Effect of Plant Growth Promoting Microorganisms on Pepper Plants Infected with Tomato Brown Rugose Fruit Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Inoculation by Beneficial Microorganisms
2.2. Virus Inoculum Preparation, Inoculation on Pepper Plants and Symptoms Evaluation
2.3. Plant Sampling, Detection of Virus by Real-Time qPCR
2.4. Staining of Roots, Fluorescence In Situ Hybridization and Microscopy Detection and Evaluation of Colonization by AMF and Azospirillum Bacterium
2.5. Statistical Analysis
3. Results
3.1. Symptoms Evaluation
3.2. Results of Real-Time qPCR Assay
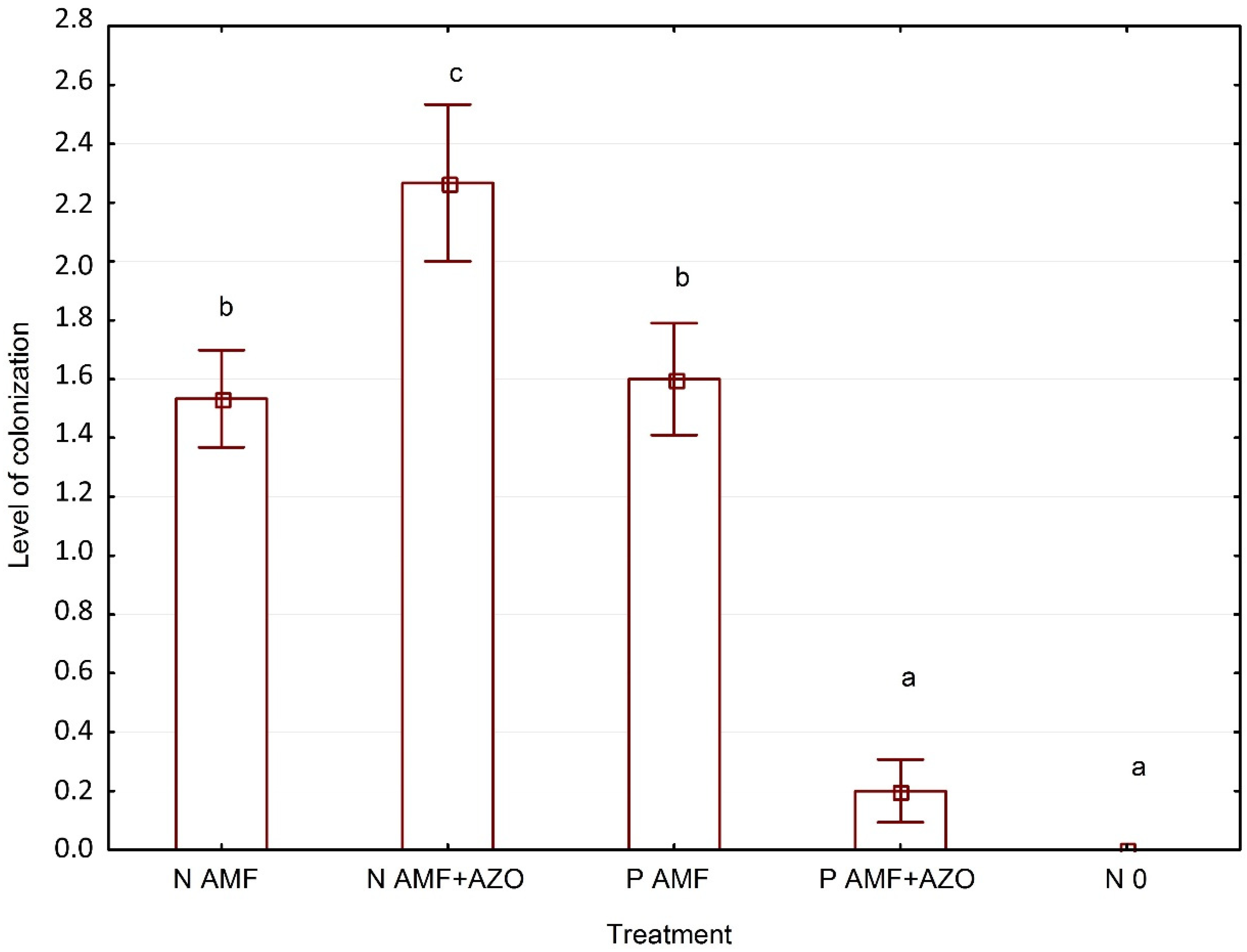
3.3. Evaluation of Funneliformis Mosseae and Azospirillum Brasilense Root System Colonization
4. Discussion
4.1. ToBRFV Symptoms Emergence and Real-Time qPCR Assay Results
4.2. Funneliformis mosseae and Azospirillum brasilense Root System Colonization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. FAOSTAT. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 December 2021).
