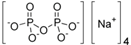
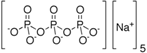
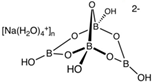
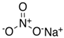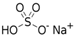Abstract
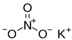
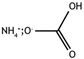
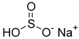
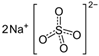
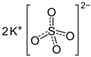
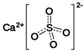
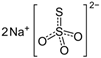
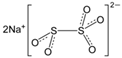
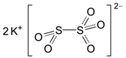
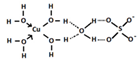
The food industry is not the only sphere of human activity where inorganic food additives are globally used. In certain concentrations, they are safe for people and agricultural animals. Nonetheless, they impose a negative impact on other classes of living organisms. Therefore, our objective was to determinine the influence of some inorganic food additives (alkalis, acids, salts) on the vitality of nematode larvae that parasitize agricultural animals: Strongyloides papillosus, Haemonchus contortus and Muellerius capillaris. We studied the effects of sodium hydroxide, potassium hydroxide, boric acid, phosphoric acid, potassium chloride, calcium chloride, sodium nitrite, potassium nitrite, sodium nitrate, potassium nitrate, ammonium bicarbonate, sodium bisulfite, sodium bisulfate, sodium sulfate, potassium sulfate, calcium sulfate, sodium thiosulfate, sodium metabisulfite, potassium metabisulfite, copper sulfate pentahydrate, tetrasodium pyrophosphate, sodium triphosphate, sodium borate decahydrate and talc. In in vitro experiments, the strongest effects were produced by alkalis sodium hydroxide and potassium hydroxide. In 24 h, 1% solutions of those substances killed 69% of larvae of S. papillosus, H. contortus and M. capillaris of various development stages. Sodium sulfate was effective against all stages of larvae of S. papillosus, and also against first-age M. capillaris. Nematocidal properties only against all stages of S. papillosus were exerted by copper sulfate pentahydrate. Non-invasive stages of S. papillosus nematodes were affected only by phosphoric acid, ammonium bicarbonate, calcium chloride, sodium nitrite, calcium sulfate, potassium metabisulfite, tetrasodium pyrophosphate, sodium triphosphate and the same stages of M. capillaris—by phosphoric acid, sodium bisulfite and potassium nitrite.
1. Introduction
Nematodes are common in agricultural and wild animals and humans, parasitizing the intestine [1], lungs [2], liver and other organs [3]. Therefore, the literature has large amounts of data regarding the struggle against the diseases those larvae cause [4,5]. The significance of those human and mammal parasites is hard to exaggerate, for many species are common in local populations of hosts, and broad use of anti-nematode drugs often leads to the spread of drug resistance among many nematode species [6,7]. The necessity of evaluation of effects of various natural [8,9] and synthesized [10,11] chemical substances on nematode larvae is of great interest in terms of ecotoxicology and evaluation of the potential of their anti-parasitic activity.
Strongyloidiasis is common in people in many countries with tropical and subtropical climates: 100–200 M people in 70 countries of the world are suffering from strongyloidiasis, including inhabitants and immigrants in countries of Southern, Eastern and Central Europe, inhabitants of the Appalachian region of the US and travelers returning from southern countries [12]. Opportunistic disseminated strongyloidiases is usually found in patients with weakened immunity. Free-living generations of nematodes of Strongyloides genus form local hotbeds of infection in soil. From eggs they lay in soil, rhabdite-like larvae (L1–2) develop. In unfavourable conditions, they transform into more resistant filariform larvae (L3), able to invade people and other mammals. They actively penetrate through the skin into the blood circulatory system or directly enter the digestive tract of vertebrates with food or water [13,14,15]. In the small intestine of mammals (in the duodenum and jejunum in people), they are able to reproduce for a long time (decades), forming more and more generations. Imbalance between the activity of eosinophils and threadworms causes serious health issues. By penetrating into the blood circulatory system, brain, cardiac muscle, lungs, kidneys, lymph nodes and other organs, nematodes cause inflammations in those organs. Strongyloidiasis is a serious problem in healthcare and veterinary medicine [16]. In Europe, 22 species of Strongyloides genus are distributed, including S. papillosus (Wedl, 1856), found in cattle, pigs, sheep, goats, rabbits, polecats, and rats [17,18,19].
Haemonchosis is spread throughout the world. Its pathogen is barber’s pole worm Haemonchus contortus (Rudolphi, 1803) Cobb, 1898. People are rarely infected by this parasite, which mainly affects ruminants, severely harming the intestine, nervous, endocrine systems and other organs [20]. Most often, mature parasites attach to the mucous membrane of the abomasums of sheep and goats, and consume blood, causing edemas and gradual development of anemia. The disease is much more common during the years with a large amount of precipitations. Haemonchosis imposes great losses on farmers across the planet [21,22]. Over recent years, barber’s pole worms have been observed to become drug-resistant. As a means of fighting this parasite, copper oxide (2 g of copper oxide in gel capsules per one goat or sheep) has begun to be introduced, but it often does not kill helminths, and only decreases the number of eggs (by 82.5% for sheep and 80.5% for goats) which are discharged into the environment [23]. The search for alternative anti-haemonchosis drugs may be very practically significant [24,25].
