Abstract
During the last decades, the number of observations of the basket star Astrospartus mediterraneus (Risso, 1826) in the Mediterranean Sea has significantly grown, thanks to SCUBA diver and ROV sightings, citizen reports, as well as particularly large catches by the artisanal fishery. Having been generally considered rare in many areas of the basin, such a long-term increase of records might assign to this basket star the putative role of a winner species in the context of climate changes. In the present study, we combined the overall literature information with the data available for the Ligurian Sea collected during extensive ROV campaigns conducted between 2012 and 2022 at a depth ranging from 20 to 123 m, to better understand the distribution and abundance of this species. The basket star was observed in almost the whole explored bathymetric range living on gorgonians (Eunicella cavolini, E. verrucosa, Paramuricea clavata, and Leptogorgia sarmentosa) and massive sponges (Aplysina cavernicola, Sarcotragus foetidus, Spongia lamella, and Axinella polypoides). In the considered period, the number of recorded specimens did not show a clear trend, but differences emerged over years and months. These variations were strongly correlated with rainfall amounts that, in oligotrophic waters, such as those of the Ligurian Sea, represent an important input of organic matter for these passive filter feeders, especially in the summertime.
1. Introduction
The basket star Astrospartus mediterraneus (Risso, 1826) is the only gorgonocephalid ophiuroid of the Mediterranean Sea [1], already known by Rondelet [2] and Bianchi [3], long before its formal description in the early 1800s.
According to the World Register of Marine Species (WORMS), it is an Atlantic-Mediterranean species with a geographic distribution that also covers the North African and Portuguese coasts. In the Mediterranean basin, the distribution of A. mediterraneus includes the Western basin, the Sicily Channel, and the Ionian Sea. The species was also rarely reported for the Aegean Sea [4,5,6,7,8,9]. The basket star is known to live mainly in mesophotic habitats, with a clear prevalence at depths ranging from 50 to 80 m [10]. Generally, A. mediterraneus is acrophilic on erect benthic organisms, such as sponges and sea fans to improve its passive filter-feeding ability [11,12]. In some cases, specimens demonstrated host fidelity, remaining on the same gorgonian for a very long time, sometimes several years [13].
The basket star has been generally considered rare [11], but in the last two decades, its records have become increasingly frequent, sometimes in the form of massive population blooms. In particular, blooms were observed from 2016 to 2019 from Cap de Creus to Banyuls sur Mer (Catalan Sea). The entity of phenomenon was such to produce a significant impact on fishermen using trammel nets, which became choked by specimens [10,14]. At the same time, A. mediterraneus was abundantly recorded also in other Western Mediterranean areas, such as the Marine Protected Area of Tavolara Punta Coda Cavallo (Nort Sardinia) [12,13].
In the last 20 years, a higher number of surveys has been conducted at mesophotic depths, and Remotely Operated Vehicles (ROVs) proved to be a powerful tool for improving knowledge of the ecology of the deep-sea macrofauna [15,16,17,18,19,20,21,22,23] and were also very useful in monitoring programs aiming to evaluate the environmental status of benthic communities [24] at depths poorly taken into account compared with shallower ones [25].
An accurate revision of the papers citing A mediterraneus, combined with records obtained by citizen sciences, was used to evaluate the distribution trend of this species within the Mediterranean Sea. In addition, thanks to numerous explorative and monitoring ROV surveys conducted in the period 2012–2022 and targeting the mesophotic benthic communities along the entire Ligurian Arc, we investigated the geographic, bathymetric, and temporal distribution patterns of this charismatic ophiuroid and the environmental drivers affecting them.
2. Materials and Methods
The overall systematic literature search and the general distribution of Astrospartus mediterraneus in the Mediterranean Sea was assessed using the World Register of Marine Species (WoRMS) database, the Global Biodiversity Information Facility (GBIF) (www.gbif.org (accessed on 1 June 2022)) and the Base pour l’inventaire des observations subaquatiques (BioObs) (www.bioobs.fr (accessed on 1 June 2022)). These datasets were also used to check the increase of observations coming from citizen science in the last 23 years (2000–2022). In addition, the mean number of scientific papers and grey literature published per decade from 1800 to the present was grouped to describe the trend of scientific reports for this species. The detailed revision published by Zibrowius [4], citing all the records of the species in the Mediterranean Sea, including the historical ones, represented an important baseline. This was incremented with an accurate review of all the available scientific literature published in the following period to date (Table S1). These data were also used to map the general distribution of the species.
The focus on the Ligurian Sea was made using data coming from ROV campaigns financed by the Ministero dell’Ambiente e della Tutela del Territorio e del Mare (MATTM) in 2012 and those carried out within the European Marine Strategy Framework Directive (MSFD) in the period 2015–2022 (Table 1). Data on the abundance of specimens (expressed as the mean number of specimens m−2) were obtained in 37 sites located in three macro-areas: the eastern macro-area (A1, 12 sites) from Montenero Cape to Genoa; the central macro-area (A2, 12 sites) comprehensive of the coast from Arenzano to the western limit of the Savona province; the western macro-area (A3, 13 sites) covering the Imperia administrative boundaries.

Table 1.
Explored sites and recorded Astrospartus mediterraneus in the three macro-areas of the Ligurian coast.
In these sites, 210 ROV dives (47, 92, and 71, respectively, for A1, A2, and A3) were performed in the depth range of 20–123 m during different seasons (Table 1). The video transects were about 200 m long, covering about 100 m2 of the seafloor, following the MSFD protocol for deep coralligenous monitoring. Further technical specifics of ROVs, tracks and video analysis used for all transects carried out can be found in Enrichetti et al. [24]. Due to the discontinuity of hard substrata in the ROV footages, the number of recorded basket stars per transect was expressed per m2 of hard bottom (Table 1). The entire dataset of the obtained records was used to build the depth distribution of A. mediterraneus in the investigated area.
