Abstract
We assessed true bugs among aboveground and soil inhabitants of four different biocoenoses of pine forests representing the intrazone flora and fauna areas of the Kazakh Upland. True bugs were collected from litter according to the methods of soil zoological studies. The results of a comparative analysis of the similarities and differences of the dominant and ecological structures of Heteroptera complexes of aboriginal and derived forest types are presented. Fourteen species and subspecies of three families were listed for the Central Kazakhstan region for the first time. The species Eremocoris podagricus was not previously registered for the territory of Kazakhstan. Fifteen species (50%) were true aboveground inhabitants or live on grass, whereas other species use soil surfaces and ground litter as temporary habitats. Drymus brunneus and Eremocoris fenestratus play a key role in the structure and function of the true bug assemblages in the studied biotopes. The ecological success of typical forest and boreal inhabitants in biotopes of arid regions is explained by the relict nature of forests and ancient connections with the taiga zone of Western Siberia and the mountain forests of the Urals and Altai.
1. Introduction
True bugs (Heteroptera) are one of the largest and most peculiar suborders of hemipterous insects (Hemiptera), playing an important role in different biogeocenoses of Kazakhstan. The annotated list of hemipterous insects in Kazakhstan includes 1250 species of 35 families and 411 genera [1]. Studies of Heteroptera in Kazakhstan started in the 1950s under the supervision of the Laboratory of Entomology at the Institute of Zoology of the Academy of Sciences of Kazakh SSR, with the main focus on the phytophagan group of insects of the southern regions of Kazakhstan [2,3,4]. Materials on true bugs of steppe, desert steppe, and desert zones of Central Kazakhstan were collected in 1957–1962 during the biocomplex expeditions of the Zoological Institute of the Academy of Sciences of the USSR and Institute of Zoology of Academy of Sciences of Kazakh SSR. Subsequently, these data were generalized and completed [5]. In Central Kazakhstan, 301 species of Heteroptera were found [6]. However, publications about the composition and structure of hemipterous insect complexes of ground litter and soil in exact ecosystems are, to date, very limited and sector nonspecific [7,8,9]. Prior to the studies of N.P. Slavchenko, it was not known, with certainty, that true bugs are aboveground inhabitants of the intrazonal pine forests of Central Kazakhstan [10,11,12,13]. Some species were subsequently published by Asanova in their review of fauna of the infraorder Pentatomomorpha [14]. Nevertheless, none of the faunistic reviews contain any information on ground litter true bugs in the native pine forests of Central Kazakhstan. The goal of this paper is to assess species composition, abundance, and the ecological peculiarities of Heteroptera in the litter of intrazonal pine forests in the Karkaraly mountain range in the eastern part of the Kazakh Upland.
2. Material and Methods
2.1. The Study Area
Studies were carried out in the most common types of forests of the Bayanaul–Karkaraly group of pine groves in the Kazakh Upland (territory of Karkaraly National Park, coordinates 49.41667° N latitude and 75.41667° E longitude). The studied area belongs to the eastern floral region of the central Kazakh Uplands and Karkaraly floral subregion [15].
Aboriginal forests here are relicts of vast mountain taiga forests of the Pleistocene age. These are the most southern variants of pine forests in subarea of dry and absinth–typchak grass steppes [16].
Haplic Kastanozems with a sodic phase are the dominant soils in dry steppe climatic zones. Stony soils characterize the central part of the Kazakh Upland. Mollie Gleysols and Kastanozems occur in depressions and river terraces [17].
Testing biotopes (Table 1) were selected in four aboriginal plant associations with Pinus sylvestris L., 1753, as the dominant tree: dry stone–lichen pine forest (I), wet herb and birch pine forest (II), wet herb and stone bramble pine forest (III), and wet herb moss and fern pine forest (IV) [18,19]. In addition, we gathered herpetobionts from the derived forest–mountain birch forest type (V).

Table 1.