Muellerius capillaris (Mueller, 1889), also known as goat lungworm, belongs to the most economically harmful parasite of ruminants. Sheep and goats accidentally swallow terrestrial gastropods Trochoidea spp., Helix spp., Theba spp., Abida spp., Zebrina spp., Limax spp., Agriolimax spp. and other genera of Gastropoda class, Stylommatophora order, and become infected by larvae of this species of nematodes [26]. In the organisms of ruminants, they migrate through the blood circulatory system from the intestine to the subpleural space of the lungs [27]. The lung tissues of dead goats and sheep are found to hold inflammation hotbeds with white 3 cm-long filariform nematodes, accumulations of eggs, often concentrated in up to 2 cm-diameter nodes with white pus. Usually, first-age larvae hatch in the bronchi and the trachea, are swallowed by sheep and goats, travel through the intestine and are discharged into the environment with feces [28]. No publications about drug resistance of this species of nematodes have emerged yet.
Inorganic food additives are broadly used in the food industry and veterinary medicine. They include some that are highly dangerous to living organisms, including nematodes, in relatively low concentrations [29,30,31].
Therefore, prior to the beginning of the study, we formulated three hypotheses. The first of our hypotheses was that inorganic food additives would in general have a lesser effect on nematode larvae in soil than the organic, since most of the studied inorganic substances we studied are present in soil in some concentration, and nematodes—in the processes of their long evolution—have encountered them.
The second hypothesis could be formulated as follows: inorganic substances that do not occur in such types of soils where nematode larvae live would kill nematodes depending on the extent of their solubility in water. The third hypothesis was that the pH of the environment would have a low effect on the vitality of nematode larvae, since it is common knowledge that nematodes are one of the most successful groups of organisms living in both acidic and alkaline environments, and in ruminants’ feces, nematode larvae can end up in wetland soil with pH of around 5 or solonchaks with pH of around 10.
The objective of this article was in vitro evaluation of the effects inorganic food additives take on the survivability of nematode larvae parasitizing farm animals.
2. Materials and Methods
In the experiment, we studied the influence of inorganic food additives: alkalis (sodium hydroxide, potassium hydroxide), acids (boric acid, phosphoric acid) and salts (potassium chloride, calcium chloride, sodium nitrite, potassium nitrite, sodium nitrate, potassium nitrate, ammonium bicarbonate, sodium bisulfite, sodium bisulfate, sodium sulfate, potassium sulfate, calcium sulfate, sodium thiosulfate, sodium metabisulfite, potassium metabisulfite, copper sulfate pentahydrate, tetrasodium pyrophosphate, sodium triphosphate, sodium borate decahydrate, talc) on nematode larvae of Strongyloides papillosus, Haemonchus contortus and Muellerius capillaris (Table 1).

Table 1.
Uses of inorganic food additives, utilized to determine the survivability of nematodes in the laboratory experiment.
The pH of manure of goats is close to neutral (Table 2). According to various authors, concentrations of phosphorus, calcium and potassium vary greatly depending on place of sample collection. While being decomposed by microorganisms with access to oxygen and in anaerobic conditions, organic substances in manure interact with inorganic substances, and therefore the concentration of acids or their salts changes over time in the same sample depending on moisture of the substrate, its temperature, dominating microorganisms and many other factors [33,34,35,36].

Table 2.
Chemical composition of manure of goats.
The larvae were cultivated in a thermostat for 10 days in 18–22 °C temperature. Larvae of S. papillosus (first–third ages—L1, L2, L3), H. contortus (third stage L3) and M. capillaris (first stage of development L1) were isolated from feces of goats, which had been naturally infected during grazing, using Baermann’s technique (Baermann test) [37]. Species of parasites were identified based on morphological peculiarities of larvae of the indicated stages [38,39]. At the same time, we took into account body size, structure of the esophagus, and also the intestine. Then, the larvae were placed in 10 mL test tubes and centrifuged at 1500 rpm for 4 min. The sediment with larvae was stirred and evenly distributed (an average S. papillosus of 14–35 larvae, H. contortus of 5–11 larvae, M. capillaris of 15–25 larvae) into 1.5 mL centrifugation tubes, 0.1 mL into each, to which then the tested substances were added in five repetitions for each variant of the experiment. In the experiment, we used three concentrations of inorganic food additives: 1%, 0.1% and 0.01%. The exposure lasted 24 h, the temperature was 22 °C;. Then, we counted live and dead (immobile nematodes that had decomposition of the intestine tissue) larvae.
