A New Locality for the Blind Loach, Eidinemacheilus smithi (Teleostei: Nemacheilidae) in Iranian Zagros: A Morpho-Molecular Approach
Abstract
1. Introduction
2. Material and Methods
2.1. Taxon Sampling
2.2. Morphological Study
2.3. Molecular Study
3. Molecular Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, W.; Culver, D. Encyclopedia of Caves, 2nd ed.; Academic Press: New York, NY, USA, 2011. [Google Scholar]
- Culver, D.C.; Deharveng, L.; Pipan, T.; Bedos, A. An Overview of Subterranean Biodiversity Hotspots. Diversity 2021, 13, 487. [Google Scholar] [CrossRef]
- Culver, D.C.; Sket, B. Hotspots of Subterranean Biodiversity in Caves and Wells. J. Cave Karst Stud. 2000, 62, 11–17. [Google Scholar]
- Pipan, T.; Deharveng, L.; Culver, D.C. Hotspots of subterranean biodiversity. Diversity 2020, 12, 209. [Google Scholar] [CrossRef]
- Brad, T.; Ieper, S.; Sarbu, S.M. The Chemoautotrophically Based Movile Cave Groundwater Ecosystem, a Hotspot of Subterranean Biodiversity. Diversity 2021, 13, 128. [Google Scholar] [CrossRef]
- Fatemi, Y.; Malek-Hosseini, M.J.; Falniowski, A.; Hofman, S.; Kuntner, M.; Grego, J. Description of a new genus and species as the first gastropod species from caves in Iran. J. Cave. Karst. Stud. 2019, 81, 233–243. [Google Scholar] [CrossRef]
- Romero, A. Cave Biology, Life in Darkness; Cambridge University Press: New York, NY, USA, 2009. [Google Scholar]
- Khalaji-Pirbalouty, V.; Fatemi, Y.; Malek-Hosseini, M.J.; Kuntner, M. A new species of Stenasellus Dollfus, 1897 from Iran, with a key to the western Asian species (Crustacea, Isopoda, Stenasellidae). ZooKeys 2018, 766, 39–50. [Google Scholar] [CrossRef]
- Culver, D.C.; Holsinger, J.R. How many species of troglobites are there? Natl. Speleol. Soc. Bull. 1992, 54, 79–80. [Google Scholar]
- Camacho, A.I. The Natural History of Biospeleology; Museo Nacional de Ciencias Naturales: Madrid, Spain, 1992. [Google Scholar]
- Proudlove, G.S. Subterranean Fishes of the World. Available online: https://cavefishes.org.uk/ (accessed on 21 March 2022).
- Bruun, A.F.; Kaiser, E.W. Iranocypris typhlops ng., n. sp., the first true cave fish from Asia. Dan. Sci. Investig. Iran Cph. 1944, 4, 1–8. [Google Scholar]
- Farashi, A.; Kaboli, M.; Rezaei, H.R.; Naghavi, M.R.; Rahimian, H.; Coad, B.W. Reassessment of the taxonomic position of Iranocypris typhlops Bruun & Kaiser, 1944 (Actinopterygii, Cyprinidae). ZooKeys 2014, 374, 69–77. [Google Scholar] [CrossRef]
- Esmaeili, H.R.; Sayyadzadeh, G.; Coad, B.W.; Eagderi, S. Review of the genus Garra Hamilton, 1822 in Iran with description of a new species: A morpho-molecular approach (Teleostei: Cyprinidae). Iran. J. Ichthyol. 2016, 3, 82–121. [Google Scholar]
- Sayyadzadeh, G.; Esmaeili, H.R.; Freyhof, J. Garra mondica, a new species from the Mond River drainage with remarks on the genus Garra from the Persian Gulf basin in Iran (Teleostei: Cyprinidae). Zootaxa 2015, 4048, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Mousavi-Sabet, H.; Eagderi, S. Garra lorestanensis, a new cave fish from the Tigris River drainage with remarks on the subterranean fishes in Iran (Teleostei: Cyprinidae). FishTaxa 2016, 1, 45–54. [Google Scholar]
