Vertical Distribution of Beetles (Coleoptera) in Pine Forests in Central European Russia
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Sampling Procedures
2.3. Identification and Taxonomic Status of Samples
2.4. Data Analysis
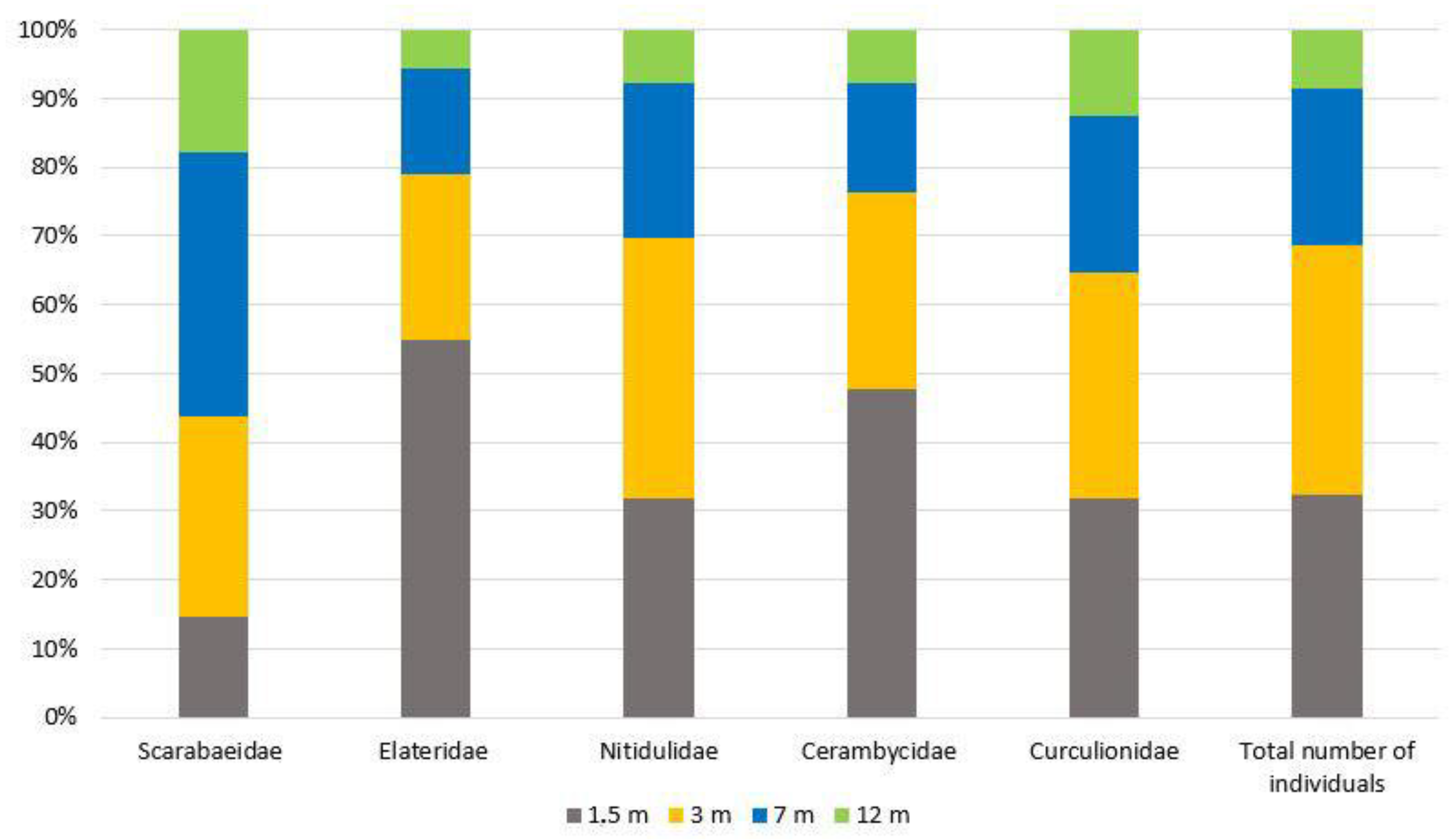
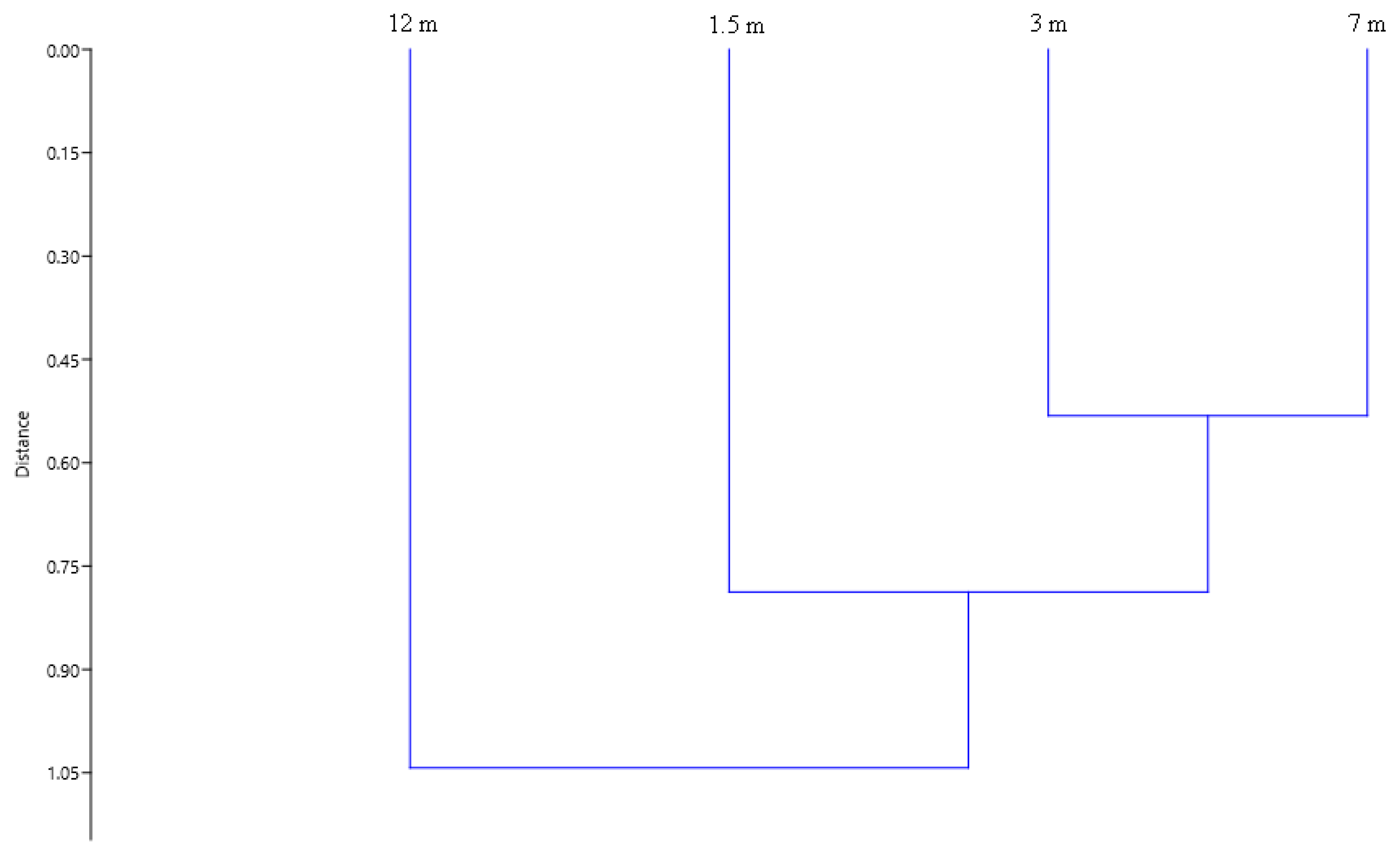
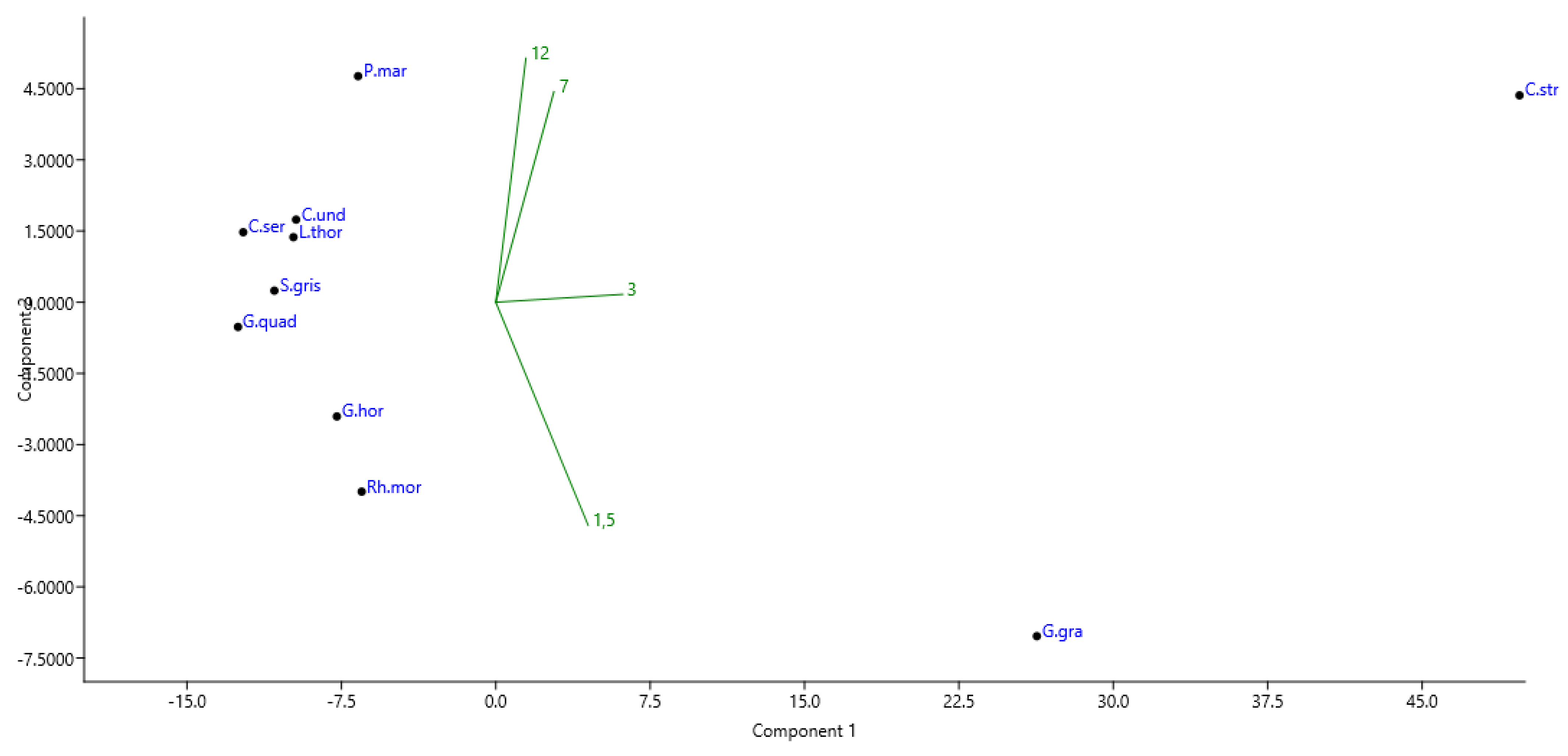
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Taxon Name | 1.5 m | 3 m | 7 m | 12 m | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | M | O | T | M | O | T | M | O | T | M | O | |
| Carabidae | ||||||||||||
| Calathus micropterus (Duftschmid, 1812) | 1 | 0.02 | 2.27 | |||||||||
| Harpalus signaticornis (Duftschmid, 1812) | 1 | 0.02 | 2.27 | |||||||||
| Histeridae | ||||||||||||
| Gnathoncus buyssoni Auzat, 1917 | 22 | 0.50 | 36.36 | 16 | 0.36 | 29.55 | 16 | 0.36 | 18.18 | 8 | 0.18 | 11.36 |
| Margarinotus striola (C.R. Sahlberg, 1819) | 24 | 0.54 | 22.73 | 1 | 0.02 | 2.27 | ||||||
| Platysoma deplanatum (Gyllenhal, 1808) | 2 | 0.05 | 4.55 | 2 | 0.05 | 4.55 | ||||||
| Platysoma elongatum (Thunberg, 1787) | 17 | 0.39 | 27.27 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | |||
| Platysoma lineare Erichson, 1834 | 20 | 0.45 | 27.27 | 2 | 0.05 | 4.55 | ||||||
| Silphidae | ||||||||||||
| Necrodes littoralis (Linnaeus, 1758) | 7 | 0.16 | 13.64 | 6 | 0.14 | 9.09 | 4 | 0.09 | 9.09 | |||
| Nicrophorus vespilloides Herbst, 1783 | 3 | 0.07 | 2.27 | |||||||||
| Oiceoptoma thoracicum (Linnaeus, 1758) | 12 | 0.27 | 18.18 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | |||
| Staphylinidae | ||||||||||||
| Staphylinidae sp. | 775 | 17.61 | 95.45 | 291 | 6.61 | 79.55 | 151 | 3.43 | 75.00 | 22 | 0.50 | 29.55 |
| Euplectus sp. | 1 | 0.02 | 2.27 | |||||||||
| Quedius dilatatus (Fabricius, 1787) | 5 | 0.11 | 6.82 | 22 | 0.50 | 15.91 | 15 | 0.34 | 13.63 | 2 | 0.05 | 4.55 |
| Scarabaeidae | ||||||||||||
| Cetonia aurata (Linnaeus, 1758) | 3 | 0.07 | 6.82 | 4 | 0.09 | 9.09 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 |
| Gnorimus variabilis (Linnaeus, 1758) | 2 | 0.05 | 2.27 | 3 | 0.07 | 4.55 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 |
| Oxythyrea funesta (Poda von Neuhaus, 1761) | 1 | 0.02 | 2.27 | |||||||||
| Protaetia fieberi (Kraatz, 1880) | 4 | 0.09 | 9.09 | 4 | 0.09 | 9.09 | 6 | 0.14 | 9.09 | |||
| Protaetia marmorata (Fabricus, 1792) | 100 | 2.27 | 68.18 | 219 | 4.98 | 68.18 | 307 | 6.98 | 79.55 | 139 | 3.16 | 72.73 |
| Potosia cuprea volhyniensis (Gory & Percheron, 1833) | 14 | 0.32 | 22.73 | 12 | 0.27 | 25.00 | 5 | 0.11 | 11.36 | |||
| Scirtidae | ||||||||||||
| Contacyphon sp. | 1 | 0.02 | 2.27 | |||||||||
| Contacyphon padi (Linnaeus, 1758) | 1 | 0.02 | 2.27 | |||||||||
| Contacyphon pubescens (Fabricius, 1792) | 1 | 0.02 | 2.27 | |||||||||
| Throscidae | ||||||||||||
| Trixagus sp. | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | ||||||
| Elateridae | ||||||||||||
| Agrypnus murinus (Linnaeus, 1758) | 2 | 0.05 | 4.55 | |||||||||
| Ampedus balteatus (Linnaeus, 1758) | 5 | 0.11 | 9.09 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | |||
| Ampedus cinnabarinus (Eschscholtz, 1829) | 1 | 0.02 | 2.27 | |||||||||
| Ampedus nigroflavus (Goeze, 1777) | 2 | 0.05 | 4.55 | 2 | 0.05 | 4.55 | ||||||
| Ampedus pomorum (Herbst, 1784) | 2 | 0.05 | 4.55 | 3 | 0.07 | 6.82 | ||||||
| Ampedus praeustus (Fabricius, 1792) | 1 | 0.02 | 2.27 | |||||||||
| Athous subfuscus (O.F. Müller, 1764) | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | ||||||
| Dalopius marginatus (Linnaeus, 1758) | 1 | 0.02 | 2.27 | 4 | 0.09 | 4.55 | 3 | 0.07 | 4.55 | 1 | 0.02 | 2.27 |
| Denticollis borealis (Paykull, 1800) | 1 | 0.02 | 2.27 | |||||||||
| Elater ferrugineus Linnaeus, 1758 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | ||||||
| Melanotus castanipes (Paykull, 1800) | 3 | 0.07 | 6.82 | 1 | 0.02 | 2.27 | ||||||
| Prosternon tesselatum (Linnaeus, 1758) | 23 | 0.52 | 15.91 | 5 | 0.11 | 9.09 | 2 | 0.05 | 4.55 | |||
| Selatosomus aeneus (Linnaeus, 1758) | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | |||
| Buprestidae | ||||||||||||
| Dircaea quadriguttata (Paykull, 1798) | 1 | 0.02 | 2.27 | |||||||||
| Cantharidae | ||||||||||||
| Cantharis nigricans O.F. Müller, 1776 | 2 | 0.05 | 4.55 | 1 | 0.02 | 2.27 | ||||||
| Cantharis pellucida Fabricius, 1792 | 1 | 0.02 | 2.27 | |||||||||
| Rhagonycha nigripes (W. Redtenbacher, 1842) | 2 | 0.05 | 4.55 | |||||||||
| Dermestidae | ||||||||||||
| Attagenus schaefferi (Herbst, 1792) | 8 | 0.18 | 9.09 | 1 | 0.02 | 2.27 | 6 | 0.14 | 9.09 | 1 | 0.02 | 2.27 |
| Ctesias serra (Fabricius, 1792) | 24 | 0.54 | 15.91 | 73 | 1.66 | 22.73 | 81 | 1.84 | 20.45 | 37 | 0.84 | 22.73 |
| Dermestes lardarius Linnaeus, 1758 | 1 | 0.02 | 2.27 | |||||||||
| Megatoma undata (Linnaeus, 1758) | 4 | 0.09 | 9.09 | 3 | 0.07 | 6.82 | ||||||