The results were statistically analyzed using a set of Statistica 8.0 (StatSoft Inc., Tulsa, OK, USA). The tables indicate mean value (x) ± standard deviation (SD). Differences between the values of the control and experimental groups were determined using the Tukey test, where the differences were considered significant at p < 0.05.
3. Results
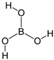
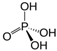
Table 3 demonstrates the results of the study of influence of alkali sodium hydroxide and potassium hydroxide. After 24 h exposure to 1% solutions of those substances, 100% of larvae of all studied species of nematodes died. However, 0.1% concentration of sodium hydroxide caused 100% death of only non-invasive larvae of S. papillosus. Over 72% of larvae of other nematode species, and also invasive larvae of S. papillosus survived the 24 h exposures to this concentration of sodium hydroxide. Lower effects against non-invasive larvae of S. papillosus were exhibited by potassium hydroxide. Moreover, the nematodes were affected by phosphoric acid: 1% solution of this acid decimated 80% of nematode larvae (except invasive larvae of H. contortus, and also S. papillosus, mortality of which did not exceed 5% and 27%, respectively). We observed no casualties among nematode larvae exposed to boric acid in any of the concentrations. At the same time, the lowest susceptibility to alkalis and acids were seen among non-invasive larvae of S. papillosus.

Table 3.
Mortality of larvae of S. papillosus, H. contortus, M. capillaris (%) during 24 h laboratory experiment under the influence of alkalis and acids used as food additives (x ± SD, n = 5).
Similar results were obtained for the use of sodium metabisulfite: 1% solution caused death to over 85% of larvae of S. papillosus, M. capillaris and also over 69% of H. contortus. This inorganic food additive in 0.1% concentration also produced death of 62% of non-invasive larvae of S. papillosus. The rest of the salts of inorganic food additives were less effective compared with alkalis. Among those compounds, sodium sulfate had the strongest effects on S. papillosus and M. capillaris. This substance in 1% solution killed 100% of S. papillosus larvae of different stages and over 66% of first-age M. capillaris larvae. At the same time, 0.1% solution of sodium sulfate had a lethal effect on 73% of non-invasive larvae (first-second ages) of S. papillosus (Table 4).

Table 4.
Mortality of larvae of S. papillosus, H. contortus and M. capillaris (%) during 24 h laboratory experiment under the influence of salts, used as food additives (x ± SD, n = 5).
Over 24 h, copper sulfate pentahydrate in 1% concentration killed all stages of S. papillosus. Ammonium bicarbonate, calcium chloride, sodium nitrite, calcium sulfate, potassium metabisulfite, tetrasodium pyrophosphate, sodium triphosphate in 1% solutions caused death to only non-invasive larvae of S. papillosus. Lower concentrations of those additives had weak effects on survivability of larvae of the studied species of nematodes. Sodium bisulfite and potassium nitrite were effective only against M. capillaris. Their 1% solutions caused death to over 94% of the larvae (Table 4).
4. Discussion
The fight against pathogens of infectious and parasitic diseases that inflict great economic losses on agricultural and livestock farms is underway all around the globe [20,40]. At the same time, agriculture is losing large amounts of meat and dairy products [25,41,42]. There are numerous publications on the use of chemically synthesized substances, nematicides and anthelmintic drugs, which have been accumulating in soil, water bodies and also tissues of living organisms [43,44]. During the migration through food chains, those substances end up in the organisms of animals and contaminate agricultural products [29,30]. Over recent years, alternative substances to combat pests have become broadly distributed: there are compounds that already exist in nature, such as medicinal plants [45,46,47], their essential oils [48,49], and also substances used in the food industry [50,51].
Some inorganic food additives, including alkalis, acids and salts may have negative effects on nematode larvae—parasites of agricultural animals. McSorley and McGovern [52] studied the effects of ammonium bicarbonate on nematode parasitizing Cucurbita pepo L. and Catharanthus roseus (L.) G. Don. According to the results of their studies, use of ammonium biocarbonate led to lower number of phytoparasitic nematodes of Belonolaimus longicaudatus Rau, 1958. In our experiments, ammonium bicarbonate also caused a significant impact on larvae of S. papillosus. Nonetheless, larvae of other species of nematodes (H. contortus, M. capillaris) were more resistant to this substance. This food additive, and also a number of other compounds used in the food industry (sodium nitrite and nitrate) have demonstrated effective fungicidal effects against Fusarium oxysporum f. sp. cucumbrum, F. oxysporum f. sp. niveum and F. oxysporum f. sp. melonis [53]. Aslam et al. [54] and Sun et al. [55] also used ammonium bicarbonate against the rot pathogen of carrot (Daucus carota L.), Pectobacterium carotovorum (Jones, 1901) Waldee, 1945. In those studies, two substances recommended as safe (GRAS) were used: sodium bicarbonate and ammonium bicarbonate. The experiments were carried out on carrots that had been inoculated and infected naturally. Carrots that had not been treated with the studied substances became infected nine days later. Carrots that had been inoculated prior to the treatment with 2% ammonium bicarbonate were observed to have 50% decrease in morbidity after nine days of storage. At the same time, during the storage of carrots, organoleptic parameters and physical–chemical properties of the treated tubers did not change.