- ÚKZÚZ. Tomato Brown Rugose Fruit Virus (ToBRFV). Rostlinolékařský Portál. 2021. Available online: https://eagri.cz/public/app/srs_pub/fytoportal/public/?key=%2218c5e01b52e298fc5d46cc452f76b389%22#rlp|so|choroby|detail:18c5e01b52e298fc5d46cc452f76b389|popis (accessed on 1 December 2021).
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A.; et al. A New Israeli Tobamovirus Isolate Infects Tomato Plants Harboring Tm-22 Resistance Genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panno, S.; Caruso, A.; Davino, S. First Report of Tomato Brown Rugose Fruit Virus on Tomato Crops in Italy. Plant Dis. 2019, 103, 1443. [Google Scholar] [CrossRef]
- Menzel, W.; Knierim, D.; Winter, S.; Hamacher, J.; Heupel, M. First report of Tomato brown rugose fruit virus infecting tomato in Germany. New Dis. Rep. 2019, 39, 2044–2588. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Beltrán, E.; Pérez-Villarreal, A.; Leyva-López, N.; Rodríguez-Negrete, E.; Ceniceros-Ojeda, E.; Méndez-Lozano, J. Occurrence of Tomato brown rugose fruit virus Infecting Tomato Crops in Mexico. Plant Dis. 2019, 103, 1440. [Google Scholar] [CrossRef]
- Alkowni, R.; Alabdallah, O.; Fadda, Z. Molecular identification of tomato brown rugose fruit virus in tomato in Palestine. J. Plant Pathol. 2019, 101, 719–723. [Google Scholar] [CrossRef]
- Ling, K.S.; Tian, T.; Gurung, S.; Salati, R.; Gilliard, A. First report of Tomato brown rugose fruit virus infecting greenhouse tomato in the United States. Plant Dis. 2019, 103, 1439. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, H.; Han, S.; Geng, C.; Tian, Y.; Li, X. First Report of Tomato brown rugose fruit virus Infecting Tomato in China. Plant Dis. 2019, 103, 2973. [Google Scholar] [CrossRef]
- Alfaro-Fernández, A.; Castillo, P.; Sanahuja, E.; Rodríguez-Salido, M.; Font, M. First Report of Tomato Brown Rugose Fruit Virus in Tomato in Spain. Plant Dis. 2021, 105, 515. [Google Scholar] [CrossRef]
- Dey, K.; Velez-Climent, M.; Soria, P.; Batuman, O.; Mavrodieva, V.; Wei, G.; Zhou, J.; Adkins, S.; McVay, J. First report of Tomato brown rugose fruit virus infecting tomato in Florida, USA. New Dis. Rep. 2021, 44, e12028. [Google Scholar] [CrossRef]
- Sabra, A.; Al-Saleh, M.; Al-Shahwan, I.; Amer, M. First Report of Tomato Brown Rugose Fruit Virus Infecting the Tomato Crop in Saudi Arabia. Plant Dis. 2022, 106, 1310. [Google Scholar] [CrossRef] [PubMed]
- Tomato brown rugose fruit virus. EPPO Bull. 2020, 50, 529–534. Available online: https://gd.eppo.int/taxon/TOBRFV/datasheet (accessed on 11 December 2020). [CrossRef]
