Land Uses for Pasture and Cacao Cultivation Modify the Odonata Assemblages in Atlantic Forest Areas
Abstract
:1. Introduction
2. Material and Methods
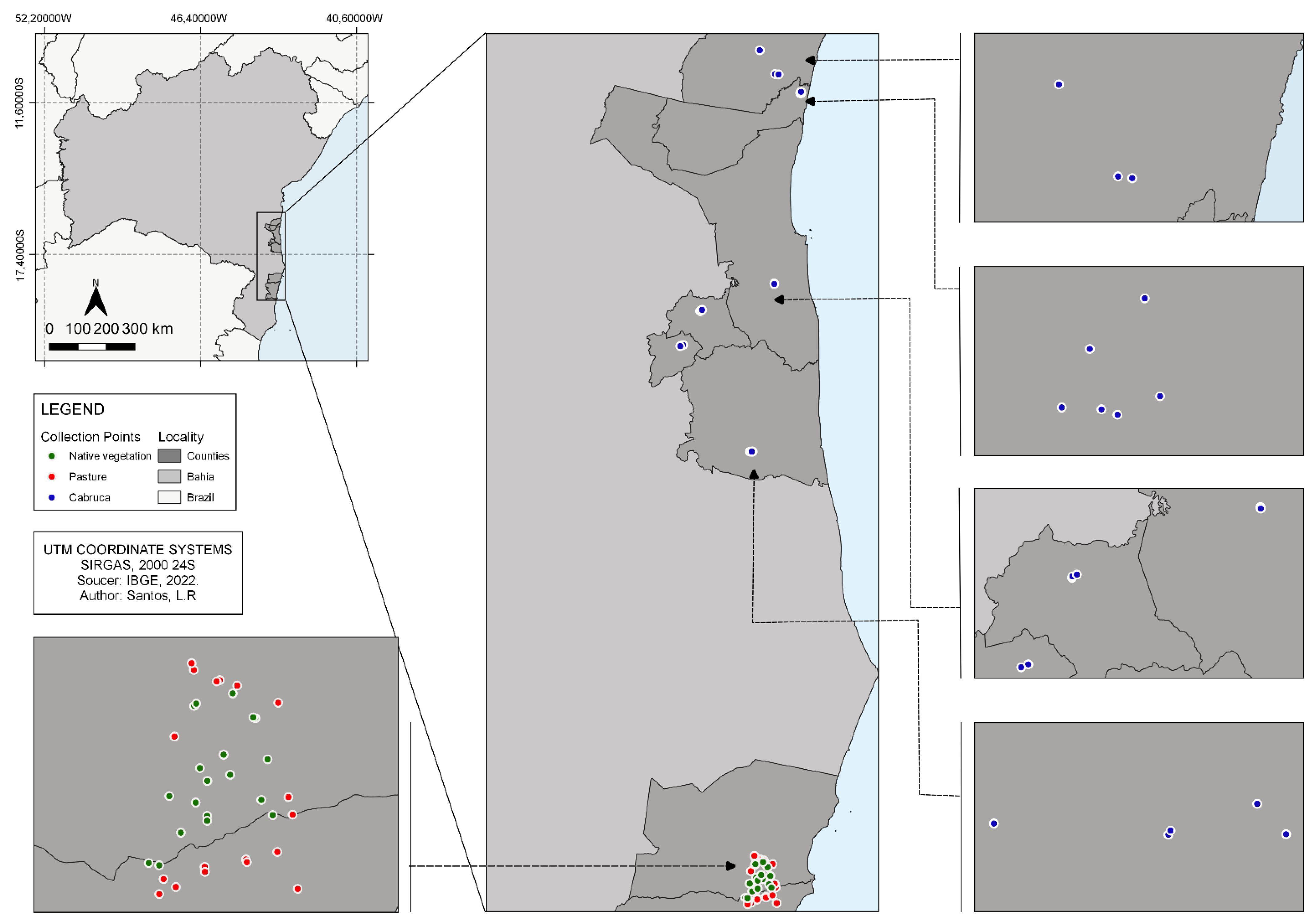
2.1. Study Area
2.2. Sampling Method
2.3. Data Analysis
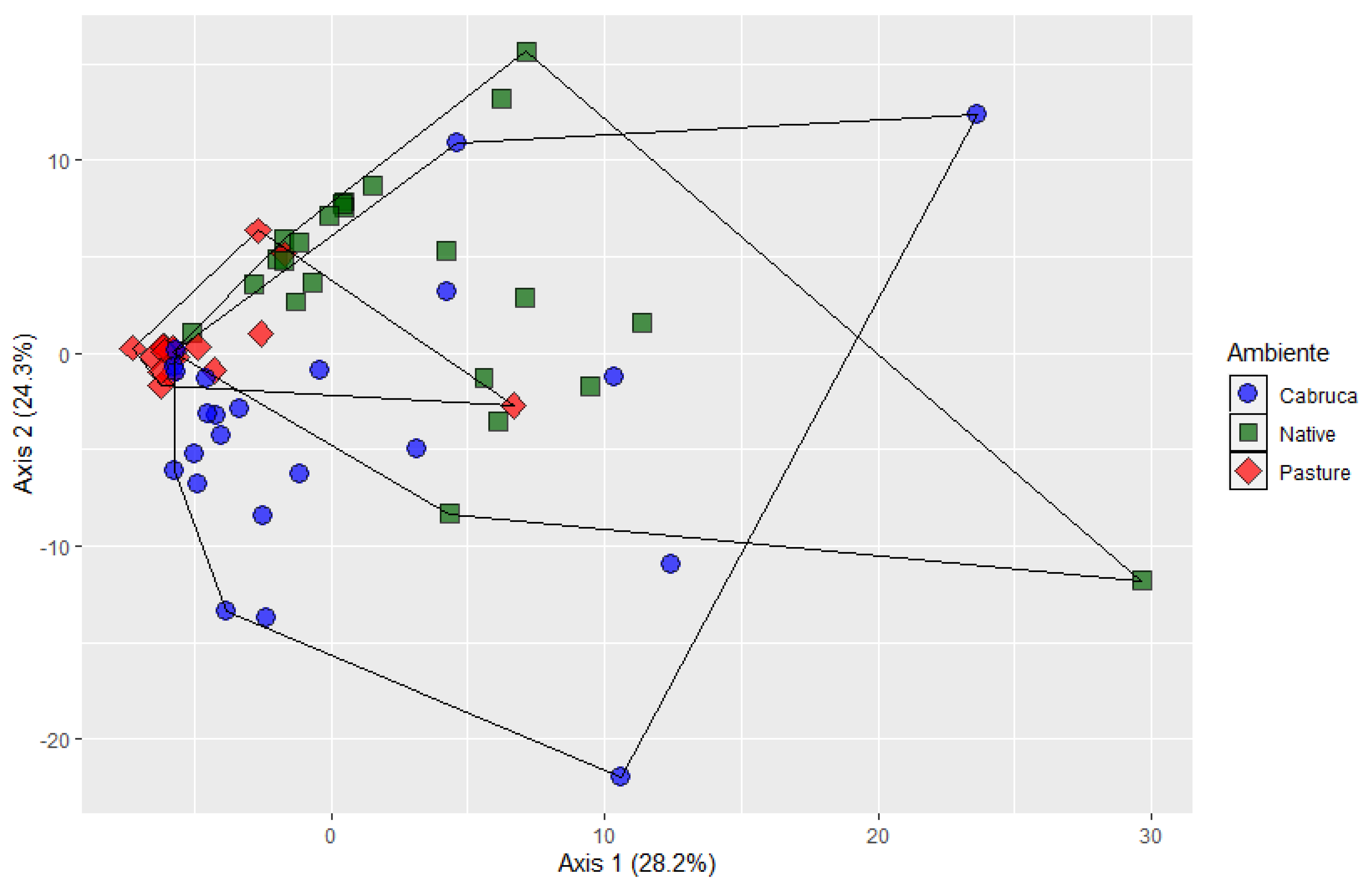
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Species Recorded for Different Land Uses in Cabruca, Native Forest, and Pasture Areas in the Sampled Streams of an Atlantic Forest Region in Southern Bahia, Brazil
| SUBORDEM | Family/Species | Abundance | |||
| ZYGOPTERA | CALOPTERYGIDAE | Cabruca | Native Forest | Pasture | Total |
| Hetaerina longipes Hagen in Selys, 1853 | 23 | 25 | 17 | 65 | |
| Hetaerina rosea Selys, 1853 | 87 | 113 | 18 | 218 | |
| COENAGRIONIDAE Acanthagrion aepiolum Tennessen, 2004 | 85 | 1 | 0 | 86 | |
| Acanthagrion cuyabae Calvert, 1909 | 0 | 0 | 2 | 2 | |
| Acanthagrion gracile (Rambur, 1842) | 1 | 0 | 5 | 6 | |
| Aceratobasis cornicauda (Calvert, 1909) | 0 | 1 | 0 | 1 | |
| Aceratobasis macilenta (Rambur, 1842) | 1 | 0 | 0 | 1 | |
| Aceratobasis nathaliae (Lencioni, 2004) | 5 | 0 | 0 | 5 | |
| Argia chapadae Calvert, 1909 | 154 | 32 | 0 | 186 | |
| Argia hasemani Calvert, 1909 | 0 | 42 | 24 | 66 | |
| Epipleoneura machadoi Rácenis, 1960 | 0 | 10 | 14 | 24 | |
| Epipleoneura metallica Rácenis, 1955 | 7 | 0 | 0 | 7 | |
| Forcepsioneura sancta (Hagen in Selys, 1860) | 1 | 4 | 3 | 8 | |
| Forcepsioneura serrabonita Pinto & Kompier, 2018 | 12 | 1 | 0 | 13 | |
| Idioneura ancilla Selys, 1860 | 6 | 1 | 4 | 11 | |
| Ischnura capreolus (Hagen, 1861) | 4 | 1 | 44 | 49 | |
| Kiautagrion acutum Santos, 1961 | 0 | 3 | 0 | 3 | |
| Leptagrion macrurum (Burmeister, 1839) | 0 | 10 | 0 | 10 | |
