The American Tribes Anypotactini and Eudiagogini (Coleoptera, Curculionidae) in Eocene of Europe as Indicators of Eocene Climate with Description a New Species
Abstract
:1. Introduction
2. Materials and Methods
3. Results
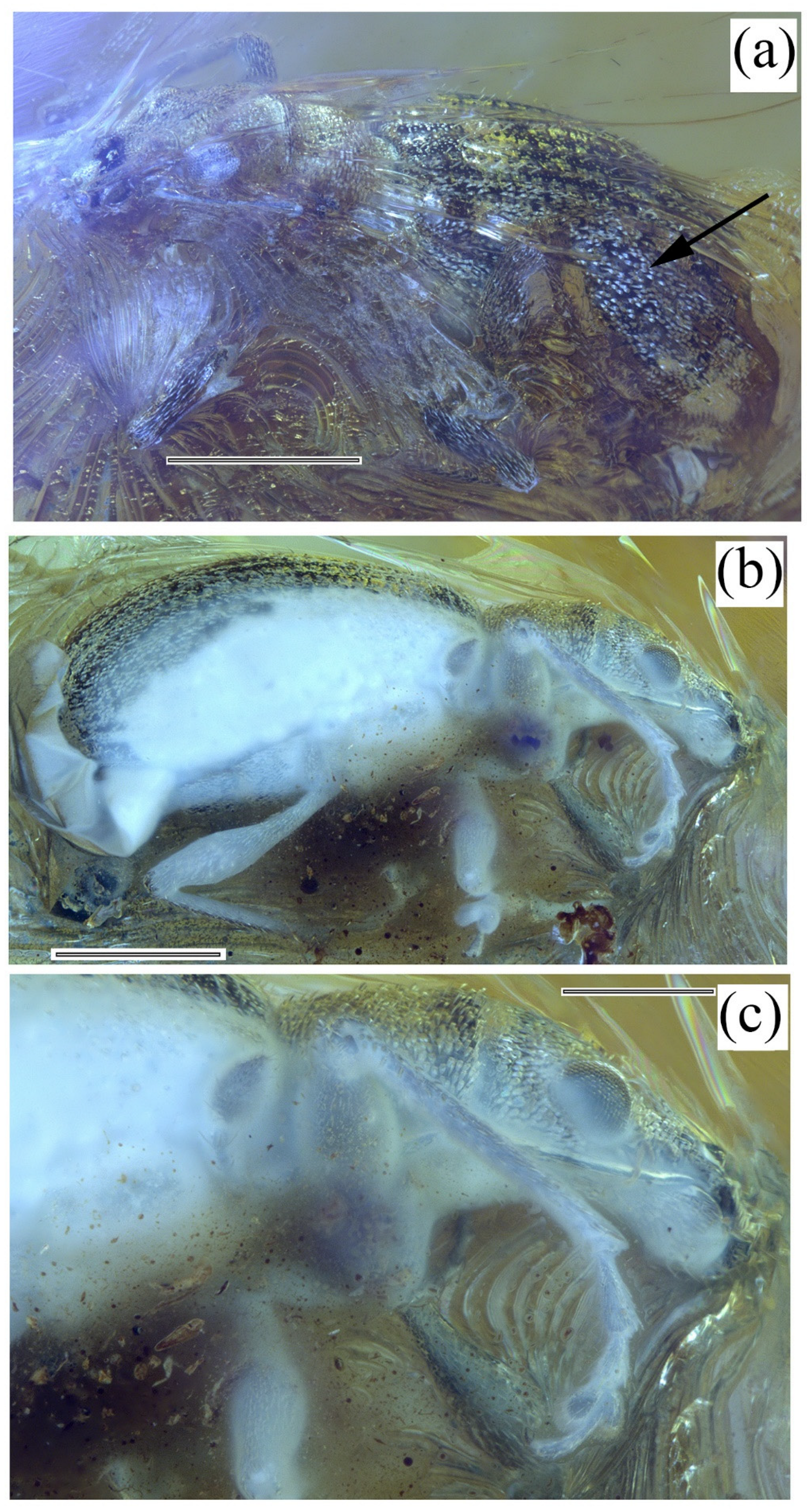
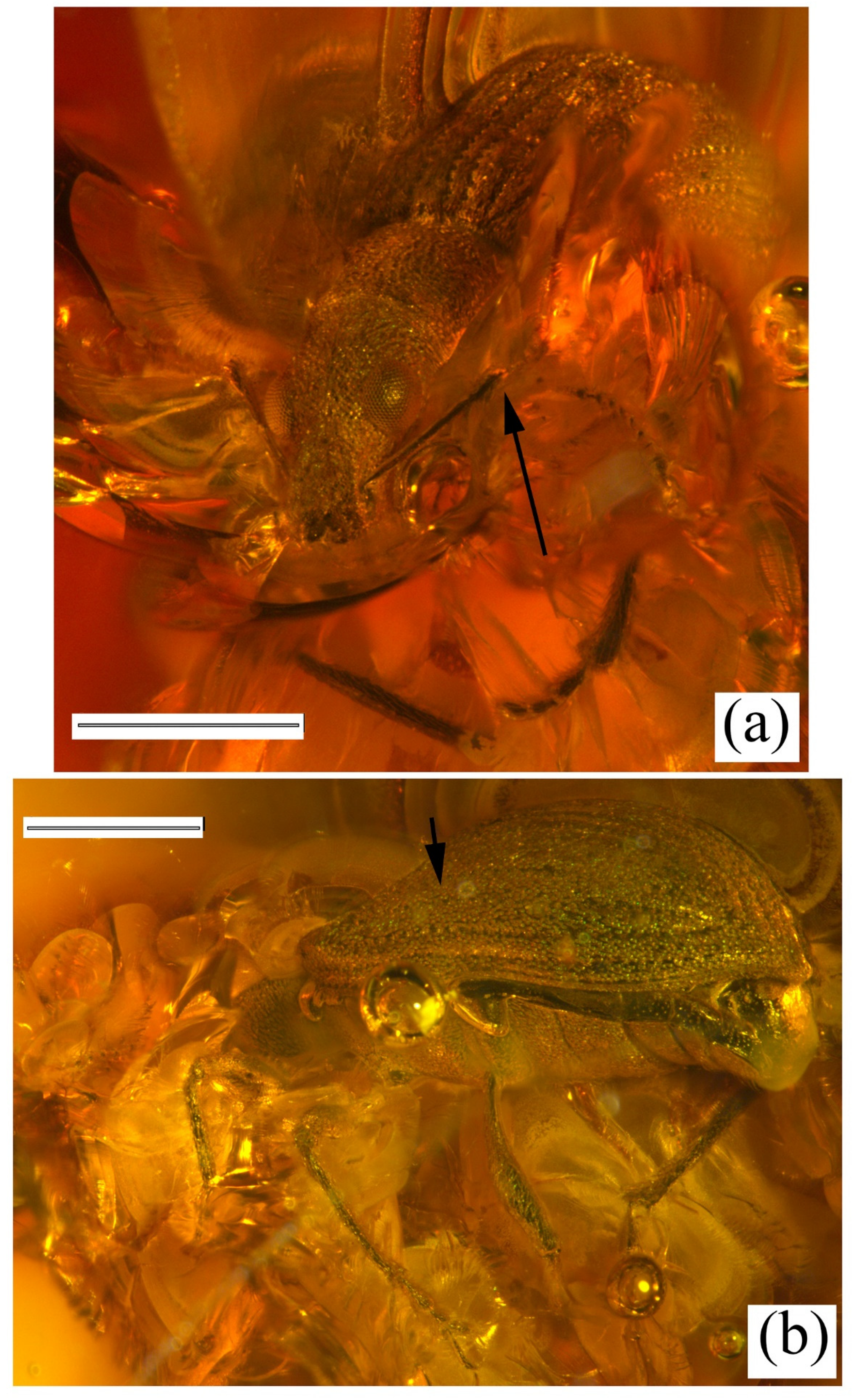
Systematic Paleontology
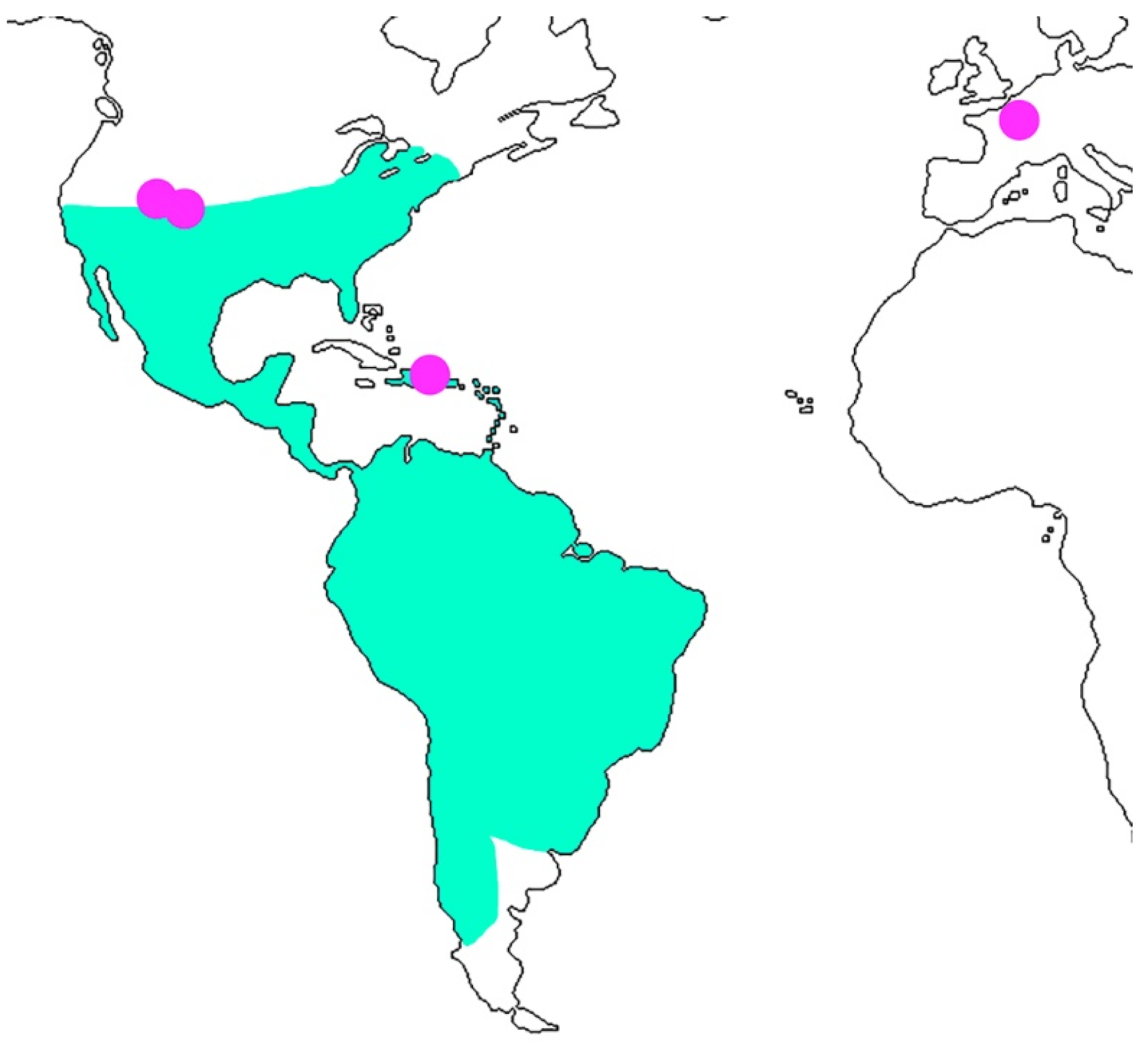
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso-Zarazaga, M.A.; Lyal, C.H.C. A World Catalogue of Families and Genera Curculionoidea (Insecta: Coleoptera) (Excepting Scolytidae and Platypodidae); Entomopraxis: Barcelona, Spain, 1999; p. 315. [Google Scholar]
- Legalov, A.A.; Nazarenko, V.Y.; Perkovsky, E.E. New weevils (Coleoptera: Curculionidae) from Rovno amber. Paleontol. J. 2019, 53, 1045–1059. [Google Scholar] [CrossRef]
- Legalov, A.A. A review of the Curculionoidea (Coleoptera) from European Eocene ambers. Geosciences 2020, 10, 16. [Google Scholar] [CrossRef]
- Legalov, A.A. Fossil history of Curculionoidea (Coleoptera) from the Paleogene. Geosciences 2020, 10, 358. [Google Scholar] [CrossRef]
- Voss, E. Einige Rhynchophoren der Bernsteinfauna (Col.). Mitt. Geol. Paläontol. Inst. Hambg. 1953, 22, 119–140. [Google Scholar]
- Voss, E. Einige Rüsselkäfer der Tertiärzeit aus Baltischen Bernstein (Coleoptera, Curculionidea). Steenstupia 1972, 2, 167–181. [Google Scholar]
- Zherikhin, V.V. On weevils (Insecta, Coleoptera) from the Baltic amber. Tr. Paleontol. Inst. Akad. Nauk. SSSR 1971, 130, 197–209. [Google Scholar]
- Wanat, M.; Borovec, L. New genus of weevil (Coleoptera, Curculionidae) from Baltic amber. Pol. Pismo Entomol. 1986, 56, 243–247. [Google Scholar]
- Yunakov, N.N.; Kirejtshuk, A.G. New genus and species of broad-nosed weevils from Baltic amber and notes on fossils of the subfamily Entiminae (Coleoptera, Curculionidae). ZooKeys 2011, 160, 73–96. [Google Scholar] [CrossRef]