- Greenwood, P.H. A new and eyeless cobitid fish (Pisces, Cypriniformes) from the Zagros Mountains, Iran. J. Zool. 1976, 180, 129–137. [Google Scholar] [CrossRef]
- Hasemzadeh-Segherloo, I.; Ghaedrahmati, N.; Freyhof, J. Eidinemacheilus, a new generic name for Noemacheilus smithi Greenwood (Teleostei; Nemacheilidae). Zootaxa 2016, 4147, 466–476. [Google Scholar] [CrossRef]
- Freyhof, J.; Abdullah, Y.S.; Ararat, K.; Ibrahim, H.; Geiger, M.F. Eidinemacheilus proudlovei, a new subterranean loach from Iraqi Kurdistan (Teleostei; Nemacheilidae). Zootaxa 2016, 4173, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Mahjoorazad, A.; Coad, B.W. A new cave fish locality for Iran. Electron. J. Ichthyol. 2009, 2, 30–33. [Google Scholar]
- Vatandoust, S.; Mousavi-Sabet, H.; Geiger, M.F.; Freyhof, J. A new record of Iranian subterranean fishes reveals the potential presence of a large freshwater aquifer in the Zagros Mountains. J. Appl. Ichthyol. 2019, 35, 1269–1275. [Google Scholar] [CrossRef]
- Mousavi-Sabet, H.; Vatandoust, S.; Fatemi, Y.; Eagderi, S. Tashan Cave a new cave fish locality for Iran; and Garra tashanensis, a new blind species from the Tigris River drainage (Teleostei: Cyprinidae). FishTaxa 2016, 1, 133–148. [Google Scholar]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Kottelat, Cornol & Freyhof: Berlin, Germany, 2007. [Google Scholar]
- Vidergar, N.; Toplak, N.; Kuntner, M. Streamlining DNA Barcoding Protocols: Automated DNA Extraction and a New cox1 Primer in Arachnid Systematics. PLoS ONE 2014, 9, e113030. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P.D.N. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.6. 2018. Available online: http://www.mesquiteproject.org (accessed on 2 June 2021).
- Huelsenbeck, J.P.; Ronquist, F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Schmidt, H.A.; Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef]
- Mobaraki, A.; Amiri, M.; Alvandi, R.; Tehrani, M.E.; Kia, H.Z.; Khoshnamvand, A.; Bali, A.; Forozanfar, E.; Browne, R.K. A conservation reassessment of the critically endangered, Lorestan newt Neurergus kaiseri (Schmidt 1952) in Iran. Amphib. Reptile Conserv. 2014, 9, 16–25. [Google Scholar]
- Ashrafzadeh, M.R.; Naghipour, A.A.; Haidarian, M.; Kusza, S.; Pilliod, D.S. Effects of climate change on habitat and connectivity for populations of a vulnerable, endemic salamander in Iran. GECCO 2019, 19, e00637. [Google Scholar] [CrossRef]
- Malek-Hosseini, M.J.; Jugovic, J.; Fatemi, Y.; Kuntner, M.; Kostanjšek, R.; Douady, C.J.; Malard, F. A new obligate groundwater species of Asellus (Isopoda, Asellidae) from Iran. Subterr. Biol. 2022, 42, 97–124. [Google Scholar] [CrossRef]
- Coad, B.W. Threatened fishes of the world: Iranocypris typhlops Bruun and Kaiser, 1944 (Cyprinidae). Environ. Biol. Fish 1996, 46, 374. [Google Scholar] [CrossRef]
- Coad, B.W.; Mehrani, R.; Najafpour, N. Threatened fishes of the world: Paracobitis smithi (Greenwood, 1976) (Balitoridae). Environ. Biol. Fish 2009, 84, 323. [Google Scholar] [CrossRef][Green Version]
- IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 21 March 2022).