| Metaleptobasis selysi Santos, 1956 | 4 | 0 | 0 | 4 | |
| Neoneura ethela Williamson, 1917 | 4 | 2 | 0 | 6 | |
| Neoneura sylvatica Hagen in Selys, 1886 | 0 | 0 | 5 | 5 | |
| Nehalennia minuta (Selys in Sagra, 1857) | 0 | 0 | 4 | 4 | |
| Telagrion longum Selys, 1876 | 3 | 1 | 1 | 5 | |
| Telebasis corollina (Selys, 1876) | 2 | 7 | 43 | 52 | |
| Telebasis willinki Fraser, 1948 | 1 | 0 | 0 | 1 | |
| DICTERIADIDAE Heliocharis amazona Selys, 1853 | 0 | 8 | 0 | 8 | |
| LESTIDAE Archilestes exoletus (Hagen in Selys, 1862) | 4 | 0 | 0 | 4 | |
| Lestes forficula Rambur, 1842 | 0 | 0 | 8 | 8 | |
| Lestes tricolor Erichson in Schomburgk, 1848 | 0 | 0 | 1 | 1 | |
| HETERAGRIONIDAE Heteragrion aurantiacum Selys, 1862 | 87 | 203 | 20 | 310 | |
| Heteragrion consors Hagens in Selys, 1862 | 34 | 0 | 0 | 34 | |
| Heteragrion gracile Machado, 2006 | 0 | 2 | 0 | 2 | |
| PERILESTIDAE Perilestes fragilis Hagen in Selys, 1862 | 6 | 4 | 2 | 12 | |
| ANISOPTERA | GOMPHIDAE | ||||
| Gomphoides praevia St. Quentin, 1967 | 1 | 0 | 0 | 1 | |
| Gomphoides sp1 | 0 | 1 | 0 | 1 | |
| Progomphus sp | 0 | 1 | 1 | 2 | |
| Progomphus montanus Belle, 1973 | 0 | 0 | 2 | 2 | |
| Phyllogomphoides sp | 0 | 1 | 1 | 2 | |
| Zonophora calippus Selys, 1869 | 0 | 1 | 2 | 3 | |
| LIBELULIDAE | |||||
| Anatya guttata (Erichson in Schomburgk, 1848) | 5 | 0 | 2 | 7 | |
| Anatya januaria Ris, 1911 | 2 | 0 | 0 | 2 | |
| Dasythemis essequiba Ris, 1919 | 1 | 0 | 0 | 1 | |
| Dasythemis venosa (Burmeister, 1839) | 1 | 0 | 0 | 1 | |
| Diastatops obscura (Fabricius, 1775) | 3 | 0 | 8 | 11 | |
| Diastatops nigra Montgomery, 1940 | 9 | 3 | 0 | 12 | |
| Elasmothemis alcebiadesi (Santos, 1945) | 6 | 0 | 0 | 6 | |
| Elasmothemis cannacrioides (Calvert, 1906) | 0 | 7 | 3 | 10 | |
