7, 201287. [Google Scholar] [CrossRef] [PubMed]
- Tolimieri, N.; Jeffs, A.; Montgomery, J. Ambient Sound as a Cue for Navigation by the Pelagic Larvae of Reef Fishes. Mar. Ecol. Prog. Ser. 2000, 207, 219–224. [Google Scholar] [CrossRef]
- Tyack, P.L.; Clark, C.W. Communication and Acoustic Behavior of Dolphins and Whales. In Hearing by Whales and Dolphins; Au, W.W.L., Fay, R.R., Popper, A.N., Eds.; Springer Handbook of Auditory Research; Springer: New York, NY, USA, 2000; Volume 12, pp. 156–224. ISBN 978-1-4612-7024-9. [Google Scholar]
- Stanley, J.A.; Radford, C.A.; Jeffs, A.G. Location, Location, Location: Finding a Suitable Home among the Noise. Proc. R. Soc. B Biol. Sci. 2012, 279, 3622–3631. [Google Scholar] [CrossRef]
- Ladich, F. Sound Communication in Fishes, 1st ed.; Animal Signals and Communication; Springer: Vienna, Austria, 2015; ISBN 978-3-7091-1846-7. [Google Scholar]
- Popper, A.N.; Hawkins, A. The Effects of Noise on Aquatic Life II; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2016; Volume 875, ISBN 978-1-4939-2980-1. [Google Scholar]
- Cox, K.; Brennan, L.P.; Gerwing, T.G.; Dudas, S.E.; Juanes, F. Sound the Alarm: A Meta-Analysis on the Effect of Aquatic Noise on Fish Behavior and Physiology. Global Chang. Biol. 2018, 24, 3105–3116. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, E.; Pierson, P.; Di Iorio, L.; Calò, A.; Cottalorda, J.M.; Derijard, B.; Di Franco, A.; Galvé, A.; Guibbolini, M.; Lebrun, J.; et al. Effects of Marine Noise Pollution on Mediterranean Fishes and Invertebrates: A Review. Mar. Pollut. Bull. 2020, 159, 111450. [Google Scholar] [CrossRef]
- Solé, M.; Lenoir, M.; Fontuño, J.M.; Durfort, M.; van der Schaar, M.; André, M. Evidence of Cnidarians Sensitivity to Sound after Exposure to Low Frequency Noise Underwater Sources. Sci. Rep. 2016, 6, 37979. [Google Scholar] [CrossRef] [PubMed]
- Weilgart, L. The Impact of Ocean Noise Pollution on Fish and Invertebrates. Doctoral Dissertation, Oceancare & Dalhousie University, Dalhousie, NS, Camada, 2018. Volume 34. [Google Scholar]
- Duarte, C.M.; Chapuis, L.; Collin, S.P.; Costa, D.P.; Devassy, R.P.; Eguiluz, V.M.; Erbe, C.; Gordon, T.A.C.; Halpern, B.S.; Harding, H.R.; et al. The Soundscape of the Anthropocene Ocean. Science 2021, 371, eaba4658. [Google Scholar] [CrossRef]
- Levin, P.S. Fine-Scale Temporal Variation in Recruitment of a Temperate Demersal Fish: The Importance of Settlement versus Post-Settlement Loss. Oecologia 1994, 97, 124–133. [Google Scholar] [CrossRef]
- Macpherson, E. Ontogenetic Shifts in Habitat Use and Aggregation in Juvenile Sparid Fishes. J. Exp. Mar. Biol. Ecol. 1998, 220, 127–150. [Google Scholar] [CrossRef]
- Leis, J.M.; McCormick, M.I. The Biology, Behavior, and Ecology of the Pelagic, Larval Stage of Coral Reef Fishes. In Coral Reef Fishes; Elsevier: Amsterdam, The Netherlands, 2002; pp. 171–199. ISBN 978-0-12-615185-5. [Google Scholar]
- Bussotti, S.; Guidetti, P. Timing and Habitat Preferences for Settlement of Juvenile Fishes in the Marine Protected Area of Torre Guaceto (South-Eastern Italy, Adriatic Sea). Ital. J. Zool. 2011, 78, 243–254. [Google Scholar] [CrossRef]
