Terrestrial and Subterranean Mammals as Reservoirs of Zoonotic Diseases in the Central Part of European Russia
Abstract
1. Introduction
2. Materials and Methods
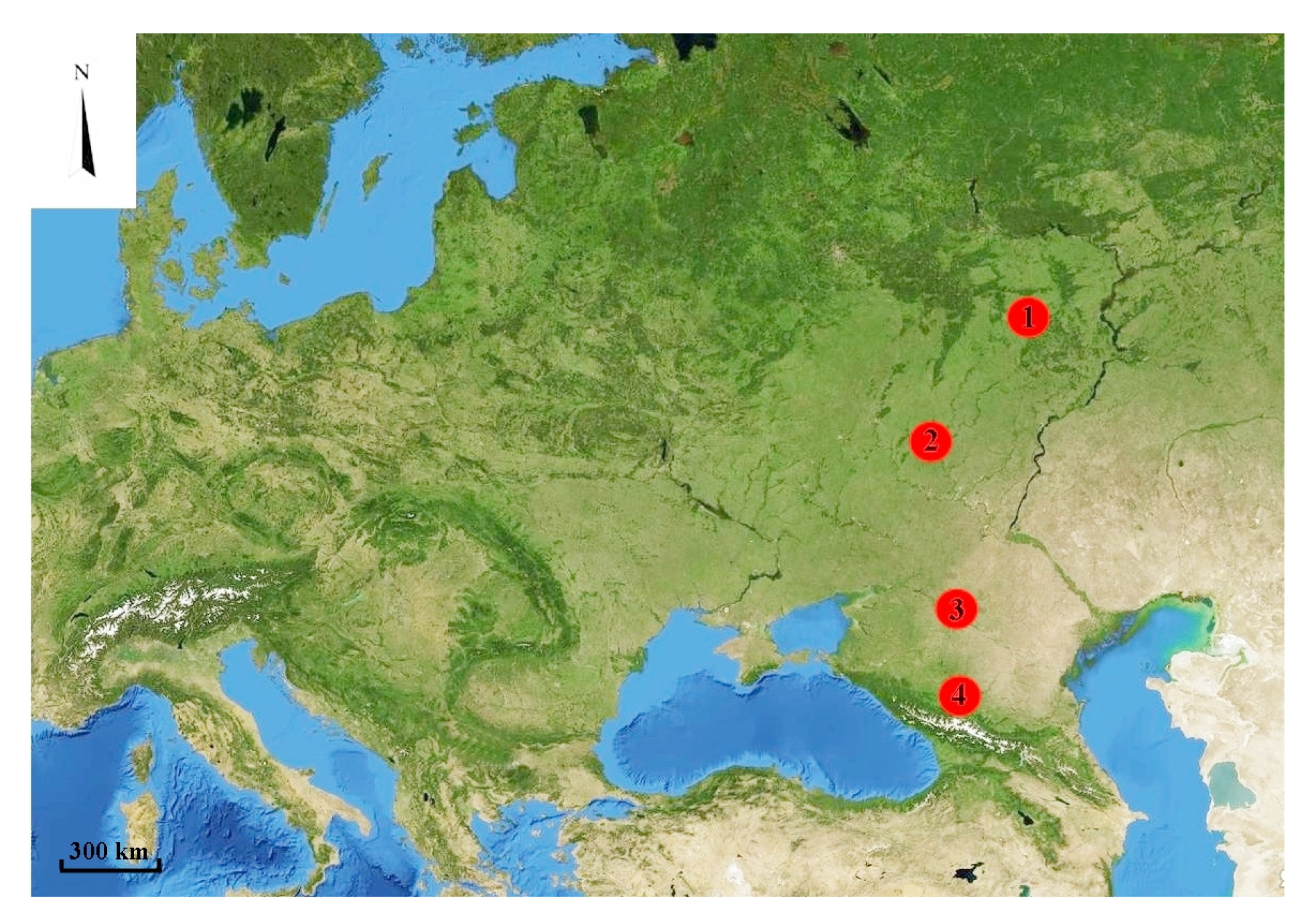
2.1. Study Area
2.2. Small Mammal Trapping Methods
2.3. Molecular and Statistical Analyses
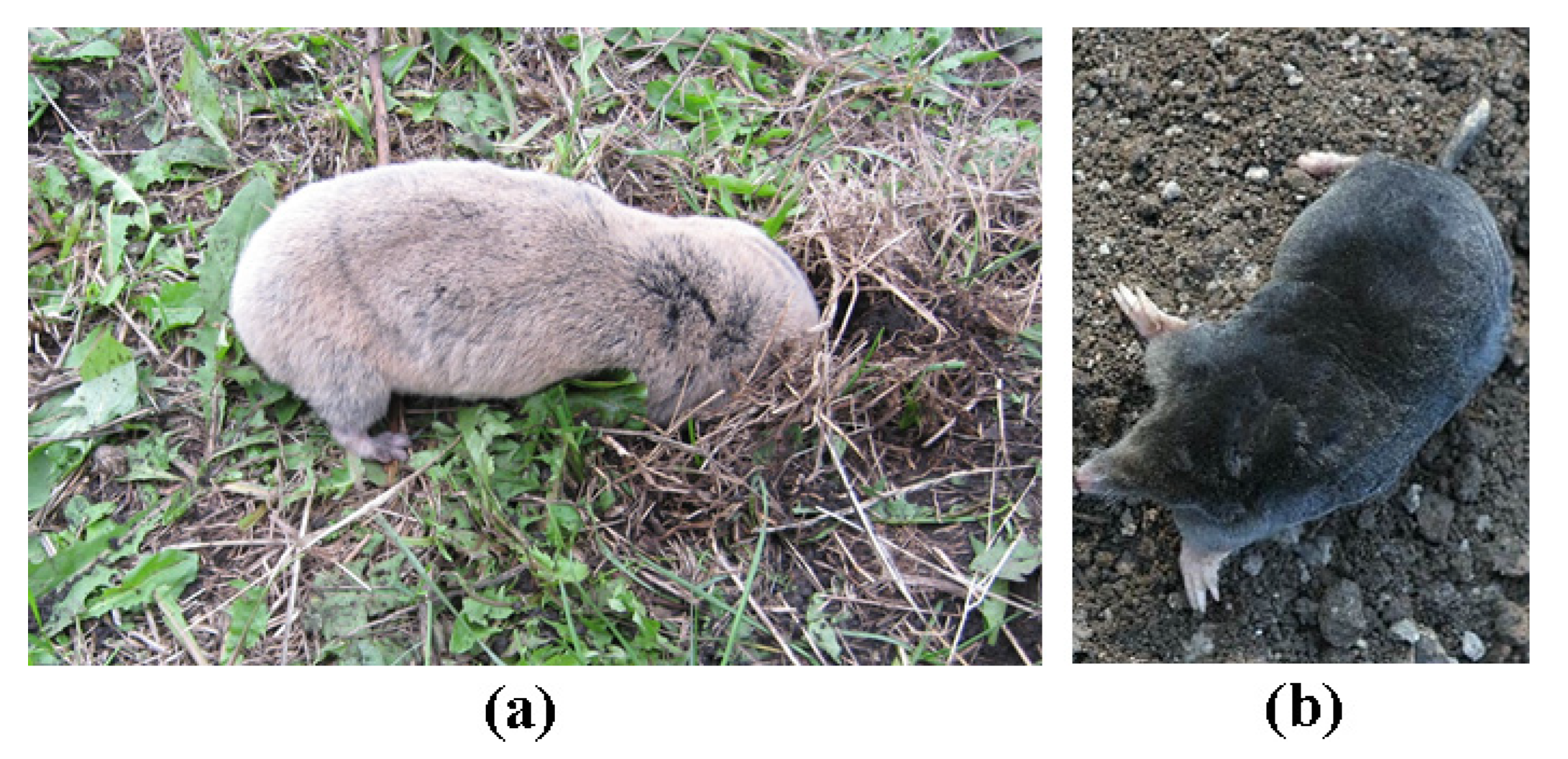
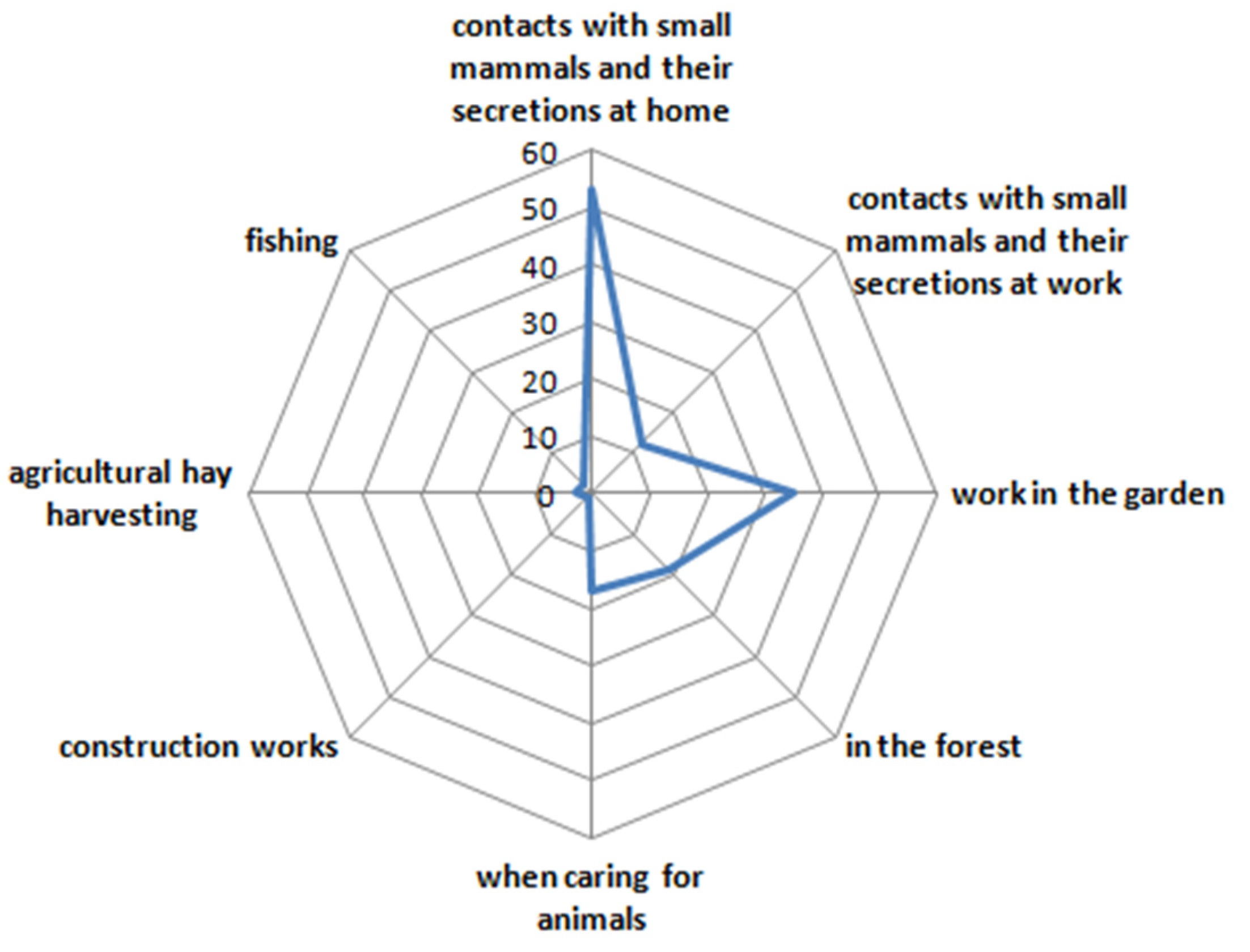
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, B.A.; Kramer, A.M.; Drake, J.M. Global patterns of zoonotic disease in mammals. Trends Parasitol. 2016, 32, 565–577. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Razgour, O. Emerging zoonotic diseases originating in mammals: A systematic review of effects of anthropogenic land-use change. Mamm. Rev. 2020, 50, 336–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rohr, J.; Cui, R.; Xin, Y.; Han, L.; Yang, X.; Gu, S.; Du, Y.; Liang, J.; Wang, X.; et al. Biological invasions facilitate zoonotic disease emergences. Nat. Commun. 2022, 13, 1762. [Google Scholar] [CrossRef] [PubMed]
- Trankvilevsky, D.V. About infection of small mammals with pathogens of zoonoses in the Russian Federation. Public Health Habitat 2016, 10, 53–56. [Google Scholar]
- Trankvilevsky, D.V.; Tsarenko, V.A.; Zhukov, V.I. The current state of epizootological monitoring of natural foci of infections in the Russian Federation. Med. Parasitol. Parasit. Dis. 2016, 2, 19–24. [Google Scholar]
- Savitskaya, T.A.; Ivanova, A.V.; Isaeva, G.S.; Reshetnikova, I.D.; Kabve, E.; Trifonov, V.A.; Ziatdinov, V.B.; Trankvilevsky, D.V.; Serova, I.V.; Popov, N.V.; et al. Review of hantavirus infections in the world, epidemiological situation on hemorrhagic fever with renal syndrome in the Russian Federation in 2020 and a forecast for 2021. Probl. Osob. Opasnykh Infektsii 2021, 2, 62–70. [Google Scholar] [CrossRef]