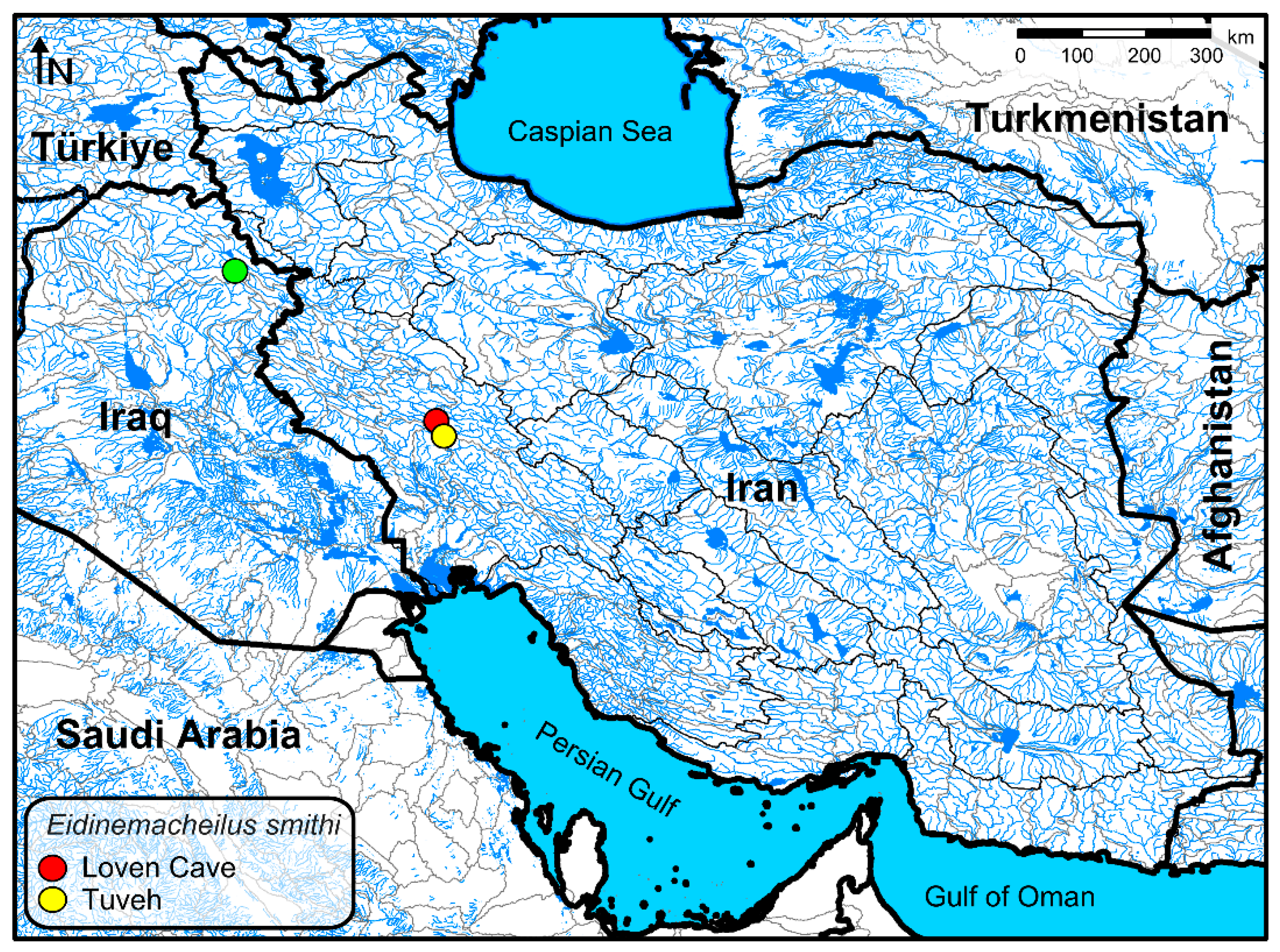

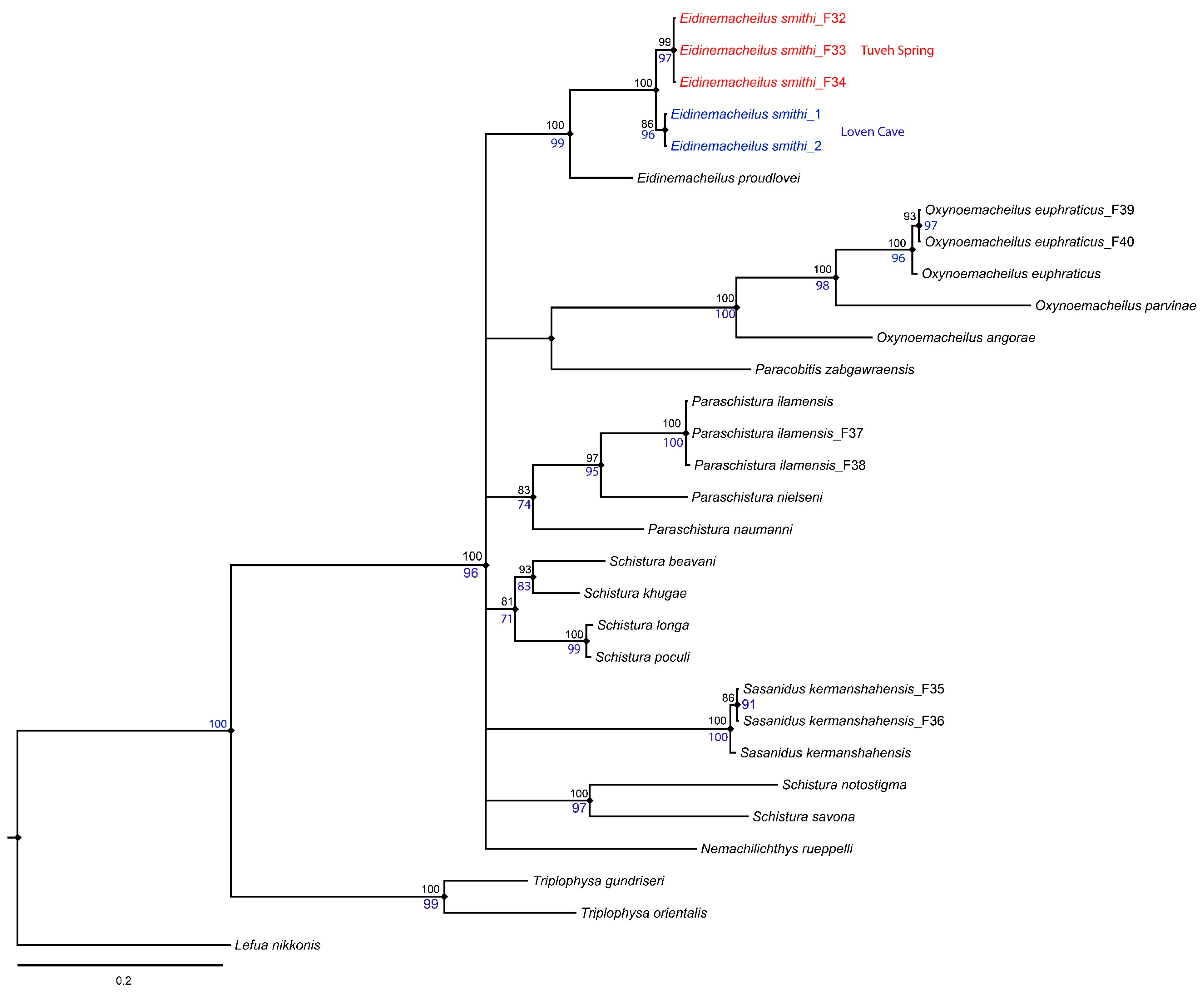

| No. | Species | Voucher Number and COI Accession Number |
|---|---|---|
| 1 | Eidinemacheilus smithi (Tuveh) | F-32: OP310812; F33: OP310813; F34: OP310814 |
| 2 | Eidinemacheilus smithi 1, 2 (Loven) | KX429660 / KX461958 |
| 3 | Eidinemacheilus proudlovei | KX774390 |
| 4 | Nemachilichthys rueppelli | KU928278 |
| 5 | Oxynoemacheilus angorae | AP011233 |
| 6 | Oxynoemacheilus euphraticus | F-39: OP310815; F-40: OP310816 |
| 7 | Oxynoemacheilus euphraticus | MK546456 |
| 8 | Oxynoemacheilus parvinae | KX980092 |
| 9 | Paracobitis zabgawraensis | MK238776 |
| 10 | Paraschistura ilamensis | F-37: OP310817; F-38: OP310818 |
| 11 | Paraschistura ilamensis | MN258032 |
| 12 | Paraschistura naumanni | KY808480. |
| 13 | Paraschistura nielseni | KY808482 |
| 14 | Sasanidus kermanshahensis | F-35: OP310819; F-36: OP310820 |
| 15 | Sasanidus kermanshahensis | KU928288 |
| 16 | Schistura beavani | HQ219200 |
| 17 | Schistura khugae | KJ909375 |
| 18 | Schistura longa | KM610912 |
| 19 | Schistura notostigma | NC_031585 |
| 20 | Schistura poculi | KM610972 |
| 21 | Schistura savona | KJ542586 |
| 22 | Triplophysa gundriseri | KX039656 |
| 23 | Triplophysa orientalis | NC_030505 |
| 24 | Lefua nikkonis | NC_027662 |
| Measured Characters | Min | Max | Mean | SD |
|---|---|---|---|---|
| Standard length (mm) | 24.6 | 25.1 | 24.8 | |
| In percent of standard length | ||||
| Head length | 24 | 25.4 | 24.7 | 1.0 |