- Simpson, S.D.; Radford, A.N.; Tickle, E.J.; Meekan, M.G.; Jeffs, A.G. Adaptive Avoidance of Reef Noise. PLoS ONE 2011, 6, e16625. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.D.; Radford, A.N.; Holles, S.; Ferarri, M.C.O.; Chivers, D.P.; McCormick, M.I.; Meekan, M.G. Small-Boat Noise Impacts Natural Settlement Behavior of Coral Reef Fish Larvae. In The Effects of Noise on Aquatic Life II; Popper, A.N., Hawkins, A., Eds.; Springer: New York, NY, USA, 2016; Volume 875, pp. 1041–1048. ISBN 978-1-4939-2980-1. [Google Scholar]
- Holles, S.; Simpson, S.; Radford, A.; Berten, L.; Lecchini, D. Boat Noise Disrupts Orientation Behaviour in a Coral Reef Fish. Mar. Ecol. Prog. Ser. 2013, 485, 295–300. [Google Scholar] [CrossRef]
- Simpson, S.; Meekan, M.; McCauley, R.; Jeffs, A. Attraction of Settlement-Stage Coral Reef Fishes to Reef Noise. Mar. Ecol. Prog. Ser. 2004, 276, 263–268. [Google Scholar] [CrossRef]
- Kingsford, M.J.; Leis, J.M.; Shanks, A.; Lindeman, K.C.; Morgan, S.G.; Pineda, J. Sensory Environments, Larval Abilities and Local Self-Recruitment. Bull. Mar. Sci. 2002, 70, 309–340. [Google Scholar]
- Gordon, T.A.C.; Harding, H.R.; Wong, K.E.; Merchant, N.D.; Meekan, M.G.; McCormick, M.I.; Radford, A.N.; Simpson, S.D. Habitat Degradation Negatively Affects Auditory Settlement Behavior of Coral Reef Fishes. Proc. Natl. Acad. Sci. USA 2018, 115, 5193–5198. [Google Scholar] [CrossRef] [PubMed]
- Beets, J. Effects of a Predatory Fish on the Recruitment and Abundance of Caribbean Coral Reef Fishes. Mar. Ecol. Prog. Ser. 1997, 148, 11–21. [Google Scholar] [CrossRef]
- Fontes, J.; Caselle, J.E.; Afonso, P.; Santos, R.S. Multi-Scale Recruitment Patterns and Effects on Local Population Size of a Temperate Reef Fish. J. Fish Biol. 2009, 75, 1271–1286. [Google Scholar] [CrossRef]
- Gordon, T.A.C.; Radford, A.N.; Davidson, I.K.; Barnes, K.; McCloskey, K.; Nedelec, S.L.; Meekan, M.G.; McCormick, M.I.; Simpson, S.D. Acoustic Enrichment Can Enhance Fish Community Development on Degraded Coral Reef Habitat. Nat. Commun. 2019, 10, 5414. [Google Scholar] [CrossRef]
- Airoldi, L.; Beck, M.W.; Firth, L.B.; Bugnot, A.B.; Steinberg, P.D.; Dafforn, K.A. Emerging Solutions to Return Nature to the Urban Ocean. Annu. Rev. Mar. Sci. 2021, 13, 445–477. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Airoldi, L.; Ballesteros, E.; Benedetti-Cecchi, L.; Boero, F.; Bulleri, F.; Cebrian, E.; Cerrano, C.; Claudet, J.; Colloca, F.; et al. Mediterranean Rocky Reefs in the Anthropocene: Present Status and Future Concerns. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 89, pp. 1–51. ISBN 978-0-12-824623-8. [Google Scholar]
- Coll, M.; Piroddi, C.; Albouy, C.; Ben Rais Lasram, F.; Cheung, W.W.L.; Christensen, V.; Karpouzi, V.S.; Guilhaumon, F.; Mouillot, D.; Paleczny, M.; et al. The Mediterranean Sea under Siege: Spatial Overlap between Marine Biodiversity, Cumulative Threats and Marine Reserves: The Mediterranean Sea under Siege. Glob. Ecol. Biogeogr. 2012, 21, 465–480. [Google Scholar] [CrossRef]
- Micheli, F.; Halpern, B.S.; Walbridge, S.; Ciriaco, S.; Ferretti, F.; Fraschetti, S.; Lewison, R.; Nykjaer, L.; Rosenberg, A.A. Cumulative Human Impacts on Mediterranean and Black Sea Marine Ecosystems: Assessing Current Pressures and Opportunities. PLoS ONE 2013, 8, e79889. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase 2021. Available online: https://www.fishbase.org (accessed on 1 January 2023).