- Kudryavtseva, T.Y.; Popov, V.P.; Mokrievich, A.N.; Kulikalova, E.S.; Kholin, A.V.; Mazepa, A.V.; Trankvilevsky, D.V.; Khramov, M.V.; Dyatlov, I.A. Epizootiological and epidemiological situation on tularemia in Russia in 2020, the forecast for 2021. Probl. Osob. Opasnykh Infektsii 2021, 1, 32–42. [Google Scholar] [CrossRef]
- Boyarova, E.; Andreychev, A.; Kozlova, I.; Kuznetsov, V. Red fox (Vulpes vulpes) as the main vector of animal’s rabies in the forest-steppe zone of Republic of Mordovia. For. Ideas 2020, 26, 355–365. [Google Scholar]
- Andreychev, A.; Boyarova, E.; Lapshin, A.; Kuznetsov, V. Detection of foci of tularemia using enzyme immunoassay for the predatory bird pellets. Period. Tche Quim. 2019, 16, 632–641. [Google Scholar] [CrossRef]
- Kang, H.J.; Bennett, S.N.; Sumibcay, L.; Arai, S.; Hope, A.G.; Mocz, G.; Song, J.W.; Cook, J.A.; Yanagihara, R. Evolutionary insights from a genetically divergent hantavirus harbored by the European common mole (Talpa europaea). PLoS ONE 2009, 4, e6149. [Google Scholar] [CrossRef]
- Tamam, O.A.S.; Refai, M. Dual mycotic pulmonary granulomas caused by Alternaria alternata and Aspergillus candidus in the wild egyptian mole rat (Spalax leucodon egyptiacus). Assiut Vet. Med. J. 2013, 59, 9–13. [Google Scholar]
- Yanagihara, R.; Gu, S.H.; Arai, S.; Kang, H.J.; Song, J.W. Hantaviruses: Rediscovery and new beginnings. Virus Res. 2014, 187, 6–14. [Google Scholar] [CrossRef]
- Gu, S.H.; Dormion, J.; Hugot, J.P.; Yanagihara, R. High prevalence of Nova hantavirus infection in the European mole (Talpa europaea) in France. Epidemiol. Infect. 2014, 142, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.H.; Kumar, M.; Sikorska, B.; Hejduk, J.; Markowski, J.; Markowski, M.; Liberski, P.P.; Yanagihara, R. Isolation and partial characterization of a highly divergent lineage of hantavirus from the European mole (Talpa europaea). Sci. Rep. UK 2016, 6, 21119. [Google Scholar] [CrossRef] [PubMed]