| Elga leptostyla Ris, 1909 | 1 | 0 | 0 | 1 | |
| Erythemis carmelita Williamson, 1923 | 1 | 0 | 0 | 1 | |
| Erythemis credula (Hagen, 1861) | 0 | 0 | 4 | 4 | |
| Erythemis vesiculosa (Fabricius, 1775) | 0 | 1 | 0 | 1 | |
| Erythrodiplax avittata Borror, 1942 | 0 | 1 | 3 | 4 | |
| Erythrodiplax castanea (Burmeister, 1839) | 14 | 0 | 0 | 14 | |
| Erythrodiplax famula (Erichson in Schomburgk, 1848) | 1 | 0 | 0 | 1 | |
| Erythrodiplax funerea (Hagen, 1861) | 0 | 3 | 0 | 3 | |
| Erythrodiplax fusca (Rambur, 1842) | 62 | 9 | 30 | 101 | |
| Erythrodiplax latimaculata Ris, 1911 | 1 | 0 | 0 | 1 | |
| Erythrodiplax leticia Machado, 1996 | 0 | 0 | 6 | 6 | |
| Erythrodiplax lygaea Ris, 1911 | 1 | 0 | 1 | 2 | |
| Erythrodiplax maculosa (Hagen, 1861) | 3 | 0 | 0 | 3 | |
| Erythrodiplax media Borror, 1942 | 4 | 0 | 0 | 4 | |
| Erythrodiplax paraguayensis (Förster, 1905) | 0 | 1 | 17 | 18 | |
| Erythrodiplax umbrata (Linnaeus, 1758) | 5 | 2 | 3 | 10 | |
| Erythrodiplax sp1 | 1 | 0 | 0 | 1 | |
| Erythrodiplax sp2 | 2 | 0 | 0 | 2 | |
| Erythrodiplax sp3 | 1 | 0 | 0 | 1 | |
| Macrothemis tenuis Hagen, 1868 | 4 | 0 | 0 | 4 | |
| Micrathyria atra (Martin, 1897) | 0 | 1 | 1 | 2 | |
| Micrathyria artemis Ris, 1911 | 8 | 0 | 2 | 10 | |
| Micrathyria catenata Calvert, 1909 | 1 | 0 | 2 | 3 | |
| Micrathyria mengeri Ris, 1919 | 0 | 0 | 1 | 1 | |
| Micrathyria ungulata Förster, 1907 | 12 | 0 | 2 | 14 | |
| Nephepeltia phryne (Perty, 1833) | 1 | 0 | 0 | 1 | |
| Oligoclada abbreviata (Rambur, 1842) | 1 | 0 | 0 | 1 | |
| Oligoclada umbricola Borror, 1931 | 1 | 0 | 2 | 3 | |
| Orthemis attenuata (Erichson in Schomburgk, 1848) | 3 | 4 | 2 | 9 | |
| Orthemis discolor (Burmeister, 1839) | 4 | 1 | 0 | 5 | |
| Perithemis lais (Perty, 1833) | 1 | 3 | 5 | 9 | |