Description of investigated localities in the Kazakh Upland (Central Kazakhstan).
We formed geobotanical descriptions according to Gortchakovskyi [16] and Kupriyanov [20] and dated the dominant species in the forest stand. The species nomenclature is given by the study of the List of Vascular Plants of Kazakhstan [21] and World Flora Online (WFO) [22].
Aboriginal and secondary plant associations form the ecological series from the less-moist-assured localities with unstable moisture regimes and germinative soil down along the profile to moderately moist-assured localities with more stable moisture regimes and mature soil. Soil is mostly moisturized as a result of atmospheric precipitation and surface flow. Optimal humidification is due to moist inflow from slopes and groundwater.
2.2. Taking Censuses of Soil Surface Dwelling True Bugs
The samples of soil fauna and were taken over a long period starting from 1980 up to now. Soil zoological studies were carried out from May to October. Litter samples of 0.0625 m2 in size were taken at depths of 0–(10) cm within each biotope in 10-fold replications and every 3–4 years (in total, more than 600 samples) [23]. True bugs were collected from litter and upper layer of soil 0–5 cm by hand and sorted onto a plastic sheet and with the help of an exhauster. Materials were fixed in 80% ethanol [24]. Abundance data were calculated according to 1 m2 of surface as specimen/m2 = sp/m2.
2.3. Ecology-Faunistic Analysis of True Bug Assemblages
Trophic preferences, ecological groups of recorded heteropterans according to the basis position among vegetation tiers, and objective attitude toward soil moisture were defined according to pertinent scientific reports [1,5,25,26,27,28,29,30].
True bug assemblages were characterized according to (1) dominance, determined according to the scale of O. Renkonen [31]; (2) index of abundance: K = PB/100, where P is the share of the total number of specimens in the samples, in which the given species was registered, and B is the relative number of samples in which the species occurs; and (3) Margalef’s diversity index (DMg) [32]. The coefficients of similarity in heteropterofauna of the biogeocenosis—Jaccard faunistic coefficient (Kj), coefficient of common specific abundance of species (Kn), and total Vainstein’s coefficient (Kv)—were calculated according to [33]. The nomenclature of species follows the Catalogues of Heteroptera of the Palaearctic Region [34,35,36,37] and the Asian part of Russia [38]. The classification of the area of species is based upon the scheme of A.F. Emeljanov [39].
3. Results
3.1. Species Diversity, Density, and Dominance
In total, 680 specimens of imago and nymphs were collected in litter and soil samples during the study period. The true bugs recorded in the studied forests belong to 30 species and subspecies and eight families. A specific complex of species is registered for each biotope (Table 2).

Table 2.
Species composition of true bugs in a pine forest litter and derived phytocenoses of the Kazakh Upland (Central Kazakhstan).
Fourteen species were found in the dry stone–lichen pine forest. The total number of hemipterous insects in the litter is 1.8 ± 0.9 sp/m2, with an index of abundance of 0.55. Prevalent here are mesophile polyphytophages Eremocoris plebejus plebejus (19.0% of the total number) and Dolycoris baccarum (14.4%), peculiar representatives of the herb and forest complex of species. The largest share (55.3%) of rare species is encountered in the severe conditions of pine forests. Moreover, these are distinct from the dominating species of other forest types. Hemipterous insects dwelling here prefer warm and dry habitat conditions and are generally not encountered in other biotopes.
Drymus brunneus is the superdominating member and comprises 64% of all hemipterous insects in wet herb and birch pine forest, where six species of true bugs are found. Less simple, but related to dominants, Eremocoris fenestratus (9.7% of the total number), meso-xerophile oligophytophage, prevailingly dwell in the litter under pine or juniper. The total number of Heteroptera in the litter is 3.5 ± 1.1 sp/m2, with an index of abundance of 0.95.