| Body depth at dorsal-fin origin | 12.6 | 13.0 | 12.8 | 0.2 |
| Body width at dorsal-fin origin | 4.6 | 5.5 | 5.1 | 0.6 |
| Pre-dorsal length | 46.5 | 48.5 | 47.5 | 1.4 |
| Post-dorsal length | 41.8 | 41.8 | 41.8 | 0.01 |
| Pre-pelvic length | 51.8 | 51.9 | 51.8 | 0.7 |
| Pre-anal length | 73.3 | 74.3 | 73.8 | 0.7 |
| Distance between pectoral and pelvic-fin origins | 26.1 | 27.7 | 26.9 | 1.1 |
| Distance between pelvic and anal-fin origins | 20.9 | 23.5 | 22.2 | 1.9 |
| Depth of caudal peduncle | 9.8 | 9.9 | 9.8 | 0.1 |
| Length of caudal peduncle | 14.6 | 16.0 | 15.3 | 0.9 |
| Dorsal-fin length | 23.2 | 24.7 | 24.0 | 1.1 |
| Pectoral-fin length | 21.8 | 22.0 | 21.9 | 0.1 |
| Pelvic-fin length | 12.6 | 14.4 | 13.5 | 1.2 |
| In percent of head length | ||||
| Head depth at nape | 44.0 | 45.8 | 44.9 | 1.2 |
| Maximum head width | 45.6 | 48.3 | 46.9 | 1.9 |
| Inner rostral–barbel length | 17.9 | 18.9 | 18.4 | 0.7 |
| Outer rostral–barbel length | 39.6 | 41.5 | 40.6 | 1.3 |
| Maxillary–barbel length | 25.5 | 28.2 | 26.8 | 1.9 |
| NO | Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Eidinemacheilus smithi F32-Tuveh | |||||||||||||||||||
| 2 | E. smithi 1- Loven | 1.39 | ||||||||||||||||||
| 3 | E. proudlovei | 8.06 | 7.67 | |||||||||||||||||
| 4 | Nemachilichthys rueppelli | 18.05 | 18.49 | 16.52 | ||||||||||||||||
| 5 | Oxynoemacheilus angorae | 19.30 | 18.85 | 19.16 | 18.76 | |||||||||||||||
| 6 | O. euphraticus_F39 | 20.07 | 19.62 | 18.97 | 19.82 | 13.15 | ||||||||||||||