- Hao, L.; Zhang, Z.; Hao, B.; Diao, F.; Zhang, J.; Bao, Z.; Guo, W. Arbuscular mycorrhizal fungi alter microbiome structure of rhizosphere soil to enhance maize tolerance to La. Ecotox. Environ. Saf. 2021, 212, 111996. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.; Paulitz, T.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2008, 321, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Pokluda, R.; Ragasová, L.; Jurica, M.; Kalisz, A.; Komorowska, M.; Niemiec, M.; Sekara, A. Effects of growth promoting microorganisms on tomato seedlings growing in different media conditions. PLoS ONE 2021, 16, e0259380. [Google Scholar] [CrossRef]
- Fukami, J.; Nogueira, M.; Araujo, R.; Hungria, M. Accessing inoculation methods of maize and wheat with Azospirillum brasilense. AMB Express 2016, 6, 3. [Google Scholar] [CrossRef]
- Jung, S.; Martinez-Medina, A.; Lopez-Raez, J.; Pozo, M. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Miozzi, L.; Vaira, A.; Brilli, F.; Casarin, V.; Berti, M.; Ferrandino, A.; Nerva, L.; Accotto, G.; Lanfranco, L. Arbuscular Mycorrhizal Symbiosis Primes Tolerance to Cucumber Mosaic Virus in Tomato. Viruses 2020, 12, 675. [Google Scholar] [CrossRef]
- Lozano-Durán, R.; Rosas-Díaz, T.; Gusmaroli, G.; Luna, A.; Taconnat, L.; Deng, X.; Bejarano, E. Geminiviruses Subvert Ubiquitination by Altering CSN-Mediated Derubylation of SCF E3 Ligase Complexes and Inhibit Jasmonate Signaling in Arabidopsis thaliana. Plant Cell 2011, 23, 1014–1032. [Google Scholar] [CrossRef] [Green Version]
- Maffei, G.; Miozzi, L.; Fiorilli, V.; Novero, M.; Lanfranco, L.; Accotto, G. The arbuscular mycorrhizal symbiosis attenuates symptom severity and reduces virus concentration in tomato infected by Tomato yellow leaf curl Sardinia virus (TYLCSV). Mycorrhiza 2013, 24, 179–186. [Google Scholar] [CrossRef]
- Stolyarchuk, I.M.; Shevchenko, T.P.; Polischuk, V.P.; Kripka, A.V. Virus infection course in different plant species under influenceof arbuscular mycorrhiza. Microbiol. Biotechnol. 2009, 6, 70–75. [Google Scholar]
- Thiem, D.; Szmidt-Jaworska, A.; Baum, C.; Muders, K.; Niedojadło, K.; Hrynkiewicz, K. Interactive physiological response of potato (Solanum tuberosum L.) plants to fungal colonization and Potato virus Y (PVY) infection. Acta Mycol. 2014, 49, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Miozzi, L.; Catoni, M.; Fiorilli, V.; Mullineaux, P.; Accotto, G.; Lanfranco, L. Arbuscular Mycorrhizal Symbiosis Limits Foliar Transcriptional Responses to Viral Infection and Favors Long-Term Virus Accumulation. Mol. Plant-Microbe Interact. 2011, 24, 1562–1572. [Google Scholar] [CrossRef] [Green Version]