- Perkovsky, E.E.; Nel, A. A new Rovno amber termite genus (Isoptera, Rhinotermitidae) from Styr River basin. Palaeont. Electron. 2021, 24, a05. [Google Scholar] [CrossRef]
- Matalin, A.V.; Perkovsky, E.E.; Vasilenko, D.V. First record of tiger beetles (Coleoptera, Cicindelidae) from Rovno amber with the description of a new genus and species. Zootaxa 2021, 5016, 243–256. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Beutel, R.G.; Leschen, R.A.B.; Slipinsky, S.A. Chapter 2. Glossary of Morphological Terms. In Handbook of Zoology. Arthropoda: Insecta. Coleoptera, Beetles. Volume 2: Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia partim); Kristensen, N.P., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 2010; pp. 9–20. [Google Scholar]
- Bukejs, A.; Háva, J.; Alekseev, V.I. A new fossil species of Attagenus Latreille (Coleoptera: Dermestidae) in Rovno and Baltic ambers, with a brief review of known fossil beetles from the Rovno amber Lagerstätte. Foss. Rec. 2020, 23, 95–104. [Google Scholar] [CrossRef]
- Bukejs, A.; Alekseev, V.I.; Kairišs, K. The first record of Cupedidae (Coleoptera: Archostemata) from Eocene Rovno amber: Cupes groehni Kirejtshuk, 2005 examined using X-ray microtomography. Balt. J. Coleopterol. 2021, 21, 111–116. [Google Scholar]
- Lyubarsky, G.Y.; Perkovsky, E.E. New findings of Cryptophagidae (Coleoptera: Clavicornia) from Baltic amber in the unbiased collection of the Paleontological Institute of RAS. Russ. Entomol. J. 2021, 30, 282–287. [Google Scholar] [CrossRef]
- Telnov, D.; Perkovsky, E.E.; Vasilenko, D.V.; Yamamoto, S. The first fossil Coleoptera record from the Volyn Region, Ukraine, with description of a new Glesoconomorphus (Coleoptera, Mycteridae) in syninclusion with Winterschmidtiidae (Acari) and a key to species. ZooKeys 2021, 1068, 189–201. [Google Scholar] [CrossRef]