- Laenen, L.; Vergote, V.; Kafetzopoulou, L.E.; Wawina, T.B.; Vassou, D.; Cook, J.A.; Hugot, J.P.; Deboutte, W.; Kang, H.J.; Witkowski, P.T.; et al. A novel hantavirus of the European mole, Bruges virus, is involved in frequent Nova virus coinfections. Genome Biol. Evol. 2018, 10, 45–55. [Google Scholar] [CrossRef]
- Hubálek, Z.; Burda, H.; Scharff, A.; Heth, G.; Nevo, E.; Šumbera, R.; Peško, J.; Zima, J. Emmonsiosis of subterranean rodents (Bathyergidae, Spalacidae) in Africa and Israel. Med. Mycol. 2005, 43, 691–697. [Google Scholar] [CrossRef]
- Pichurina, N.L.; Moskvitina, E.A.; Orekhov, I.V. Carriers of tularemia etiological agent in natural foci of the Rostov Region. Epidemiol. Vaccine Prev. 2011, 5, 21–24. [Google Scholar]
- Tarasov, M.A.; Porshakov, A.M.; Kazakova, L.V.; Kresova, U.A.; Romanov, R.A.; Sludsky, A.A. Modern cadastre of species of tularemia microbe carriers habitant in tularemia foci of different types, situated in the territory of Russia. Izv. Saratov Univ. 2019, 19, 70–78. [Google Scholar]
- Zaitsev, M.V.; Voita, L.L.; Sheftel, B.I. Mammals of the fauna of Russia and Adjacent Territories. Insectivores; Science Press: St. Petersburg, Russia, 2014; pp. 1–391. [Google Scholar]
- Andreychev, A.V.; Kuznetsov, V.A. Checklist of rodents and insectivores of the Mordovia, Russia. ZooKeys 2020, 1004, 129–139. [Google Scholar] [CrossRef]
- Stepanova, I.; Andreychev, A.; Kulakhmetov, R.; Lobachev, E. Commensals of underground mammals: European mole (Talpa europaea, Eulipotyphla, Talpidae) and the greater mole-rat (Spalax microphthalmus, Rodentia, Spalacidae). Biodiversitas 2021, 22, 4665–4670. [Google Scholar] [CrossRef]
- Andreychev, A.; Kuznetsov, V.; Lapshin, A.; Alpeev, M. Activity of the Russian desman Desmana moschata (Talpidae, Insectivora) in its burrow. Therya 2020, 11, 161–167. [Google Scholar] [CrossRef]