| Trogoderma glabrum (Herbst, 1783) | 12 | 0.27 | 15.91 | 14 | 0.32 | 20.45 | 19 | 0.43 | 18.18 | 1 | 0.02 | 2.27 |
| Cleridae | ||||||||||||
| Thanasimus femoralis (Zetterstedt, 1828) | 1 | 0.02 | 2.27 | 2 | 0.05 | 4.55 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 |
| Thanasimus formicarius (Linnaeus, 1758) | 4 | 0.09 | 9.09 | |||||||||
| Melyridae | ||||||||||||
| Aplocnemus nigricornis (Fabricius, 1792) | 2 | 0.05 | 4.55 | 1 | 0.02 | 2.27 | ||||||
| Dasytes fusculus (Illiger, 1801) | 1 | 0.02 | 2.27 | |||||||||
| Dasytes niger (Linnaeus, 1761) | 1 | 0.02 | 2.27 | |||||||||
| Malachius bipustulatus (Linnaeus, 1758) | 2 | 0.05 | ||||||||||
| Erotylidae | ||||||||||||
| Dacne bipustulata (Thunberg, 1781) | 1 | 0.02 | 2.27 | |||||||||
| Triplax russica (Linnaeus, 1758) | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | ||||||
| Monotomidae | ||||||||||||
| Rhizophagus bipustulatus (Fabricius, 1792) | 1 | 0.02 | 2.27 | |||||||||
| Rhizophagus cribratus (Gyllenhal, 1827) | 1 | 0.02 | 2.27 | |||||||||
| Rhizophagus fenestralis (Linnaeus, 1758) | 8 | 0.18 | 9.09 | 9 | 0.20 | 13.63 | 6 | 0.14 | 4.55 | |||
| Nitidulidae | ||||||||||||
| Carpophilus hemipterus (Linnaeus, 1758) | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | ||||||
| Carpophilus marginellus Motschulsky, 1858 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | ||||||
| Cryptarcha strigata (Fabricius, 1787) | 1476 | 33.55 | 100 | 2074 | 47.14 | 97.72 | 1608 | 24.27 | 95.45 | 651 | 14.80 | 90.91 |
| Cryptarcha undata (G.-A. Olivier, 1790) | 75 | 1.70 | 61.36 | 162 | 3.68 | 77.27 | 123 | 2.80 | 79.55 | 63 | 1.43 | 68.18 |
| Cychramus luteus (Fabricius, 1787) | 22 | 0.50 | 18.18 | 9 | 0.20 | 11.36 | ||||||
| Cychramus variegatus (Herbst, 1792) | 2 | 0.05 | 4.55 | 1 | 0.02 | 2.27 | ||||||
| Epuraea sp. | 161 | 3.66 | 79.55 | 90 | 2.05 | 63.64 | 26 | 0.59 | 27.27 | 8 | 0.18 | 13.63 |
| Epuraea guttata (G.-A. Olivier, 1811) | 4 | 0.09 | 9.09 | 3 | 0.07 | 6.82 | 1 | 0.02 | 2.27 | |||
| Glischrochilus grandis (Tournier, 1872) | 1136 | 25.82 | 68.18 | 1345 | 30.57 | 54.55 | 489 | 11.11 | 43.18 | 58 | 1.32 | 38.64 |
| Glischrochilus hortensis (Geoffroy, 1785) | 277 | 6.30 | 56.82 | 173 | 3.93 | 56.82 | 62 | 1.41 | 27.27 | 7 | 0.16 | 13.63 |
| Glischrochilus quadriguttatus (Fabricius, 1777) | 48 | 1.09 | 6.82 | 3 | 0.07 | 6.82 | 1 | 0.02 | 2.27 | |||
| Glischrochilus quadripunctatus (Linnaeus, 1758) | 95 | 2.16 | 40.91 | 41 | 0.93 | 27.27 | 15 | 0.34 | 13.63 | 19 | 0.43 | 6.82 |
| Glischrochilus quadrisignatus (Say, 1835) | 2 | 0.05 | 4.55 | 2 | 0.05 | 4.55 | 1 | 0.02 | 2.27 | |||
| Ipidia binotata Reitter, 1875 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | ||||||
| Nitidula bipunctata (Linnaeus, 1758) | 2 | 0.05 | 2.27 | |||||||||
| Omosita depressa (Linnaeus, 1758) | 3 | 0.07 | 6.82 | |||||||||
| Omosita discoidea (Fabricius, 1775) | 2 | 0.05 | 4.55 | |||||||||
| Pityophagus ferrugineus (Linnaeus, 1761) | 1 | 0.02 | 2.27 | |||||||||
| Soronia grisea (Linnaeus, 1758) | 85 | 1.93 | 50.00 | 131 | 2.98 | 54.55 | 60 | 1.36 | 40.91 | 22 | 0.50 | 31.82 |
| Soronia punctatissima (Illiger, 1794) | 6 | 0.14 | 9.09 | 1 | 0.02 | 2.27 | ||||||
| Silvanidae | ||||||||||||
| Silvanus bidentatus (Fabricius, 1792) | 2 | 0.05 | 4.55 | |||||||||
| Cucujidae | ||||||||||||
| Pediacus depressus (Herbst, 1797) | 31 | 0.70 | 27.27 | 9 | 0.20 | 20.45 | 1 | 0.02 | 2.27 | |||
| Laemophloeidae | ||||||||||||
| Cryptolestes sp. | 1 | 0.02 | 2.27 | |||||||||
| Endomychidae | ||||||||||||
| Mycetina cruciata (Schaller, 1783) | 1 | 0.02 | 2.27 | |||||||||
| Coccinellidae | ||||||||||||
| Anatis ocellata (Linnaeus, 1758) | 3 | 0.07 | 4.55 | 4 | 0.09 | 6.82 | ||||||
| Calvia quatuordecimguttata (Linnaeus, 1758) | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | |||
| Halyzia sedecimguttata (Linnaeus, 1758) | 1 | 0.02 | 2.27 | 7 | 0.16 | 15.91 | 5 | 0.11 | 11.36 | 1 | 0.02 | 2.27 |
| Harmonia quadripunctata (Pontoppidan, 1763) | 1 | 0.02 | 2.27 | |||||||||
| Mycetophagidae | ||||||||||||
| Litargus connexus (Geoffroy, 1785) | 5 | 0.11 | 9.09 | 6 | 0.14 | 11.36 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 |
| Mycetophagus piceus (Fabricius, 1777) | 1 | 0.02 | 2.27 | |||||||||
| Mycetophagus quadripustulatus (Linnaeus, 1761) | 1 | 0.02 | 2.27 | |||||||||
| Ciidae | ||||||||||||
| Orthocis alni (Gyllenhal, 1813) | 1 | 0.02 | 2.27 | |||||||||
| Melandryidae | ||||||||||||
| Dircaea quadriguttata (Paykull, 1798) | 1 | 0.02 | 2.27 | |||||||||
| Orchesia fasciata (Illiger, 1798) | 4 | 0.09 | 6.82 | |||||||||
| Mordellidae | ||||||||||||
| Mordellistena humeralis (Linnaeus, 1758) | 1 | 0.02 | 2.27 | |||||||||
| Tomoxia bucephala A. Costa, 1854 | 1 | 0.02 | 2.27 | |||||||||
| Tenebrionidae | ||||||||||||
| Bolitophagus reticulatus (Linnaeus, 1767) | 1 | 0.02 | 2.27 | |||||||||