- Tshernyshev, S.E.; Perkovsky, E.E. Protomauroania mikhailovi—A new species of malachite beetles (Coleoptera, Dasytidae) in Rovno amber. Zootaxa 2021, 5006, 189–194. [Google Scholar] [CrossRef]
- Alekseev, V.I.; Bukejs, A. A new fossil genus of minute-clubbed beetles (Coleoptera: Cucujoidea: Monotomidae) from Paleogene amber of Europe. Hist. Biol. 2022. [Google Scholar] [CrossRef]
- Alekseev, V.I.; Bukejs, A. A new extinct species of Zavaljus Reitter (Coleoptera: Erotylidae) from Rovno amber: Boreal distribution range since the Eocene. Hist. Biol. 2022. [Google Scholar] [CrossRef]
- Bukejs, A.; Alekseev, V.I. Extant genus of flat bark beetle (Coleoptera: Silvanidae) with a present-day Australian-southern South American disjunction discovered in Eocene Rovno amber. Zootaxa 2022, 5129, 137–144. [Google Scholar] [CrossRef]
- Kazantsev, S.V.; Perkovsky, E.E. Imprint of a Helcophorus Fairmaire, 1881: The first net-winged beetle (Coleoptera: Lycidae) from Rovno amber. Zootaxa 2022, 5128, 84–90. [Google Scholar] [CrossRef]
- Kirichenko-Babko, M.; Perkovsky, E.E.; Vasilenko, D.V. A new genus and species of Lebiini (Coleoptera: Carabidae) from late Eocene Rovno amber. Hist. Biol. 2022, 34, 436–442. [Google Scholar] [CrossRef]
- Kirichenko-Babko, M.; Perkovsky, E.E.; Vasilenko, D.V. †Antephilorhizus zerovae sp. nov. (Carabidae: Lebiini), the second ground beetle species from Rovno amber. Hist. Biol. 2022. [Google Scholar] [CrossRef]
- Legalov, A.A.; Nazarenko, V.Y.; Perkovsky, E.E. A new species of the genus Glaesotropis Gratshev and Zherikhin, 1995 (Coleoptera, Anthribidae) from Rovno amber. Foss. Rec. 2021, 24, 1–7. [Google Scholar] [CrossRef]
- Legalov, A.A.; Nazarenko, V.Y.; Perkovsky, E.E. A new species of the genus Dorytomus Germar, 1817 (Coleoptera, Curculionidae) from Rovno amber. Zootaxa 2021, 5006, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Legalov, A.A.; Nazarenko, V.Y.; Vasilenko, D.V.; Perkovsky, E.E. Ceutorhynchus Germar (Coleoptera, Curculionidae) as proxy for Eocene Brassicaceae: First record of the genus from Rovno amber. J. Paleontol. 2022, 96, 379–386. [Google Scholar] [CrossRef]