The temporal variation of the densities of the basket stars was tentatively correlated with the parameters of the water column. The food supply for a coastal passive filter-feeder in oligotrophic waters such as those of the Ligurian Sea [26] is considered correlated with rainfall events [27]. Rainfall data in the different localities of the Ligurian Sea were obtained from the ARPAL platform (https://ambientepub.regione.liguria.it/ (accessed on 1 June 2022)). Although water temperature anomalies were not recorded in mesophotic habitats so far, we also checked for a possible influence of Sea Surface Temperature (SST) variation using data derived from NOAA (US National Oceanic and Atmospheric Administration) satellite records, available at www.esrl.noaa.gov/psd/cgi-bin/data/timeseries/timeseries1.pl (accessed on 1 June 2022). In particular, data about A. mediterraneus density were correlated with the total amount of rainfall and the mean value of SST in the three months before each sampling.
The density variations were also correlated with the amount of rainfall in the different macro-areas. It is indeed well known that Liguria’s rainfall regime is highly variable along a West–East gradient [28]. In this way, the mean densities of A. mediterraneus within each macro area were plotted vs. the corresponding mean annual rainfall (mean of the last 20 years). The significativity of correlation was tested through the Pearson correlation coefficient (r).
A. mediterraneus is an acrophilic species, mainly recorded on sea fans. The influence of sea fans on the distribution of the basket star was tested by comparing, in each macro-area, the density of the coral forests (expressed as the number of colonies m−2) in sites where A. mediterraneus was recorded with sites where the species was absent (data obtained from Enrichetti et al. [24]). The results were tested by Permutational Analysis of Variance (PERMANOVA) (factor “macro-area” fixed, three levels; factors “presence-absence”, fixed, two levels).
3. Results
The review of the scientific literature mentioning Astrospartus mediterraneus indicated that the number of works remained low (<1 paper y−1) from 1800 to 2000, with a clear increase in the last two decades, with values reaching 4 papers y−1 after 2020 (Figure 1a). At the same time, the number of observations coming from citizen science exponentially increased from 2000 to present (Figure 1b).
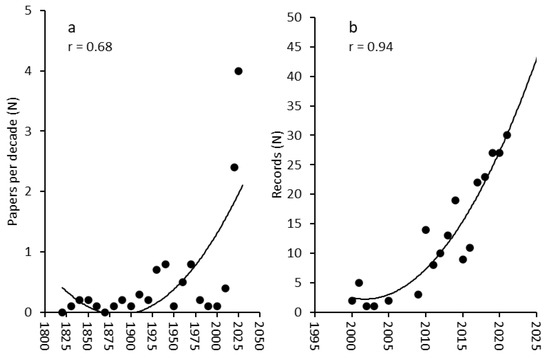
Figure 1.
(a) Trends in the number of published scientific papers mentioning Astrospartus mediterraneus per decade from 1800 to the present and (b) of the number of records obtained from the citizen science databases divided per year from 2000 to the present.
Based on scientific literature and citizen records, the geographic distribution of the species was updated (Table S1, Figure 2a), confirming the wide presence of A. mediterraneus in the western basin, particularly in the Alboran Sea, the Catalan Sea, the Gulf of Lion the Ligurian Sea and the Tyrrhenian Sea and in the Sicily Channel. The scarcity of records for the Balearic Sea, Western Sardinian and Western Corse coasts was remarkable. At the same time, the records were very scarce for the Eastern Mediterranean basin, with scattered observations in the Ionian Sea, the Adriatic Sea, and the Sirte Gulf (Figure 2a; Table S1).
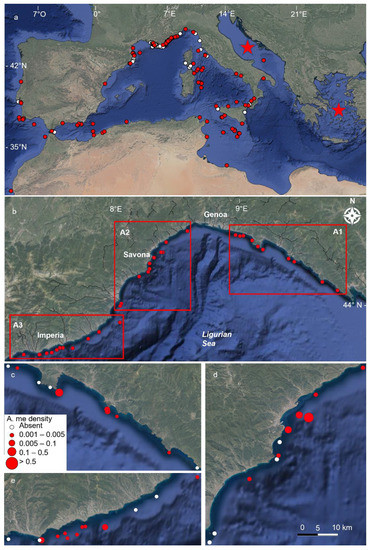
Figure 2.
(a) Distribution of Astrospartus mediterraneus in the Mediterranean Sea and Atlantic waters. Red circles, scientific papers; white spots, citizen science observations; red stars, presence without indication of geographic coordinates. (b) Map of the Ligurian Sea with indicated the studied sites in the three macro-areas (A1, Eastern Liguria; A2, Central Liguria; A3, Western Liguria). (c–e) Enlargements of the three macro-areas. Red circles are sites with A. mediterraneus (the size of the circle is proportional to the density of the specimens); white circles are sites without recorded specimens.
The occurrence of A. mediterraneus in the Ligurian Sea was assessed along the entire coastline (Table 1; Figure 2b). The species was recorded in 28 out of the 37 explored sites (75.6%), with 563 specimens counted in total. From a geographic point of view, in the Eastern Ligurian Riviera (A1), A. mediterraneus was recorded in 66.6% of the sites and 45% of the dives, with a total of 267 specimens; 98% of these specimens were recorded in the two close sites of Portofino Cape and Manara Cape (Figure 2c and Figure 3). In the central portion (A2), the species was noted in 82% of the sites and 43% of the dives with 203 specimens. More than half of these were observed at the Mantice Shoal (Figure 2d and Figure 3). Finally, along the Western Ligurian coast (A3), 77% of the sites and 28% of the dives hosted 93 specimens. 84% of these specimens were recorded at St. Stefano Shoal and Bordighera (Figure 2e and Figure 3).