| Perithemis thais Kirby, 1889 | 18 | 1 | 0 | 19 | |
| Planiplax phoenicura Ris, 1912 | 0 | 0 | 9 | 9 | |
| Tauriphila argo (Hagen, 1869) | 0 | 0 | 1 | 1 | |
| Uracis infumata (Rambur, 1842) | 2 | 0 | 0 | 2 | |
| Zenithoptera viola Ris, 1910 | 0 | 0 | 5 | 5 | |
| Total Abundance | 712 | 514 | 332 | 1558 | |
| Zygoptera Abundance | 531 | 471 | 215 | 1217 | |
| Anisoptera Abundance | 181 | 43 | 117 | 341 | |
References
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global Biodiversity Conservation: The Critical Role of Hotspots. In Biodiversity Hotspots; Zachos, F., Habel, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar] [CrossRef]
- Barlow, J.; França, F.; Gardner, T.A.; Hicks, C.; Lennox, G.D.; Berenguer, E.; Castello, L.; Economo, E.P.; Ferreira, J.; Guénard, B.; et al. The future of hyperdiverse tropical ecosystems. Nature 2018, 559, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Bogunovic, I.; Muñoz-Rojas, M.; Brevik, E.C. Soil ecosystem services, sustainability, valuation and management. Curr. Opin. Environ. Sci. Health 2018, 5, 7–13. [Google Scholar] [CrossRef]
- de Mello, K.; Taniwaki, R.H.; de Paula, F.R.; Valente, R.A.; Randhir, T.O.; Macedo, D.R.; Leal, C.G.; Rodrigues, C.B.; Hughes, R.M. Multiscale land use impacts on water quality: Assessment, planning, and future perspectives in Brazil. J. Environ. Manag. 2020, 270, 110879. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.S. Recuperação Ambiental da Mata Atlêntica, 3rd ed.; Editus: Luxembourg, 2016. [Google Scholar]
- SOS Mata Atlântica, 2018. Relatório Anual 2018. São Paulo—SP. Available online: https://www.sosma.org.br/wpcontent/uploads/2019/07/RA_SOSMA_2018DIGITAL.pdf (accessed on 12 August 2019).