- Legalov, A.A.; Vasilenko, D.V.; Perkovsky, E.E. New proxy for Moraceae in Priabonian of Europe: First record of the genus Demimaea Pascoe, 1870 (Coleoptera: Curculionidae) from Eocene Rovno amber. Hist. Biol. 2022. [Google Scholar] [CrossRef]
- Vitali, F.; Perkovsky, E.E. Poliaenus europaeus n. sp., the first cerambycid from Rovno amber (Coleoptera Cerambycidae). Hist. Biol. 2022. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nazarenko, V.Y.; Vasilenko, D.V.; Perkovsky, E.E. First fossil species of ship-timber beetles (Coleoptera: Lymexylidae) from Eocene Rovno amber (Ukraine). Foss. Rec. 2022, 25, 65–74. [Google Scholar] [CrossRef]
- Perkovsky, E.E.; Odnosum, V.K.; Nazarenko, V.Y.; Vasilenko, D.V. First record of the genus Cyrtanaspis Emery (Insecta: Coleoptera: Scraptiidae) from Baltic amber. Paleontol. J. 2022, 56, 208–212. [Google Scholar] [CrossRef]
- Perkovsky, E.E. Rovno amber caddisflies (Insecta, Trichoptera) from different localities, with information about three new sites. Vestn. Zool. 2017, 51, 15–22. [Google Scholar] [CrossRef]
- Melnitsky, S.I.; Ivanov, V.D.; Perkovsky, E.E. A new species of Plectrocnemia (Trichoptera: Polycentropodidae) from Rovno amber. Zootaxa 2021, 5006, 106–109. [Google Scholar] [CrossRef]
- Melnitsky, S.I.; Ivanov, V.D.; Perkovsky, E.E. A new species the fossil genus Electrotrichia (Insecta: Trichoptera: Hydroptilidae) from Rovno amber (Zhytomyr region, Olevsk amber locality). Palaeoentomology 2021, 4, 421–424. [Google Scholar] [CrossRef]
- Melnitsky, S.I.; Ivanov, V.D.; Perkovsky, E.E. A new species of the genus Holocentropus (Trichoptera: Polycentropodidae) from Rovno amber. Zoosystematica Ross. 2021, 30, 298–302. [Google Scholar] [CrossRef]