Ignatowicz and Pankiewicz-Nowicka [56] studied the effects of inorganic salts on biology and development of Acari, particularly the influence of calcium chloride on fertility and the development of eggs of Acarus siro Linnaeus, 1758. Addition of this substance in 1.5–6.0% amounts to the diet of the Acari led to over 50% decrease in their fertility. The life span of A. siro decreased as well. As alternative compounds against mites Polyphagotarsonemus latus (Banks, 1904) and aphids Myzus persicae (Sulzer, 1776), salts and inorganic acids were used, including boric and ascorbic acids, and also potassium sorbate. Those same substances used in the food industry were studied in field experiments against Acari and aphids infesting potatoes. Populations of these pests were observed to decrease [57,58].
Boric acid is used in spheres other than the food industry. This acid is quite often used against helminths of cattle which parasitize animals’ eyes. According to Karmaliyev et al. [59], solution of boric acid is used in order to wash Thelazia sp. off of the conjunctival sac. To remove helminths of this genus, Singh and Khindria [60] also used 2–3% solution of boric acid. However, boric acid was ineffective at combatting nematode larvae—parasites of the gastrointestinal tract and respiratory system of ruminants—in the environment. Twenty-four-hour exposure of its 1% solution led to death of larvae of all researched species of helminths. Orthophosphate acid is also recorded not only as a food additive, but also as an insecticide [61]. We also observed its action against non-invasive larvae of S. papillosus. However, no effects were observed against other stages of S. papillosus, and also larvae of H. contortus and M. capillaries.
Substances allowed in organic arable farming and processing include many organic food additives. In most cases, use of inorganic chemically synthesized compounds that may be a source of contamination of organic products or environmental objects is prohibited. Potassium metabisulfite belongs to inorganic food additives allowed by the IFOAM Standards Committee (International Federation of Organic Agriculture Movements) to be used in organic arable farming [62]. In our experiment, this substance had the greatest impact on non-invasive stages of S. papillosus: over 80% of first–second stage larvae died when exposed to its 1% solution. Therefore, potassium metabisulfite may be further studied for purposes of combating nematode larvae at early stages of development in the environment of organic farms.
Thus, food additives introduced into the environment together with unused food products of humans or as a result of utilization in various spheres of human activity (in agricultural farms, construction, chemical industries, etc.) cause changes in life of soil stages of the development of parasitic nematode larvae, and also other invertebrates [63]. High concentrations of the substances (1%) we studied are likely to be found in soil only locally, near the places of non-sorted solid municipal wastes. However, some studied substances increase the mortality of nematode larvae even in 0.1% concentrations, indicating significant risk from their use to maintenance of the normal quantity of soil nematodes—normal component of communities of soil invertebrates.
Regarding the hypotheses we formulated in the Introduction, we should emphasize the following. A high occurrence of inorganic substance in the wild did not turn out to be a guarantee of its safety for larvae of parasitic nematodes: for example, 1% concentration of phosphorus acid killed L1–2 of S. papillosus and L1 of M. capillaris (Table 3); 1% concentration of ammonium bicarbonate caused death to L1–2 of S. papillosus; and 0.1% and 1% concentrations of sodium sulfate were lethal to L1–2 and L3 of S. papillosus.
On the other hand, the presumption that substances that do not occur in natural soils could cause death to soil larvae of parasitic nematodes was not found to be correct entirely and in general was not confirmed for such compounds as boric acids (Table 3), sodium thiosulphate (Table 4), which did not increase mortality of any of the three species of nematodes we studied. However, sodium metabisulfite—which does not occur in natural soil—was efficient against L1–2 and L3 of S. papillosus in 1% concentration, as well as L1 of M. capillaris; potassium metabisulfite produced death of L1–2 of S. papillosus in 1% concentrations; copper (II) sulfate pentahydrate, which is not found in natural soils, exerted a lethal effect for L1–2 and L3 of S. papillosus in 100% of the cases, but caused no significant increase in mortality of L1 of M. capillaris and L3 of H. contortus; sodium borate decahydrate in natural soils does not occur as well, but killed no nematode larvae. Substances that do not occur in natural soils and are highly soluble in water can cause death of nematodes or have no effect on their vitality at all.
As with our third hypothesis that pH would poorly affect the survivability of the nematodes, we turned out to be partly right. Alkaline reaction of the environment increased mortality of all the studied species of nematodes in one-percent concentration (for sodium hydroxide and potassium hydroxide in Table 3), whereas acids had a lower effect on survivability of the species of nematodes we studied.