- Ovchinnikova, S.L. Distribution of the greater mole rat (Spalax microphthalmus Guld.) in the south-eastern part of the Chernozem Center. In Book Proceedings of the Voronezh University; Lakomkin, A.I., Skufin, K.V., Lakomkin, A.I., Skufin, K.V., Eds., Eds.; Voronezh University Press: Voronezh, Russia, 1971; Volume 93, pp. 80–83. [Google Scholar]
- Puzachenko, A.Y. Space pattern of the mirco groupings in subterranean mole rat Spalax microphthalmus (Rodentia, Spalacidae) populations. Mammalia 1993, 57, 619–648. [Google Scholar]
- Andreychev, A.V. Daily and seasonal feeding activity of the greater mole-rat (Spalax microphthalmus, Rodentia, Spalacidae). Biol. Bull. 2019, 46, 1172–1181. [Google Scholar] [CrossRef]
- Rossow, H.; Sissonen, S.; Koskela, K.A.; Kinnunen, P.M.; Hemmila, H.; Niemimaa, J.; Huitu, O.; Kuusi, M.; Vapalahti, O.; Henttonen, H.; et al. Detection of Francisella tularensis in voles in Finland. Vector-Borne Zoonot. 2014, 14, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Andreychev, A.; Boyarova, E. Forest dormouse (Dryomys nitedula, Rodentia, Gliridae)—A highly contagious rodent in relation to zoonotic diseases. For. Ideas 2020, 26, 262–269. [Google Scholar]
- Maydanov, M.; Andreychev, A.; Boyarova, E.; Kuznetsov, V.; Ilykaeva, E. Small mammals as reservoirs of tularemia and HFRS in the forest zone of Saransk. For. Ideas 2021, 27, 128–135. [Google Scholar]
- Yamashkin, A.A. Physico-Geographical Conditions and Landscapes of Mordovia; Mordovian University Press: Saransk, Russia, 1998; pp. 1–156. [Google Scholar]
- Akhtyrtsev, B.P.; Akhtyrtsev, A.B. Soil Cover of the Central Russian Chernozem Region; Voronezh University Press: Voronezh, Russia, 1993; pp. 1–216. [Google Scholar]
- Kazeev, K.S.; Strelkova, V.I. Physical Geography; Southern Federal University Press: Rostov-on-Don, Russia, 2008; pp. 1–249. [Google Scholar]