- Albert, J.S.; Destouni, G.; Duke-Sylvester, S.M.; Magurran, A.E.; Oberdorff, T.; Reis, R.E.; Winemiller, K.O.; Ripple, W.J. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 2020, 50, 85–94. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and Riverscapes: The Influence of Land Use on Stream Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Yu, S.; Xu, Z.; Wu, W.; Zuo, D. Effect of land use types on stream water quality under seasonal variation and topographic characteristics in the Wei River basin, China. Ecol. Indic. 2016, 60, 202–212. [Google Scholar] [CrossRef]
- Dala-Corte, R.B.; Melo, A.S.; Siqueira, T.; Bini, L.M.; Martins, R.T.; Cunico, A.M.; Pes, A.M.; Magalhães, A.L.B.; Godoy, B.S.; Leal, C.G.; et al. Thresholds of freshwater biodiversity in response to riparian vegetation loss in the Neotropical region. J. Appl. Ecol. 2020, 57, 1391–1402. [Google Scholar] [CrossRef]
- Lobão, D.E.; Setenta, W.C.; Valle, R.R. Sistema agrossilvicultural cacaueiro-modelo de agricultura sustentável. Agrossilvicultura 2004, 1, 163–173. [Google Scholar]
- Cassano, C.R.; Schroth, G.P.; De Faria, D.E.; Delabie, J.H.C.; Bede, L. Landscape and farm scale management to enhance biodiversity conservation in the cocoa producing region of southern Bahia, Brazil. Biodivers. Conserv. 2009, 18, 577–603. [Google Scholar] [CrossRef]
- Kersul, M.G.; Costa, N.A.; Boullosa, R.G.; Silva, A.A.; Rios, O.; Munhoz, A.D.; Andrade-Silva, B.E.; Maldonado, A.; Gentile, R.; Alvarez, M.R. Helminth communities of sigmonontine rodents in cocoa agroforestry systems in Brazil. Int. J. Parasitol. Parasites Wildl. 2020, 11, 62–71. [Google Scholar] [CrossRef]
- Vanwalleghem, T.; Gómez, J.; Amate, J.I.; de Molina, M.G.; Vanderlinden, K.; Guzmán, G.; Laguna, A.; Giráldez, J. Impact of historical land use and soil management change on soil erosion and agricultural sustainability during the Anthropocene. Anthropocene 2017, 17, 13–29. [Google Scholar] [CrossRef]
- Leal, C.G.; Lennox, G.D.; Ferraz, S.F.B.; Ferreira, J.; Gardner, T.A.; Thomson, J.R.; Berenguer, E.; Lees, A.C.; Hughes, R.M.; Mac Nally, R.; et al. Integrated terrestrial-freshwater planning doubles conservation of tropical aquatic species. Science 2020, 370, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Tonello, G.; Decian, V.S.; Restello, R.M.; Hepp, L.U. The conversion of natural riparian forests into agricultural land affects ecological processes in Atlantic forest streams. Limnologica 2021, 91, 125927. [Google Scholar] [CrossRef]
- Teresa, F.B.; Casatti, L. Importância da vegetação ripária degradada em região intensamente desmatada no sudeste do Brasil: Um estudo com peixes de riacho. Panamjas 2010, in press. [Google Scholar]
- Lange, M.; Weisser, W.W.; Gossner, M.M.; Kowalski, E.; Türke, M.; Joner, F.; Fonseca, C.R. The impact of forest management on litter-dwelling invertebrates: A subtropical–temperate contrast. Biodivers. Conserv. 2011, 20, 2133–2147. [Google Scholar] [CrossRef]
- Ometo, J.P.H.B.; Martinelli, L.A.; Ballester, M.V.; Gessner, A.; Krusche, A.V.; Victoria, R.L.; Williams, M. Effects of land use and water chemistry and macroinvertebrates in two streams of the Piracicaba river basin, south-east Brazil. Freshw. Biol. 2000, 44, 327–337. [Google Scholar] [CrossRef]
- Benstead, J.P.; Douglas, M.M.; Pringle, C.M. Relationships of Stream Invertebrate Communities to Deforestation in Eastern Madagascar. Ecol. Appl. 2003, 13, 1473–1490. [Google Scholar] [CrossRef]
- Rodrigues, M.E.; Roque, F.D.O.; Guillermo-Ferreira, R.; Saito, V.S.; Samways, M.J. Egg-laying traits reflect shifts in dragonfly assemblages in response to different amount of tropical forest cover. Insect Conserv. Divers. 2018, 12, 231–240. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Ferreira, W.; Hepp, L.; Ligeiro, R.; Macedo, D.; Hughes, R.; Kaufmann, P.; Callisto, M. Partitioning taxonomic diversity of aquatic insect assemblages and functional feeding groups in neotropical savanna headwater streams. Ecol. Indic. 2017, 72, 365–373. [Google Scholar] [CrossRef]
- Casatti, L. Alterações no Código Florestal Brasileiro: Impactos potenciais sobre uma ictiofauna. Biota Neotropica 2010, 10, 31–34. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.P.; Faria, D.; Morante-Filho, J.C. Landscape composition is more important than local vegetation structure for understory birds in cocoa agroforestry systems. For. Ecol. Manag. 2021, 481, 118704. [Google Scholar] [CrossRef]
- Brazil. Lei nº 12.651—Institui o novo Código Florestal Brasileiro. 2012. Available online: http://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/lei/l12651.htm (accessed on 27 January 2022).
