The ‘Edge Effect’ Phenomenon in Plants: Morphological, Biochemical and Mineral Characteristics of Border Tissues
Abstract
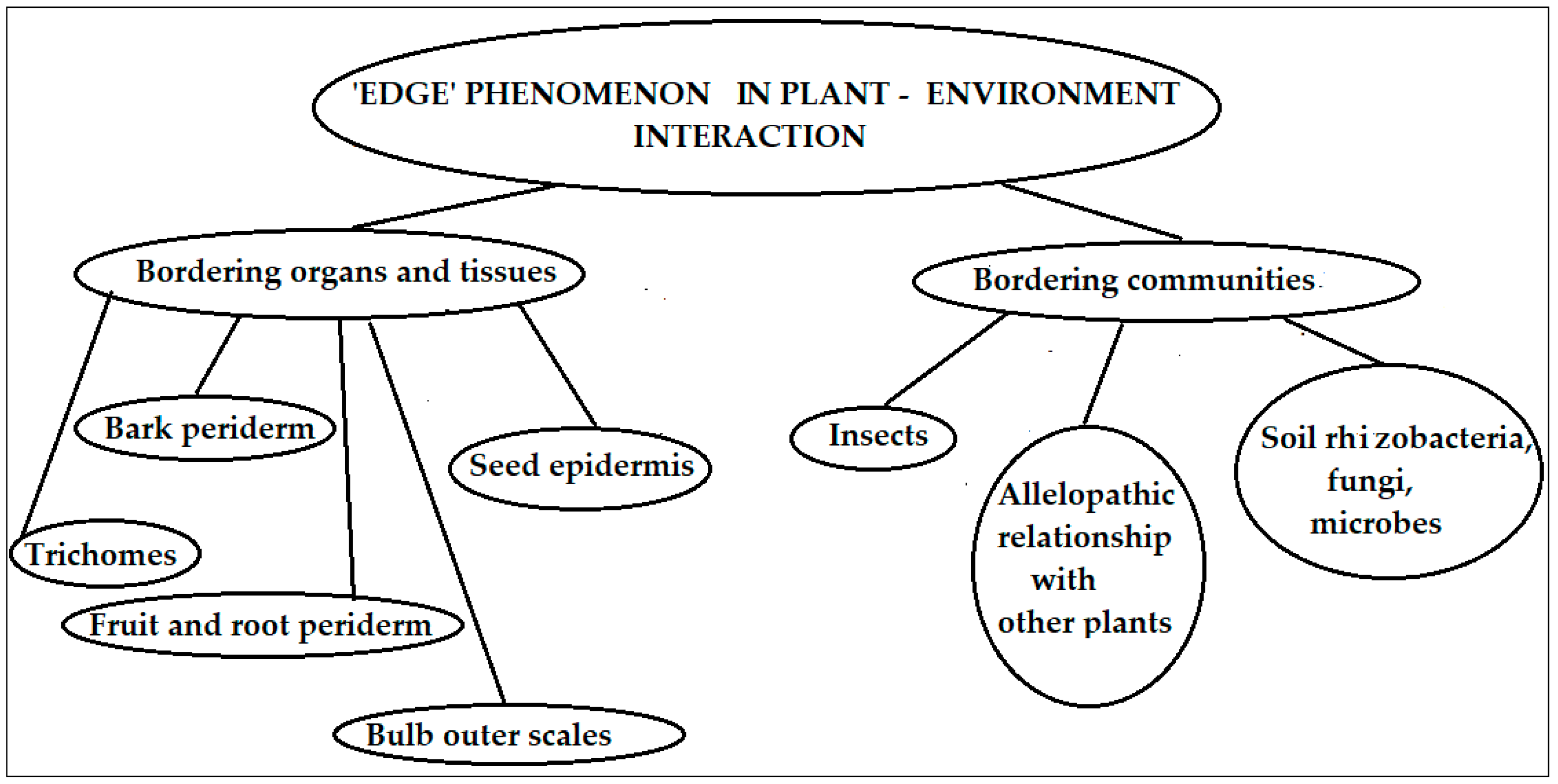
1. Introduction
- (1)
- intensive oxidant stress;
- (2)
- high biodiversity of organisms in bordering areas and of biologically active compounds in plant border tissues;
- (3)
- damper effect of bordering areas/tissues necessary to withstand the unfavorable environmental conditions;
- (4)
- high adsorption capacity of plant bordering tissues and intensive energy accumulation in ecosystem bordering areas.