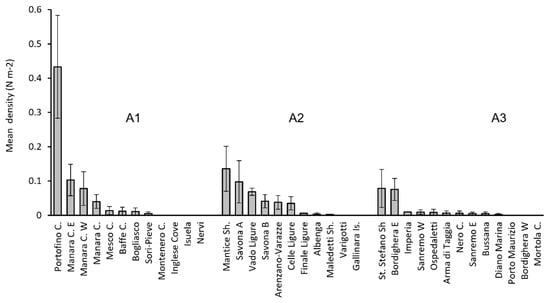
Figure 3.
Sites in the three explored macro-areas (A1, Eastern Liguria; A2, Central Liguria; A3, Western Liguria) were sorted according to decreasing order of recorded mean density (±SE).
Regarding the depth range, the species was observed between 42 and 101 m, with a peak between 60 and 75 m (Figure 4). All the individuals were found on gorgonians (Eunicella cavolini (Koch, 1887), E. verrucosa (Pallas, 1766), Paramuricea clavata (Risso, 1827), and Leptogorgia sarmentosa (Esper, 1791)) (Figure 5a–d) and on massive sponges (Aplysina cavernicola (Vacelet, 1959), Sarcotragus foetidus Schmidt, 1862, Spongia lamella (Schultze, 1879), and Axinella polypoides Schmidt, 1862) (Figure 5e).
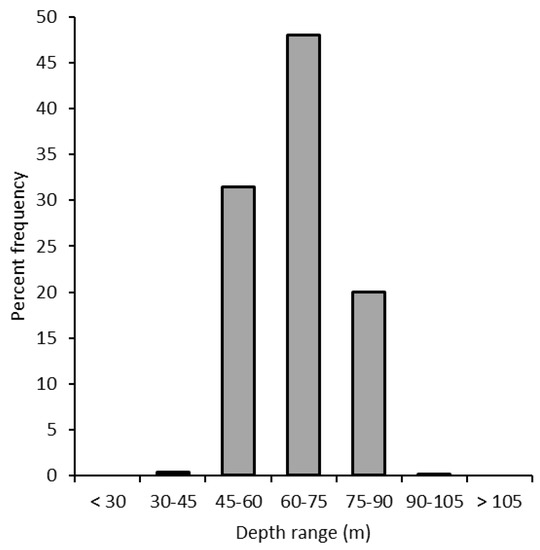
Figure 4.
Percentage frequency distribution of the recorded specimens with depth.

Figure 5.
(a,b) Specimens of Astrospartus mediterraneus acrophilic on the sea fans Eunicella verrucosa, (c,d) Paramuricea clavata, (e) E. cavolini (arrows) and (f) the sponge Sarcotragus foetidus. Scale bars: 10 cm.
The density of the sea fan forests significantly affected the presence of A. mediterraneus. In fact, in all the three considered macro-areas, the basket star occurred in forests with a mean density of about five colonies m−2, while it was absent in more scattered gorgonian aggregations (Figure 6). The statistical analysis (PERMANOVA) confirmed significant differences according to forest density, while differences among macro-areas were not significant (Table 2).
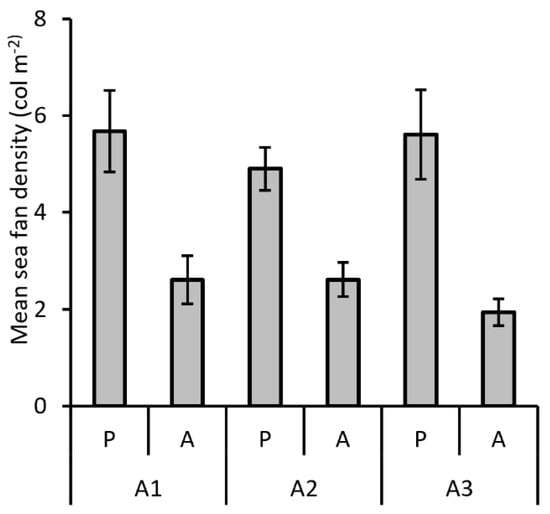
Figure 6.
Mean density (±SE) of the gorgonian forests in sites with the presence (P) and absence (A) of Astrospartus mediterraneus in the three studied macro-areas.

Table 2.
PERMANOVA was performed on sea fan density of the sites where Astrospartus mediterraneus was found in the investigated period. Bray-Curtis similarity index used for the resemblance matrix construction; permutation n = 9999. Significant values are in bold.
The maximal mean density of basket stars in the macro-areas A2 and A3 was about 0.1 specimen m−2. In comparison, in the macro-area A1, the species density reached 0.43 ± 0.15 specimen m−2 in the Portofino Cape (Figure 3). In this site, the maximal recorded density was 1.24 specimen m−2 in July 2020.
At the regional scale, the temporal trend of the mean densities was variable, with a main peak in July 2020 (Figure 7a). While data did not show any significant correlation with a mean SST (Figure 7b), a strong relationship (r = 0.87) was evident with the amount of rainfall in the three months before each survey (Figure 7c). The correlation with rainfall was more robust in the summer months (r = 0.96) than in winter ones (r = 0.81). The correlation between rainfall and basket star density during summer months was also very strong when considering the three macro-areas separately (Figure 7d(A1–A3)).
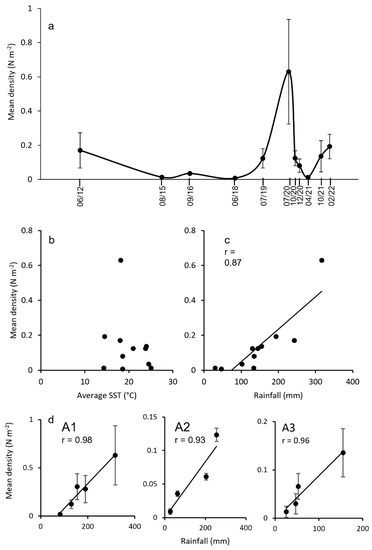
Figure 7.