5. Conclusions
Inorganic food additives such as alkalis, acids and salts have various effects on the gastrointestinal tract and respiratory system of ruminants. The strongest effects on survivability of larvae in in vitro conditions were caused by sodium hydroxide, potassium hydroxide and also sodium metabisulfite. Twenty-four-hour exposure to their 1% solutions killed over 69% of larvae of S. papillosus, H. contortus and M. capillaris of various stages of the development. Sodium sulfate was effective against larvae of various ages of S. papillosus, and also first-stage larvae of M. capillaris. Copper sulfate pentahydrate in 1% concentration caused the death of all development stages of S. papillosus. Thus, the results we obtained revealing the strong effect of alkalis on nematode larvae could be of not only scientific but also practical significance for farms in combating parasites of ruminants. The data we obtained are relevant for future ecologic studies of the effects alkalis have on other soil nematodes, structural elements of natural biocenoses.
Author Contributions
Conceptualization, O.B. and V.B.; methodology, O.B.; validation, V.B.; formal analysis, V.B.; investigation, O.B.; resources, O.B. and V.B.; data curation, O.B. and V.B.; writing—original draft preparation, O.B. and V.B.; writing—review and editing, O.B. and V.B.; visualization, O.B. and V.B.; supervision, O.B. and V.B.; project administration, O.B.; funding acquisition, O.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Education and Science of Ukraine, grant number 0120U102384 “Ecological evaluation of use of food and fodder additives in animal husbandry”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Albuquerque, A.; Almeida, F.; Bassetto, C.; Lins, J.; Amarante, A. Influence of breed and parasite challenge on the immune response to naturally acquired intestinal nematode infection in sheep. J. Helminthol. 2022, 96, E27. [Google Scholar] [CrossRef] [PubMed]
- Gudata, D. Prevalence of ovine lung worm at Jucavm open air veterinary clinic, Southwest Ethiopia. Int. J. Res. Inform. Sci. Appl. Techn. 2019, 3, 51–67. [Google Scholar] [CrossRef]
- Gugero, B.C.; Abisso, T.G. Major trematode infections of sheep in Lemo woreda and associated economic loss due to liver condemnation at Hossana Town, Southern Ethiopia. Int. J. Dev. Res. 2019, 9, 25229–25235. [Google Scholar]
- Aboelhadid, S.M.; Arafa, W.M.; El-Ashram, S.; Noaman, A.F.; Shokier, K.A.; Darwish, A.B.; Mahmoud, M.M.; Gadelhaq, S.M. Haemonchus contortus susceptibility and resistance to anthelmintics in naturally infected Egyptian sheep. Acta Parasitol. 2020, 66, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Kießler, J.; Amadesi, A.; Varady, M.; Hinney, B.; Inniello, D.; Maurelli, M.P.; Cringoli, G.; Rinaldi, L. The threat of reduced efficacy of anthelmintics against gastrointestinal nematodes in sheep from an area considered anthelmintic resistance-free. Parasites Vectors 2020, 13, 457. [Google Scholar] [CrossRef]
- Flávia da Silva, F.; Bezerra, H.M.F.F.; Feitosa, T.F.; Vilela, V.L.R. Nematode resistance to five anthelmintic classes in naturally infected sheep herds in Northeastern Brazil. Rev. Bras. De Parasitol. Vet. 2018, 27, 423–429. [Google Scholar] [CrossRef]
- Hennessey, M.; Whatford, L.; Payne-Gifford, S.; Johnson, K.F.; Van Winden, S.; Barling, D.; Häsler, B. Antimicrobial and antiparasitic use and resistance in British sheep and cattle: A systematic review. Prev. Vet. Med. 2020, 185, 105174. [Google Scholar] [CrossRef]
- Boyko, A.A.; Brygadyrenko, V.V. Influence of water infusion of medicinal plants on larvae of Strongyloides papillosus (Nematoda, Strongyloididae). Visn. Dnipropetr. Univ. Biol. Ecol. 2016, 24, 519–525. [Google Scholar] [CrossRef][Green Version]
- Štrbac, F.; Bosco, A.; Maurelli, M.P.; Ratajac, R.; Stojanović, D.; Simin, N.; Orčić, D.; Pušić, I.; Krnjajić, S.; Sotiraki, S.; et al. Anthelmintic properties of essential oils to control gastrointestinal nematodes in sheep—In vitro and in vivo studies. Vet. Sci. 2022, 9, 93. [Google Scholar] [CrossRef]