- Vigliola, L.; Harmelin-Vivien, M. Post-Settlement Ontogeny in Three Mediterranean Reef Fish Species of the Genus Diplodus. Bull. Mar. Sci. 2001, 68, 16. [Google Scholar]
- Di Franco, A.; Calò, A.; Pennetta, A.; De Benedetto, G.; Planes, S.; Guidetti, P. Dispersal of Larval and Juvenile Seabream: Implications for Mediterranean Marine Protected Areas. Biol. Conserv. 2015, 192, 361–368. [Google Scholar] [CrossRef]
- Isnard, E.; Tournois, J.; McKenzie, D.J.; Ferraton, F.; Bodin, N.; Aliaume, C.; Darnaude, A.M. Getting a Good Start in Life? A Comparative Analysis of the Quality of Lagoons as Juvenile Habitats for the Gilthead Seabream Sparus aurata in the Gulf of Lions. Estuaries Coasts 2015, 38, 1937–1950. [Google Scholar] [CrossRef]
- Vandenbussche, P.S.P.; Spennato, G.; Pierson, P.M. Juvenile Oblada melanura (L. 1758) Otolith Shape Is Impacted near Recreational Harbours or Due to Settlement Position in Nearby Sites. Cont. Shelf Res. 2020, 208, 104239. [Google Scholar] [CrossRef]
- Di Franco, A.; Di Lorenzo, M.; Guidetti, P. Spatial Patterns of Density at Multiple Life Stages in Protected and Fished Conditions: An Example from a Mediterranean Coastal Fish. J. Sea Res. 2013, 76, 73–81. [Google Scholar] [CrossRef]
- Biagi, F.; Gambaccini, S.; Zazzetta, M. Settlement and Recruitment in Fishes: The Role of Coastal Areas. Ital. J. Zool. 1998, 65, 269–274. [Google Scholar] [CrossRef]
- Vigliola, L.; Harmelin-Vivien, M.; Biagi, F.; Galzin, R.; Garcia-Rubies, A.; Harmelin, J.; Jouvenel, J.; Le Direach-Boursier, L.; Macpherson, E.; Tunesi, L. Spatial and Temporal Patterns of Settlement among Sparid Fishes of the Genus Diplodus in the Northwestern Mediterranean. Mar. Ecol. Prog. Ser. 1998, 168, 45–56. [Google Scholar] [CrossRef]
- Di Franco, A.; Guidetti, P. Patterns of Variability in Early-Life Traits of Fishes Depend on Spatial Scale of Analysis. Biol. Lett. 2011, 7, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Harmelin-Vivien, M.L.; Harmelin, J.G.; Leboulleux, V. Microhabitat Requirements for Settlement of Juvenile Sparid Fishes on Mediterranean Rocky Shores. Hydrobiologia 1995, 300–301, 309–320. [Google Scholar] [CrossRef]
- Meinesz, A.; Blanfuné, A.; Chancollon, O.; Javel, F.; Longepierre, S.; Markovic, L.; Vaugelas de, J.; Garcia, D. Côtes Méditerranéennes Françaises: Inventaire et Impacts des Aménagements Gagnés sur la mer; Lab. ECOMERS, Université de Nice Sophia Antipolis: Nice, France, 2013; p. 156, Et Pubblication Electronique. [Google Scholar]
- Maglio, A.; Soares, C.; Bouzidi, M.; Zabel, F.; Souami, Y.; Pavan, G. Mapping Shipping Noise in the Pelagos Sanctuary (French Part) through Acoustic Modelling to Assess Potential Impacts on Marine Mammals. Sci. Rep. Port-Cros. Natl. Park 2015, 185, 167–185. [Google Scholar]
- Ourmieres, Y.; Mansui, J.; Molcard, A.; Galgani, F.; Poitou, I. The Boundary Current Role on the Transport and Stranding of Floating Marine Litter: The French Riviera Case. Cont. Shelf Res. 2018, 155, 11–20. [Google Scholar] [CrossRef]
- Di Franco, A.; Gianni, F.; Guidetti, P. Mismatch in Early Life Traits between Settlers and Recruits in a Mediterranean Fish: Clue of the Relevance of the Settlement Tail? Acta Ichthyol. Et Piscat. 2015, 45, 153–159. [Google Scholar] [CrossRef]
- Wright, K.J.; Higgs, D.M.; Cato, D.H.; Leis, J.M. Auditory Sensitivity in Settlement-Stage Larvae of Coral Reef Fishes. Coral Reefs 2010, 29, 235–243. [Google Scholar] [CrossRef]
- Ainslie, M.A. Standard for Measurement and Monitoring of Underwater Noise, Part I; Physical Quantities and Their Units; 2011; Retrieved Online on 10 January 2023. Available online: https://www.noordzeeloket.nl/publish/pages/122320/standard_for_measurement_and_monitoring_of_underwater_noise_part_i_648.pdf (accessed on 8 January 2023).