2. Trichomes
3. Seed Epidermis
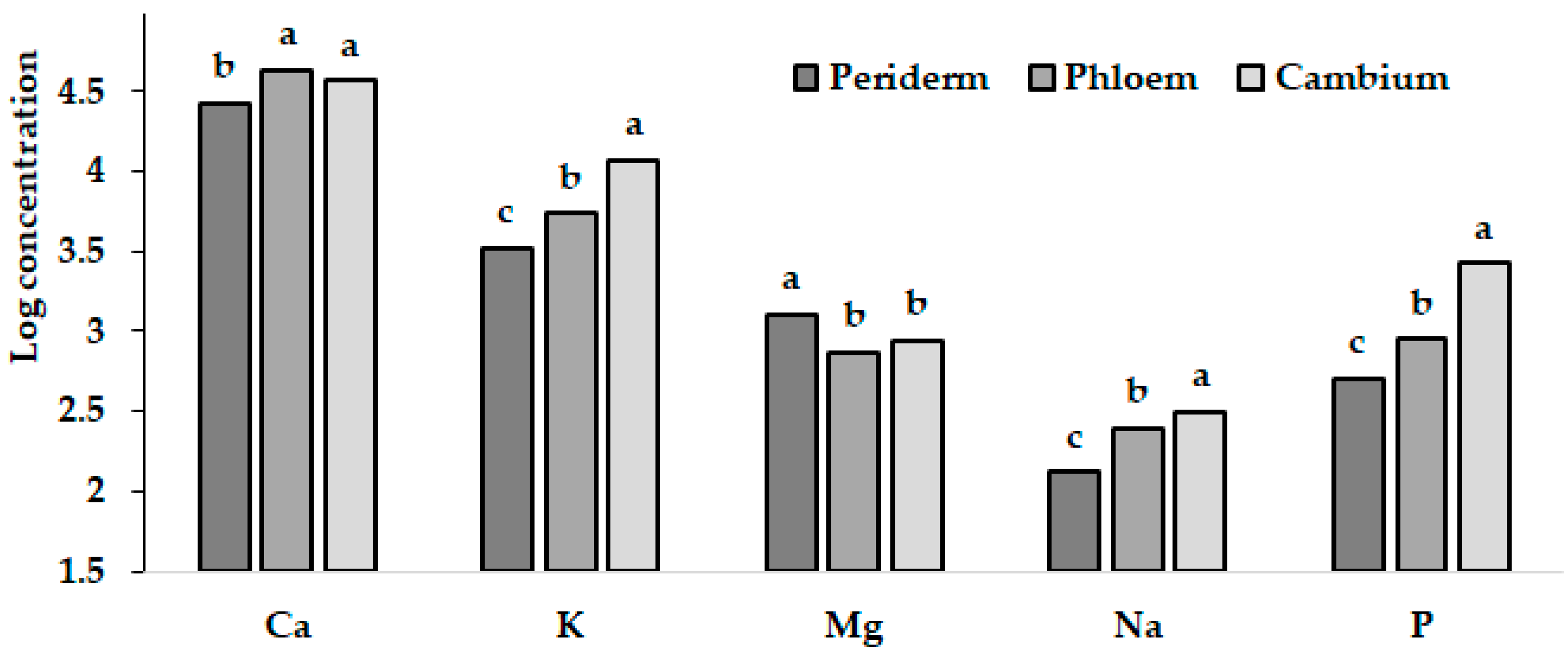
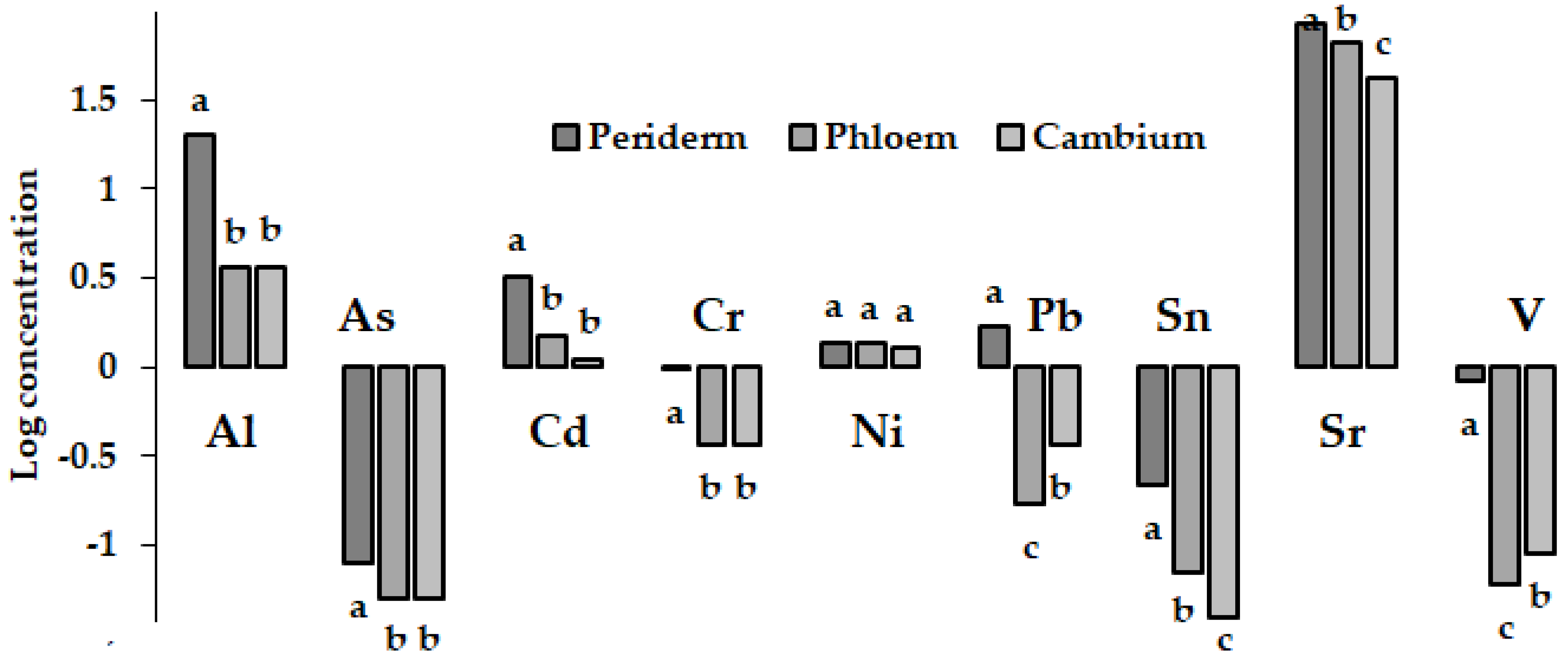
4. Tree Bark
5. Vegetable Root Periderm
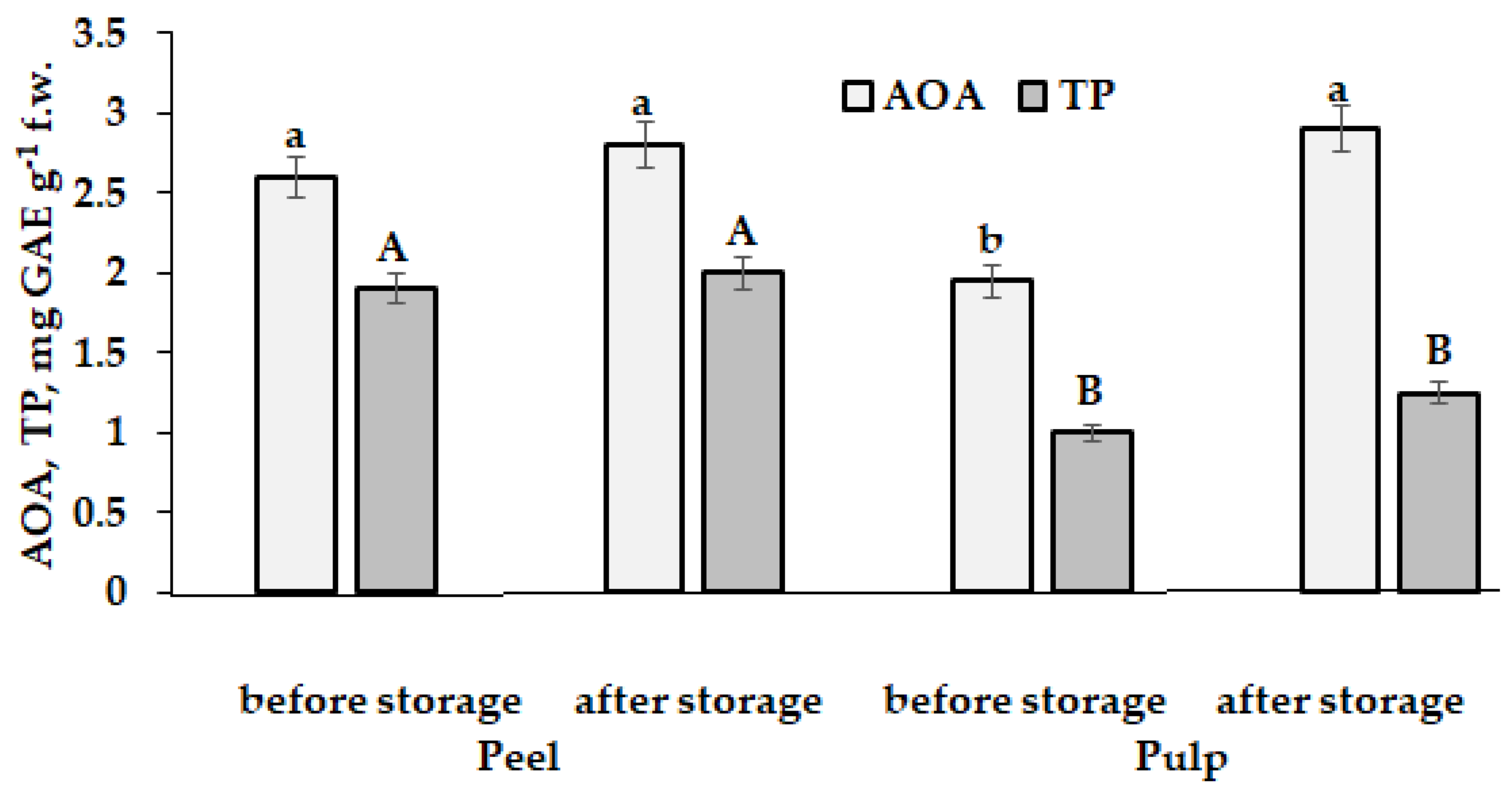
5.1. Potato (Solanum tuberosum L.)
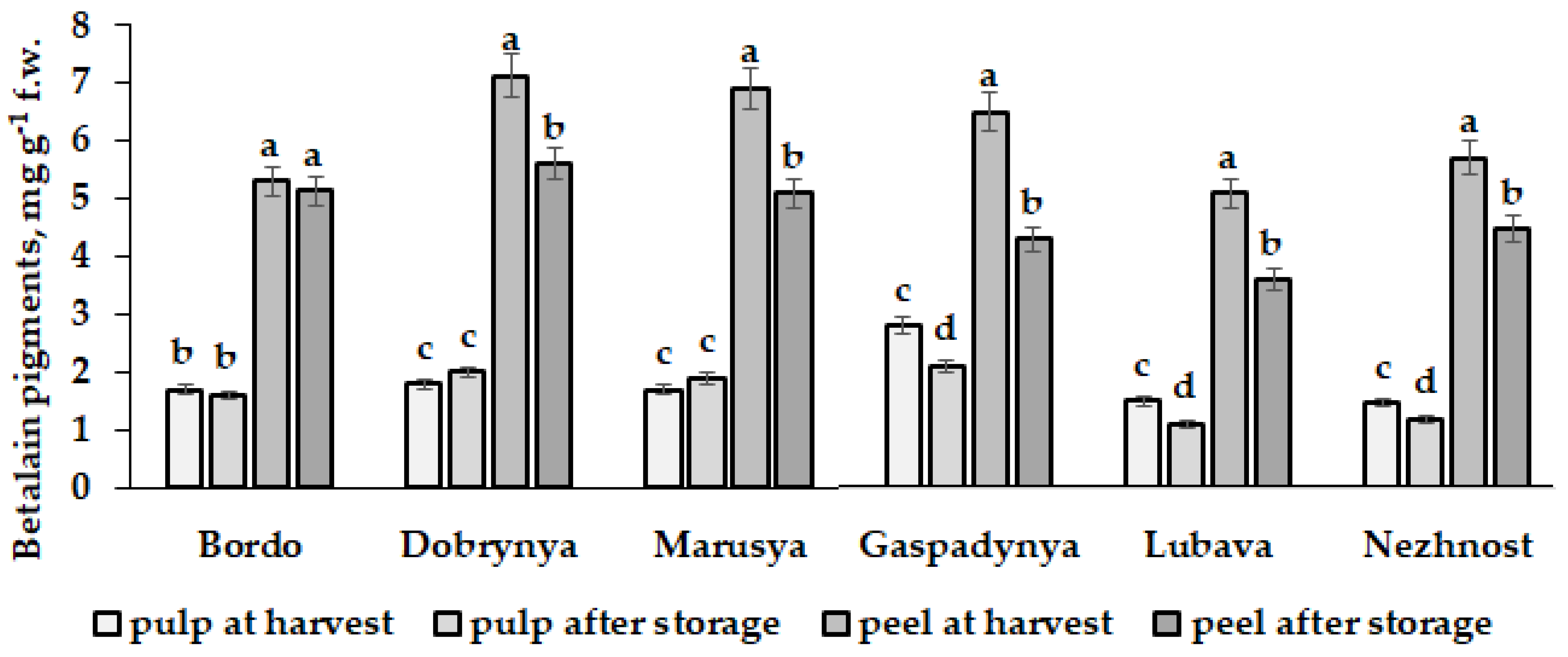
5.2. Beta vulgaris L.
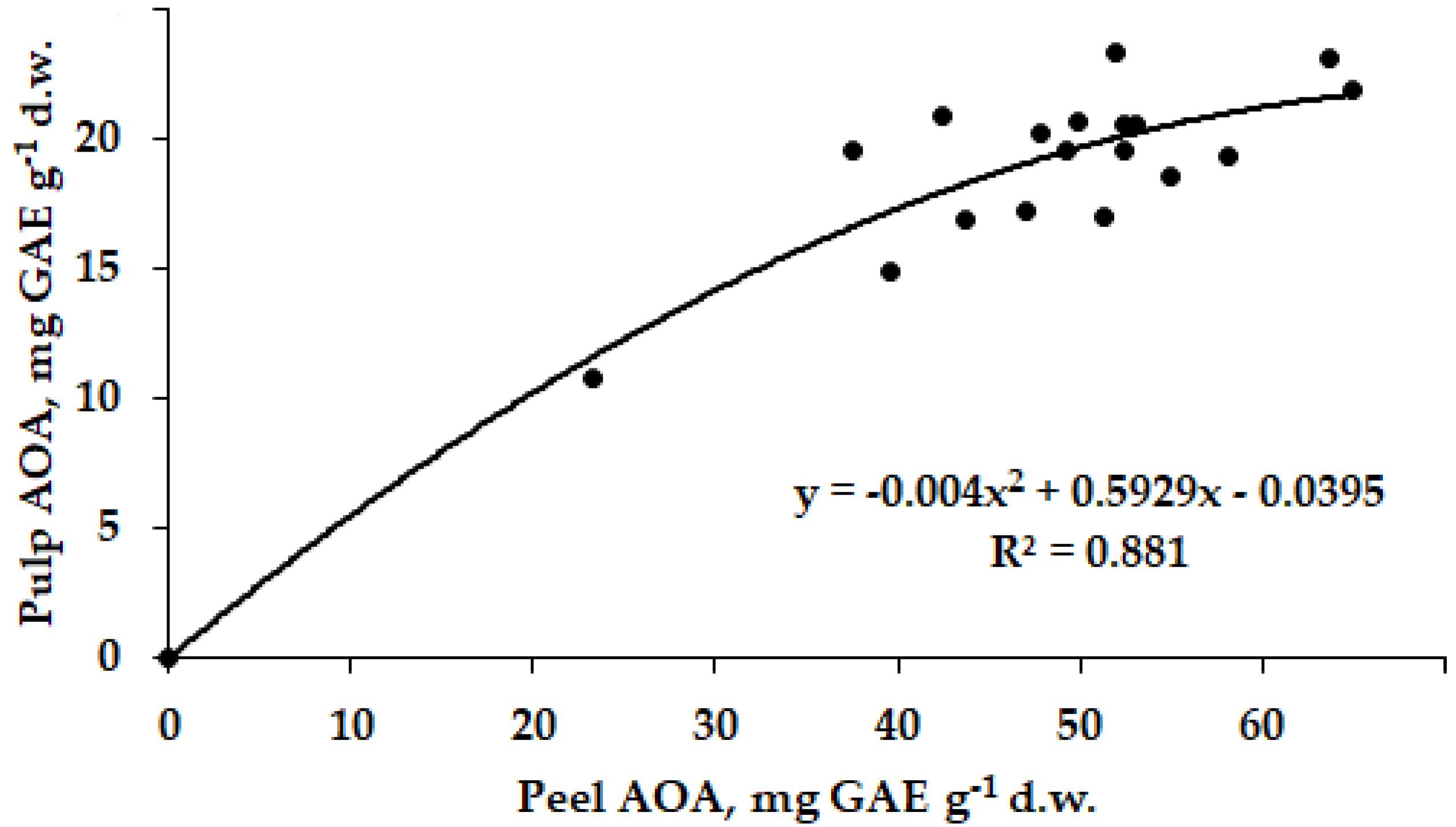
5.3. Raphanus sativus L.
5.4. Daucus carota subsp. sativus
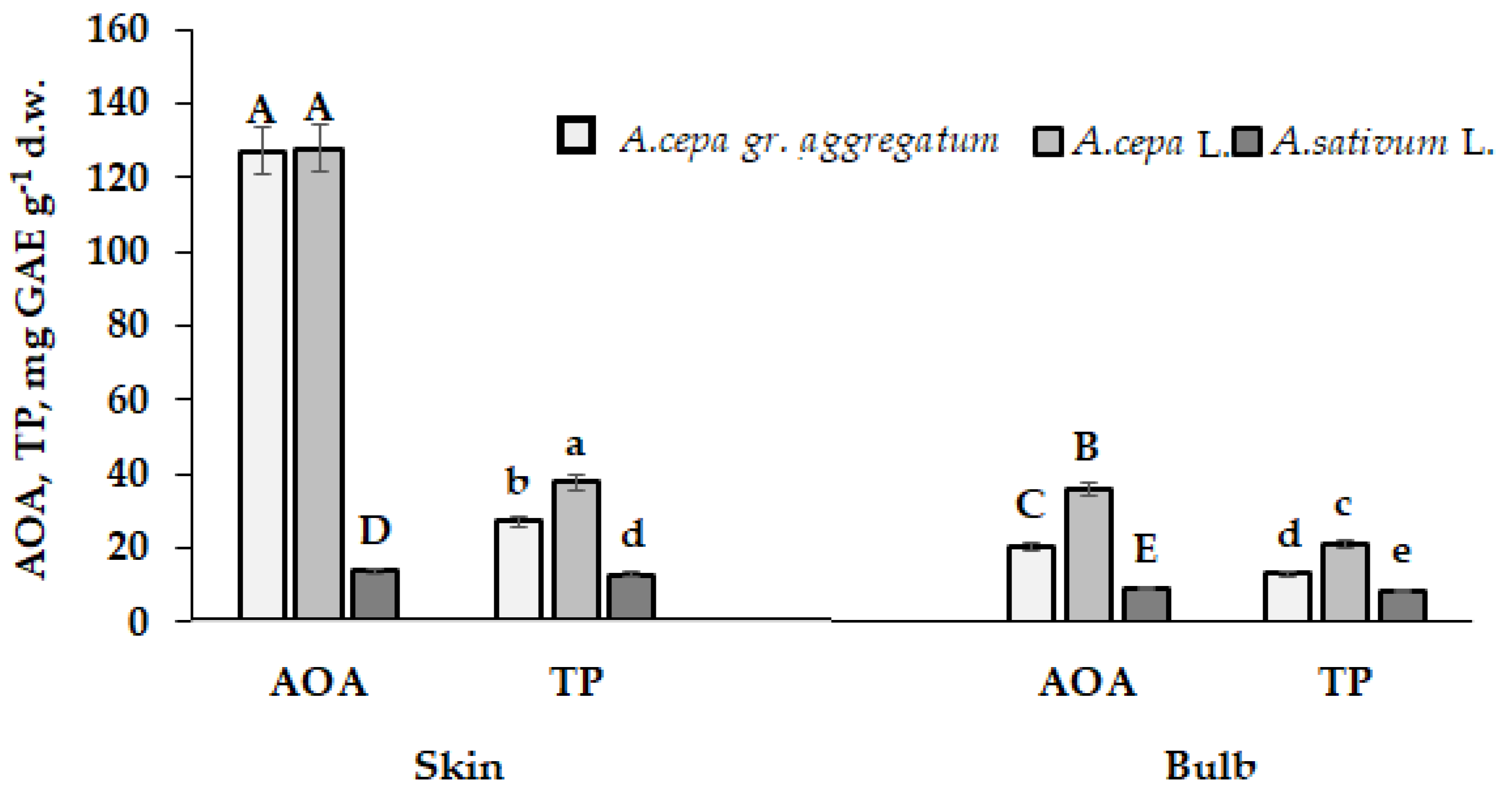
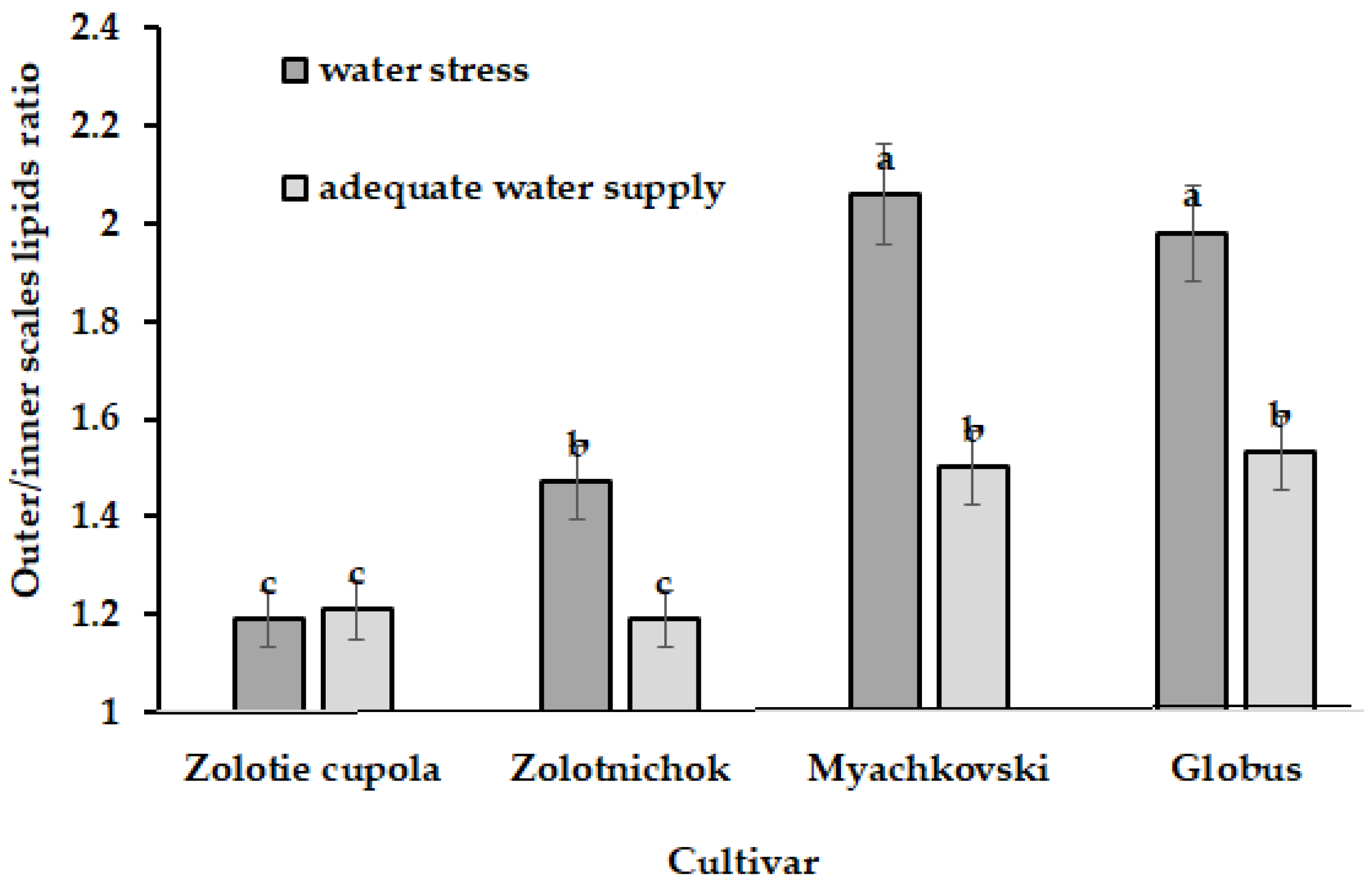
5.5. Allium Species
5.6. Cucurbita Species
6. Other Agricultural Crops and Nanoparticle Production
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levin, S.A. The Princeton Guide to Ecology; Princeton University Press: Princeton, NJ, USA, 2009; p. 780. [Google Scholar]
- Fonseca, M.S. Edge effect. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: London, UK, 2008; pp. 1207–1211. [Google Scholar]
- Porensky, L.M.; Young, T.P. Edge-effect interactions in fragmented and patchy landscapes. Conserv. Biol. 2013, 27, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Uphoff, N. Symbiotic root-endophytic soil microbes improve crop productivity and provide environmental benefits. Hindawi Scientifica 2019, 2019, 9106395. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.M.; Ahmad, A.; Ullah, E.; Kamal, A.; Nawaz, M.Y.; Ali, H.H. Plant allelopathy in agriculture and Its environmental and functional mechanisms: A review. Int. J. Food Sci. Agr. 2021, 5, 623–626. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, L.; Han, X.; Yang, Y. Research advances in allelopathy of volatile organic compounds (VOCs) of plants. Horticulturae 2021, 7, 278. [Google Scholar] [CrossRef]
- Ashra, H.; Nair, S. Review: Trait plasticity during plant-insect interactions: From molecular mechanisms to impact on community dynamics. Plant Sci. 2022, 317, 111188. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, M.; Bruin, S.; Luckerhoff, L.; van Lontestijn, R.S.P.; Schlaeppi, K. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J. 2016, 10, 389–399. [Google Scholar] [CrossRef]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of arbuscular mycorrhizal fungi on soil fertility: Contribution in the improvement of physical, chemical, and biological properties of the soil. Front. Fungal Biol. 2022, 3, 723892. [Google Scholar] [CrossRef]
- Geisen, S.; Heinen, R.; Andreou, E.; van Lent, T.; ten Hooven, F.C.; Thakur, M.P. Contrasting effects of soil microbial interactions on growth–defense relationships between early- and mid-successional plant communities. New Phytol. 2022, 233, 1345–1357. [Google Scholar] [CrossRef]
- Golubkina, N.; Krivenkov, L.; Sekara, A.; Vasileva, V.; Tallarita, A.; Caruso, G. Prospects of arbuscular mycorrhizal fungi utilization in production of Allium plants. Plants 2020, 9, 279. [Google Scholar] [CrossRef]
- Dyer, L.A.; Philbin, C.S.; Ochsenrider, K.M.; Richards, L.A.; Massad, T.J.; Smilanich, A.M.; Forister, M.L.; Parchman, T.L.; Galland, L.M.; Hurtado, P.J.; et al. Modern approaches to study plant–insect interactions in chemical ecology. Nat. Rev. Chem. 2018, 2, 50–64. [Google Scholar] [CrossRef]
- Annegowda, H.V.; Majumder, P. Chapter 5—Valuable bioactives from vegetable wastes. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: London, UK, 2021; pp. 83–109. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.M.A. Some natural antioxidants sources from foods and tree barks. Int. J. Sci. Technol. Res. 2019, 8, 93–98. [Google Scholar]
- Celano, R.; Docimo, T.; Piccinelli, A.L.; Gazzerro, P.; Tucci, M.; Sanzo, R.D.; Carabetta, S.; Campone, L.; Russo, M.; Rastrelli, L. Onion peel: Turning a food waste into a resource. Antioxidants 2021, 10, 304. [Google Scholar] [CrossRef]
- Prasadi, N.V.P.; Joye, I.J. Dietary fibre from whole grains and their benefits on metabolic health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Yucetepe, A.; Altin, G.; Ozcelik, B. A novel antioxidant source: Evaluation of in vitro bioaccessibility, antioxidant activity and polyphenol profile of phenolic extract from black radish peel wastes (Raphanus sativus L. var. niger) during simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2021, 56, 1376–1384. [Google Scholar] [CrossRef]
- Gebrechristos, H.Y.; Chen, W. Utilization of potato peel as eco-friendly products: A review. Food Science and Nutrition. Food Sci. Nutr. 2018, 6, 1352–1356. [Google Scholar] [CrossRef]
- Staichok, A.C.B.; Mendonça, K.R.B.; dos Santos, P.G.A.; Garcia, L.G.C.; Damiani, C. Pumpkin peel flour (Cucurbita máxima L.)—Characterization and technological applicability. J. Food Nutr. Res. 2016, 4, 327–333. [Google Scholar] [CrossRef]
- Aschenbrenner, A.-K.; Horakh, S.; Spring, O. Linear glandular trichomes of Helianthus (Asteraceae): Morphology, localization, metabolite activity and occurrence. AoB Plants 2013, 5, plt028. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Jiang, R.; Dong, B.; Fang, S.; Li, Q.; Lv, Z.; Chen, W. Phytohormone-based regulation of trichome development. Front. Plant Sci. 2021, 12, 734776. [Google Scholar] [CrossRef]
- Judd, R.; Bagley, M.C.; Li, M.L.; Zhu, Y.; Lei, C.; Yuzuak, S.; Ekelöf, M.; Pu, G.; Zhao, X.; Muddiman, D.C.; et al. Artemisinin biosynthesis in non-glandular trichome cells of Artemisia Annua. Mol. Plant. 2019, 12, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Schilmiller, A.L.; Last, R.L.; Pichersky, E. Harnessing plant trichome biochemistry for the production of useful compounds. Plant J. 2008, 54, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Huchelmann, A.; Boutry, M.; Hachez, C. Plant glandular trichomes: Natural cell factories of high biotechnological interest. Plant Physiol. 2017, 175, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.K.; Foster, A.J.; Aung, T.; Mahmoud, S.S. Essential oil production: Relationship with abundance of glandular trichomes in aerial surface of plants. Acta Physiol. Plant 2009, 31, 13–19. [Google Scholar] [CrossRef]
- Fernandes, Y.S.; Trindade, L.M.P.; Rezende, H.; Paula, J.R.; Gonçalves, L.A. Trichomes and chemical composition of the volatile oil of Trichogonia cinerea (Gardner) R.M.King; H.Rob (Eupatorieae, Asteraceae). Annals Braz. Acad. Sci. 2016, 88, 309–322. [Google Scholar] [CrossRef]
- Freeman, J.L.; Zhang, L.H.; Marcus, M.A.; Fakra, S.; McGrath, S.P.; Pilon-Smits, E.A. Spatial imaging, speciation, and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant Physiol. 2006, 142, 124–134. [Google Scholar] [CrossRef]
- Hare, J.D.; Elle, E. Variable impact of diverse insect herbivores on dimorphic Datura wrightil. Ecology 2002, 83, 2711–2720. [Google Scholar] [CrossRef]
- Lehnebach, C.A.; Robertson, A.W. Pollination ecology of four epiphytic orchids of New Zealand. Ann. Bot. 2004, 93, 773–781. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D. Protective and defensive roles of non-glandular trichomes against multiple stresses: Structure–function coordination. J. For. Res. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Duan, Q.; Zhu, Z.; Wang, B.; Chen, M. Recent progress on the salt tolerance mechanisms and application of tamarisk. Int. J. Mol. Sci. 2022, 23, 3325. [Google Scholar] [CrossRef]