- Shaul, O.; Galili, S.; Volpin, H.; Ginzberg, I.; Elad, Y.; Chet, I.; Kapulnik, Y. Mycorrhiza-Induced Changes in Disease Severity and PR Protein Expression in Tobacco Leaves. Mol. Plant-Microbe Interact. 1999, 12, 1000–1007. [Google Scholar] [CrossRef] [Green Version]
- Pii, Y.; Aldrighetti, A.; Valentinuzzi, F.; Mimmo, T.; Cesco, S. Azospirillum brasilense inoculation counteracts the induction of nitrate uptake in maize plants. J. Exp. Bot. 2019, 70, 1313–1324. [Google Scholar] [CrossRef]
- Chanda, B.; Shamimuzzaman, M.; Gilliard, A.; Ling, K. Effectiveness of disinfectants against the spread of tobamoviruses: Tomato brown rugose fruit virus and Cucumber green mottle mosaic virus. Virol. J. 2021, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Jewehan, A.; Salem, N.; Tóth, Z.; Salamon, P.; Szabó, Z. Screening of Solanum (sections Lycopersicon and Juglandifolia) germplasm for reactions to the tomato brown rugose fruit virus (ToBRFV). J. Plant Dis. Prot. 2022, 129, 117–123. [Google Scholar] [CrossRef]
- Eichmeier, A.; Baránek, M.; Pidra, M. Analysis of genetic diversity and phylogeny of partial coat protein domain in Czech and Italian GFLV isolates. Plant Prot. Sci. 2010, 46, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.; Schmittgen, T. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Menzel, W.; Winter, S. Identification of novel and known tobamoviruses in tomato and other solanaceous crops using a new pair of generic primers and development of a specific RT-qPCR for ToBRFV. Acta Hortic. 2021, 1316, 143–148. [Google Scholar] [CrossRef]
- Christensen, N.; Nicolaisen, M.; Hansen, M.; Schulz, A. Distribution of Phytoplasmas in Infected Plants as Revealed by Real-Time PCR and Bioimaging. Mol. Plant-Microbe Interact. 2004, 17, 1175–1184. [Google Scholar] [CrossRef] [Green Version]
- Johansen, D. Plant Microtechnique; McGraw Hill: New York, NY, USA, 1940. [Google Scholar]
- Vierheilig, H.; Schweiger, P.; Brundrett, M. An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol. Plant. 2005, 125, 393–404. [Google Scholar] [CrossRef]