| Pseudocistela ceramboides (Linnaeus, 1758) | 1 | 0.02 | 2.27 | |||||||||
| Oedemeridae | ||||||||||||
| Chrysanthia geniculata W.L.E. Schmidt, 1846 | 1 | 0.02 | 2.27 | |||||||||
| Chrysanthia viridissima (Linnaeus, 1758) | 5 | 0.11 | 6.82 | |||||||||
| Oedemera lurida (Marsham, 1802) | 1 | 0.02 | 2.27 | |||||||||
| Pythidae | ||||||||||||
| Pytho depressus (Linnaeus, 1767) | 1 | 0.02 | 2.27 | |||||||||
| Pyrochroidae | ||||||||||||
| Schizotus pectinicornis (Linnaeus, 1758) | 1 | 0.02 | 2.27 | |||||||||
| Anthicidae | ||||||||||||
| Notoxus monoceros (Linnaeus, 1761) | 1 | 0.02 | 2.27 | |||||||||
| Scraptiidae | ||||||||||||
| Anaspis sp. | 3 | 0.07 | 6.82 | |||||||||
| Cerambycidae | ||||||||||||
| Anastrangalia reyi (Heyden, 1889) | 1 | 0.02 | 2.27 | |||||||||
| Aromia moschata (Linnaeus, 1758) | 2 | 0.05 | 4.55 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | |||
| Cortodera femorata (Fabricius, 1787) | 1 | 0.02 | 2.27 | |||||||||
| Dinoptera collaris (Linnaeus, 1758) | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | ||||||
| Leptura quadrifasciata Linnaeus, 1758 | 34 | 0.77 | 31.82 | 17 | 0.39 | 20.45 | 9 | 0.20 | 6.82 | 1 | 0.02 | 2.27 |
| Leptura thoracica Creutzer, 1799 | 111 | 2.52 | 38.64 | 128 | 2.91 | 40.91 | 116 | 2.64 | 34.09 | 77 | 1.75 | 36.36 |
| Lepturalia nigripes (De Geer, 1775) | 5 | 0.11 | 9.09 | 2 | 0.05 | 4.55 | 2 | 0.05 | 4.55 | 1 | 0.02 | 2.27 |
| Molorchus minor (Linnaeus, 1758) | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | ||||||
| Necydalis major Linnaeus, 1758 | 5 | 0.11 | 6.82 | 3 | 0.07 | 4.55 | 4 | 0.09 | 9.09 | |||
| Obrium cantharinum (Linnaeus, 1767) | 1 | 0.02 | 2.27 | |||||||||
| Pachyta quadrimaculata (Linnaeus, 1758) | 4 | 0.09 | 9.09 | 8 | 0.18 | 13.63 | 4 | 0.09 | 6.82 | 1 | 0.02 | 2.27 |
| Phymatodes testaceus (Linnaeus, 1758) | 1 | 0.02 | 2.27 | |||||||||
| Plagionotus detritus (Linnaeus, 1758) | 1 | 0.02 | 2.27 | |||||||||
| Rhagium inquisitor (Linnaeus, 1758) | 10 | 0.23 | 9.09 | 6 | 0.14 | 9.09 | 1 | 0.02 | 2.27 | |||
| Rhagium mordax (De Geer, 1775) | 389 | 8.84 | 52.27 | 169 | 3.84 | 40.91 | 46 | 1.05 | 34.09 | 11 | 0.25 | 15.91 |
| Rutpela maculata (Poda von Neuhaus, 1761) | 1 | 0.02 | 2.27 | |||||||||
| Stenocorus meridianus (Linnaeus, 1758) | 1 | 0.02 | 2.27 | |||||||||
| Stenurella melanura (Linnaeus, 1758) | 1 | 0.02 | 2.27 | |||||||||
| Stictoleptura maculicornis (De Geer, 1775) | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | 1 | 0.02 | 2.27 | |||
| Chrysomelidae | ||||||||||||
| Cryptocephalus flavipes Fabricius, 1781 | 1 | 0.02 | 2.27 | |||||||||
| Phyllotreta atra (Fabricius, 1775) | 1 | 0.02 | 2.27 | |||||||||
| Anthribidae | ||||||||||||
| Gonotropis dorsalis (Gyllenhal, 1813) | 1 | 0.02 | 2.27 | |||||||||
| Tropideres albirostris (Schaller, 1783) | 2 | 0.05 | 4.55 | |||||||||
| Nemonychidae | ||||||||||||
| Cimberis attelaboides (Fabricius, 1787) | 1 | 0.02 | 2.27 | 2 | 0.05 | 4.55 | 6 | 0.14 | 11.36 | |||
| Curculionidae | ||||||||||||
| Anisandrus dispar (Fabricius, 1792) | 149 | 3.39 | 20.45 | 152 | 3.45 | 25.00 | 104 | 2.36 | 15.91 | 58 | 0.32 | 11.36 |
| Anthonomus phyllocola (Herbst, 1795) | 1 | 0.02 | 2.27 | |||||||||
| Hylastes brunneus (Erichson, 1836) | 1 | 0.02 | 2.27 | |||||||||
| Phyllobius argentatus (Linnaeus, 1758) | 1 | 0.02 | 2.27 | |||||||||
| Polydrusus cervinus (Linnaeus, 1758) | 1 | 0.02 | 2.27 | |||||||||
| Tomicus minor (Hartig, 1834) | 1 | 0.02 | 2.27 | |||||||||
| Xyleborinus saxesenii (Ratzeburg, 1837) | 1 | 0.02 | 2.27 | |||||||||
| Total of individuals | 5281 | 5273 | 3335 | 1228 | ||||||||
References
- Brygadyrenko, V.V. Effect of canopy density on litter invertebrate community structure in pine forests. Ekológia 2016, 35, 90–102. [Google Scholar] [CrossRef]
- Connor, S.E.; Araújo, J.; Boski, T.; Gomes, A.; Gomes, S.D.; Leira, M.; Freitas, M.C.; Andrade, C.; Morales-Molino, C.; Franco-Múgica, F.; et al. Drought, fire and grazing precursors to large-scale pine forest decline. Divers. Distrib. 2021, 27, 1138–1151. [Google Scholar] [CrossRef]
- Popkova, T.V.; Zryanin, V.A.; Ruchin, A.B. The ant fauna (Hymenoptera: Formicidae) of the Mordovia State Nature Reserve, Russia. Nat. Conserv. Res. 2021, 6, 45–57. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Shashkov, M.P.; Shanin, V.N. Study of pine forest stand structure in the Priosko-Terrasny State Nature Biosphere Reserve (Russia) based on aerial photography by quad-rocopter. Nat. Conserv. Res. 2021, 6, 1–14. [Google Scholar] [CrossRef]
- Ganopoulos, I.; Aravanopoulos, F.; Madesis, P.; Pasentsis, K.; Bosmali, I.; Ouzounis, C.; Tsaftaris, A. Taxonomic identification of Mediterranean pines and their hybrids based on the high resolution melting (HRM) and trnL approaches: From cytoplasmic inheritance to timber tracing. PLoS ONE 2013, 8, e60945. [Google Scholar] [CrossRef] [PubMed]