- Li, S.; Tosens, T.; Harley, P.C.; Jiang, Y.; Kanagendran, A.; Grosberg, M.; Jaamets, K.; Niinemets, Ü. Glandular trichomes as a barrier against atmospheric oxidative stress: Relationships with ozone uptake, leaf damage, and emission of LOX products across a diverse set of species. Plant Cell Environ. 2018, 41, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Pan, J.; An, L.; Gan, Y.; Feng, H. The responses of trichome mutants to enhanced ultraviolet-B radiation in Arabidopsis thaliana. J. Photochem. Photobiol. B Biol. 2012, 113, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Logvinenko, L.; Golubkina, N.; Fedotova, I.; Bogachuk, M.; Fedotov, M.; Kataev, V.; Alpatov, A.; Shevchuck, O.; Caruso, G. Effect of foliar sodium selenate and nano selenium supply on biochemical characteristics, essential oil accumulation and mineral composition of Artemisia annua L. Plants 2022, 27, 8246. [Google Scholar] [CrossRef]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [CrossRef]
- Dardick, C.; Callahan, A.M. Evolution of the fruit endocarp: Molecular mechanisms underlying adaptations in seed protection and dispersal strategies. Front. Plant Sci. 2014, 5, 284. [Google Scholar] [CrossRef] [PubMed]
- Okafor, J.N.C.; Meyer, M.; Le Roes-Hill, M.; Jideani, V.A. Flavonoid and phenolic acid profiles of dehulled and whole Vigna subterranea (L.) Verdc seeds commonly consumed in South Africa. Molecules 2022, 27, 5265. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, P.; Zhou, T.; Rong, J.; Jia, H.; Liu, Z. Transcriptome analysis of the effects of shell removal and exogenous gibberellin on germination of Zanthoxylum seeds. Sci. Rep. 2017, 7, 8521. [Google Scholar] [CrossRef]
- Golubkina, N.; Kharchenko, V.; Moldovan, A.; Zayachkovsky, V.; Stepanov, V.; Pivovarov, V.; Sekara, A.; Tallarita, A.; Caruso, G. Nutritional value of Apiaceae veeds as affected by 11 species and 43 sultivars. Horticulturae 2021, 7, 57. [Google Scholar] [CrossRef]
- Mangueze, A.V.J.; Pessoa, M.F.G.; Silva, M.J.; Ndayiragije, A.; Magaia, H.E.; Cossa, V.S.I.; Reboredo, F.H.; Carvalho, M.L.; Santos, J.P.; Guerra, M.; et al. Simultaneous zinc and selenium biofortification in rice. Accumulation, localization and implications on the overall mineral content of the flour. J. Cereal Sci. 2018, 82, 34–41. [Google Scholar] [CrossRef]
- Moore, K.L.; Schröder, M.; Lombi, E.; Zhao, F.-J.; McGrath, S.P.; Hawkesford, M.J.; Shewry, P.R.; Grovenor, C.R.M. Nano SIMS analysis of arsenic and selenium in cereal grain. New Phytol. 2010, 185, 434–445. [Google Scholar] [CrossRef]
- Lima, L.W.; Stonehouse, G.C.; Walters, C.; El Mehdawi, A.F.; Fakra, S.C.; Pilon-Smits, E.A.H. Selenium accumulation, speciation and localization in Brazil nuts (Bertholletia excelsa H.B.K.). Plants 2019, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Bradford, K.; Nonogaki, H. (Eds.) Seed development, dormancy and germination. Ann. Plant Rev. 2007, 27, 164. [Google Scholar]
- Golubkina, N.A.; Papazyan, T.T. Selenium in Nutrition. Plants, Animals, Human Beings; Moscow Pechatny Gorod: Moscow, Russia, 2006. (In Russian) [Google Scholar]
- Lyons, G.H.; Genc, Y.; Stangoulis, J.C.R.; Palmer, L.T.; Graham, R.D. Selenium distribution in wheat grain, and the effect of postharvest processing on wheat selenium content. Biol. Trace Elem. Res. 2005, 103, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Olorunwa, O.J.; Barickman, T.C. Seed priming enhances seed germination and morphological traits of Lactuca sativa L. under salt stress. Seeds 2022, 1, 74–86. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, J.; Piskurewicz, U.; Loubery, S.; Utz-Pugin, A.; Bailly, C.; Mène-Saffrané, L.; Lopez-Molina, L. An endosperm-associated cuticle ss required for Arabidopsis seed viability, dormancy and early control of germination. PLoS Genet. 2015, 11, e1005708. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef]
- Azabou, S.; Sebii, H.; Taheur, F.B.; Abid, Y.; Iridi, M.; Nasri, M. Phytochemical profile and antioxidant properties of tomato by-products as affected by extraction colvents abnd potential application in refined olive oils. Food Biosci. 2020, 36, 100664. [Google Scholar] [CrossRef]
- Shao, D.; Venkitasamy, C.; Li, X.; Pan, Z.; Shi, J.; Wang, B.; The, H.E.; McHugh, T.H. Thermal and storage characteristics of tomato seed oil. LWT Food Sci. Technol. 2015, 63, 191–197. [Google Scholar] [CrossRef]
- Taveira, M.; Silva, L.R.; Vale-Silva, L.A.; Pinto, E.; Valentầo, P.; Ferreres, F.; de Pinho, P.G.; Andrade, P.B. Lycopersicom esculentum seeds: An industrial byproduct as an antimicrobial agent. J. Agric. Food Chem. 2010, 58, 9529–9536. [Google Scholar] [CrossRef]
- Allaqaband, S.; Dar, A.H.; Patel, U.; Kumar, N.; Nayik, G.A.; Khan, S.A.; Ansari, M.J.; Alabdallah, N.M.; Kumar, P.; Pandey, V.K.; et al. Utilization of fruit seed-based bioactive compounds for formulating the nutraceuticals and functional food: A review. Front. Nutr. 2022, 9, 902554. [Google Scholar] [CrossRef] [PubMed]
- Giannelos, P.N.; Sxizas, S.; Lois, E.; Zannikos, F.; Anastopoulos, G. Physical, chemical and fuel related properties of tomato seeds oil for evaluating its direct use in diesel engines. Ind. Crop Prod. 2005, 22, 193–199. [Google Scholar] [CrossRef]
- Alene, A.N.; Abate, G.Y.; Habte, A.T.; Getahun, D.M. Utilization of a novel low-cost gibto (Lupinus Albus) seed peel waste for the removal of malachite green dye: Equilibrium, kinetic, and thermodynamic studies. J. Chem. 2021, 2021, 6618510. [Google Scholar] [CrossRef]
- Obi, A.; Ejikeme, P.M.; Onukwuli, O.D. Lead removal from waste water by fluted pumpkin seed shell activated carbon: Adsorption modeling and kinetics. Int. J. Environ. Sci. Technol. 2010, 7, 793–800. [Google Scholar] [CrossRef]
- Kowalkowska, A.; Jóźwiak, T. Utilization of pumpkin (Cucurbita pepo) seed husks as a low-cost sorbent for removing anionic and cationic dyes from aqueous solutions. Desalin. Water Treat. 2019, 171, 397–407. [Google Scholar] [CrossRef]
- Alakabei, I. Antibiotic removal from the aquatic environment with activated carbon produced from pumpkin seeds. Molecules 2022, 27, 1380. [Google Scholar] [CrossRef]
- Bulut, Y.; Baysal, Z. Removal of Pb(II) from wastewater using wheat bran. J. Environ. Manag. 2006, 78, 107–113. [Google Scholar] [CrossRef]
- Chung, W.J.; Shim, J.; Ravindran, B. Application of wheat bran based biomaterials and nano-catalyst in textile wastewater. J. King Saud Univ. Sci. 2022, 34, 101775. [Google Scholar] [CrossRef]
- Reddy, M.C.S.; Ashwini, V.N.C. Bengal gram seed husk as an adsorbent for the removal of dye from aqueous solutions—Batch studies. Arab. J. Chem. 2017, 10, S2554–S2566. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Ndlovu, S.S.; Odiyo, J.O. Characterization of pulverized marula seed husk and its potential for the sequestration of methylene blue from aqueous solution. BMC Chem. 2019, 13, 10. [Google Scholar] [CrossRef]