(a) Trend of the basket star density (±SE) in the entire sampling period. (b) Correlation of Astrospartus mediterraneus density vs. the mean SST in the three months before the sampling. (c) Correlation of A. mediterraneus density vs. total rainfall in the three months before the sampling. (d) A1–A3, Correlation of the density vs. rainfall during the summer months (June-September) in the three macro-areas.
At the geographic level, rainfall amount also seemed to drive the differences among macro-areas: a very strong correlation (r = 0.92) emerged between the mean density of specimens per macro-area and annual rainfall (Figure 8).
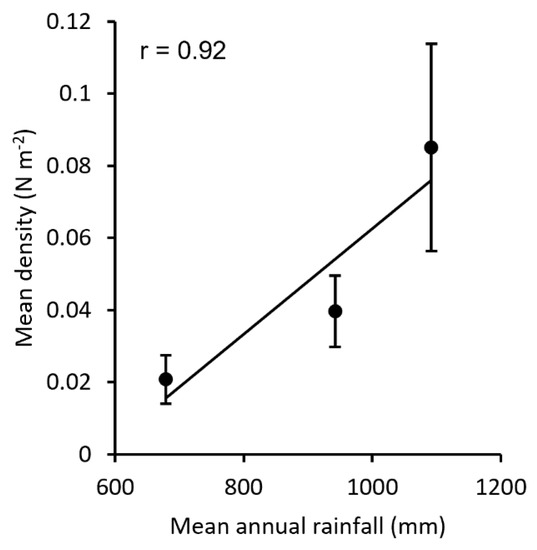
Figure 8.
Correlation of the A. mediterraneus mean density (±SE) in the three considered macro-areas vs. the mean annual rainfall in the area.
4. Discussion
In the last two decades, the records of Astrospartus mediterraneus coming from both scientific papers and citizen science strongly increased. Although these two trends are partially overlapping, they are attributed to different causes. Among them, the citizen science records, in their totality due to diving activity, could be biased by the increment of census projects involving diver volunteers and diving associations registered in the last decades [29,30]. Nevertheless, these records well represent an increase in the abundance of this charismatic species because divers typically re-visit the same dive spots over time, and A. mediterraneus is among one of the most interesting encounters and, therefore, represents a noticeable species. Observational data of one of us (GB) deriving from at least 40 years of diving activity in the Portofino MPA indicate that before 2010 no specimen of this basket star occurred in the standard depth range of scientific diving (0–40 m).
The records of the basket star in scientific studies have been influenced by the rapid growth of research targeting the mesophotic communities in the Mediterranean Sea in the last 20 years [31] and references herein] thanks to the now routine use of ROVs, although sightings might be locally affected by variations of the species abundance. These considerations agree with the high abundances observed in this present study for the Ligurian coast, together with the impressive outbreak recorded from 2013 to present by fishermen in the trammel nets in the area of Cap de Creus (Catalan Sea) [10,14]. In this last site, the number of specimens was so high that the species represented a problem for artisanal fishermen, wasting much time disentangling the ophiuroids from the fishing gear [32].
In an age of global changes, several species have drastically varied their abundance in the Mediterranean Sea, acting respectively as winners or losers in terms of contribution to the benthic communities [33,34]. Generally, the species showing the highest positive or negative variations are organisms living in shallow waters, which are more prone to temperature increases and human impacts [34,35]. Some of these shallow-water species, in response to these stresses, have widened their bathymetric distribution to colonize deeper waters, as in the case of algae, hydrozoans and several other benthic groups [34,36]. Mesophotic species (living between 40 and 200 m) are so far considered less influenced by environmental changes. The case of A. mediterraneus is peculiar because there are no other typically mesophotic species known to have increased their abundance in the longterm. In this regard, no bathymetric changes are reported, and our data agree with all the recent papers indicating a higher abundance between 50 and 80 m [10,32].
It is not easy to hypothesize a possible driver influencing the abundance of A. mediterraneus. Generally speaking, environmental parameters shaping the structure and dynamics of echinoderms populations are poorly understood [37], although some data indicate the involvement of physical and biological factors [38], such as temperature [39,40] or availability of food resources [41].
Temperature is considered an important variable determining the timing of reproduction, with higher temperatures usually being triggers of gametogenesis and spawning [42,43,44,45]. As part of the climate change effects, the seawater temperature is globally rising [34,35], and this phenomenon may extend the reproductive periods and enhance the reproductive performance in northern populations of basket stars, such as those of the Catalan Sea [32] and the Ligurian Sea. The megabenthic communities of the mesophotic zone are indirectly influenced by atmospheric and shallow-water environmental factors, as plumes of warm waters and turbid currents have been demonstrated to seasonally or occasionally reach mesophotic depths [46,47]. However, so far, generalized temperature anomalies have not been registered in the Mediterranean basin at these depths. In this study, no correlation was found between basket star occurrence and SST, but the lack of mesophotic long-term series might introduce a putative bias in this picture.
Regarding food availability for passive filter feeders, an increase in the productivity of the Ligurian Sea with a clear shift in the period 1995–2007 was demonstrated [48]. This increase in food availability temporally overlapped with the intensification of basket star records in citizen sciences observations.
Although this species was considered rare in the Northern Mediterranean Sea [10,11], in the decade 2012–2022, it was frequently recorded during our surveys. For this period, we do not have a recorded, defined trend, but the number of specimens was found to be highly variable among localities, years, and months.
In oligotrophic waters such as those of the Ligurian Sea [26], food availability for a suspension-feeder organism is a strong constraint on its growth and reproduction. The hydrological regime of the area varies from vertically isothermal winter conditions to strong thermal stratification in summer and fall. Nutrients are depleted in the surface water layer during summer oligotrophic conditions and re-injected during winter mixing. During summer, strong rainfall events can increase the availability of nutrients in the surface layer [27] but also in deeper waters, determining the sedimentation of coarse organic detritus able to “pierce” the water stratification and reach the mesophotic zone [47,49,50], especially along rocky cliffs where coastal gorgonian forests are present, together with their acrophilic epibionts.