- Belikov, G.A. The Road from the Past: Entertaining Pages of the History of Stavropol; Book Press: Stavropol, Russia, 1991; pp. 1–267. [Google Scholar]
- Karaseva, E.V.; Telicina, A.Y. Methods for Studying Rodents in the Field; Science Press: Moscow, Russia, 1996; pp. 1–227. [Google Scholar]
- Balahonov, S.V.; Innokentyeva, T.I.; Chesnokova, M.V.; Mazepa, A.V.; Tatarnikov, S.A. The Order of Organization and Conduct of Laboratory Diagnosis of Tularemia for Laboratories of Territorial, Regional and Federal Levels; Federal Center of Hygieneand Epidemiology of Rospotrebnadzor Press: Moscow, Russia, 2011; pp. 1–45. [Google Scholar]
- Sergiev, V.P.; Morozov, E.N.; Morozova, L.F. Laboratory Diagnostics of Dangerous Infectious Diseases; Shico Press: Moscow, Russia, 2013; pp. 1–560. [Google Scholar]
- Reiczigel, J.; Marozzi, M.; Fabian, I.; Rozsa, L. Biostatistics for parasitologists—A primer quantitative parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Hervé, M. RV Aide Memoire: Testing and Plotting Procedures for Biostatistics. R Package Version 0.9-81-2. 2022. Available online: https://CRAN.R-project.org/package=RVAideMemoire (accessed on 30 November 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R. Foundation for Statistical Computing: Viena, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 20 August 2022).
- Lin, X.D.; Wang, W.; Guo, W.P.; Zhang, X.H.; Xing, J.G.; Chen, S.Z.; Li, M.H.; Chen, Y.; Xu, J.; Plyusnin, A.; et al. Cross-species transmission in the speciation of the currently known murinae-associated hantaviruses. J. Virol. 2012, 86, 11171–11182. [Google Scholar] [CrossRef]
- Svobodová, P.; Pejčoch, M.; Heroldová, M.; Pavlíček, T.; Nevo, E.; Šumbera, R.; Hubálek, Z. Examination of rodents (Rodentia) for emmonsiosis in the Czech Republic, Israel and Africa. Czech Mycol. 2009, 61, 99–106. [Google Scholar] [CrossRef]