- Houston Durrant, T.; de Rigo, D.; Caudullo, G. Pinus sylvestrisin Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 132–133. [Google Scholar]
- Horák, J.; Materna, J.; Halda, J.P.; Mladenović, S.; Bogusch, P.; Pech, P. Biodiversity in remnants of naturalmountain forests under conservation-oriented management. Sci. Rep. 2019, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Lust, N.; Muys, B.; Nachtergale, L. Increase of biodiversity in homogeneous Scots pine stands by anecologically diversified management. Biodivers. Conserv. 1998, 7, 249–260. [Google Scholar] [CrossRef]
- Urbanavichus, G.P.; Urbanavichene, I.N.; Vondrák, J.; Ismailov, A.B. Epiphytic lichen biota of Prielbrusie National Park (Northern Caucasus, Russia). Nat. Conserv. Res. 2021, 6, 77–94. [Google Scholar] [CrossRef]
- Filimonova, L.V. Vegetation dynamics in the Kostomuksha State Nature Reserve (Russia) and surroundings against changes in the natural environment during the Holocene. Nat. Conserv. Res. 2021, 6 (Suppl. S1), 98–115. [Google Scholar] [CrossRef]
- Başkent, E.Z. Assessment and valuation of key ecosystem services provided by two forest ecosystems in Turkey. J. Environ. Manag. 2021, 285, 112135. [Google Scholar] [CrossRef]
- Weiss, M.; Didham, R.K.; Procházka, J.; Schlaghamerský, J.; Basset, Y.; Odegaard, F.; Tichechkin, A.; Schmidl, J.; Floren, A.; Curletti, G.; et al. Saproxylic beetles in tropical and temperate forests—A standardized comparison of vertical stratification patterns. For. Ecol. Manag. 2019, 444, 50–58. [Google Scholar] [CrossRef]
- Teshome, M.; Asfaw, Z.; Mohammed, M. Pattern of functional diversity along the elevation gradient in the dry evergreen Afromontane forest of Hararghe Highland, Southeast Ethiopia. Biosyst. Divers. 2020, 28, 257–264. [Google Scholar] [CrossRef]
- Sabattini, J.A.; Sabattini, R.A.; Cian, J.C.; Sabattini, I.A. Carbon stock in subtropical native forests in a South American Protected Area. Nat. Conserv. Res. 2021, 6, 66–79. [Google Scholar] [CrossRef]
- Ivanov, D.G.; Kotlov, I.P.; Minayeva, T.Y.; Kurbatova, J.A. Estimation of carbon dioxide fluxes on a ridge-hollow bog complex using a high resolution orthophotoplan. Nat. Conserv. Res. 2021, 6, 16–28. [Google Scholar] [CrossRef]
- Kim, J. Subdivision design and landscape structure: Case study of The Woodlands, Texas, US. Urban For. Urban Green. 2019, 38, 232–241. [Google Scholar] [CrossRef]
- Kharitonova, A.O.; Kharitonova, T.I. The effect of landscape pattern on the 2010 wildfire spread in the Mordovia State Nature Reserve, Russia. Nat. Conserv. Res. 2021, 6, 29–41. [Google Scholar] [CrossRef]
- Kirstová, M.; Pyszko, P.; Šipoš, J.; Drozd, P.; Kočárek, P. Vertical distribution of earwigs (Dermaptera: Forficulidae) in a temperate lowland forest, based on sampling with a mobile aerial lift platform. Entomol. Sci. 2017, 20, 57–64. [Google Scholar] [CrossRef]
- Giovanni, F.; Mei, M.; Cerretti, P. Vertical stratification of selected Hymenoptera in a remnant forest of the Po Plain (Italy, Lombardy) (Hymenoptera: Ampulicidae, Crabronidae, Sphecidae). Fragm. Entomol. 2017, 49, 71–77. [Google Scholar] [CrossRef][Green Version]
- Dvořák, L.; Dvořáková, K.; Oboňa, J.; Ruchin, A.B. Selected Diptera families caught with beer traps in the Republic of Mordovia (Russia). Nat. Conserv. Res. 2020, 5, 65–77. [Google Scholar] [CrossRef]
- Ruchin, A.B. Seasonal dynamics and spatial distribution of lepidopterans in selected locations in Mordovia, Russia. Biodiversitas 2021, 22, 2569–2575. [Google Scholar] [CrossRef]
- Urban-Mead, K.R.; Muñiz, P.; Gillung, J.; Espinoza, A.; Fordyce, R.; van Dyke, M.; McArt, S.H.; Danforth, B.N. Bees in the trees: Diverse spring fauna in temperate forest edge canopies. For. Ecol. Manag. 2021, 482, 118903. [Google Scholar] [CrossRef]
- Makarkin, V.N.; Ruchin, A.B. Materials on the Neuroptera and Raphidioptera fauna in Mordovia and adjacent regions of European Russia. Proc. Mordovia State Nat. Reserve 2020, 24, 161–181. [Google Scholar]
- Haack, N.; Borges, P.A.V.; Grimm-Seyfarth, A.; Schlegel, M.; Wirth, C.; Bernhard, D.; Brunk, I.; Henle, K.; Pereira, H.M. Response of common and rare beetle species to tree species and vertical stratification in a floodplain forest. Insects 2022, 13, 161. [Google Scholar] [CrossRef]
- Davis, A.J.; Sutton, S.L.; Brendell, M.J.D. Vertical distribution of beetles in a tropical rainforest in Sulawesi: The role of the canopy in contributing to biodiversity. Sepilok Bull. 2011, 13, 59–83. [Google Scholar]
- Puker, A.; Ferreira, K.R.; Correa, C.M.A. Sampling flower chafer beetles (coleoptera: Cetoniidae) in the Amazon rainforest: The role of bait types and trap installation heights get access arrow. Environ. Entomol. 2020, 49, 1096–1104. [Google Scholar] [CrossRef]