- Stoffels, M.; Castellanos, T.; Hartmann, A. Design and Application of New 16S rRNA-targeted Oligonucleotide Probes for the Azospirillum-Skermanella-Rhodocista-Cluster. Syst. Appl. Microbiol. 2001, 24, 83–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neef, A.; Amann, R.; Schlesner, H.; Schleifer, K. Monitoring a widespread bacterial group: In situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology 1998, 144, 3257–3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alarcón, C.; Cuenca, G. Arbuscular mycorrhizas in coastal sand dunes of the Paraguanä Peninsula, Venezuela. Mycorrhiza 2005, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Daft, M.; Okusanya, B. Effect of Endogone Mycorrhiza on Plant Growth V. Influence of Infection on the Multiplication of Viruses in Tomato, Petunia and Strawberry. New Phytol. 1973, 72, 975–983. [Google Scholar] [CrossRef]
- Hao, Z.; Xie, W.; Chen, B. Arbuscular Mycorrhizal Symbiosis Affects Plant Immunity to Viral Infection and Accumulation. Viruses 2019, 11, 534. [Google Scholar] [CrossRef] [Green Version]
- Borer, E.; Seabloom, E.; Mitchell, C.; Power, A. Local context drives infection of grasses by vector-borne generalist viruses. Ecol. Lett. 2010, 13, 810–818. [Google Scholar] [CrossRef]
- Lima, A.; Venturoso, L.; Silva, B.; Gomes, A.; Schimidt, O. Eficiência da inoculação de Azospirillum brasilense associado com enraizador no crescimento e na produção de alface. Rev. Verde Agroecol. Desenvol. Sustent. 2017, 12, 233. [Google Scholar] [CrossRef]
- Al-Ani, R.A.; Adhab, M.A.; El-Muadhidi, M.A.; Al-Fahad, M.A. Induced systemic resistance and promotion of wheat and barley plants growth by biotic and non-biotic agents against barley yellow dwarf virus. Afr. J. Biotechnol. 2011, 10, 12078–12084. [Google Scholar]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.; Anli, M.; Meddich, A.; Oufdou, K. Use of Rhizobacteria and Mycorrhizae Consortium in the Open Field as a Strategy for Improving Crop Nutrition, Productivity and Soil Fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zsögön, A.; Lambais, M.; Benedito, V.; Figueira, A.; Peres, L. Reduced arbuscular mycorrhizal colonization in tomato ethylene mutants. Sci. Agric. 2008, 65, 259–267. [Google Scholar] [CrossRef]
- Santoyo, G.; Gamalero, E.; Glick, B. Mycorrhizal-Bacterial Amelioration of Plant Abiotic and Biotic Stress. Front. Sustain. Food Syst. 2021, 5, 672881. [Google Scholar] [CrossRef]
- Aseel, D.; Rashad, Y.; Hammad, S. Arbuscular Mycorrhizal Fungi Trigger Transcriptional Expression of Flavonoid and Chlorogenic Acid Biosynthetic Pathways Genes in Tomato against Tomato Mosaic Virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef] [Green Version]

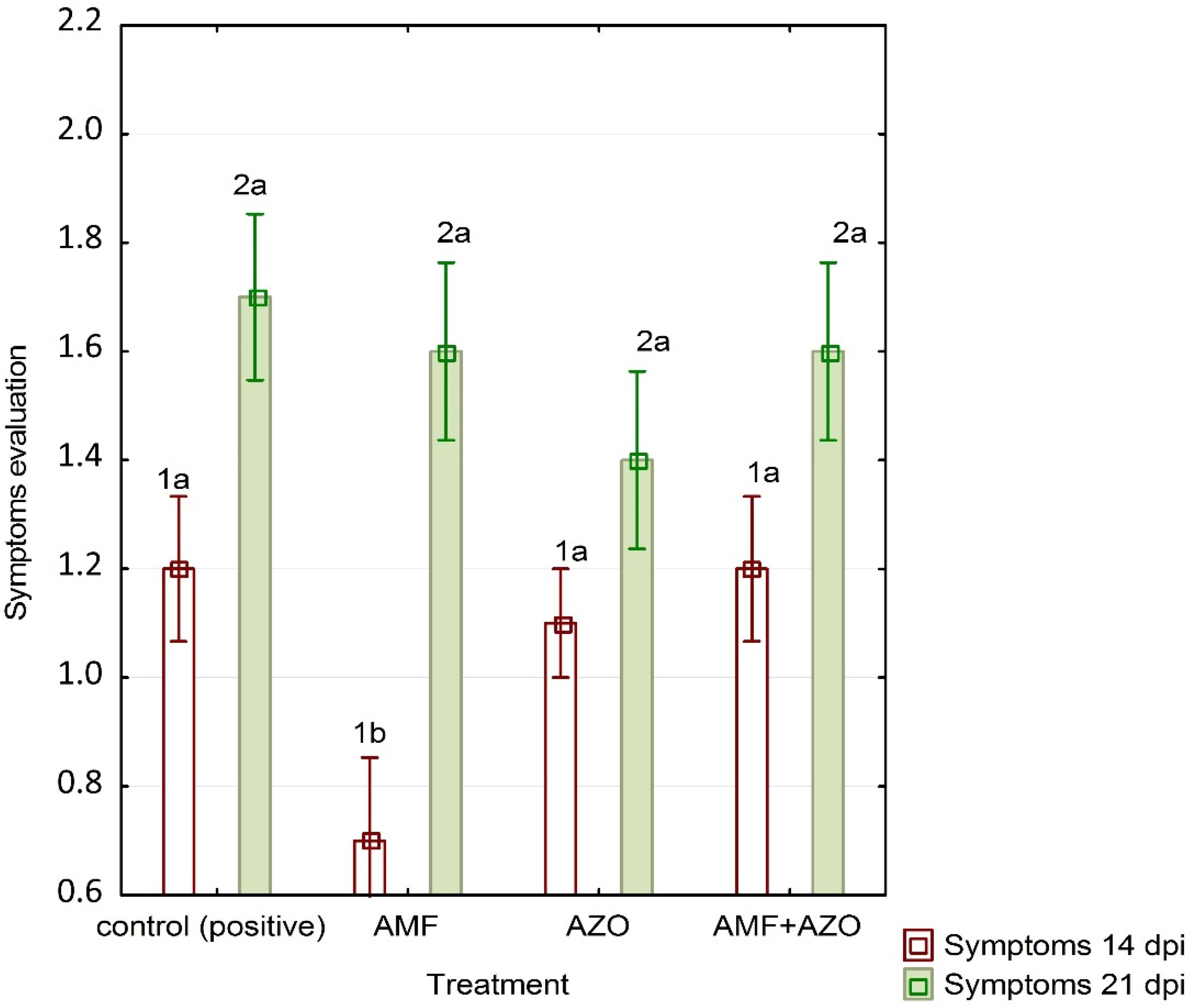