- Rahimdokht, M.; Pajootan, E.; Arami, M. Application of melon seed shell as a natural low-cost adsorbent for the removal pf methylene blue from dye-bearing wastewaters: Optimization, isothem, kinetic, and thermodynamic. Desalination Water Treat. 2016, 57, 18049–18061. [Google Scholar] [CrossRef]
- De Oliveira Brito, S.M.; Andrade, H.M.C.; Soares, L.F.; de Azevedo, R.P. Brazil nut shells as a new biosorbent to remove methylene blue and indigo carmine from aqueous solutions. J. Hazard. Mater. 2010, 174, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.T.; Keng, P.S.; Lee, S.L.; Leong, M.H.; Hung, Y.T. Equilibrium studies for the removal of basic dye by sunflower seed husk (Helianthus annuus). Int. J. Phys. Sci. 2010, 5, 1270–1276. [Google Scholar]
- Jackson, J.F.; Adams, D.C.; Jackson, U.B. Allometry of constitutive defense: A model and a comparative test with tree bark and fire regime. Am. Nat. 1999, 153, 614–632. [Google Scholar] [CrossRef] [PubMed]
- Rosell, J.A. Bark thickness across the angiosperms: More than just fire. New Phytol. 2016, 211, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Rosell, J.A. Bark in woody plants: Understanding the diversity of a multifunctional structure. Integr. Comp. Biol. 2019, 59, 535–547. [Google Scholar] [CrossRef]
- Biswas, S.; Gupta, K.; Talapatra, S.N. A digitized database of bark morphology for identification of common tree species and literature study of bark phytochemicals and therapeutic usage. WSN 2016, 42, 143–155. [Google Scholar]
- Sakai, K. Chemistry of bark. In Wood and Cellulosic Chemistry, 2nd ed.; Hon, D.N.S., Shiraishi, N., Dekker, M., Eds.; American Chemical Society: New York, NY, USA, 2001. [Google Scholar]
- Paine, C.E.T.; Stahl, C.; Courtois, E.A.; Patiño, S.; Sarmiento, C.; Baraloto, C. Functional explanations for variation in bark thickness in tropical rain forest trees. Funct. Ecol. 2010, 24, 1202–1210. [Google Scholar] [CrossRef]
- Romero, C. Bark: Structure and functional ecology. Adv. Econ. Bot. 2014, 17, 5–25. Available online: http://www.jstor.org/stable/43932771 (accessed on 24 November 2022).
- Golubkina, N.; Plotnikova, U.; Lapchenko, V.; Lapchenko, H.; Sheshnitsan, S.; Amagova, Z.; Matsadze, V.; Naumenko, T.; Bagrikova, N.; Logvinenko, L. Evaluation of factors affecting tree and shrub bark’s antioxidant status. Plants 2022, 11, 2609. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G. Adsorption of pollutants by plant bark derived adsorbents: An empirical review. J. Water Process. Eng. 2020, 35, 101228. [Google Scholar] [CrossRef]
- Parvina, S.; Rahmana, W.; Sahaa, I.; Alama, J.; Khan, M.R. Coconut tree bark as a potential low-cost adsorbent for the removal of methylene blue from wastewater. Desalin. Water Treat. 2019, 146, 385–392. [Google Scholar] [CrossRef]
- şen, A.; Pereira, H.; Olivella, M.A. Heavy metals removal in aqueous environments using bark as a biosorbent. Int. J. Environ. Sci. Technol. 2015, 12, 391–404. [Google Scholar] [CrossRef]
- Au, J.; Youngentob, K.N.; Clark, R.G.; Phillips, R.; Foley, W.J. Bark chewing reveals a nutrient limitation of leaves for a specialist folivore. J. Mammal. 2017, 98, 1185–1192. [Google Scholar] [CrossRef]
- Krutul, D.; Zielenkiewicz, T.; Radomski, A.; Zawadzki, J.; Antczak, A.; Drożdżek, M.; Makowski, T. Metals accumulation on Scots pine (Pinus sylvestris L.) wood and bark affectes with environmental pollution. Wood Res. 2017, 62, 353–364. [Google Scholar]
- Enzmann, J.W.; Goodrich, R.D.; Meiske, J.C. Chemical composition and nutritive value of poplar bark. J. Anim. Sci. 1969, 29, 653–660. [Google Scholar] [CrossRef]
- Skrypnik, L.; Grigorev, N.; Michailov, D.; Antipina, M.; Danilova, M.; Pungin, A. Comparative study on radical scavenging activity and phenolic compounds content in water bark extracts of alder (Alnus glutinosa (L.) Gaertn.), oak (Quercus robur L.) and pine (Pinus sylvestris L.). Eur. J. Wood Wood Prod. 2019, 77, 879–890. [Google Scholar] [CrossRef]
- Brennan, M.; Fritsch, C.; Cosgun, S.; Dumarcay, S.; Colin, F.; Gérardin, P. Quantitative and qualitative composition of bark polyphenols changes longitudinally with bark maturity in Abies alba Mill. Ann. For. Sci. 2020, 77, 9. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Chang, S.-T. Variation in antioxidant activity of extracts of Acacia confusa of different ages. Nat. Prod. Commun. 2010, 5, 73–76. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Cen, Z.; Fu, Y.; Zhu, X.; He, H.; Kong, D.; Wu, H. The variation in essential oils composition, phenolic acids and flavonoids is correlated with changes in antioxidant activity during Cinnamomum loureirii bark growth. Arab. J. Chem. 2021, 14, 1032149. [Google Scholar] [CrossRef]
- Dogan, K.; Akman, P.K.; Tornuk, F. Tree barks as potential sources of value-added components for the food industry. Int. J. Food Technol. Nutr. 2019, 2, 25–35. [Google Scholar]
- Aoyama, M.; Kishino, M.; Jo, T.S. Biosorption of Cr(VI) on Japanese cedar bark. Sep. Sci. Technol. 2005, 39, 1149–1162. [Google Scholar] [CrossRef]
- Tanase, C.; Coșarcă, S.; Muntean, D.-L. A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef] [PubMed]
- Ciesla, W.M. Non-Wood Forest Products from Temperate-Leved Trees; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002; 125p. [Google Scholar]
- Salih, E.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Hiltunen, R.; Vuorela, H.; Julkunen-Tiitto, R.; Fyhrquist, P. Tannins, flavonoids and stilbenes in extracts of african savanna woodland trees Terminalia brownii, Terminalia laxiflora and Anogeissus leiocarpus showing promising antibacterial potential. South Afr. J. Bot. 2017, 108, 370–386. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, antioxidant and antimicrobial activities of leaf and bark extracts of Solidago canadensis L. Ind. Crops Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Enkhtaivan, G.; John, K.M.; Ayyanar, M.; Sekar, T.; Jin, K.-J.; Kim, D.H. Anti-influenza (H1N1) potential of leaf and stem bark extracts of selected medicinal plants of south India. Saudi J. Biol. Sci. 2015, 22, 532–538. [Google Scholar] [CrossRef]
- Kadir, A.A. Drugs from plants. In Forest Products Biotechnology; Taylor & Francis: London, UK, 1998; pp. 209–234. [Google Scholar]
- Martini, S.; Afroze, S.; Roni, K.A. Modified eucalyptus bark as a sorbent for simultaneous removal of COD, oil, and Cr(III) from industrial wastewater. Alex. Eng. J. 2020, 59, 1637–1648. [Google Scholar] [CrossRef]
- Akar, S.; Lorestani, B.; Sobhanardakani, S.; Cheraghi, M.; Moradi, O. Surveying the efficiency of Platanus orientalis bark as biosorbent for Ni and Cr(VI) removal from plating wastewater as a real sample. Environ. Monit. Assess. 2019, 191, 373. [Google Scholar] [CrossRef]