- Colleye, O.; Kéver, L.; Lecchini, D.; Berten, L.; Parmentier, E. Auditory Evoked Potential Audiograms in Post-Settlement Stage Individuals of Coral Reef Fishes. J. Exp. Mar. Biol. Ecol. 2016, 483, 1–9. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package_. R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 8 January 2023).
- Wang, Y.; Naumann, U.; Wright, S.; Warton, D.I. Mvabund: An R Package for Model-Based Analysis of Multivariate Abundance Data. Methods Ecol. Evol. 2012, 3, 471–474. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. Permanova+ for Primer: Guide to Software and Statistical Methods. J. Oceanogr. 2008, 67, 589–599. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; Benthem, K.J.; van Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2014. [Google Scholar]
- Chahouri, A.; Elouahmani, N.; Ouchene, H. Recent Progress in Marine Noise Pollution: A Thorough Review. Chemosphere 2022, 291, 132983. [Google Scholar] [CrossRef]
- Caraka, R.E.; Yusra, Y.; Toharudin, T.; Chen, R.-C.; Basyuni, M.; Juned, V.; Gio, P.U.; Pardamean, B. Did Noise Pollution Really Improve during COVID-19? Evidence from Taiwan. Sustainability 2021, 13, 5946. [Google Scholar] [CrossRef]
- Ryan, J.P.; Joseph, J.E.; Margolina, T.; Hatch, L.T.; Azzara, A.; Reyes, A.; Southall, B.L.; DeVogelaere, A.; Peavey Reeves, L.E.; Zhang, Y.; et al. Reduction of Low-Frequency Vessel Noise in Monterey Bay National Marine Sanctuary During the COVID-19 Pandemic. Front. Mar. Sci. 2021, 8, 656566. [Google Scholar] [CrossRef]
- Bertucci, F.; Lecchini, D.; Greeven, C.; Brooker, R.M.; Minier, L.; Cordonnier, S.; René-Trouillefou, M.; Parmentier, E. Changes to an Urban Marina Soundscape Associated with COVID-19 Lockdown in Guadeloupe. Environ. Pollut. 2021, 289, 117898. [Google Scholar] [CrossRef] [PubMed]
- Karkarey, R.; Theo, A.H. Homeward Bound: Fish Larvae Use Dispersal Corridors When Settling on Coral Reefs. Front. Ecol. Environ. 2016, 14, 569–570. [Google Scholar] [CrossRef]
- Azzurro, E.; Matiddi, M.; Fanelli, E.; Guidetti, P.; Mesa, G.L.; Scarpato, A.; Axiak, V. Sewage Pollution Impact on Mediterranean Rocky-Reef Fish Assemblages. Mar. Environ. Res. 2010, 69, 390–397. [Google Scholar] [CrossRef]
- Lee, J.-W.; Choi, H.; Hwang, U.-K.; Kang, J.-C.; Kang, Y.J.; Kim, K.I.; Kim, J.-H. Toxic Effects of Lead Exposure on Bioaccumulation, Oxidative Stress, Neurotoxicity, and Immune Responses in Fish: A Review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fortibuoni, T.; Giovanardi, O.; Pranovi, F.; Raicevich, S.; Solidoro, C.; Libralato, S. Analysis of Long-Term Changes in a Mediterranean Marine Ecosystem Based on Fishery Landings. Front. Mar. Sci. 2017, 4, 33. [Google Scholar] [CrossRef]
- Kantoussan, J.; Laë, R.; Tine, M. Review of the Fisheries Indicators for Monitoring the Impacts of Fishing on Fish Communities. Rev. Fish. Sci. Aquac. 2018, 26, 460–478. [Google Scholar] [CrossRef]