Along the Ligurian coast, the amount of rainfall is a good driver at a geographic level in solving the different abundances of A. mediterraneus in the three considered macro-areas. In fact, the mean density of specimens progressively decreases from East to West, and this trend is completely in agreement with that of the mean annual amount of rainfall. The influence of rainfall reaching the sea through the rivers’ mouth is clarified considering, for example, the situation of the Tigullio Gulf, in the center of the Ligurian Arc, the area with the higher amount of recorded basket stars. The presence of A. mediterraneus at Manara Cape is related to the input of the close Petronio River (mean flow rate 1.24 m3 s−1). The high basket star density recorded on the Portofino Cape is driven by the Entella River (mean flow rate, 15.88 m3 s−1), the plumes of which reach this area thanks to the surface current of the Gulf [51,52]. It is remarkable that in other sites present on the Promontory (Inglesi Cove and Isuela Shoal) that are sheltered by Portofino Cape, the species was never found (Figure 9).
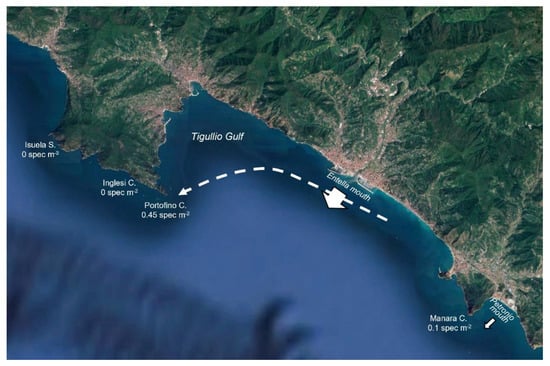
Figure 9.
Map of the Tigullio Gulf, the zone characterized by the highest recorded densities of Astrospartus mediterraneus. The arrows indicate the inputs of the two main rivers of the area (Entella and Petronio). The arrow thickness is proportional to the mean flow rates. The dashed line represents the direction of the main current in the Gulf.
Other ecological parameters could be invoked to explain the different local abundances of the species, sometimes in very close sites. Very likely, geomorphological features, such as the occurrence of topographic rocky reliefs exposed to moderate to strong currents enhance the presence and the density of the gorgonian forests, which, in turn, may play an important role in the A. mediterraneus presence and abundance. In the Ligurian Sea, gorgonian forests are abundant although irregularly distributed [18]. Our data clearly indicate that the density of the forest is an important driver in conditioning the presence of A. mediterraneus. It is intriguing that, generally, the species was recorded in forests with a colony density of around 5 colonies m−2. This evidence strongly suggests a “forest effect” that could, for example, determine a level of water movement ideal for this passive filter feeder [53,54].
At the temporal level, the comparison of the data of abundance with the amount of rainfall in the three months before each survey indicates a strong linear correlation. The evidence that the correlation between rainfall and the occurrence of basket stars is stronger in summer months is probably related to the particularly oligotrophic waters of this period. In these conditions, a TOM (total organic matter content) input due to heavy rainfall produces a drastic change in the trophic conditions of a site. In winter, the effect of precipitation is probably softer due to a higher basal level of TOM.
The variations of the specimens’ density in a specific site in relation to a putative food input remain an unresolved issue. A possible hypothesis could be related to reproductive phenomena triggered by an increase in trophic resources. Unfortunately, information about the reproduction and ontogeny of A. mediterraneus is very scarce. Hendler [55] described for this species very large oocytes, which strongly suggest direct development and hence limited dispersal. ROV footage does not allow the observation of small post-metamorphic stages, and due to their high complexity, the size measurement of adults is difficult; therefore, we were not able to document a true reproductive event.
Contemporaneously (or alternatively), the sudden increase in food availability at the base of the cliffs may attract individuals from the surroundings, which may move towards the source of the food input. This latter hypothesis seems the most plausible but disagrees with the already suggested relatively low mobility and host fidelity of this basket star [12,13]. Although a single datum is not conclusive, it is suggestive that, in the station of Manara Cape W, the density of basket stars drastically declined in one month (October–November 2020) from 0.22 to 0.02 specimens m−2 (Table 1).
In conclusion, this study strongly suggests that the distribution of A. mediterraneus is related to the food supply and the presence of the hosting species. Probably, food variation is also the driver of the differences recorded at the temporal level, putatively attributed to aggregation and dispersion of the individuals in relation to local increases in food availability due to terrestrial inputs related to rainfall. At a more general level, scientific reports and citizen science data indicate an expansion of this basket star in the Northern Ligurian Sea in the last decades, probably due to an observed TOM increase.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15010122/s1, Table S1: Canessa et al., 2022.
Author Contributions
Conceptualization, G.B. and M.C.; methodology, F.E.; validation, M.C., F.E. and M.T; formal analysis, M.C., F.E. and M.T; investigation, F.E. and M.T.; resource, G.B. and M.B.; data curation, G.B. and M.B.; writing—original draft preparation, G.B., F.B., M.B., M.C., F.E. and M.T.; writing—review and editing, G.B., M.B., F.B., M.C., F.E. and M.T.; visualization, M.C. and F.B.; supervision, M.B. and G.B.; project administration, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
ROV surveys were financed by ARPAL (grants 2015–2021 within the Marine Strategy Framework Monitoring Program) and Ministero delle Politiche Agricole, Alimentari e Forestali (Project 2012, “Use of ROV in the management of deep Corallium rubrum populations”; L.R. 7 Agosto 2007, no. 7). This research was also supported by the National Biodiversity Future Center.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
The authors would like to thank Alessandro Dagnino and Valentina Queirolo (Agenzia Regionale per la Protezione dell’Ambiente Ligure–ARPAL, Istituto Superiore per la Ricerca e la Protezione Ambientale (ISPRA) and all crew members involved in the campaigns for their help in field activities and data collection. We would like to thank the Reviewers for their help in the manuscript improvement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ocaña, A.; Pérez-Ruzafa, A. Los equinodermos de las costas andaluzas. Acta Granatense 2004, 3, 83–136. [Google Scholar]
- Rondelet’s “Libri de Piscibus Marinis”: A Book About Science, Sea Monks and Sea Bishops; Macé Bonhomme: Lyon, France, 1554.