- Sweeney, J.; Hughes, C.; Webster, V.; Kostanowicz, C.; Webster, R.; Mayo, P.; Allison, J.D. Impact of horizontal edge–interior and vertical canopy–understory gradients on the abundance and diversity of bark and woodboring beetles in survey traps. Insects 2020, 11, 573. [Google Scholar] [CrossRef]
- Foit, J.; Čermák, V.; Gaar, V.; Hradil, K.; Nový, V.; Rolincová, P. New insights into the life history of Monochamus galloprovincialis can enhance surveillance strategies for the pinewood nematode. J. Pest Sci. 2019, 92, 1203–1215. [Google Scholar] [CrossRef]
- Francese, J.A.; Oliver, J.B.; Fraser, I.; Lance, D.R.; Youssef, N.; Sawyer, A.J.; Mastro, V.C. Influence of trap placement and design on capture of the emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2008, 101, 1831–1837. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Q.H.; Wang, Y.; Liu, G.T.; Zhou, X.; Niu, J.; Schlyter, F. Catching Ips duplicatus (Sahlberg) (Coleoptera: Scolytidae) with pheromone-baited traps: Optimal trap type, colour, height and distance to infestation. Pest Manag. Sci. 2010, 66, 213–219. [Google Scholar] [CrossRef]
- Miller, D.R.; Crowe, C.M.; Sweeney, J.D. Trap height affects catches of bark and woodboring beetles (Coleoptera: Curculionidae, Cerambycidae) in baited multiple-funnel traps in Southeastern United States. J. Econ. Entomol. 2020, 113, 273–280. [Google Scholar] [CrossRef]
- Vance, C.C.; Kirby, K.R.; Malcolm, J.R.; Smith, S.M. Community composition of longhorned beetles (Coleoptera: Cerambycidae) in the canopy and understorey of sugar maple and white pine stands in south-central Ontario. Environ. Entomol. 2003, 32, 1066–1074. [Google Scholar] [CrossRef]
- Bouget, C.; Brin, A.; Brustel, H. Exploring the “last biotic frontier”: Are temperate forest canopies special for saproxylic beetles? For. Ecol. Manag. 2011, 261, 211–220. [Google Scholar] [CrossRef]
- Leksono, A.S.; Takada, K.; Koji, S.; Nakagoshi, N.; Anggraeni, T.; Nakamura, K. Vertical and seasonal distribution of flying beetles in a suburban temperate deciduous forest collected by water pan trap. Insect Sci. 2005, 12, 199–206. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Khapugin, A.A. Red Data Book Invertebrates in a Protected Area of European Russia. Acta Zool. Acad. Sci. Hung. 2019, 65, 349–370. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Makarkin, N.V. Neuroptera and Raphidioptera in the Mordovia State Nature Reserve. Nat. Conserv. Res. 2017, 2, 38–46. [Google Scholar] [CrossRef][Green Version]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A.; Vikhrev, N.E.; Esin, M.N. The use of simple crown traps for the insects collection. Nat. Conserv. Res. 2020, 5, 87–108. [Google Scholar] [CrossRef]
- Bouchard, P.; Bousquet, Y.; Davies, A.E.; Alonso-Zarazaga, M.A.; Lawrence, J.F.; Lyal, C.H.C.; Newton, A.F.; Ried, C.A.M.; Schmitt, M.; Ślipiński, S.A.; et al. Family-group names in Coleoptera (Insecta). ZooKeys 2011, 88, 1–972. [Google Scholar] [CrossRef]
- Bouchard, P.; Bousquet, Y. Additions and corrections to “Family-group names in Coleoptera (Insecta)”. ZooKeys 2020, 922, 65–139. [Google Scholar] [CrossRef]
- Löbl, I.; Smetana, A. (Eds.) Catalogue of Palaearctic Coleoptera. In Curculionoidea I; Apollo Books: Stenstrup, Denmark, 2011; Volume 7, p. 373. [Google Scholar]
- Löbl, I.; Smetana, A. (Eds.) Catalogue of Palaearctic Coleoptera. In Curculionoidea II; Apollo Books: Stenstrup, Denmark, 2013; Volume 8, p. 707. [Google Scholar]
- Löbl, I.; Löbl, D. (Eds.) Catalogue of Palaearctic Coleoptera. Revised and Updated Version. Hydrophiloidea–Staphylinoidea; Brill: Leiden, The Netherlands; Boston, MA, USA, 2015; Volume 2/1, p. 1702. [Google Scholar]
- Löbl, I.; Löbl, D. (Eds.) Catalogue of Palaearctic Coleoptera. Revised and Updated Version. Scarabaeoidea–Scirtoidea–Dascilloidea–Buprestoidea–Byrrhoidea; Brill: Leiden, The Netherlands; Boston, MA, USA, 2016; Volume 3, p. 983. [Google Scholar]
- Löbl, I.; Löbl, D. (Eds.) Catalogue of Palaearctic Coleoptera. Revised and Updated Version. Archostemata–Adephaga–Myxophaga; Brill: Leiden, The Netherlands; Boston, MA, USA, 2017; Volume 1, p. 1443. [Google Scholar]
- Iwan, D.; Löbl, I. (Eds.) Catalogue of Palaearctic Coleoptera. Revised and Updated Second Edition. Tenebrionoidea; Brill: Leiden, The Netherlands; Boston, MA, USA, 2020; Volume 5, p. 945. [Google Scholar]
- Danilevsky, M. (Ed.) Catalogue of Palaearctic Coleoptera. Updated and Revised Second Edition. Chrysomeloidea I (Vesperidae, Disteniidae, Cerambycidae); Brill: Leiden, The Netherlands; Boston, MA, USA, 2020; Volume 6/1, p. 712. [Google Scholar]
- Robertson, J.; Ślipiński, A.; Moulton, M.; Shockley, F.W.; Giorgi, A.; Lord, N.P.; McKenna, D.D.; Tomaszewska, W.; Forrester, J.; Miller, K.B.; et al. Phylogeny and classification of Cucujoidea and the recognition of a new superfamily Coccinelloidea (Coleoptera: Cucujiformia). Syst. Entomol. 2015, 40, 745–778. [Google Scholar] [CrossRef]