| Scale | Symptoms |
|---|---|
| 0 | No symptoms |
| 1 | Mild mosaic or mottling, followed by recovery |
| 2 | Mild mosaic or mottling with leaf deformation |
| 3 | Moderate mosaic or mottling and leaf deformation, followed by rolling, necrotic spots and stunting |
| 4 | Severe mosaic or mottling and leaf deformity, extended necrotic spots |
| 5 | Severe mosaic or mottling, leaf deformity, shoestring |
| Value | Proportion of Root Colonized by Funneliformis mosseae/Azospirillum brasilense |
|---|---|
| 0 | Without colonization |
| 1 | Colonization trace |
| 2 | Less than 10% |
| 3 | From 11 to 50% |
| 4 | From 51% to 90% |
| 5 | More than 90% |
| LSD Test; Variable Mean Ct (qPCR), Homogenous Groups, Alfa = 0.05000 Error: Mean Sum of sq. = 9.8032, Degrees of Freedom = 16.000 | ||
|---|---|---|
| Treatment | Mean Ct (Mean) | 1 |
| P AMF | 13.33800 | **** |
| Control (positive) | 13.39800 | **** |
| P AMF + AZO | 14.11600 | **** |
| P AZO | 17.27400 | **** |
| Colonization intensity (M) by Azospirillum | |||||
| Treatment | N AZO | N AMF + AZO | P AZO | P AMF + AZO | control (negative) |
| Intensity of colonization (M) | 20% | 28% | 41% | 33% | 0.4% |
| Colonization intensity (M) by funneliformis mosseae | |||||
| Treatment | N AMF | N AMF + AZO | P AMF | P AMF + AZO | control (negative) |
| Intensity of colonization (M) | 4.5% | 19.3% | 6.2% | 0.2% | 0.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragasová, L.; Hakalová, E.; Ferby, V.; Čechová, J.; Klapcová, G.; Pokluda, R. Effect of Plant Growth Promoting Microorganisms on Pepper Plants Infected with Tomato Brown Rugose Fruit Virus. Diversity 2022, 14, 635. https://doi.org/10.3390/d14080635
Ragasová L, Hakalová E, Ferby V, Čechová J, Klapcová G, Pokluda R. Effect of Plant Growth Promoting Microorganisms on Pepper Plants Infected with Tomato Brown Rugose Fruit Virus. Diversity. 2022; 14(8):635. https://doi.org/10.3390/d14080635
Chicago/Turabian StyleRagasová, Lucia, Eliška Hakalová, Vojtěch Ferby, Jana Čechová, Gabriela Klapcová, and Robert Pokluda. 2022. "Effect of Plant Growth Promoting Microorganisms on Pepper Plants Infected with Tomato Brown Rugose Fruit Virus" Diversity 14, no. 8: 635. https://doi.org/10.3390/d14080635
APA StyleRagasová, L., Hakalová, E., Ferby, V., Čechová, J., Klapcová, G., & Pokluda, R. (2022). Effect of Plant Growth Promoting Microorganisms on Pepper Plants Infected with Tomato Brown Rugose Fruit Virus. Diversity, 14(8), 635. https://doi.org/10.3390/d14080635