- Caruso, A.A. Lettere inedite di Domenico Cirillo a Giovanni Bianchi (Jano Planco). Nuova Riv. Stor. Med. 2021, 2, 15–34. [Google Scholar]
- Zibrowius, H. Nouvelles observations de l’ophiure gorgonocephale Astrospartus mediterraneus sur la côte méditerranéenne de France. Bibliographie annotee et repartition. Trav. Sci. Parc Natl. Port Cros 1978, 4, 157–169. Available online: http://pascal-francis.inist.fr/vibad/index.php? (accessed on 1 July 2022).
- Stöhr, S.; O’Hara, T.; Thuy, B. (Eds.) World Ophiuroidea Database. Astrospartus mediterraneus (Risso, 1826). 2022. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=124963#distributions (accessed on 1 July 2022).
- Tortonese, E. Distribution and Ecology of Endemic Elements in the Mediterranean Fauna (Fishes and Echinoderms). In Mediterranean Marine Ecosystems; Moraitou-Apostolopoulou, M., Kiortsis, V., Eds.; NATO Conference Series; Springer: Boston, MA, USA, 1985; Volume 8. [Google Scholar] [CrossRef]
- Hussein, K.B.; Bensahla Talet, L. A preliminary inventory of biodiversity and benthic habitats of “Plane” Island (Paloma) in Oran Bay, north western Algeria (western Mediterranean). J. Black Sea/Mediterr. Environ. 2019, 25, 49–72. Available online: http://hdl.handle.net/1834/40685 (accessed on 1 July 2022).
- Chammem, H.; Souissi, J.B.; Pérez-Ruzafa, A. Checklist with first records for the Echinoderms of northern Tunisia (central Mediterranean Sea). Sci. Mar. 2019, 83, 277–288. [Google Scholar] [CrossRef]
- Fitori, A.; Fituri, A.E.; Aguilar, R.; Badreddine, A. First Record of Two Species of Echinodermata for Libyan Waters. J. Fish. Livest Prod. 2022, 10, 325. [Google Scholar]
- Montasell Bartrés, M. Astrospartus mediterraneus (Echinodermata: Ophiuroidea) in the Cap de Creus Marine Area: Ecological Characterization of an Emblematic Species. Final Grade. Bachelor’s Thesis, Universitat de Vic—Universitat Central de Catalunya, Facultat de Ciències i Tecnologia, Barcelona, Spain, 2022. Available online: http://hdl.handle.net/10854/6142 (accessed on 1 July 2022).
- Zibrowius, H. Observations biologiques au large du Lavandou (côte méditerranéenne de France) à l’aide du sous-marin Griffon de la marine nationale. Trav. Sci. Parc Natl. Port Cros 1978, 4, 171–176. [Google Scholar]
- Canessa, M.; Bavestrello, G.; Bo, M.; Enrichetti, F.; Trainito, E. Filling a Gap: A Population of Eunicella verrucosa (Pallas, 1766) (Anthozoa, Alcyonacea) in the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Italy). Diversity 2022, 14, 405. [Google Scholar] [CrossRef]
- Canessa, M.; Bavestrello, G.; Bo, M.; Enrichetti, F.; Trainito, E. Leptogorgia sarmentosa (Anthozoa: Octocorallia) in NE Sardinia: Distribution and growth patterns. Mar. Biodivers. 2022; in press. [Google Scholar] [CrossRef]
- Santín, A.; Grinyó, J.; Ambroso, S.; Baena, P.; Biel Cabanelas, M.; Corbera, G.; Salazar, J.; Montseny, M.; Gili, J.M. Fishermen and scientists: Synergies for the exploration, conservation and sustainability of the marine environment. In The Ocean We Want: Inclusive and Transformative Ocean Science; Consejo Superior de Investigaciones Científicas (España): Madrid, Spain, 2022; pp. 77–79. [Google Scholar] [CrossRef]
- Bo, M.; Canese, S.; Spaggiari, C.; Pusceddu, A.; Bertolino, M.; Angiolillo, M.; Giusti, M.; Loreto, M.F.; Salvati, E.; Greco, S.; et al. Deep coral oases in the South Tyrrhenian Sea. PLoS ONE 2012, 7, e49870. [Google Scholar] [CrossRef]
- Bo, M.; Coppari, M.; Betti, F.; Enrichetti, F.; Bertolino, M.; Massa, F.; Bava, S.; Gay, G.; Cattaneo-Vietti, R.; Bavestrello, G. The high biodiversity and vulnerability of two Mediterranean bathyal seamounts support the need for creating offshore protected areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 543–566. [Google Scholar] [CrossRef]
- Cau, A.; Follesa, M.C.; Moccia, D.; Alvito, A.; Bo, M.; Angiolillo, M.; Canese, S.; Paliaga, E.M.; Orrù, P.E.; Sacco, F.; et al. Deepwater corals biodiversity along roche du large ecosystems with different habitat complexity along the south Sardinia continental margin (CW Mediterranean Sea). Mar. Biol. 2015, 162, 1865–1878. [Google Scholar] [CrossRef]
- Enrichetti, F.; Dominguez-Carrió, C.; Toma, M.; Bavestrello, G.; Betti, F.; Canese, S.; Bo, M. Megabenthic communities of the Ligurian deep continental shelf and shelf break (NW Mediterranean Sea). PLoS ONE 2019, 14, e0223949. [Google Scholar] [CrossRef]
- Angiolillo, M.; La Mesa, G.; Giusti, M.; Salvati, E.; Di Lorenzo, B.; Rossi, L.; Canese, S.; Tunesi, L. New records of scleractinian cold-water coral (CWC) assemblages in the southern Tyrrhenian Sea (western Mediterranean Sea): Human impacts and conservation prospects. Prog. Oceanogr. 2021, 197, 102656. [Google Scholar] [CrossRef]