- Alonso-Zarazaga, M.A.; Barrios, H.; Borovec, R.; Bouchard, P.; Caldara, R.; Colonnelli, E.; Gültekin, L.; Hlaváč, P.; Korotyaev, B.; Lyal, C.H.C.; et al. Cooperative Catalogue of Palaearctic Coleoptera Curculionoidea. Monogr. Electrón. SEA 2017, 8, 1–729. [Google Scholar]
- Löbl, I.; Smetana, A. (Eds.) Catalogue of Palaearctic Coleoptera. Elateroidea–Derodontoidea–Bos-trichoidea–Lymexyloidea–Cleroidea–Cucujoidea; Apollo Books: Stenstrup, Denmark, 2007; Volume 4, p. 935. [Google Scholar]
- Löbl, I.; Smetana, A. (Eds.) Catalogue of Palaearctic Coleoptera. Chrysomeloidae; Apollo Books: Stenstrup, Denmark, 2010; Volume 6, p. 924. [Google Scholar]
- Bousquet, Y. Litteratura Coleopterologica (1758–1900): A guide to selected books related to the taxonomy of Coleoptera with publication dates and notes. ZooKeys 2016, 583, 1–776. [Google Scholar] [CrossRef]
- Speight, M.C.D. Saproxylic invertebrates and their conservation. In Nature and Environment Series 42; Council of Europe: Strasbourg, France, 1989; pp. 1–79. [Google Scholar]
- Lachat, T.; Wermelinger, B.; Gossner, M.M.; Bussler, H.; Isacsson, G.; Müller, J. Saproxylic beetles as indicator species for dead-wood amount and temperature in European beech forests. Ecol. Indic. 2012, 23, 323–331. [Google Scholar] [CrossRef]
- Carpaneto, G.M.; Baviera, C.; Biscaccianti, A.B.; Brandmayr, P.; Mazzei, A.; Mason, F.; Battistoni, A.; Teofili, C.; Rondinini, C.; Fattorini, S.; et al. A red list of Italian saproxylic beetles: Taxonomic overview, ecological features and conservation issues (Coleoptera). Fragm. Entomol. 2015, 47, 53–126. [Google Scholar] [CrossRef]
- Papis, M.; Mokrzycki, T. Saproxylic beetles (Coleoptera) of the strictly protected area Bukowa Góra in the Roztoczański National Park. For. Res. Pap. 2015, 76, 229–239. [Google Scholar] [CrossRef][Green Version]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423, 623–659. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Chapman & Hall: London, UK, 1996; p. 179. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics soft-ware package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Moore, D.S.; Notz, W.I.; Flinger, M.A. The Basic Practice of Statistics, 6th ed.; W. H. Freeman and Company: New York, NY, USA, 2013; p. 745. [Google Scholar]
- Ulyshen, M.D. Arthropod vertical stratification in temperate deciduous forests: Implications for conservation-oriented management. For. Ecol. Manag. 2011, 261, 1479–1489. [Google Scholar] [CrossRef]
- Touroult, J.; Dalens, P.H. Beetles vertical stratification in French Guiana’ forests: Study using aerial fruit traps. In Coléoptères de Guyane; ACOREP-France: Paris, France, 2012; pp. 16–24. [Google Scholar]
- Hirao, T.; Murakami, M.; Kashizaki, A. Importance of the understory stratum to entomofaunal diversity in a temperate deciduous forest. Ecol. Res. 2009, 24, 263–272. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Usage of fermental traps for studying the species diversity of Coleoptera. Insects 2021, 12, 407. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Hanula, J.L. A comparison of the beetle (Coleoptera) fauna captured at two heights above the ground in a North American temperate deciduous forest. Am. Midl. Nat. 2007, 158, 260–278. [Google Scholar] [CrossRef]
- Kato, M.; Inque, T.; Hamid, A.A.; Nagamitsu, T.; Merdek, M.B.; Nona, A.R.; Itino, T.; Yamane, S.; Yumoto, T. Seasonality and vertical structure of light-attracted insect communities in a dipterocarp forest in Sarawak. Res. Popul. Ecol. 1995, 37, 59–79. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V. Vertical stratification of beetles in deciduous forest communities in the Centre of European Russia. Diversity 2021, 13, 508. [Google Scholar] [CrossRef]
- Lange, M.; Türke, M.; Pašalić, E.; Boch, S.; Hessenmöller, D.; Müller, J.; Prati, D.; Socher, S.A.; Fischer, M.; Weisser, W.W.; et al. Effects of forest management on ground-dwelling beetles (Coleoptera; Carabidae, Staphylinidae) in Central Europe are mainly mediated by changes in forest structure. For. Ecol. Manag. 2014, 329, 166–176. [Google Scholar] [CrossRef]
- Porhajašová, J.I.; Babošová, M.; Noskovič, J.; Ondrišík, P. Long-term developments and biodiversity in carabid and staphylinid (Coleoptera: Carabidae and Staphylinidae) fauna during the application of organic fertilizers under agroecosystem conditions. Pol. J. Environ. Stud. 2018, 27, 2229–2235. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Semishin, G.B. Fauna of click beetles (Coleoptera: Elateridae) in the interfluve of Rivers Moksha and Sura, Republic of Mordovia, Russia. Biodiversitas 2018, 19, 1352–1365. [Google Scholar] [CrossRef]