- Simutnik, S.A.; Perkovsky, E.E.; Khomych, M.R.; Vasilenko, D.V. Two new genera of Encyrtidae (Hymenoptera, Chalcidoidea) with reduced ovipositor sheaths. J. Hymenopt. Res. 2022, 89, 47–60. [Google Scholar] [CrossRef]
- Simutnik, S.A.; Perkovsky, E.E.; Vasilenko, D.V. Protaphycus shuvalikovi Simutnik gen. et sp. n. (Chalcidoidea, Encyrtidae, Encyrtinae) from Rovno amber. J. Hymenopt. Res. 2022, 91, 1–9. [Google Scholar] [CrossRef]
- Olmi, M.; Eggs, B.; Capradossi, L.; van de Kamp, T.; Perkovsky, E.E.; Guglielmino, A.; Vasilenko, D.V. A new species of Bocchus from upper Eocene Rovno amber (Hymenoptera: Dryinidae). J. Hymenopt. Res. 2022, 92, 257–272. [Google Scholar] [CrossRef]
- Olmi, M.; Guglielmino, A.; Vasilenko, D.V.; Perkovsky, E.E. Discovery of the first apterous pincer wasp from amber, with description of a new tribe, genus and species of Apodryininae (Hymenoptera, Dryinidae). Zootaxa 2022, 5162, 54–66. [Google Scholar] [CrossRef]
- Dlussky, G.M.; Rasnitsyn, A.P. Ants (Insecta: Vespida: Formicidae) in the Upper Eocene amber of Central and Eastern Europe. Paleontol. J. 2009, 43, 1024–1042. [Google Scholar] [CrossRef]
- Perkovsky, E.E.; Rasnitsyn, A.P.; Vlaskin, A.P.; Rasnitsyn, S.P. Contribution to the study of the structure of amber forest communities based on analysis of syninclusions in the Rovno amber (late Eocene of Ukraine). Paleontol. J. 2012, 46, 293–301. [Google Scholar] [CrossRef]
- Kosmowska-Ceranowicz, B. The Tadeusz Giecewicz’s Collection at the Museum of the Earth, Polish Academy of Sciences, Warsaw; Oficyna Wydawnicza Sadyba: Warsaw, Poland, 2001; pp. 1–97. [Google Scholar]
- Perkovsky, E.E. Tropical and Holarctic ants in Late Eocene ambers. Vestn. Zool. 2016, 50, 111–122. [Google Scholar] [CrossRef]
- Legalov, A.A. New species of weevils (Coleoptera, Curculionidae) in Dominican amber. Paleontol. J. 2019, 53, 511–521. [Google Scholar] [CrossRef]