- Edgar, G.J.; Stuart-Smith, R.D.; Willis, T.J.; Kininmonth, S.; Baker, S.C.; Banks, S.; Barrett, N.S.; Becerro, M.A.; Bernard, A.T.F.; Berkhout, J.; et al. Global Conservation Outcomes Depend on Marine Protected Areas with Five Key Features. Nature 2014, 506, 216–220. [Google Scholar] [CrossRef]
- Giakoumi, S.; Scianna, C.; Plass-Johnson, J.; Micheli, F.; Grorud-Colvert, K.; Thiriet, P.; Claudet, J.; Di Carlo, G.; Di Franco, A.; Gaines, S.D.; et al. Ecological Effects of Full and Partial Protection in the Crowded Mediterranean Sea: A Regional Meta-Analysis. Sci. Rep. 2017, 7, 8940. [Google Scholar] [CrossRef]
- Sievert, N.A.; Paukert, C.P.; Tsang, Y.-P.; Infante, D. Development and Assessment of Indices to Determine Stream Fish Vulnerability to Climate Change and Habitat Alteration. Ecol. Indic. 2016, 67, 403–416. [Google Scholar] [CrossRef]
- Sabetian, A.; Zhang, J.; Campbell, M.; Walter, R.; Allen, H.; Reid, M.; Wijenayake, K.; Lilkendey, J. Fish Nearshore Habitat-Use Patterns as Ecological Indicators of Nursery Quality. Ecol. Indic. 2021, 131, 108225. [Google Scholar] [CrossRef]
- Guidetti, P.; Baiata, P.; Ballesteros, E.; Di Franco, A.; Hereu, B.; Macpherson, E.; Micheli, F.; Pais, A.; Panzalis, P.; Rosenberg, A.A.; et al. Large-Scale Assessment of Mediterranean Marine Protected Areas Effects on Fish Assemblages. PLoS ONE 2014, 9, e91841. [Google Scholar] [CrossRef]
- Di Franco, E.; Di Franco, A.; Calò, A.; Di Lorenzo, M.; Mangialajo, L.; Bussotti, S.; Bianchi, C.N.; Guidetti, P. Inconsistent Relationships among Protection, Benthic Assemblage, Habitat Complexity and Fish Biomass in Mediterranean Temperate Rocky Reefs. Ecol. Indic. 2021, 128, 107850. [Google Scholar] [CrossRef]
- Vargas, C.A.; Lagos, N.A.; Lardies, M.A.; Duarte, C.; Manríquez, P.H.; Aguilera, V.M.; Broitman, B.; Widdicombe, S.; Dupont, S. Species-Specific Responses to Ocean Acidification Should Account for Local Adaptation and Adaptive Plasticity. Nat. Ecol. Evol. 2017, 1, 0084. [Google Scholar] [CrossRef]
- Audzijonyte, A.; Richards, S.A.; Stuart-Smith, R.D.; Pecl, G.; Edgar, G.J.; Barrett, N.S.; Payne, N.; Blanchard, J.L. Fish Body Sizes Change with Temperature but Not All Species Shrink with Warming. Nat. Ecol. Evol. 2020, 4, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.R.; Simning, D.; Serafin, J.; Sepúlveda, M.S.; Griffitt, R.J. Acute Exposure to Oil Induces Age and Species-Specific Transcriptional Responses in Embryo-Larval Estuarine Fish. Environ. Pollut. 2020, 263, 114325. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Whitfield, A.K.; Cowley, P.D.; Järnegren, J.; Næsje, T.F. Does Boat Traffic Cause Displacement of Fish in Estuaries? Mar. Pollut. Bull. 2013, 75, 168–173. [Google Scholar] [CrossRef]
- Engås, A.; Løkkeborg, S.; Ona, E.; Soldal, A.V. Effects of Seismic Shooting on Local Abundance and Catch Rates of Cod (Gadus Morhua) and Haddock (Melanogrammus Aeglefinus). Can. J. Fish. Aquat. Sci. 1996, 53, 2238–2249. [Google Scholar] [CrossRef]