- Gómez-Tolosa, M.; Rivera-Velázquez, G.; Rioja-Paradela, T.M.; Mendoza-Cuenca, L.F.; Tejeda-Cruz, C.; López, S. The use of Odonata species for environmental assessment: A meta-analysis for the Neotropical region. Environ. Sci. Pollut. Res. 2021, 28, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.M.B., Jr.; Shimano, Y.; Gardner, T.A.; Hughes, R.M.; De Marco, P.D., Jr.; Juen, L. Neotropical dragonflies (Insecta: Odonata) as indicators of ecological condition of small streams in the eastern Amazon. Austral Ecol. 2015, 40, 733–744. [Google Scholar] [CrossRef]
- Rodrigues, M.; Roque, F.D.O.; Quintero, J.M.O.; Pena, J.C.C.; de Sousa, D.C.; De Marco, P., Jr. Nonlinear responses in damselfly community along a gradient of habitat loss in a savanna landscape. Biol. Conserv. 2016, 194, 113–120. [Google Scholar] [CrossRef]
- De Oliveira, J.M.B., Jr.; De Marco, P., Jr.; Dias-Silva, K.; Leitão, R.P.; Leal, C.; Pompeu, P.S.; Gardner, T.A.; Hughes, R.M.; Juen, L. Effects of human disturbance and riparian conditions on Odonata (Insecta) assemblages in eastern Amazon basin streams. Limnogical 2017, 66, 31–39. [Google Scholar] [CrossRef]
- Miguel, T.B.; Oliveira-Junior, J.M.B.; Ligeiro, R.; Juen, L. Odonata (Insecta) as a tool for the biomonitoring of environmental quality. Ecol. Indic. 2017, 81, 555–566. [Google Scholar] [CrossRef]
- Carvalho, F.G.; Roque, F.D.O.; Barbosa, L.; Montag, L.F.D.A.; Juen, L. Oil palm plantation is not a suitable environment for most forest specialist species of Odonata in Amazonia. Anim. Conserv. 2018, 21, 526–533. [Google Scholar] [CrossRef]
- Corbet, P.S.; May, M.L. Fliers and perchers among Odonata: Dichotomy or multidimensional continuum? A provisional reappraisal the flier/percher template. Int. J. Odonatol. 2008, 11, 155–171. [Google Scholar] [CrossRef]
- De Marco, P., Jr.; Batista, J.D.; Cabette, H.S.R. Community Assembly of Adult Odonates in Tropical Streams: An Ecophysiological Hypothesis. PLoS ONE 2015, 10, e0123023. [Google Scholar] [CrossRef] [PubMed]
- Valente-Neto, F.; Roque, F.D.O.; Rodrigues, M.; Juen, L.; Swan, C.M. Toward a practical use of Neotropical odonates as bioindicators: Testing congruence across taxonomic resolution and life stages. Ecol. Indic. 2016, 61, 952–959. [Google Scholar] [CrossRef]
- Júnior, C.D.S.M.; Juen, L.; Hamada, N. Analysis of urban impacts on aquatic habitats in the central Amazon basin: Adult odonates as bioindicators of environmental quality. Ecol. Indic. 2015, 48, 303–311. [Google Scholar] [CrossRef]
- Calvão, L.B.; Juen, L.; Junior, J.M.B.D.O.; Batista, J.D.; Júnior, P.D.M. Land use modifies Odonata diversity in streams of the Brazilian Cerrado. J. Insect Conserv. 2018, 22, 675–685. [Google Scholar] [CrossRef]
- Rodrigues, M.E.; Moura, E.B.; Roque, F.D.O. Dragonflies as indicators of the environmental conditions of veredas in a region of central-western Brazil. Oecol. Aust. 2019, 23, 969–978. [Google Scholar] [CrossRef]
- Batista, J.D.; Ferreira, V.R.S.; Cabette, H.S.R.; de Castro, L.A.; De Marco, P.; Juen, L. Sampling efficiency of a protocol to measure Odonata diversity in tropical streams. PLoS ONE 2021, 16, e0248216. [Google Scholar] [CrossRef]
- Garrison, R.W.; von Ellenrieder, N.; Louton, J.A. Dragonfly genera of the New World: An illustrated and annotated key to the Anisoptera. In Encyclopedia of South American Aquatic Insects: Odonata—Anisoptera; Heckman, C.W., Ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2006. [Google Scholar]