- Poinar, G., Jr.; Legalov, A.A. Five new species from the subfamily Entiminae (Coleoptera: Curculionidae) in Dominican amber. Palaeont. Electron. 2017, 20, 1–13. [Google Scholar] [CrossRef]
- Colombo, W.D.; Perkovsky, E.E.; Vasilenko, D.V. The first sclerodermine flat wasp (Hymenoptera: Bethylidae) from the upper Eocene Rovno amber Ukraine. Alcheringa 2021, 45, 429–434. [Google Scholar] [CrossRef]
- Ramos, M.S.; Perkovsky, E.E.; Rasnitsyn, A.P.; Azevedo, C.O. Revision of Bethylinae fossils (Hymenoptera: Bethylidae) from Baltic, Rovno and Oise amber, with comments on the Tertiary fauna of the subfamily. Neues Jahrb. Fur Geol. Palaontol. Abh. 2014, 271, 203–228. [Google Scholar] [CrossRef]
- Perkovsky, E.E.; Olmi, M.; Vasilenko, D.V.; Capradossi, L.; Guglielmino, A. First Bocchus Ashmead (Hymenoptera: Dryinidae) from Upper Eocene Rovno amber: B. schmalhauseni sp. nov. Zootaxa 2020, 4819, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.E. The genus Eudiagogus (Coleoptera, Curculionidae, Leptopiinae), with two new species on the weed Sesbania (Leguminosae). Proc. Entomol. Soc. Wash. 1979, 81, 304–320. [Google Scholar]
- Ward, R.; O’Brien, C.W.; O’Brien, L.B.; Foster, D.E.; Huddleston, E.W. Annotated checklist of New World insects associated with Prosopis (mesquite). U.S. Dep. Agrie. Tech. Bull. 1977, 1557, 1–115. [Google Scholar]
- Jossang, J.; Bel-Kassaoui, H.; Jossang, A.; Seuleiman, M.; Nel, A. Quesnoin, a novel pentacyclic ent-diterpene from 55 million years old Oise amber. J. Org. Chem. 2008, 73, 412–417. [Google Scholar] [CrossRef]
- Czeczott, H. The flora of the Baltic amber and its age. Prace Muz. Ziemi. 1961, 4, 119–145. [Google Scholar]
- Pimenova, N.V. The Flora of the Tertiary Sandstones of the Western Bank-Region of the Dnieper in the Ukr; Institute of Geological Science, Academy of Sciences of Ukrainian SSR: Kiev, Ukraine, 1937; pp. 1–135. (In Ukrainian) [Google Scholar]