- Consoli, P.; Altobelli, C.; Perzia, P.; Bo, M.; Rosso, A.; Alongi, G.; Serio, D.; Canese, S.; Romeo, T.; Andaloro, F. Species and habitats of conservation interest in the Ecologically and Biologically Significant Area of the Strait of Sicily: A contribution towards the creation of a Specially Protected Areas of Mediterranean Importance. Mediterr. Mar. Sci. 2021, 22, 297–316. [Google Scholar] [CrossRef]
- Moccia, D.; Cau, A.; Bramanti, L.; Carugati, L.; Canese, S.; Follesa, M.C.; Cannas, R. Spatial distribution and habitat characterization of marine animal forest assemblages along nine submarine canyons of Eastern Sardinia (central Mediterranean Sea). Deep. Sea Res. Part I Oceanogr. Res. Pap. 2020, 167, 103422. [Google Scholar] [CrossRef]
- Toma, M.; Bo, M.; Cattaneo-Vietti, R.; Canese, S.; Canessa, M.; Cannas, R.; Cardone, F.; Carugati, L.; Cau, A.; Corriero, G.; et al. Basin-scale occurrence and distribution of mesophotic and upper bathyal red coral forests along the Italian coasts. Med. Mar. Sci. 2022, 23, 484–498. [Google Scholar] [CrossRef]
- Toma, M.; Betti, F.; Bavestrello, G.; Cattaneo-Vietti, R.; Canese, S.; Cau, A.; Andaloro, F.; Greco, S.; Bo, M. Diversity and abundance of heterobranchs (Mollusca, Gastropoda) from the mesophotic and bathyal zone of the Mediterranean Sea. Eur. Zool. J. 2022, 89, 167–189. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bo, M.; Morri, C.; Montefalcone, M.; Toma, M.; Bavestrello, G.; Tunesi, L.; Canese, S.; Giusti, M.; Salvati, E.; et al. Assessing the environmental status of temperate mesophotic reefs: A new, integrated methodological approach. Ecol. Ind. 2019, 102, 218–229. [Google Scholar] [CrossRef]
- Danovaro, R.; Corinaldesi, C.; Dell’Anno, A.; Snelgrove, P.V. The deep-sea under global change. Curr. Biol. 2017, 27, R461–R465. [Google Scholar] [CrossRef]
- Cattaneo-Vietti, R.; Albertelli, G.; Aliani, S.; Bava, S.; Bavestrello, G.; Cecchi, L.B.; Bianchi, C.N.; Bozzo, E.; Capello, M.; Castellano, M.; et al. The Ligurian Sea: Present status, problems and perspectives. Chem. Ecol. 2010, 26, 319–340. [Google Scholar] [CrossRef]
- Ruggieri, N.; Castellano, M.; Misic, C.; Gasparini, G.; Cattaneo-Vietti, R.; Povero, P. Seasonal and interannual dynamics of a coastal ecosystem (Portofino, Ligurian Sea) in relation to meteorological constraints. Geophys. Res. Abstr. 2006, 8, 07774. [Google Scholar]
- Agrillo, G.; Bonati, V. Atlante Climatico della Liguria; ARPAL-Centro Funzionale Meteoidrologico di Protezione Civile: Genova, Italy, 2013; Volume 128. [Google Scholar]
- Hughes, R.N.; Hughes, D.J.; Smith, I.P. Citizen scientists and marine research: Volunteer participants, their contributions, and projection for the future. Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 257–314. [Google Scholar]
- Sandahl, A.; Tøttrup, A.P. Marine Citizen Science: Recent Developments and Future Recommendations. Citiz. Sci. Theory Pract. 2020, 5, 24. [Google Scholar] [CrossRef]
- Rossi, S.; Bramanti, L.; Gori, A.; Orejas, C. (Eds.) Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 1–1366. [Google Scholar] [CrossRef]
- Biel-Cabalenas, M.; Monastrell, M.; Santín, A.; Salazar, J.; Baena, P.; Vilandrich, N.; Gori, A.; Montseny, M.; Corbera, G.; Ambroso, S.; et al. “Can emblematic species become a pest? The case of Astrospartus mediterraneus (Risso, 1826) (Echinodermata: Ophiuroidea) in the artisanal fishing grounds of the Cap de Creus area (NW Mediterranean Sea)” UNEP/MAP—SPA/RAC, 2022. In Proceedings of the 4th Mediterranean Symposium on the Conservation of Coralligenous & Other Calcareous Bio-Concretions, Genova, Italy, 20–21 September 2022; Bouafif, C., Ouerghi, A., Eds.; SPA/RAC: Tunis, Tunisia, 2022; p. 184. [Google Scholar]
- Gatti, G.; Bianchi, C.N.; Parravicini, V.; Rovere, A.; Peirano, A.; Montefalcone, M.; Massa, F.; Morri, C. Ecological change, sliding baselines and the importance of historical data: Lessons from combing observational and quantitative data on a temperate reef over 70 years. PLoS ONE 2015, 10, e0118581. [Google Scholar] [CrossRef]
- Gatti, G.; Bianchi, C.N.; Montefalcone, M.; Venturini, S.; Diviacco, G.; Morri, C. Observational information on a temperate reef community helps understanding the marine climate and ecosystem shift of the 1980–90s. Mar. Pollut. Bull. 2017, 114, 528–538. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Chiantore, M.; Montefalcone, M.; Parravicini, V.; Rovere, A. Mediterranean Sea biodiversity between the legacy from the past and a future of change. In Life in the Mediterranean Sea: A Look at Habitat Changes; Stambler, N., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 1–55. [Google Scholar]