- Brás, I.; Lemos, L.T.; Alves, A.; Pereira, M.F.R. Application of pine bark as a sorbent for organic pollutants in effluents management of environmental quality. Manag. Environ. Qual. 2004, 15, 491–501. [Google Scholar] [CrossRef]
- Salazar-Rabago., J.; Leyva-Ramos., R.; Rivera-Utrilla, J.; Ocampo-Perez, R.; Cerino-Cordova, F.J. Biosorption mechanism of methylene blue from aqueous solution onto white pine (Pinus durangensis) sawdust: Effect of operating conditions. Sustain. Environ. Res. 2017, 27, 32–40. [Google Scholar] [CrossRef]
- Ansari, R.; Mosayebzade, Z. Removal of basic dye methylene blue from aqueous solutions using sawdust and sawdust coated with polypyrrole. J. Iran Chem. Soc. 2010, 7, 339–350. [Google Scholar] [CrossRef]
- Jauberty, L.; Gloaguen, V.; Astier, C.; Krausz, P.; Delpechi, V.; Berland, A.; Granger, V.; Niort, I.; Royer, A.; Decossas, J.-L. Bark, a suitable biosorbent for the removal of uranium from wastewater—From laboratory to industry. Radioprotection 2011, 46, 443. [Google Scholar] [CrossRef]
- Haussard, M.; Gaballah, I.; de Donato, P.; Barrès, O.; Mourey, A. Removal of hydrocarbons from wastewater using treated bark. J. Air Waste Manag. Assoc. 2001, 51, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Şen, A.; Olivella, M.A.; Fiol, N.; Miranda, I.; Villaescusa, I.; Pereira, H. Removal of chromium (VI) in aqueous environments using cork and heat treated cork samples from Quercus cerris and Quercus suber. BioResources 2012, 7, 4843–4857. [Google Scholar] [CrossRef]
- Di Wu Recycle technology for potato peel waste processing: A review. Procedia Environ. Sci. 2016, 31, 103–107. [CrossRef]
- Kot, A.M.; Pobiega, K.; Piwowarek, K.; Kieliszek, M.; Błażejak, S.; Gniewosz, M.; Lipińska, E. Biotechnological methods of management and utilization of potato industry waste—A review. Potato Res. 2020, 63, 431–447. [Google Scholar] [CrossRef]
- Wu, Z.G.; Xu, H.Y.; Ma, Q.; Cao, Y.; Ma, J.N.; Ma, C.M. Isolation, identification and quantification of unsaturated fatty acids, amides, phenolic compounds and glycoalkaloids from potato peel. Food Chem. 2012, 135, 2425–2429. [Google Scholar] [CrossRef]
- Friedman, M.; Kozukue, N.; Kim, H.J.; Choi, S.H.; Mizuno, M. Glycoalkaloid, phenolic, and flavonoid content and antioxidative activities of conventional nonorganic and organic potato peel powders from commercial gold, red, and Russet potatoes. J. Food Comp. Anal. 2017, 62, 69–75. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Askari, S.; Siddiqui, A.; Kaleem, M. Potato peel mediated improvement in organic substances of vigna mungo growing under copper stress. J. Pharmacogn. Phytochem. 2017, 6, 1373–1378. [Google Scholar]
- Albishi, T.; John, J.; Al-Khalifa, A.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Al-Weshahy, A.; Venket Rao, A. Isolation and characterization of functional components from peel samples of six potatoes varieties growing in Ontario. Food Res. Int. 2009, 42, 1062–1066. [Google Scholar] [CrossRef]
- Külen, O.; Stushnoff, C.; Holm, D. Effect of cold storage on total phenolics content, antioxidant activity and vitamin c level of selected potato clones. J. Sci. Food Agric. 2013, 93, 2437–2444. [Google Scholar] [CrossRef]
- Ezekiel, R.; Singh, N.; Sharma, S.; Kaur, A. Beneficial phytochemicals in potato—A review. Food Res. Int. 2013, 50, 487–496. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Gullón, B.; Yáñez, R. Identification and recovery of valuable bioactive compounds from potato peels: A comprehensive review. Antioxidants 2021, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Gonçalves, I.; Nunes, C.; Teixeira, B.; Mendes, R.; Ferreira, P.; Coimbra, M.A. Potato peel phenolics as additives for developing active starch-based films with potential to pack smoked fish fillets. Food Packag. Shelf Life 2021, 28, 100644. [Google Scholar] [CrossRef]
- Mohdaly, A.; Sarhan, M.; Smetanska, I.; Mahmoud, A. Antioxidant properties of various solvent extracts of potato peel, sugar beet pulp and sesame cake. J. Sci. Food Agric. 2010, 90, 218–226. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidative phenolic constituents of skins of onion varieties and their activities. J. Funct. Foods 2013, 5, 1191–1203. [Google Scholar] [CrossRef]
- Habeebullah, S.F.K.; Grejsen, H.D.; Jacobsen, C. Potato peel extract as a natural antioxidant in chilled storage of minced horse mackerel (Trachurus trachurus): Effect on lipid and protein oxidation. Food Chem. 2012, 131, 843–851. [Google Scholar]
- Kanatt, S.; Chander, R.; Radhakrishna, P.; Sharma, A. Potato peel extracta natural antioxidant for retarding lipid peroxidation in radiation processed lamb meat. J. Agric. Food Chem. 2005, 53, 1499–1500. [Google Scholar] [CrossRef]
- Manjunath, K.S.; Bhandage, S.; Kamat, S. Potato peel dressing: A novel adjunctive in the management of necrotizing fasciitis. J. Maxillofac Oral. Surg. 2015, 14, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Mamoini, F.Z.; Eazouk, R.; Kajji, A.; Daoui, K.; El Ouali, A.; Boukhlifi, F. Characterization of organic waste used as biofertilizers: Case of potato peelings, almond hulls and shrimp shells. Int. J. Agric. Innov. Res. 2016, 4, 993–997. [Google Scholar]
- Javed, A.; Ahmad, A.; Tahir, A.; Shabbir, U.; Nouman, M.; Hameed, A. Potato peel waste—Its nutraceutical, industrial and biotechnological applacations. AIMS Agric. Food 2019, 4, 807–823. [Google Scholar] [CrossRef]
- Mohammed, N.M.S.; Salim, H.A.M. Adsorption of Cr (V) ion from aqueous solutions by solid waste of potato peels. Sci. J. Uni Zakho 2017, 5, 254–258. [Google Scholar] [CrossRef]
- Mutongo, F.; Kuipa, O.; Kuipa, P.K. Removal of Cr (VI) from aqueous solutions using powder of potato peelings as a low cost sorbent. Bioinorg. Chem. Appl. 2014, 2014, 973153. [Google Scholar] [CrossRef]
- Bibi, S.; Farooqi, A.; Yasmin, A.; Kamran, M.A.; Niazi, N.K. Arsenic and fluoride removal by potato peel and rice husk (PPRH) ash in aqueous environments. Int. J. Phytoremediat. 2017, 19, 1029–1036. [Google Scholar] [CrossRef]
- Guechi, E.K.; Hamdaoui, O. Evaluation of potato peel as a novel adsorbent for the removal of Cu (II) from aqueous solutions: Equilibrium, kinetic, and thermodynamic studies. Desalin Water Treat 2016, 57, 10677–10688. [Google Scholar] [CrossRef]
- Mahale, K.K.; Mokhasi, H.R.; Ashoka, H. Biosorption of nickel (II) from aqueous solutions using potato peel. Res. J. Chem. Environ. Sci. 2016, 4, 96–101. [Google Scholar]
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M. Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arab. J. Chem. 2016, 9, S707–S716. [Google Scholar] [CrossRef]
- Nathan, R.J.; Martin, C.E.; Barr, D.; Rosengren, R.J. Simultaneous removal of heavy metals from drinking water by banana, orange and potato peel beads: A study of biosorption kinetics. Appl. Water Sci. 2021, 11, 116. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Issa, A.A.; Al-Sulaiti, M.; Al-Yafie, J.; Shomar, B.; Al-Saad, K. Potato peels as an adsorbent for heavy metals from aqueous solutions: Eco-structuring of a green adsorbent operating plackett–burman design. J. Chem. 2019, 2019, 4926240. [Google Scholar] [CrossRef]