- Procházka, J.; Cizek, L.; Schlaghamerský, J. Vertical stratification of scolytine beetles in temperate forests. Insect Conserv. Divers. 2018, 11, 534–544. [Google Scholar] [CrossRef]
- Nikitsky, N.B.; Legalov, A.A. The ant-like stone beetles (Scydmaenidae) and the fungus weevils (Anthribidae) of the Moscow region. Euroasian Entomol. J. 2016, 15, 219–227. [Google Scholar]
- Zemoglyadchuk, A.V.; Ruchin, A.B.; Egorov, L.V. An annotated checklist of the tumbling flower beetles (Coleoptera, Mordellidae) of the Republic of Mordovia, with a short review of the family in European Russia. Entomol. Rev. 2020, 100, 771–787. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Polumordvinov, O.A. Coleoptera of the Penza region, Russia based on fermental crown trap. Biodiversitas 2021, 22, 1946–1960. [Google Scholar] [CrossRef]
- Bajerlein, D.; Matuszewski, S.; Konwersk, S. Insect succession on carrion: Seasonality, habitat preference and residency of histerid beetles (Coleoptera: Histeridae) visiting pig carrion exposed in various Forests (Western Poland). Pol. J. Ecol. 2011, 59, 787–797. [Google Scholar]
- Marković, Č.; Stojanović, A. Fauna of phloemo-xylophagous insects, their parasitoids and predators on Ulmus minor in Serbia. Biologia 2012, 67, 584–589. [Google Scholar] [CrossRef]
- Krivets, S.A.; Kerchev, I.A. Insects inhabiting the galleries of the four-eyed fir bark beetle Polygraphus proximus Blandf. (Coleoptera, Curculionidae: Scolytinae) in Siberia. Entomol. Rev. 2016, 96, 545–558. [Google Scholar] [CrossRef]
- Kadej, M. Larva and pupa of Ctesias (s. str.) serra (Fabricius, 1792) with remarks on biology and economic importance, and larval comparison of co-occurring genera (Coleoptera, Dermestidae). ZooKeys 2018, 758, 115–135. [Google Scholar] [CrossRef]
- Vodka, Š.; Cizek, L. The effects of edge-interior and understorey-canopy gradients on the distribution of saproxylic beetles in a temperate lowland forest. For. Ecol. Manag. 2013, 304, 33–41. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V. Fauna of longicorn beetles (Coleoptera: Cerambycidae) of Mordovia. Russ. Entomol. J. 2018, 27, 161–177. [Google Scholar] [CrossRef]
- Egorov, L.V.; Ruchin, A.B.; Semenov, V.B.; Semionenkov, O.I.; Semishin, G.B. Checklist of the Coleoptera of Mordovia State Nature Reserve, Russia. ZooKeys 2020, 962, 13–122. [Google Scholar] [CrossRef]
- Egorov, L.V.; Ruchin, A.B.; Semishin, G.B. Some data on the Coleoptera fauna of the Mordovia State Nature Reserve. Report 10. Proc. Mordovia State Nat. Reserve 2021, 26, 96–128. [Google Scholar]
- Ruzzier, E.; Galli, A.; Bani, L. Monitoring exotic beetles with inexpensive attractants: A case study. Insects 2021, 12, 462. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Seasonal activity of Coleoptera attracted by fermental crown traps in forest ecosystems of Central Russia. Ecol. Quest. 2021, 32, 37–53. [Google Scholar] [CrossRef]
- Alekseev, V.I.; Nikitsky, N.B. Rare and new for the fauna of the Baltic States beetles (Coleoptera) from the Kaliningrad Region. Acta Zool. Litu. 2008, 18, 254–259. [Google Scholar] [CrossRef]
- Lasoń, A.; Holly, M. Glischrochilus grandis Tournier, 1872—New species of beetle for the Polish fauna and new data on the occurrence of genus Glischrochilus Reitter, 1873 (Coleoptera: Nitidulidae: Cryptarchinae). Acta Entomol. Sil. 2015, 23, 1–4. [Google Scholar]
- Gorshkova, V.P.; Volodchenko, A.N. The specific assemblage structure of longhorn beetles (Coleoptera, Cerambycidae) in floodplain forests of the western part of Saratov oblast. Biol. Bull. 2016, 43, 1416–1421. [Google Scholar] [CrossRef]
- Sheehan, T.N.; Ulyshen, M.D.; Horn, S.; Hoebeke, E.R. Vertical and horizontal distribution of bark and woodboring beetles by feeding guild: Is there an optimal trap location for detection? J. Pest Sci. 2019, 92, 327–341. [Google Scholar] [CrossRef]
- Holuša, J.; Fiala, T.; Foit, J. Ambrosia beetles prefer closed canopies: A case study in oak forests in Central Europe. Forests 2021, 12, 1223. [Google Scholar] [CrossRef]
- Danilevsky, M.L.; Ruchin, A.B.; Egorov, L.V. Mass collection of two rare longicorn-species (Coleoptera, Cerambycidae) in Central Russia. Humanit. Space 2019, 8, 1179–1183. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V.; MacGowan, I.; Makarkin, V.N.; Antropov, A.V.; Gornostaev, N.G.; Khapugin, A.A.; Dvořák, L.; Esin, M.N. Post-fire insect fauna explored by crown fermental traps in forests of the European Russia. Sci. Rep. 2021, 11, 21334. [Google Scholar] [CrossRef]
- Sama, G. Atlas of the Cerambycidae of Europe and the Mediterranean Area. Northern, Western, Central and Eastern Europe British Isles and Continental Europe from France (Excl. Corsica) to Scandinavia and Urals. Kabourek, Zlín; Coronet Books Incorporated: London, UK, 2002; Volume 1, p. 173. [Google Scholar]