- Puce, S.; Bavestrello, G.; Di Camillo, C.G.; Boero, F. Long-term changes in hydroid (Cnidaria, Hydrozoa) assemblages: Effect of Mediterranean warming? Mar. Ecol. 2009, 30, 313–326. [Google Scholar] [CrossRef]
- Calero, B.; Ramos, A.; Ramil, F. Distribution of suspension-feeder brittle stars in the Canary Current upwelling ecosystem (Northwest Africa). Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 142, 1–15. [Google Scholar] [CrossRef]
- Barry, J.P.; Dayton, P.K. Physical Heterogeneity and the Organization of Marine Communities. In Ecological Heterogeneity; Kolasa, J., Pickett, S.T.A., Eds.; Springer: New York, NY, USA, 1991; pp. 270–320. [Google Scholar]
- Drouin, G.; Himmelman, J.H.; Béland, P. Impact of tidal salinity fluctuations on echinoderm and mollusc populations. Can. J. Zool. 1985, 63, 1377–1387. [Google Scholar] [CrossRef]
- Tyler, P.A.; Young, C.M.; Clarke, A. Temperature and pressure tolerances of embryos and larvae of the Antarctic sea urchin Sterechinus neumayeri (Echinodermata: Echinoidea): Potential for deep-sea invasion from high latitudes. Mar. Ecol. Progr. Ser. 2020, 192, 173–180. [Google Scholar] [CrossRef]
- Menge, B.A. Community Regulation: Under What Conditions Are Bottom-Up Factors Important on Rocky Shores? Ecology 1992, 73, 755–765. [Google Scholar] [CrossRef]
- Strathmann, R.R. Larval Feeding in Echinoderms. Am. Zool. 1975, 15, 717–730. [Google Scholar] [CrossRef]
- Lessios, H.A. Adaptation and phylogeny as determinants of egg size in echinoderms from the two sides of the Isthmus of Panama. Am. Nat. 1990, 135, 1–13. [Google Scholar] [CrossRef]
- Herrero-Pérezrul, M.D.; Reyes Bonilla, H.; García-Domínguez, F.; Cintra-Buenrostro, C.E. Reproduction and growth of Isostichopus fuscus (Echinodermata: Holothuroidea) in the southern Gulf of California, Mexico. Mar. Biol. 1999, 135, 521–532. [Google Scholar] [CrossRef]
- Brodie, J.; Fabricius, K.; De’ath, G.; Okaji, K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar. Pollut. Bull. 2005, 51, 266–278. [Google Scholar] [CrossRef]
- Coppari, M.; Zanella, C.; Rossi, S. The importance of coastal gorgonians in the blue carbon budget. Sci. Rep. 2019, 9, 13550. [Google Scholar] [CrossRef]
- Coppari, M.; Ferrier-Pages, C.; Castellano, M.; Massa, F.; Olivari, E.; Bavestrello, G.; Povero, P.; Bo, M. Seasonal variation of the stable C and N isotopic composition of the mesophotic black coral Antipathella subpinnata (Ellis & Solander, 1786). Estuar. Coast. Shelf Sci. 2020, 233, 106520. [Google Scholar] [CrossRef]
- Enrichetti, F.; Baldrighi, E.; Bavestrello, G.; Betti, F.; Canese, S.; Costa, A.; del Pasqua, M.; Giangrande, A.; Langeneck, J.; Misic, C.; et al. Ecological role and phylogenetic position of a new habitat-forming species (Canalipalpata, Sabellidae) from the Mediterranean mesophotic soft bottoms. Estuar. Coast. Shelf Sci. 2021, 265, 107737. [Google Scholar] [CrossRef]
- Marty, J.C.; Chiavérini, J. Hydrological changes in the Ligurian Sea (NW Mediterranean, DYFAMED site) during 1995–2007 and biogeochemical consequences. Biogeosciences 2010, 7, 2117–2128. [Google Scholar] [CrossRef]
- Bavestrello, G.; Cattaneo-Vietti, R.; Cerrano, C.; Danovaro, R.; Fabiano, M. Annual sedimentation rates and role of the resuspension processes along a vertical cliff (Ligurian Sea, Italy). J. Coast Res. 1995, 11, 690–696. Available online: https://www.jstor.org/stable/4298372 (accessed on 1 July 2022).
- Bavestrello, G.; Cattaneo-Vietti, R.; Danovaro, R.; Fabiano, M. Detritus rolling down a vertical cliff of the Ligurian Sea (Italy): The ecological role in hard bottom communities. Mar. Ecol. 1991, 12, 281–292. [Google Scholar] [CrossRef]
- Corradi, N.; Fanucci, F.; Firpo, M.; Piccazzo, M.; Traverso, M. L’Olocene della Piattaforma Continentale Ligure da Portofino alla Spezia; Istituto Idrografico della Marina, Università degli Studi di Genova: Genova, Italy, 1980. [Google Scholar]
- Cerrano, C.; Danovaro, R.; Gambi, C.; Pusceddu, A.; Riva, A.; Schiaparelli, S. Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodivers. Conserv. 2010, 19, 153–167. [Google Scholar] [CrossRef]
- Gori, A.; Bavestrello, G.; Grinyó, J.; Dominguez-Carrió, C.; Ambroso, S.; Bo, M. Animal Forests in Deep Coastal Bottoms and Continental Shelf of the Mediterranean Sea. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–27. [Google Scholar] [CrossRef]
- Hendler, G. Echinodermata: Ophiuroidea. In Reproduction of Marine Invertebrates, Vol. VI, Echinoderms and Lophophorates; Giese, A., Pearse, J., Pearse, V.B., Eds.; Boxwood Press: Pacific Grove, CA, USA, 1991; pp. 355–511. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).