- Paxton, A.B.; Taylor, J.C.; Nowacek, D.P.; Dale, J.; Cole, E.; Voss, C.M.; Peterson, C.H. Seismic Survey Noise Disrupted Fish Use of a Temperate Reef. Mar. Policy 2017, 78, 68–73. [Google Scholar] [CrossRef]
- Slotte, A.; Hansen, K.; Dalen, J.; Ona, E. Acoustic Mapping of Pelagic Fish Distribution and Abundance in Relation to a Seismic Shooting Area off the Norwegian West Coast. Fish. Res. 2004, 67, 143–150. [Google Scholar] [CrossRef]
- Amorim, M.C.P.; Vieira, M.; Meireles, G.; Novais, S.C.; Lemos, M.F.L.; Modesto, T.; Alves, D.; Zuazu, A.; Lopes, A.F.; Matos, A.B.; et al. Boat Noise Impacts Lusitanian Toadfish Breeding Males and Reproductive Outcome. Sci. Total Environ. 2022, 830, 154735. [Google Scholar] [CrossRef]
- Faria, A.; Fonseca, P.J.; Vieira, M.; Alves, L.M.F.; Lemos, M.F.L.; Novais, S.C.; Matos, A.B.; Vieira, D.; Amorim, M.C.P. Boat Noise Impacts Early Life Stages in the Lusitanian Toadfish: A Field Experiment. Sci. Total Environ. 2022, 811, 151367. [Google Scholar] [CrossRef]
- Jain-Schlaepfer, S.; Fakan, E.; Rummer, J.L.; Simpson, S.D.; McCormick, M.I. Impact of Motorboats on Fish Embryos Depends on Engine Type. Conserv. Physiol. 2018, 6, coy014. [Google Scholar] [CrossRef]
- Nedelec, S.L.; Radford, A.N.; Gatenby, P.; Davidson, I.K.; Velasquez Jimenez, L.; Travis, M.; Chapman, K.E.; McCloskey, K.P.; Lamont, T.A.C.; Illing, B.; et al. Limiting Motorboat Noise on Coral Reefs Boosts Fish Reproductive Success. Nat. Commun. 2022, 13, 2822. [Google Scholar] [CrossRef] [PubMed]
- Voellmy, I.K.; Purser, J.; Flynn, D.; Kennedy, P.; Simpson, S.D.; Radford, A.N. Acoustic Noise Reduces Foraging Success in Two Sympatric Fish Species via Different Mechanisms. Anim. Behav. 2014, 89, 191–198. [Google Scholar] [CrossRef]
- Fakan, E.P.; McCormick, M.I. Boat Noise Affects the Early Life History of Two Damselfishes. Mar. Pollut. Bull. 2019, 141, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Radford, A.N.; Lèbre, L.; Lecaillon, G.; Nedelec, S.L.; Simpson, S.D. Repeated Exposure Reduces the Response to Impulsive Noise in European Seabass. Glob. Chang. Biol. 2016, 22, 3349–3360. [Google Scholar] [CrossRef]
- Mauro, M.; Pérez-Arjona, I.; Perez, E.J.B.; Ceraulo, M.; Bou-Cabo, M.; Benson, T.; Espinosa, V.; Beltrame, F.; Mazzola, S.; Vazzana, M.; et al. The Effect of Low Frequency Noise on the Behaviour of Juvenile Sparus aurata. J. Acoust. Soc. Am. 2020, 147, 3795–3807. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.J.; Lecchini, D.; Beck, H.J.; Cadiou, G.; Lecellier, G.; Booth, D.J.; Nakamura, Y. Sediment Pollution Impacts Sensory Ability and Performance of Settling Coral-Reef Fish. Oecologia 2016, 180, 11–21. [Google Scholar] [CrossRef]
- Cuadros, A.; Basterretxea, G.; Cardona, L.; Cheminée, A.; Hidalgo, M.; Moranta, J. Settlement and Post-Settlement Survival Rates of the White Seabream (Diplodus sargus) in the Western Mediterranean Sea. PLoS ONE 2018, 13, e0190278. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.D.; Radford, A.N.; Nedelec, S.L.; Ferrari, M.C.O.; Chivers, D.P.; McCormick, M.I.; Meekan, M.G. Anthropogenic Noise Increases Fish Mortality by Predation. Nat. Commun. 2016, 7, 10544. [Google Scholar] [CrossRef] [PubMed]