- Feizi, M.; Jalali, M. Removal of heavy metals from aqueous solutions using sunflower, potato, canola and walnut shell residues. J. Taiwan Inst. Chem. Eng. 2015, 54, 125–136. [Google Scholar] [CrossRef]
- Kaur, D.; Wani, A.A.; Singh, D.P.; Sogi, D.S. Shelf life enhancement of butter, ice-cream, and mayonnaise by addition of lycopene. Int. J. Food Prop. 2011, 14, 1217–1231. [Google Scholar] [CrossRef]
- Imamura, T.; Isozumi, N.; Higashimura, Y.; Koga, H.; Segawa, T.; Desaka, N.; Takagi, H.; Matsumoto, K.; Ohki, S.; Mori, M. Red-beet betalain pigments inhibit amyloid-β aggregation and toxicity in amyloid-β expressing caenorhabditis elegans. Plant Foods Human Nutr. 2022, 77, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D.; et al. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017, 110, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.J.; Cebović, T.N.; Canadanović-Brunet, J.M.; Cetković, G.S.; Canadanović, V.M.; Djilas, S.M.; Tumbas Šaponjac, V.T. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Biological properties and applications of betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef]
- Smječanin, N.; Nuhanović, M.; Sulejmanović, J.; Grahet, Ž.; Odobašić, A.; Grahet, Ž.; Odobašić, A. Study of uranium biosorption process in aqueous solution by red beet peel. J. Radioanal. Nucl. Chem. 2022, 331, 1459–1471. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; El-Mogy, M.M.; Parmar, A.; Mansour, A.T.; Shalaby, T.A.; Ali, M.R. Phytochemical characterization and utilization of dried red beetroot (Beta vulgaris) peel extract in maintaining the quality of Nile Tilapia fish fillet. Antioxidants 2022, 11, 906. [Google Scholar] [CrossRef]
- Maqbool, H.; Safeena, M.P.; Abubacker, Z.; Azhar, M.; Kumar, S. Effect of beetroot peel dip treatment on the quality preservation of Deccan mahseer (Tor khudree) steaks during frozen storage (−18 °C). LWT 2021, 151, 112222. [Google Scholar] [CrossRef]
- Lazăr, S.; Constantin, O.E.; Horincar, G.; Andronoiu, D.G.; Stănciuc, N.; Muresan, C.; Râpeanu, G. Beetroot by-product as a functional ingredient for obtaining value-added mayonnaise. Processes 2022, 10, 227. [Google Scholar] [CrossRef]
- Melo, N.; Wolff, G.H.; Costa-da-Silva, A.L.; Arribas, R.; Triana, M.F.; Gugger, M.; Riffell, J.A.; DeGennaro, M.; Stensmyr, M.C. Geosmin attracts Aedes aegypti mosquitoes to oviposition sites. Current Biol. 2020, 30, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hamauzu, Y. Phenolic compounds and their antioxidant properties in different tissues of carrots (Daucus carota L.). J. Food Agr. Environ. 2004, 2, 95–100. [Google Scholar]
- Leja, M.; Kamińska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The content of phenolic compounds and radical scavenging activity varies with carrot origin and root color. Plant Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef]
- Hellström, J.; Granato, D.; Mattila, P.H. Accumulation of phenolic acids during storage over differently handled fresh carrots. Foods 2020, 9, 1515. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Sarengaowa, G.Y.; Feng, K. Biosynthesis of phenolic compounds and antioxidant activity in fresh-cut fruits and vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) peels: A review on bioactive compounds and biomedical activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef]
- Chope, G.A.; Cools, K.; Hammond, J.P.; Thompson, A.J.; Terry, L.A. Physiological, biochemical and transcriptional analysis of onion bulbs during storage. Ann. Bot. 2012, 109, 819–831. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Romaszko, J.; Bucinski, A.; Szawara- Nowak, D.; Honke, J.; Zielinski, H.; Piskula, M.K. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J. Nutr. 2008, 138, 885–888. [Google Scholar] [CrossRef]
- Nemtinov, V.I.; Golubkina, N.; Koshevarov, A.; Konstanchuk, Y.; Molchanova, A.; Nadezhkin, S.; Sellito, V.M.; Caruso, G. Health–beneficial compounds from edible and waste bulb components of sweet onion genotypes organically grown in northern Europe. Banat’s J. Biotechnol. 2019, 10, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Shabir, I.; Pandey, V.K.; Dar, A.H.; Pandiselvam, R.; Manzoor, S.; Mir, S.A.; Shams, R.; Dash, K.K.; Fayaz, U.; Khan, S.A. Nutritional profile, phytochemical compounds, biological activities, and utilization of onion peel for food applications: A review. Sustainability 2022, 14, 11958. [Google Scholar] [CrossRef]
- Phanthuwongpakdee, J.; Babel, S.; Laohhasurayotin, K.; Sattayaporn, S.; Kaneko, T. Anthocyanin based agricultural wastes as bio-adsorbents for scavenging radioactive iodide from aqueous environment. J. Environ. Chem. Eng. 2020, 8, 104147. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.W.; Hwang, K.E.; Song, D.H.; Choi, M.S.; Ham, Y.K.; Choi, Y.S.; Lee, J.W.; Lee, S.K.; Kim, C.J. Combined effect of kimchi powder and onion peel extract on quality characteristics of emulsion sausages prepared with irradiated pork. Korean J. Food Sci. Animal Res. 2015, 35, 277–285. [Google Scholar] [CrossRef]
- Cebin, A.V.; Šeremet, D.; Mandura, A.; Martinić, A.; Komes, D. Onion solid waste as a potential source of functional food ingredients. Power Eng. 2020, 15, 7–13. [Google Scholar]
- Kostecka-Gugala, A.; Kruczek, M.; Ledwożyw-Smoleń, I.; Kaszycki, P. Antioxidants and health-beneficial nutrients in fruits of eighteen Cucurbita cultivars: Analysis of diversity and dietary implications. Molecules 2020, 25, 1792. [Google Scholar] [CrossRef]
- Goncharov, A.; Golubkina, N.; Pivovarov, V.; Gasparian, I.; Caruso, G. Comparative evaluation of biochemical parameters and mineral composition of Cucurbita ficifolia, C.maxima and C.maschata fruit, grown in the northern hemisphere. Veg. Crops Russ. 2022, 4, 46–54. [Google Scholar] [CrossRef]
- Šehović, E.; Memić, M.; Sulejmanović, J.; Huremovic, J. Sorption of metals on pulverized pumpkin (Cucurbita pepo L.) peels. Anal. Lett. 2016, 49, 2446–2460. [Google Scholar] [CrossRef]
- Bal, D.; Özer, Ç.; İmamoğlu, M. Green and ecofriendly biochar preparation from pumpkin peel and its usage as an adsorbent for methylene blue removal from aqueous solutions. Water Air Soil Pollut. 2021, 232, 457. [Google Scholar] [CrossRef]
- Rashid, J.; Tehreem, F.; Rehman, A.; Kumar, R. Synthesis using natural functionalization of activated carbon from pumpkin peels for decolourization of aqueous methylene blue. Sci. Tot. Environ. 2019, 671, 369–376. [Google Scholar] [CrossRef]
- Salami, A.; Asefi, N.; Kenari, R.E.; Garekhani, M. Extraction of pumpkin peel extract, using supercritical CO2 and subcritical water technology: Enhancing oxidative stability of canola oil. J. Food Sci. Technol. 2021, 58, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Jarungjitaree, P.; Naradisorn, M. Evaluation of antioxidant and antifungal activities of pumpkin by-product and its application in banana. J. Food Sci. Agr. Technol. 2018, 4, 129–133. Available online: http://rs.mfu.ac.th/ojs.index.php/jfat (accessed on 24 November 2022).