- Halvarsson, P.; Höglund, J. Sheep nemabiome diversity and its response to anthelmintic treatment in Swedish sheep herds. Parasites Vectors 2021, 14, 114. [Google Scholar] [CrossRef]
- Moon, C.D.; Carvalho, L.; Kirk, M.R.; McCulloch, A.F.; Kittelmann, S.; Young, W.; Janssen, P.H.; Leathwick, D.M. Effects of long-acting, broad spectra anthelmintic treatments on the rumen microbial community compositions of grazing sheep. Sci. Rep. 2021, 11, 3836. [Google Scholar] [CrossRef] [PubMed]
- Genta, R.M. Global prevalence of strongyloidiasis: Critical review with epidemiologic insights into the prevention of disseminated disease. Rev. Infect. Dis. 1989, 11, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Viney, M.E. Exploiting the life cycle of Strongyloides ratti. Parasitol. Today 1999, 15, 231–235. [Google Scholar] [CrossRef]
- Viney, M.E.; Lok, J.B. The biology of Strongyloides spp. WormBook 2015, 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Boyko, O.O.; Gugosyan, Y.A.; Shendryk, L.I.; Brygadyrenko, V.V. Intraspecific morphological variation in free-living stages of Strongyloides papillosus (Nematoda, Strongyloididae) parasitizing various mammal species. Vestn. Zool. 2019, 53, 313–324. [Google Scholar] [CrossRef]
- Page, W.; Judd, J.; Bradbury, R. The unique life cycle of Strongyloides stercoralis and implications for public health action. Trop. Med. Infect. Dis. 2018, 3, 53. [Google Scholar] [CrossRef]
- Gugosyan, Y.A.; Boyko, O.O.; Brygadyrenko, V.V. Morphological variation of four species of Strongyloides (Nematoda, Rhabditida) parasitising various mammal species. Biosyst. Divers. 2019, 27, 85–98. [Google Scholar] [CrossRef]
- Mpofu, T.J.; Nephawe, K.A.; Mtileni, B. Prevalence of gastrointestinal parasites in communal goats from different agro-ecological zones of South Africa. Vet. World 2020, 13, 26–32. [Google Scholar] [CrossRef]
- Romero, A.; García, J.A.; Castells, D.; Gayo, V.; Quintela, F.D. Strongyloidiasis (Strongyloides papillosus) in lambs in Uruguay. Vet. Parasitol. Reg. Stud. Rep. 2022, 31, 100737. [Google Scholar] [CrossRef]
- Naeem, M.; Iqbal, Z.; Roohi, N. Ovine haemonchosis: A review. Trop. Anim. Health Prod. 2021, 53, 19. [Google Scholar] [CrossRef]
- Hassan, E.E.A.M. Extraintestinal helminths. In Infectious Diseases of Dromedary Camels; Khalafalla, A.I., Hussein, M.F., Eds.; Springer: Cham, Switzerland, 2021; pp. 257–261. [Google Scholar] [CrossRef]
- Hassan, E.E.A.M. Gastrointestinal helminths (haemonchosis). In Infectious Diseases of Dromedary Camels; Khalafalla, A.I., Hussein, M.F., Eds.; Springer: Cham, Switzerland, 2021; pp. 245–255. [Google Scholar] [CrossRef]
- Soli, F.; Terrill, T.H.; Shaik, S.A.; Getz, W.R.; Miller, J.E.; Vanguru, M.; Burke, J.M. Efficacy of copper oxide wire particles against gastrointestinal nematodes in sheep and goats. Vet. Parasitol. 2010, 168, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Boyko, O.O.; Brygadyrenko, V.V. The viability of Haemonchus contortus (Nematoda, Strongylida) and Strongyloides papillosus (Nematoda, Rhabditida) larvae exposed to various flavourings and source materials used in food production. Vestn. Zool. 2019, 53, 433–442. [Google Scholar] [CrossRef]
- Hassan, N.M.F.; Zaghawa, A.A.; Abu-Elezz, N.M.T.; Nayel, M.A.; Salama, A.A. Efficacy of some Egyptian native plant extracts against Haemonchus contortus in vitro and in experimentally infected sheep along with the associated haematological and biochemical alterations. Bull. Natl. Res. Cent. 2021, 45, 180. [Google Scholar] [CrossRef]
- Rose, J.H. Observations on the larval stages of Muellerius capillaris within the intermediate hosts Agriolimax agrestis and A. reticulatus. J. Helminthol. 1957, 31, 1–16. [Google Scholar] [CrossRef]
- de Macedo, L.O.; Lima, T.A.R.F.; Verocai, G.G.; Alves, L.C.; de Carvalho, G.A.; Ramos, R.A.N. Lungworms in ruminants from Brazil: A retrospective epidemiological study over four decades. Vet. Parasitol. Reg. Stud. Rep. 2021, 26, 100645. [Google Scholar] [CrossRef]