- Bártová, E.; Kučerová, H.L.; Žákovská, A.; Budíková, M.; Nejezchlebová, H. Coxiella burnetii and Francisella tularensis in wild small mammals from the Czech Republic. Ticks Tick-Borne Dis. 2020, 11, 101350. [Google Scholar] [CrossRef]
- Jeske, K.; Schulz, J.; Tekemen, D.; Balčiauskas, L.; Balčiauskienė, L.; Hiltbrunner, M.; Drewes, S.; Mayer-Scholl, A.; Heckel, G.; Ulrich, R.G. Cocirculation of Leptospira spp. and multiple orthohantaviruses in rodents, Lithuania, Northern Europe. Transbound. Emerg. Dis. 2022, 69, 3196–3201. [Google Scholar] [CrossRef]
- Špitalská, E.; Minichová, L.; Hamšíková, Z.; Stanko, M.; Kazimírová, M. Bartonella, Rickettsia, Babesia, and Hepatozoon Species in Fleas (Siphonaptera) Infesting Small Mammals of Slovakia (Central Europe). Pathogens 2022, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Borşan, S.D.; Ionică, A.M.; Galon, C.; Toma-Naic, A.; Peştean, C.; Sándor, A.D.; Moutailler, S.; Mihalca, A.D. High diversity, prevalence, and co-infection rates of tick-borne pathogens in ticks and wildlife hosts in an urban area in Romania. Front. Microbiol. 2021, 12, 645002. [Google Scholar] [CrossRef] [PubMed]
- Balakirev, A.E.; Van Chau, N. Investigation of multiple infections with zoonotic pathogens of rodents in northern Vietnam. J. Vector Dis. 2021, 58, 47. [Google Scholar]
- Milholland, M.T.; Castro-Arellano, I.; Suzán, G.; Garcia-Peña, G.E.; Lee, T.E., Jr.; Rohde, R.E.; Aguirre, A.A.; Mills, J.N. Global diversity and distribution of hantaviruses and their hosts. Ecohealth 2018, 15, 163–208. [Google Scholar] [CrossRef] [PubMed]
- Drewes, S.; Jeske, K.; Straková, P.; Balčiauskas, L.; Ryll, R.; Balčiauskienė, L.; Kohlhause, D.; Schnidrig, G.A.; Hiltbrunner, M.; Špakova, A.; et al. Identification of a novel hantavirus strain in the root vole (Microtus oeconomus) in Lithuania, Eastern Europe. Infect. Genet. Evol. 2021, 90, 104520. [Google Scholar] [CrossRef] [PubMed]