- Karpiński, L.; Szczepański, W.T.; Boldgiv, B.; Walczak, M. New data on the longhorn bee-tles of Mongolia with particular emphasis on the genus Eodorcadion Breuning, 1947 (Coleoptera, Cerambycidae). ZooKeys 2018, 739, 107–150. [Google Scholar] [CrossRef]
- Haeler, E.; Bergamini, A.; Blaser, S.; Ginzler, C.; Hindenlang, K.; Keller, C.; Kiebacher, T.; Kormann, U.G.; Scheidegger, C.; Schmidt, R.; et al. Saproxylic species are linked to the amount and isolation of dead wood across spatial scales in a beech forest. Landsc. Ecol. 2021, 36, 89–104. [Google Scholar] [CrossRef]
- Ekström, A.L.; Bergmark, P.; Hekkala, A.M. Can multifunctional forest landscapes sustain a high diversity of saproxylic beetles? For. Ecol. Manag. 2021, 490, 119107. [Google Scholar] [CrossRef]
- Parisi, F.; Innangi, M.; Tognetti, R.; Lombardi, F.; Chirici, G.; Marchetti, M. Forest stand structure and coarse woody debris determine the biodiversity of beetle communities in Mediterranean mountain beech forests. Glob. Ecol. Conserv. 2021, 28, e01637. [Google Scholar] [CrossRef]
- Preisser, E.; Smith, D.C.; Lowman, M.D. Canopy and ground level insect distribution in a temperate forest. Selbyana 1998, 19, 141–146. [Google Scholar]
- Lettenmaier, L.; Seibold, S.; Bässler, C.; Brandl, R.; Gruppe, A.; Müller, J.; Hagge, J. Beetle diversity is higher in sunny forests due to higher microclimatic heterogeneity in deadwood. Oecologia 2022, 198, 825–834. [Google Scholar] [CrossRef]
- Pakeman, R.J.; Stockan, J.A. Drivers of carabid functional diversity: Abiotic environment, plant functional traits, or plant functional diversity? Ecology 2014, 95, 1213–1224. [Google Scholar] [CrossRef]
- Penone, C.; Allan, E.; Soliveres, S.; Felipe-Lucia, M.R.; Gossner, M.M.; Seibold, S.; Simons, N.K.; Schall, P.; Van Der Plas, F.; Manning, P.; et al. Specialisation and diversity of multiple trophic groups are promoted by different forest features. Ecol. Lett. 2019, 22, 170–180. [Google Scholar] [CrossRef]
- Schneider, A.; Blick, T.; Köhler, F.; Pauls, S.U.; Römbke, J.; Zub, P.; Dorow, W.H.O. Animal diversity in beech forests—An analysis of 30 years of intense faunistic research in Hessian strict forest reserves. For. Ecol. Manag. 2021, 499, 119564. [Google Scholar] [CrossRef]
- Tinya, F.; Kovács, B.; Bidló, A.; Dima, B.; Király, I.; Kutszegi, G.; Lakatos, F.; Mag, Z.; Márialigeti, S.; Nascimbene, J.; et al. Environmental drivers of forest biodiversity in temperate mixed forests—A multi-taxon approach. Sci. Total Environ. 2021, 795, 148720. [Google Scholar] [CrossRef]
- Maguire, D.Y.; Robert, K.; Brochu, K.; Larrivée, M.; Buddle, C.M.; Wheeler, T.A. Vertical stratification of beetles (Coleoptera) and flies (Diptera) in temperate forest canopies. Environ. Entomol. 2014, 43, 9–17. [Google Scholar] [CrossRef]
- Gosling, D.C.L.; Gosling, N.M. An annotated list of the Cerambycidae of Michigan (Coleoptera) Part II, the subfamilies Lepturinae and Lamiinae. Great Lakes Entomol. 1977, 10, 1–37. [Google Scholar]
- Prévosto, B.; Helluy, M.; Gavinet, J.; Fernandez, C.; Balandier, P. Microclimate in Mediterranean pine forests: What is the influence of the shrub layer? Agric. For. Meteorol. 2020, 282–283, 107856. [Google Scholar] [CrossRef]
- Kovács, B.; Tinya, F.; Ódor, P. Stand structural drivers of microclimate in mature temperate mixed forests. Agric. For. Meteorol. 2017, 234–235, 11–21. [Google Scholar] [CrossRef]


| Parameters | Height (m) | |||
|---|---|---|---|---|
| 1.5 | 3 | 7 | 12 | |
| Total of families | 28 | 24 | 17 | 18 |
| Total of individuals | 5281 | 5273 | 3335 | 1228 |
| Mean number of individuals per trap | 120 | 120 | 76 | 28 |
| Number of species (excluding unidentified insects) | 84 | 69 | 59 | 44 |
| Number of saproxylic species | 56 | 52 | 48 | 33 |
| Proportion (%) of saproxylic species | 66.7 | 75.4 | 81.4 | 75.0 |
| Number of anthophilic species | 34 | 34 | 26 | 24 |
| Proportion (%) of anthophilic species | 40.5 | 49.3 | 44.1 | 54.5 |
| Shannon index | 2.24 | 1.91 | 1.85 | 1.81 |
| Simpson index | 0.20 | 0.26 | 0.30 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Vertical Distribution of Beetles (Coleoptera) in Pine Forests in Central European Russia. Diversity 2022, 14, 622. https://doi.org/10.3390/d14080622
Ruchin AB, Egorov LV, Khapugin AA. Vertical Distribution of Beetles (Coleoptera) in Pine Forests in Central European Russia. Diversity. 2022; 14(8):622. https://doi.org/10.3390/d14080622
Chicago/Turabian StyleRuchin, Alexander B., Leonid V. Egorov, and Anatoliy A. Khapugin. 2022. "Vertical Distribution of Beetles (Coleoptera) in Pine Forests in Central European Russia" Diversity 14, no. 8: 622. https://doi.org/10.3390/d14080622
APA StyleRuchin, A. B., Egorov, L. V., & Khapugin, A. A. (2022). Vertical Distribution of Beetles (Coleoptera) in Pine Forests in Central European Russia. Diversity, 14(8), 622. https://doi.org/10.3390/d14080622