- Rose, J. Site of development of the lungworm Muellerius capillaris in experimentally infected lambs. J. Comp. Pathol. Ther. 1958, 68, 359–362. [Google Scholar] [CrossRef]
- Martynov, V.O.; Brygadyrenko, V.V. The influence of synthetic food additives and surfactants on the body weight of larvae of Tenebrio molitor (Coleoptera, Tenebrionidae). Biosyst. Divers. 2017, 25, 236–242. [Google Scholar] [CrossRef]
- Martynov, V.O.; Brygadyrenko, V.V. The influence of the synthetic food colourings tartrazine, allura red and indigo carmine on the body weight of Tenebrio molitor (Coleoptera, Tenebrionidae) larvae. Regul. Mech. Biosyst. 2018, 9, 479–484. [Google Scholar] [CrossRef]
- Boyko, O.O.; Brygadyrenko, V.V. The impact of acids approved for use in foods on the vitality of Haemonchus contortus and Strongyloides papillosus (Nematoda) larvae. Helminthologia 2019, 56, 202–210. [Google Scholar] [CrossRef]
- Branen, A.L.; Davidson, P.M.; Salminen, S.; Thorngate, J. Food Additives; Marcel Dekker Inc.: New York, NY, USA, 2002; 938p. [Google Scholar]
- Harun, I.G.; Benson, E.M.; Benjamin, O.D. Effect of lime and goat manure on soil acidity and maize (Zea mays) growth parameters at Kavutiri, Embu County—Central Kenya. J. Soil Sci. Environ. Manag. 2015, 6, 275–283. [Google Scholar] [CrossRef]
- Osuhor, C.U.; Alawa, J.P.; Akpa, G.N. Research note: Manure production by goats grazing native pasture in Nigeria. Trop. Grassl. 2002, 36, 123–125. [Google Scholar]
- Wuta, M.; Nyamugafata, P. Management of cattle and goat manure in Wedza smallholder farming area, Zimbabwe. Afr. J. Agric. Res. 2012, 7, 3853–3859. [Google Scholar] [CrossRef]
- Touray, N.; Tsai, W.T.; Chen, H.R.; Liu, S.C. Thermochemical and pore properties of goat-manure-derivedbiochars prepared from different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2014, 109, 116–122. [Google Scholar] [CrossRef]
- Zajac, A.M.; Conboy, G.A. Veterinary Clinical Parasitology, 8th ed.; Willey-Blackwell: New York, NY, USA, 2011; 354p. [Google Scholar]
- Van Wyk, A.; Cabaret, J.; Michael, L.M. Morphological identification of nematode larvae of small ruminants and cattle simplified. Vet. Parasitol. 2004, 119, 277–306. [Google Scholar] [CrossRef]
- Van Wyk, J.A.; Mayhew, E. Morphological identifcation of parasitic nematode infective larvae of small ruminants and cattle: A practical lab guide. Onderstepoort J. Vet. Res. 2013, 80, 1–14. [Google Scholar] [CrossRef]
- Suresh, K.P.; Sengupta, P.P.; Jacob, S.S.; Sathyanarayana, M.K.G.; Patil, S.S.; Swarnkar, C.P.; Singh, D. Exploration of machine learning models to predict the environmental and remote sensing risk factors of haemonchosis in sheep flocks of Rajasthan, India. Acta Trop. 2022, 233, 106542. [Google Scholar] [CrossRef]
- Arsenopoulos, K.V.; Fthenakis, G.C.; Katsarou, E.I.; Papadopoulos, E. Haemonchosis: A challenging parasitic infection of sheep and goats. Animals 2021, 11, 363. [Google Scholar] [CrossRef]
- Rahmani, M.M. Study of helminths in sheep in the Laghouat region in Southern Algeria. Vet. Stanica 2023, 54, 59–67. [Google Scholar] [CrossRef]
- Chen, S.; Gan, Z.; Li, Z.; Li, Y.; Ma, X.; Chen, M.; Qu, B.; Ding, S.; Su, S. Occurrence and risk assessment of anthelmintics in Tuojiang River in Sichuan, China. Ecotoxicol. Environ. Saf. 2021, 220, 112360. [Google Scholar] [CrossRef]
- Langhansová, L.; Navrátilová, M.; Skálová, L.; Moťková, K.; Podlipná, R. The effect of the manure from sheep treated with anthelmintics on clover (Trifolium pratense). Agronomy 2021, 11, 1892. [Google Scholar] [CrossRef]
- Boyko, O.O.; Brygadyrenko, V.V. Nematocidial activity of aqueous solutions of plants of the families Cupressaceae, Rosaceae, Asteraceae, Fabaceae, Cannabaceae and Apiaceae. Biosyst. Divers. 2019, 27, 227–232. [Google Scholar] [CrossRef]
- Komáromyová, M.; Mravčáková, D.; Petrič, D.; Kucková, K.; Babják, M.; Dolinská, M.U.; Königová, A.; Maďarová, M.; Pruszyńska-Oszmałek, E.; Cieslak, A.; et al. Effects of medicinal plants and organic selenium against ovine haemonchosis. Animals 2021, 11, 1319. [Google Scholar] [CrossRef] [PubMed]