- Garrison, R.W.; Von Ellenrieder, N.; Louton, J.A. Damselfly Genera of the New World: An Illustrated and Annotated Key to the Zygoptera; The Johns Hopkins University Press: Baltimore, MD, USA, 2010. [Google Scholar]
- Lencioni, F.A.A. The Damselflies of Brazil: An illustrated Guide—The Non Coenagrionidae Families; All Print Editora: São Paulo, Brazil, 2005. [Google Scholar]
- Lencioni, F.A.A. The Damselflies of Brazil: An Illustrated Guide—Coenagrionidae; All Print Editora: São Paulo, Brazil, 2006. [Google Scholar]
- Lencioni, F.A.A. Damselflies of Brazil; An Illustrated Identification Guide—Southeast Region; Camara Brazileira do Livro: Jacareí, Brazil, 2017. [Google Scholar]
- Gotelli, N.J.; Ellison, A.M. Princípios de Estatística Em Ecologia; Artmed Editora: Porto Alegre, Brazil, 2011; p. 528. ISBN 8536324325. [Google Scholar]
- Anderson, M.J.; Walsh, D.C.I. Permanova, Anosim, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- De Cáceres, M.; Sol, D.; Lapiedra, O.; Legendre, P. A framework for estimating niche metrics using the resemblance between qualitative resources. Oikos 2011, 120, 1341–1350. [Google Scholar] [CrossRef]
- Oksanen, J.F.; Blanchet, G.; Friendly, M.; Kindt, R.; Dan McGlinn, P.L.; Minchin, P.R.; OHara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; et al. Vegan: Community Ecology Package 2017. R Package Version 2.4-3. Available online: https://CRANR-project.org/package=vegan (accessed on 21 March 2022).
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Loiola, G.R.; De Marco, P. Behavioral ecology of Heteragrion consors Hagen (Odonata, Megapodagrionidae): A shade-seek Atlantic forest damselfly. Rev. Bras. Èntomol. 2011, 55, 373–380. [Google Scholar] [CrossRef]
- Calvão, L.B.; Nogueira, D.; Montag, L.; Lopes, M.A.; Juen, L. Are Odonata communities impacted by conventional or reduced impact logging? For. Ecol. Manag. 2016, 382, 143–150. [Google Scholar] [CrossRef]
- Pinto, P.; Kompier, T. In honor of conservation of the Brazilian Atlantic Forest: Description of two new damselflies of the genus Forcepsioneura discovered in private protected areas (Odonata: Coenagrionidae). Zoologia 2018, 35, 1–19. [Google Scholar] [CrossRef]
- Faria, D.; Laps, R.R.; Baumgarten, J.; Cetra, M. Bat and Bird Assemblages from Forests and Shade Cacao Plantations in Two Contrasting Landscapes in the Atlantic Forest of Southern Bahia, Brazil. Biodivers. Conserv. 2006, 15, 587–612. [Google Scholar] [CrossRef]
- Ribeiro, C.; Firme, B.; Araujo, A.S.; Sá, A.; Zander, F.; Teixeira, K.; Santos, L.R.; Rodrigues, M.E. Check-list of Odonata from the state of Bahia, Brazil: Ecological information, distribution, and new state records. Odonatologica 2021, 50, 161–186. [Google Scholar] [CrossRef]
- Santos, C.; Santos, L.R.; Rodrigues, M.E. New records of the Critically Endangered Leptagrion acutum Santos, 1961 (Odonata, Coenagrionidae) from southern Bahia, Brazil. Check List. 2021, 17, 59–62. [Google Scholar] [CrossRef]
- ICMBio; Brasília, D.F. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção, 1st ed.; 2018. Available online: https://www.icmbio.gov.br/portal/images/stories/comunicacao/publicacoes/publicacoes-diversas/livro_vermelho_2018_vol7.pdf (accessed on 27 January 2022).