| 7 | O. parvinae | 20.49 | 20.02 | 18.54 | 22.56 | 15.92 | 12.71 | |||||||||||||
| 8 | Paracobitis zabgawraensis | 17.41 | 17.19 | 17.66 | 15.98 | 16.70 | 19.10 | 20.00 | ||||||||||||
| 9 | Paraschistura ilamensis_F37 | 13.75 | 13.97 | 15.03 | 17.77 | 18.12 | 20.55 | 21.96 | 17.21 | |||||||||||
| 10 | P. naumanni | 13.97 | 13.55 | 13.56 | 15.78 | 18.84 | 17.13 | 20.80 | 16.13 | 11.43 | ||||||||||
| 11 | P. nielseni | 13.62 | 13.84 | 13.80 | 18.31 | 18.62 | 19.21 | 22.98 | 18.87 | 7.88 | 12.53 | |||||||||
| 12 | Sasanidus kermanshahensis_F35 | 16.75 | 16.53 | 16.82 | 18.85 | 19.61 | 18.12 | 21.19 | 19.68 | 14.61 | 16.15 | 14.95 | ||||||||
| 13 | Schistura beavani | 14.11 | 14.11 | 12.88 | 15.95 | 16.05 | 15.92 | 16.58 | 16.49 | 13.66 | 12.01 | 12.75 | 14.56 | |||||||
| 14 | S. khugae | 13.08 | 13.29 | 12.07 | 13.57 | 16.33 | 15.15 | 17.35 | 15.21 | 12.62 | 11.04 | 12.12 | 15.14 | 6.48 | ||||||
| 15 | S. longa | 14.13 | 13.92 | 11.81 | 14.05 | 17.44 | 16.42 | 15.98 | 16.92 | 13.88 | 12.07 | 13.55 | 14.75 | 7.66 | 7.27 | |||||
| 16 | S. notostigma | 16.69 | 16.91 | 16.05 | 16.51 | 18.71 | 19.76 | 20.42 | 17.56 | 18.69 | 16.14 | 18.42 | 17.31 | 17.19 | 14.56 | 16.76 | ||||
| 17 | S. poculi | 14.53 | 14.32 | 12.03 | 13.61 | 17.41 | 16.61 | 15.95 | 16.47 | 14.07 | 12.46 | 13.53 | 15.15 | 7.84 | 7.07 | 0.52 | 16.52 | |||
| 18 | S. savona | 15.83 | 14.99 | 16.17 | 16.33 | 17.11 | 18.54 | 22.19 | 17.74 | 15.87 | 14.60 | 16.46 | 18.59 | 14.38 | 15.85 | 16.31 | 14.71 | 16.50 | ||