- Cheminée, A.; Francour, P.; Harmelin-Vivien, M. Assessment of Diplodus spp. (Sparidae) Nursery Grounds along the Rocky Shore of Marseilles (France, NW Mediterranean). Sci. Mar. 2011, 75, 181–188. [Google Scholar] [CrossRef]
- Ventura, D.; Jona Lasinio, G.; Ardizzone, G. Temporal Partitioning of Microhabitat Use among Four Juvenile Fish Species of the Genus Diplodus (Pisces: Perciformes, Sparidae). Mar. Ecol. 2015, 36, 1013–1032. [Google Scholar] [CrossRef]
- Di Franco, A.; Gillanders, B.M.; De Benedetto, G.; Pennetta, A.; De Leo, G.A.; Guidetti, P. Dispersal Patterns of Coastal Fish: Implications for Designing Networks of Marine Protected Areas. PLoS ONE 2012, 7, e31681. [Google Scholar] [CrossRef] [PubMed]
- Spiga, I.; Aldred, N.; Caldwell, G.S. Anthropogenic Noise Compromises the Anti-Predator Behaviour of the European Seabass, Dicentrarchus labrax (L.). Mar. Pollut. Bull. 2017, 122, 297–305. [Google Scholar] [CrossRef]
- Kok, A.C.M.; van Hulten, D.; Timmerman, K.H.; Lankhorst, J.; Visser, F.; Slabbekoorn, H. Interacting Effects of Short-Term and Long-Term Noise Exposure on Antipredator Behaviour in Sand Gobies. Anim. Behav. 2021, 172, 93–102. [Google Scholar] [CrossRef]
- Filiciotto, F.; Giacalone, V.M.; Fazio, F.; Buffa, G.; Piccione, G.; Maccarrone, V.; Di Stefano, V.; Mazzola, S.; Buscaino, G. Effect of Acoustic Environment on Gilthead Sea Bream (Sparus aurata): Sea and Onshore Aquaculture Background Noise. Aquaculture 2013, 414–415, 36–45. [Google Scholar] [CrossRef]
- Nichols, T.A.; Anderson, T.W.; Širović, A. Intermittent Noise Induces Physiological Stress in a Coastal Marine Fish. PLoS ONE 2015, 10, e0139157. [Google Scholar] [CrossRef]
- Celi, M.; Filiciotto, F.; Maricchiolo, G.; Genovese, L.; Quinci, E.M.; Maccarrone, V.; Mazzola, S.; Vazzana, M.; Buscaino, G. Vessel Noise Pollution as a Human Threat to Fish: Assessment of the Stress Response in Gilthead Sea Bream (Sparus aurata, Linnaeus 1758). Fish Physiol. Biochem. 2016, 42, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance. J. Mar. Biol. Ass. 1997, 77, 572. [Google Scholar] [CrossRef]
- Verling, E.; Miralles Ricós, R.; Bou-Cabo, M.; Lara, G.; Garagouni, M.; Brignon, J.-M.; O’Higgins, T. Application of a Risk-Based Approach to Continuous Underwater Noise at Local and Subregional Scales for the Marine Strategy Framework Directive. Mar. Policy 2021, 134, 104786. [Google Scholar] [CrossRef]
- Sala, E.; Boudouresque, C.F.; Harmelin-Vivien, M. Fishing, Trophic Cascades, and the Structure of Algal Assemblages: Evaluation of an Old but Untested Paradigm. Oikos 1998, 82, 425. [Google Scholar] [CrossRef]
- Guidetti, P. Marine Reserves Reestablish Lost Predatory Interactions And Cause Community Changes In Rocky Reefs. Ecol. Appl. 2006, 16, 963–976. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).