- Hably, L. Distribution of legumes in the Tertiary of Hungary. In Advances in Legume Systematics: Part 4. The Fossil Record; Herendeen, P.S., Dilcher, D.L., Eds.; The Royal Botanic Gardens: Kew, UK, 1992; pp. 169–187. [Google Scholar]
- Morrone, J.J. The species of Entiminae (Coleoptera: Curculionidae) ranged in America south of the United States. Anales Inst. Biol. Univ. Nac. Autón. México Zool. 1999, 70, 99–168. [Google Scholar]
- Alberdi, M.; Fernández, J.; Cristo, R.; Romero, M.; Rios, D. Lipid changes in cold hardened leaves of Nothofagus dombeyi. Phytochemistry 1991, 29, 2467–2471. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legalov, A.A.; Vasilenko, D.V.; Perkovsky, E.E. The American Tribes Anypotactini and Eudiagogini (Coleoptera, Curculionidae) in Eocene of Europe as Indicators of Eocene Climate with Description a New Species. Diversity 2022, 14, 767. https://doi.org/10.3390/d14090767
Legalov AA, Vasilenko DV, Perkovsky EE. The American Tribes Anypotactini and Eudiagogini (Coleoptera, Curculionidae) in Eocene of Europe as Indicators of Eocene Climate with Description a New Species. Diversity. 2022; 14(9):767. https://doi.org/10.3390/d14090767
Chicago/Turabian StyleLegalov, Andrei A., Dmitry V. Vasilenko, and Evgeny E. Perkovsky. 2022. "The American Tribes Anypotactini and Eudiagogini (Coleoptera, Curculionidae) in Eocene of Europe as Indicators of Eocene Climate with Description a New Species" Diversity 14, no. 9: 767. https://doi.org/10.3390/d14090767
APA StyleLegalov, A. A., Vasilenko, D. V., & Perkovsky, E. E. (2022). The American Tribes Anypotactini and Eudiagogini (Coleoptera, Curculionidae) in Eocene of Europe as Indicators of Eocene Climate with Description a New Species. Diversity, 14(9), 767. https://doi.org/10.3390/d14090767