- Dutra, S.; De Marco, P. Bionomic differences in odonates and their influence on the efficiency of indicator species of environmental quality. Ecol. Indic. 2015, 49, 132–142. [Google Scholar] [CrossRef]
- Pires, M.M.; Müller, N.F.D.; Stenert, C.; Maltchik, L. Influence of different riparian vegetation widths and substrate types on the communities of larval Odonata (Insecta) in southern Brazilian streams. Acta Limnol. Bras. 2020, 32, e301. [Google Scholar] [CrossRef]
- Oliveira-Junior, J.M.B.; Juen, L. The Zygoptera/Anisoptera Ratio (Insecta: Odonata): A New Tool for Habitat Alterations Assessment in Amazonian Streams. Neotrop. Èntomol. 2019, 48, 552–560. [Google Scholar] [CrossRef]
- Ribeiro, C.; Juen, L.; Rodrigues, M.E. The Zygoptera/Anisoptera ratio as a tool to assess anthropogenic changes in Atlantic Forest streams. Biodivers. Conserv. 2021, 30, 1315–1329. [Google Scholar] [CrossRef]
- Cassano, C.R.; Schroth, G.; Faria DDelabie, J.H.; Bede, L.; Oliveira, L.C.; Mariano-Neto, E. Desafios e Recomendações Para a Conservação da Biodiversidade na Região Cacaueira do sul da Bahia; Boletim Tecnico nº 205; CEPLAC/CEPEC: Ilhéus, Brazil, 2014. [Google Scholar]
- De Almeida-Rocha, J.M.; Peres, C.A.; Monsalvo, J.A.B.; Oliveira, L.D.C. Habitat determinants of golden-headed lion tamarin (Leontopithecus chrysomelas) occupancy of cacao agroforests: Gloomy conservation prospects for management intensification. Am. J. Primatol. 2020, 82, e23179. [Google Scholar] [CrossRef]

| Species | Cabruca | Native Forest | Pasture | Index Value | p-Value | Specificity (A) | Fidelity (B) |
|---|---|---|---|---|---|---|---|
| Acanthagrion aepiolum | x | 0.718 | 0.001 | 0.9889 | 0.5217 | ||
| Acanthagrion gracile | x | 0.453 | 0.024 | 0.8712 | 0.2353 | ||
| Aceratobasis nathaliae | x | 0.417 | 0.017 | 1.000 | 0.1739 | ||
| Argia chapadae | x | 0.830 | 0.001 | 0.8339 | 0.8261 | ||
| Argia hasemani | x | x | 0.733 | 0.001 | 1.000 | 0.5366 | |
| Epipleoneura machadoi | x | x | 0.494 | 0.027 | 1.000 | 0.2439 | |
| Epipleoneura metallica | x | 0.417 | 0.011 | 1.000 | 0.1739 | ||
| Erythrodiplax castanea | x | 0.417 | 0.024 | 1.000 | 0.1739 | ||
| Erythemis credula | x | 0.420 | 0.024 | 1.000 | 0.1765 | ||
| Erythrodiplax fusca | x | x | 0.712 | 0.003 | 0.9224 | 0.5500 | |
| Erythrodiplax leticia | x | 0.485 | 0.004 | 1.000 | 0.2353 | ||
| Erythrodiplax paraguayensis | x | 0.531 | 0.002 | 0.9600 | 0.2941 | ||
| Heliocharis amazona | x | 0.456 | 0.025 | 1.000 | 0.2083 | ||
| Heteragrion aurantiacum | x | x | 0.824 | 0.001 | 0.9123 | 0.7447 | |
| Heteragrion consors | x | 0.659 | 0.001 | 1.000 | 0.4348 | ||
| Ischnura capreolus | x | 0.737 | 0.001 | 0.9231 | 0.5882 | ||
| Perithemis lais | x | 0.432 | 0.0216 | 0.6358 | 0.2941 | ||
| Perithemis thais | x | 0.674 | 0.001 | 0.9495 | 0.4783 | ||
| Planiplax phoenicura | x | 0.485 | 0.005 | 1.000 | 0.2353 | ||
| Telebasis corallina | x | 0.554 | 0.003 | 0.8698 | 0.3529 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, L.R.; Rodrigues, M.E. Land Uses for Pasture and Cacao Cultivation Modify the Odonata Assemblages in Atlantic Forest Areas. Diversity 2022, 14, 672. https://doi.org/10.3390/d14080672
Santos LR, Rodrigues ME. Land Uses for Pasture and Cacao Cultivation Modify the Odonata Assemblages in Atlantic Forest Areas. Diversity. 2022; 14(8):672. https://doi.org/10.3390/d14080672
Chicago/Turabian StyleSantos, Laís R., and Marciel E. Rodrigues. 2022. "Land Uses for Pasture and Cacao Cultivation Modify the Odonata Assemblages in Atlantic Forest Areas" Diversity 14, no. 8: 672. https://doi.org/10.3390/d14080672
APA StyleSantos, L. R., & Rodrigues, M. E. (2022). Land Uses for Pasture and Cacao Cultivation Modify the Odonata Assemblages in Atlantic Forest Areas. Diversity, 14(8), 672. https://doi.org/10.3390/d14080672