| 19 | Triplophysa gundriseri | 20.75 | 21.92 | 22.64 | 21.41 | 22.18 | 24.80 | 26.46 | 22.52 | 21.36 | 19.52 | 20.58 | 23.95 | 17.91 | 19.03 | 19.46 | 22.15 | 19.65 | 20.65 | |
| 20 | T. orientalis | 21.96 | 22.91 | 23.73 | 21.62 | 22.56 | 25.47 | 27.57 | 19.92 | 18.44 | 21.40 | 20.72 | 22.91 | 21.77 | 21.52 | 22.42 | 24.31 | 22.62 | 20.19 | 10.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malek-Hosseini, M.J.; Fatemi, Y.; Esmaeili, H.R.; Lokovšek, T.; Kuntner, M. A New Locality for the Blind Loach, Eidinemacheilus smithi (Teleostei: Nemacheilidae) in Iranian Zagros: A Morpho-Molecular Approach. Diversity 2022, 14, 724. https://doi.org/10.3390/d14090724
Malek-Hosseini MJ, Fatemi Y, Esmaeili HR, Lokovšek T, Kuntner M. A New Locality for the Blind Loach, Eidinemacheilus smithi (Teleostei: Nemacheilidae) in Iranian Zagros: A Morpho-Molecular Approach. Diversity. 2022; 14(9):724. https://doi.org/10.3390/d14090724
Chicago/Turabian StyleMalek-Hosseini, Mohammad Javad, Yaser Fatemi, Hamid Reza Esmaeili, Tjaša Lokovšek, and Matjaž Kuntner. 2022. "A New Locality for the Blind Loach, Eidinemacheilus smithi (Teleostei: Nemacheilidae) in Iranian Zagros: A Morpho-Molecular Approach" Diversity 14, no. 9: 724. https://doi.org/10.3390/d14090724
APA StyleMalek-Hosseini, M. J., Fatemi, Y., Esmaeili, H. R., Lokovšek, T., & Kuntner, M. (2022). A New Locality for the Blind Loach, Eidinemacheilus smithi (Teleostei: Nemacheilidae) in Iranian Zagros: A Morpho-Molecular Approach. Diversity, 14(9), 724. https://doi.org/10.3390/d14090724