| Species | n | TUL | HFRS | TUL + HFRS |
|---|---|---|---|---|
| Clethrionomys glareolus | 206 | 33 (16.1) | 23 (11.2) | 5 (2.4) |
| Microtus arvalis | 27 | 0 | 0 | 0 |
| Rattus norvegicus | 5 | 0 | 0 | 0 |
| Arvicola amphibius | 2 | 2 (100) | 0 | 0 |
| Dryomys nitedula | 2 | 1 (50) | 2 (100) | 1 (50) |
| Cricetus cricetus | 3 | 0 | 0 | 0 |
| Apodemus flavicollis | 39 | 15 (38.5) | 5 (12.8) | 0 |
| Apodemus uralensis | 11 | 0 | 0 | 0 |
| Apodemus agrarius | 60 | 0 | 1 (1.6) | 0 |
| Mus musculus | 35 | 0 | 3 (8.5) | 0 |
| Spalax microphthalmus | 3 (M) | 3 (100) | 0 | 0 |
| Spalax microphthalmus | 3 (R,S,V) | 2 (66.7) (R,S) | 1 (33.3) (V) | 0 |
| Micromys minutus | 1 | 0 | 1 (100) | 0 |
| Sorex araneus | 24 | 0 | 0 | 0 |
| Talpa europaea | 17 | 0 | 0 | 0 |
| Neomys fodiens | 2 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreychev, A.; Boyarova, E.; Brandler, O.; Tukhbatullin, A.; Kapustina, S. Terrestrial and Subterranean Mammals as Reservoirs of Zoonotic Diseases in the Central Part of European Russia. Diversity 2023, 15, 39. https://doi.org/10.3390/d15010039
Andreychev A, Boyarova E, Brandler O, Tukhbatullin A, Kapustina S. Terrestrial and Subterranean Mammals as Reservoirs of Zoonotic Diseases in the Central Part of European Russia. Diversity. 2023; 15(1):39. https://doi.org/10.3390/d15010039
Chicago/Turabian StyleAndreychev, Alexey, Ekaterina Boyarova, Oleg Brandler, Andrei Tukhbatullin, and Svetlana Kapustina. 2023. "Terrestrial and Subterranean Mammals as Reservoirs of Zoonotic Diseases in the Central Part of European Russia" Diversity 15, no. 1: 39. https://doi.org/10.3390/d15010039
APA StyleAndreychev, A., Boyarova, E., Brandler, O., Tukhbatullin, A., & Kapustina, S. (2023). Terrestrial and Subterranean Mammals as Reservoirs of Zoonotic Diseases in the Central Part of European Russia. Diversity, 15(1), 39. https://doi.org/10.3390/d15010039