- Gungor, K.K.; Torun, M. Pumpkin peel valorization using green extraction technology to obtain β-carotene fortified mayonnaise. Waste Biomass Valor. 2022, 13, 4375–4388. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Sehar, S.; Sarwar, A.; Ashraf, A.H.; Jamil, M.A.; Noreen, S.; Rafique, A.; Iftikhar, K.; Quddoos, M.Y.; et al. A comprehensive review of functional ingredients, especially bioactive compounds present in pumpkin peel, flesh and seeds, and their health benefits. Food Chem. Adv. 2022, 1, 100067. [Google Scholar] [CrossRef]
- Sharma, M.; Bhat, R. Extraction of carotenoids from pumpkin peel and pulp: Comparison between innovative green extraction technologies (ultrasonic and microwave-assisted extractions using corn oil. Foods 2021, 10, 787. [Google Scholar] [CrossRef]
- Grassino, A.N.; Halambek, S.; Djaković, S.; Rimac, M.; Grabarić, Z.D. Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll. 2016, 52, 265–274. [Google Scholar] [CrossRef]
- Grassino, A.N.; Djaković, S.; Bosiljkov, T.; Halambek, J.; Zorić, Z.; Dragović-Uzelac, V.; Petrović, M.; Brnčić, S.R. Valorisation of tomato peel waste as a sustainable source for pectin, polyphenols and fatty acids recovery using sequential extraction. Waste Biomass Valor. 2020, 11, 4593–4611. [Google Scholar] [CrossRef]
- Shinde, M.; Sonawane, S.K.; Patil, S. Fruit peel utilization in food packaging. IFI Mag. 2019, 1, 19–24. [Google Scholar]
- Elkodous, M.A.; El-Husseiny, H.M.; El-Sayyad, G.S.; Hashem, A.H.; Doghish, A.S.; Elfadil, D.; Radwan, Y.; El-Zeiny, H.M.; Bedair, H.; Ikhdair, O.A.; et al. Recent advances in waste-recycled nanomaterials for biomedical applications:waste-to-wealth. Nanotechnol. Rev. 2021, 10, 1662–1739. [Google Scholar] [CrossRef]
- Omar, R.A.; Chauhan, D.; Talreja, N.; Mangalaraja, R.V.; Ashfaq, M. Nano Particles Reducing and Stabilizing Agents Chapter 12—Vegetables Waste for Biosynthesis of Various Nanoparticles. In Nanobiotechnology for Plant Protection, Agri-Waste and Microbes for Production of Sustainable Nanomaterials; Abd-Elsalam, K.A., Periakaruppan, S.R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 281–298. [Google Scholar] [CrossRef]
- Deokar, G.K.; Ingale, A.G. Unveiling an unexpected potential of beetroot waste in green synthesis of single crystalline gold nanoplates: A mechanistic study. Arab. J. Chem. 2018, 11, 950–958. [Google Scholar] [CrossRef]
- Bahram, M.; Mohammadzadeh, E. Green synthesis of gold nanoparticles with willow tree bark extract: A sensitive colourimetric sensor for cysteine detection. Anal. Methods 2014, 6, 6916–6924. [Google Scholar] [CrossRef]
- Phukan, K.; Devi, R.; Chowdhury, D. Green synthesis of gold nano-bioconjugates from onion peel extract and evaluation of their antioxidant, anti-inflammatory, and cytotoxic studies. ACS Omega 2021, 6, 17811–17823. [Google Scholar] [CrossRef]
- Babu, S.; Devadiga, A.; Shetty, K.V.; Saidutta, M.B. Synthesis of silver nanoparticles using medicinal Zizyphus xylopyrus bark extract. Appl. Nanosci. 2014, 5, 755–762. [Google Scholar] [CrossRef]
- Erdem, İ.; Çakır, Ş. Green synthesis of silver nanoparticles using walnut shell powder and Cynara sp. and their antibacterial activities. Hacettepe J. Biol. Chem. 2022, 50, 335–347. [Google Scholar] [CrossRef]
- Nandhini, S.; Sheeba, D. Vegetable peel extract mediated synthesis of silver nanoparticles and its antimicrobial activities. Res. J. Chem. Environ. 2020, 24, 39–44. [Google Scholar]
- Sharma, K.; Kaushik, S.; Jyoti, A. Green synthesis of silver nanoparticles by using waste vegetable peel and its antibacterial activities. J. Pharm. Sci. Res. 2016, 8, 313–316. [Google Scholar]
- Deepa, A.F.; Amirul Islam, M.; Dhanker, R. Green synthesis of silver nanoparticles from vegetable waste of pea Pisum sativum and bottle gourd Lagenaria siceraria: Characterization and antibacterial properties. Front. Environ. Sci. 2022, 10, 941554. [Google Scholar] [CrossRef]
- Iravani, S.; Zolfaghari, B. Green synthesis of silver nanoparticles using Pinus eldarica bark extract. Biomed. Res. Int. 2013, 2013, 639725. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, A.; Theertha, V.; Prakash, P.; Chandran, S.S. From waste to a value added product: Green synthesis of silver nanoparticles from onion peels together with its diverse applications. Mater. Today Proc. 2021, 46, 4460–4463. [Google Scholar] [CrossRef]
- Puri, A.; Patil, S. Biogenic synthesis of selenium nanoparticles using Diospyros montana bark extract: Characterization, antioxidant, antibacterial, and antiproliferative activity. Biosci. Biotechnol. Res. Asia 2022, 19, 423–441. [Google Scholar] [CrossRef]
- Pattanayak, S.; Masud, M.; Mollick, R.; Maity, D.; Chakraborty, S.; Dash, S.K.; Chattopadhyay, S.; Roy, S.; Chattopadhyay, D.; Chakraborty, M. Butea monosperma bark extract mediated green synthesis of silver nanoparticles: Characterization and biomedical applications. J. Saudi Chem. Soc. 2017, 21, 673–684. [Google Scholar] [CrossRef]
- Ullah, H.; Ullah, Z.; Fazal, A.; Irfan, M. Use of vegetable waste extracts for controlling microstructure of CuO nanoparticles: Green synthesis, characterization, and photocatalytic applications. J. Chem. 2017, 2017, 2721798. [Google Scholar] [CrossRef]
- Surendra, T.V.; Roopan, S.M.; Al-Dhabi, N.A.; Arasu, M.V.; Sarkar, G.; Suthindhiran, K. Vegetable peel waste for the production of ZnO nanoparticles and its toxicological efficiency, antifungal, hemolytic, and antibacterial activities. Nanoscale Res. Lett. 2016, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Yadav, V.K.; Choudhary, N.; Alswieleh, A.M.; Sharma, A.K.; Bhardwaj, A.K.; Khan, S.H.; Yadav, K.K.; Cheon, J.K.; Jeon, B.H. Onion peel waste mediated-green synthesis of sinc oxide nanoparticles and their phytotoxicity on mung bean and wheat plant growth. Materials 2022, 15, 2393. [Google Scholar] [CrossRef] [PubMed]
- Akintelu, S.A.; Oyebamiji, A.K.; Olugbeko, S.C.; Folorunso, A.S. Green synthesis of iron oxide nanoparticles for biomedical application and environmental remediation: A review. Eclética Química 2021, 46, 17–37. [Google Scholar] [CrossRef]
- Miri, A.; Khatami, M.; Sarani, M. Biosynthesis, magnetic and cytotoxic studies of hematite nanoparticles. J. Inorg. Organomet. Polym. 2020, 30, 767–774. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Chan, K.W.; Zia-Ul-Haq, M.; Ismail, M. Chemical composition of Artemisia annua L. leaves and antioxidant potential of extracts as a function of extraction solvents. Molecules 2012, 17, 6020–6032. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Antoshkina, M.S.; Nadezhkin, S.M.; Soldatenko, A.V. Plants Antioxidants and Methods of Their Determination; Infra-M: Moscow, Russia, 2020; pp. 155–164. (In Russian) [Google Scholar]