- Boyko, O.O.; Kabar, A.M.; Brygadyrenko, V.V. Nematicidal activity of aqueous tinctures of medicinal plants against larvae of the nematodes Strongyloides papillosus and Haemonchus contortus. Biosyst. Divers. 2020, 28, 119–123. [Google Scholar] [CrossRef]
- Martynov, V.O.; Titov, O.G.; Kolombar, T.M.; Brygadyrenko, V.V. Influence of essential oils of plants on the migration activity of Tribolium confusum (Coleoptera, Tenebrionidae). Biosyst. Divers. 2019, 27, 177–185. [Google Scholar] [CrossRef]
- Boyko, O.; Shendryk, L.; Shaban, O.; Brygadyrenko, V. Influence of essential oils on sporulation of Eimeria magna oocysts. Ann. Parasitol. 2021, 67, 11–17. [Google Scholar] [CrossRef]
- Boyko, O.O.; Brygadyrenko, V.V. The impact of certain flavourings and preservatives on the survivability of larvae of nematodes of Ruminantia. Regul. Mech. Biosyst. 2018, 9, 118–123. [Google Scholar] [CrossRef]
- Boyko, O.O.; Brygadyrenko, V.V. The impact of certain flavourings and preservatives on the survivability of eggs of Ascaris suum and Trichuris suis. Regul. Mech. Biosyst. 2020, 11, 344–348. [Google Scholar] [CrossRef]
- McSorley, R.; McGovern, R.J. Effects of solarization and ammonium amendments on plant-parasitic nematodes. J. Nematol. 2000, 32, 537–541. [Google Scholar]
- Li, S.; Song, S.; XuHui, D.; YiFei, S.; ChunYan, W.; NaNa, L.; Rong, L.; QiRong, S. Inhibition mechanism of ammonium bicarbonate on Fusarium oxysporum. J. Nanjing Agric. Univ. 2015, 38, 295–303. [Google Scholar]
- Aslam, H.D.M.; Ratnayake, R.M.R.N.K.; Jayawardana, N.W.I.A.; Thilakarathne, B.M.K.S.; Ginigaddara, G.A.S. Effect of sodium and ammonium bicarbonate in managing soft rot pathogen (Erwinia carotovora) of carrot (Daucus carota). In Proceedings of the International Symposium on Agriculture and Environment, Matara, Sri Lanka, 29 November 2012; pp. 289–292. [Google Scholar]
- Sun, L.; Song, S.; Fu, L.; Deng, X.; Wang, D.; Liang, X.; Li, R.; Shen, Q. Exploring a soil fumigation strategy based on ammonium bicarbonate to control Fusarium wilts of cucurbits. Crop Prot. 2015, 70, 53–60. [Google Scholar] [CrossRef]
- Ignatowicz, S.; Pankiewicz-Nowicka, D. Effect of inorganic salts upon biology and development of acarid mites. VI. Effect of tricalcium phosphate and calcium chloride surplus in food upon fecundity, life span and egg viability of flour mite, Acarus siro L. (Acarina, Acaridae). Pol. Pismo Entomol. 1980, 50, 541–546. [Google Scholar]
- Nour El-Deen, M.A.; Abo-Zid, A.E.; Azouz, H.A. Use of some environmentally safe materials as alternatives to the chemical pesticides in controlling Polyphagotarsonimus lauts (Banks) mite & Myzus persica (Koch) aphid which attack potatoes crop. Middle East J. Agric. Res. 2014, 3, 32–41. [Google Scholar]
- Nassar, A.M.K. Pesticide alternatives use in Egypt: The concept and potential. In Sustainability of Agricultural Environment in Egypt: Part II. The Handbook of Environmental Chemistry; Negm, A., Abu-hashim, M., Eds.; Springer: Cham, Switzerland, 2018; Volume 77. [Google Scholar] [CrossRef]
- Karmaliyev, R.S.; Ussenov, Z.T.; Sidikhov, B.M.; Yertleuova, B.O.; Gabdullin, D.E.; Akhmedenov, K.M. Epizootic monitoring for helminthoses in cattle in the West Region of Kazakhstan. Adv. Anim. Vet. Sci. 2020, 8, 56–62. [Google Scholar] [CrossRef]
- Singh, K.; Khindria, A. First case of human ocular thelaziasis from India caused by Thelazia californiensis: A case report. IOSR J. Dent. Med. Sci. 2018, 17, 24–27. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F. Current-use pesticides. In Bioremediation of Agricultural Soils; Sanches-Hernandez, J.C., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 3–29. [Google Scholar] [CrossRef]
- Schmid, O.; Beck, A.; Baker, B. Comparison of Materials Standards for Organic Food; Organic Materials Review Institute: Eugene, OR, USA, 2003. [Google Scholar]
- Menezes-Oliveira, V.; Loureiro, S.; Amorim, M.J.B.; Wrona, F.; Soares, A.M.V.M. Hazard assessment of the veterinary pharmaceuticals monensin and nicarbazin using a soil test battery. Environ. Toxicol. Chem. 2018, 37, 3145–3153. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).