| Species | Pollutant | References |
|---|---|---|
| Lupinus albus L. | Malachite green dye | [57] |
| Cucurbita sp. | Pb | [58] |
| Dyes | [59] | |
| Antibiotics | [60] | |
| Triticum L. grain bran | Pb | [61] |
| Dye from textile wastewater | [62] | |
| Cicer arietinum L. | Dyes | [63] |
| Sclerocarya birrea (A.Rich.) Hochst. | Methylene blue | [64] |
| Cucumis melo L. | Methylene blue | [65] |
| Bertholletia excelsa Humb. & Bonpl. shells | Methylene blue, indigo carmine | [66] |
| Helianthus L. | Dyes | [67] |
| Active Ingredient | Pollutant | Reference |
|---|---|---|
| Eucalyptus L’Hér. bark | Cr, oil | [94] |
| Platanus orientalis L. bark | Cr, Ni | [95] |
| Pinus L. bark | Pentachlorophenol | [96] |
| Pinus durangensis Martínez sawdust | Methylene blue | [97] |
| Juglans regia L. sawdust | Methylene blue | [98] |
| Pseudotsuga menziesii (Mirb.) Franco bark | Uranium | [99] |
| Pinophyta Cronquist, Takht. & Zimmerm. ex Reveal bark | Hydrocarbons, Al, Ca, Fe, Mg, S | [100] |
| Quercus cerris L. cork | Cr6+ | [101] |
| Active Ingredient | Utilization Efficiency | References |
|---|---|---|
| Antioxidant and antiviral properties | ||
| Peel powder | Preservative in fish processing | [115,116] |
| Preservative in meat storage and processing | [117] | |
| Oil preservation | [114] | |
| Wound healing | [118] | |
| Food additive and animal feed | [20] | |
| Peel extracts | Anti-cancer, anti-inflammatory | [113] |
| Adsorption capacity for water purification | ||
| Potato peel + HCl | Cr6+ | [121,122] |
| Potato peel ash | As-, F− | [123] |
| Potato peel dry powder; pH 5 | Cu2+ | [124] |
| Potato peel | Ni2+ | [125] |
| Potato peel | Methylene blue, malachite green | [126] |
| Banana, orange, potato peel | Heavy metals | [127] |
| Potato raw and burned peel | Cd2+, Co2+, Cu2+, Fe2+, Ni2+, Pb2+ | [128] |
| Potato peel | Mn2+, Fe3+, Zn2+, Ni2+, Cu2+, Cd2+ | [129] |
| Others | ||
| Potato peel | biofertilizer | [119] |
| Active Ingredient | Properties | Ref. | |
|---|---|---|---|
| Adsorption capacity | 0.125 mm fraction of peel powder | U6+ | [135] |
| Antioxidant and antiviral effect | Peel powder | Improvement of Nile Tilapia storage | [136] |
| Peel extract | Improvement of Deccan mahseer (Tor khudree) steaks storage | [137] | |
| Medicine | Betalain pigments, fiber, polyphenols | Degenerative diseases, anti-inflammatory, anti-aging, cancer prevention | [131,132] |
| Hepatoprotective | [133] | ||
| Functional food, food additive and colorant | Peel powder | Food colorant, functional bread | [134] |
| Mayonnaise | [138] | ||
| Others | Fresh peel | Mosquito attractant | [139] |
| Species | N ** | Mean Peel AOA *** | Parameter | Regression Equation **** | r |
|---|---|---|---|---|---|
| A. cepa L. red bulbs * | 10 | 128 | AOA | Y= −0.009X2 + 0.3756X | +0.960 |
| Allium cepa gr. aggregatum | 8 | 123.4 | AOA | Y= −0.0012X2 + 0.3548X | +0.994 |
| Raphanus sativus L. | 18 | 50 | AOA | Y= −0.004X2 + 0.5929X + 0.0395 | +0.939 |
| Solanum tuberosum L. | 8 | 15 | AOA | Y= −0.0127X2 + 1.0921X−0.0064 | +0.996 |
| Beta vulgaris L. | 6 | 30 | Betacyanins | Y= −0.0128X2 + 0.5894X | +0.968 |
| 6 | Betaxanthins | Y= −0.02581X2 + 0.5961X | +0.991 |
| Active Ingredient | Utilization Efficiency | Ref. |
|---|---|---|
| Adsorption capacity | ||
| Anthocyanin containing onion peel | Removal of radioactive iodine | [150] |
| Antioxidant effect | ||
| Peel extracts | Cardioprotective, antidiabetic, anticancer, immunomodulatory, neuroprotective properties | [17,145] |
| Peel powder extract | Food preservative, pork sausages | [151] |
| Food additive and colorant | ||
| Peel powder | Food colorant, functional bread | [152] |
| Active Ingredient | Utilization Efficiency | References |
|---|---|---|
| Absorption capacity | ||
| pulverized C. pepo L. peel | Cd, Co, Cr, Fe, Mn, Ni, and Pb removal | [155] |
| Cucurbita sp. Biocar | Methylene blue removal | [156] |
| activated carbon | Methylene blue removal | [157] |
| Antioxidant effect | ||
| Peel extract | Oxidative stability of canola oil | [158] |
| Antifungal activity against anthracnose in banana | [159] | |
| Food additive and colorant | ||
| Helianthus annuus L. oil extract | β-carotene enriched mayonnaise production | [160] |
| C. maxima Lindl., peel | Pectin, polysaccharides and fiber production | [161] |
| C. maxima Lindl., peel | Bread baking | [21] |
| C. maxima Lindl. | Carotenoids extraction | [162] |
| Nanoparticles | Species Waste | Size, nm | Ref. |
|---|---|---|---|
| Au | Beta vulgaris L. waste | 50–65 | [168] |
| Salix alba L. bark | 15 | [169] | |
| Allium cepa L. peel ethyl acetate extracts | <20 nm | [170] | |
| Ag | Ziziphus xylopyrus B.Heyne ex Roth (Rhamnaceae) | 60–70 | [171] |
| Juglans regia L. shell | [172] | ||
| Cucurbita pepo L. and Trichosanthes cucumerina L. | [173] | ||
| Lagenaria siceraria (Molina) Standl., Luffa cylindrica (L.), Solanum lycopersicum L., Solanum melongena L. and Cucumis sativus L. | 20 | [174] | |
| Pinus sativum L. and Lagenaria siceraria (Molina) Standl. | [175] | ||
| Pinus eldarica (Medw.) Silba (Pinaceae)—Eldarica pine | 10–40 | [176] | |
| Allium cepa L. peel | [177] | ||
| Se | Diospyros montana Roxb. | 120–200 | [178] |
| Butea monosperma (Lam.) Taub. | 35 | [179] | |
| CuO | Brassica oleracea var. botrytis L. waste, Solanum tuberosum L. and Pisum sativum L. peel | [180] | |
| ZnO | Moringa oleifera Lam. peel | 40–45 | [181] |
| Allium cepa L. peel | 20–80 | [182] | |
| Fe2O3 | Salvadora persica L. bark | [183,184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Skrypnik, L.; Logvinenko, L.; Zayachkovsky, V.; Smirnova, A.; Krivenkov, L.; Romanov, V.; Kharchenko, V.; Poluboyarinov, P.; Sekara, A.; et al. The ‘Edge Effect’ Phenomenon in Plants: Morphological, Biochemical and Mineral Characteristics of Border Tissues. Diversity 2023, 15, 123. https://doi.org/10.3390/d15010123
Golubkina N, Skrypnik L, Logvinenko L, Zayachkovsky V, Smirnova A, Krivenkov L, Romanov V, Kharchenko V, Poluboyarinov P, Sekara A, et al. The ‘Edge Effect’ Phenomenon in Plants: Morphological, Biochemical and Mineral Characteristics of Border Tissues. Diversity. 2023; 15(1):123. https://doi.org/10.3390/d15010123
Chicago/Turabian StyleGolubkina, Nadezhda, Liubov Skrypnik, Lidia Logvinenko, Vladimir Zayachkovsky, Anna Smirnova, Leonid Krivenkov, Valery Romanov, Viktor Kharchenko, Pavel Poluboyarinov, Agnieszka Sekara, and et al. 2023. "The ‘Edge Effect’ Phenomenon in Plants: Morphological, Biochemical and Mineral Characteristics of Border Tissues" Diversity 15, no. 1: 123. https://doi.org/10.3390/d15010123
APA StyleGolubkina, N., Skrypnik, L., Logvinenko, L., Zayachkovsky, V., Smirnova, A., Krivenkov, L., Romanov, V., Kharchenko, V., Poluboyarinov, P., Sekara, A., Tallarita, A., & Caruso, G. (2023). The ‘Edge Effect’ Phenomenon in Plants: Morphological, Biochemical and Mineral Characteristics of Border Tissues. Diversity, 15(1), 123. https://doi.org/10.3390/d15010123