Seven species in a collection of 2.3 ± 0.9 sp/m2 true bugs were recorded in the moist litter of herb and stone bramble pine forest (index of abundance 0.29). D. brunneus (1.0 ± 0.3 sp/m2) is usually superdominant in the litter. Plinthisus longicollis (0.25 ± 0.1 sp/m2) is dominant and dwells in the plant detritus of dry grassy mountain shoulders with poor planting. Forest litter was found to often contain Lygaeidae nymphs.
Wet herb moss and fern pine forest is distinguished by a high number of herpetobionts (5.7 ± 1.7 sp/m2). Thirteen species of Heteroptera are present, and the index of abundance increased to 1.08. Here, the conditions for development were optimal for D. brunneus to prevail in herpetobium. The domination of Elasmucha grisea species (0.77 ± 0.2 sp/m2) is more closely connected with birch forests. Polyphytophage Stygnocoris sabulosus is also attracted to this station (0.38 ± 0.1 sp/m2). These plantings were the only ones where the species D. sylvaticus and Emblethis denticollis (0.09 ± 0.05 sp/m2) were found, as they prefer moderately moist conditions, along with hydrophilic Saldula saltatoria (0.09 ± 0.05 sp/m2).
Secondary mountain birch forest is similarly rich in species. Ecological peculiarities (nature of the litter and its turf content, hydrothermal regime) attract a vast array of Heteroptera groups. Litter includes predatory, herbivorous species, and detritophages, for which the total reaches 3.7 ± 0.2 sp/m2, with an index of abundance of 1.0. D. brunneus (0.9 ± 0.05 sp/m2) is superdominant, and E. fenestratus (0.5 ± 0.2 sp/m2) is dominant. Mountain birch forest attracts P. longicollis (0.3 ± 0.1 sp/m2) and mesophile polyphytophage Trapezonotus arenarius, which eat the plant seeds (0.3 ± 0.1 sp/m2).
3.2. Ecological Structure of True Bug Assemblages
The collected true bugs are represented in the following groups according to trophic preferences: predators (zoophages), plant eaters (phytophages), and true bugs with mixed nutrition, consuming both flesh and plants (zoophytophages) [1,5,25].
The group of phytophages unites 25 species, or 83.3% (where 12.5% of the total number of phytophage species are considered to be narrow oligophages; 12.5% are wide oligophages; and 75% are polyphages). The group of zoophages comprises 10% of the total number of Heteroptera, with zoophytophages being 6.7% overall (Figure 1).
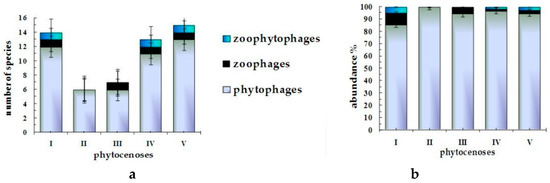
Figure 1.
Trophic structure of true bug complexes in litter of forest phytocenoses of the Kazakh Upland by, (a): number of species and (b): abundance.
The group of phytophages is represented by species of Lygaeidae, Rhopalidae, Acanthosomatidae, Cydnidae, and Pentatomidae families and, partially, of the Miridae family (12.5% of the total number of phytophage species).
Among the polyphytophages are many species of the Lygaeidae (45% of the total number of polyphytophage species), Miridae (26.25%), and Pentatomidae (15%) families.
In the group of wide oligophytophages, we may distinguish species of the Lygaeidae, Pentatomidae, and Acanthosomatidae families. The group of narrow oligophytophages is formed mainly by species of the Miridae (Lygocoris contaminatus) and Lygaeidae (Philomyrmex insignis and Eremocoris fenestratus) families.
In the Saldidae and Nabidae families, all the species found in biocenoses are related to obligate zoophages. Zoophytophages act as oligophages toward plant food. Zoophytophage species are only found in the Miridae family (Deraeocoris punctulatus and Psallus anticus; 40% of the total number of species).
The discovered hemipterous insects were divided into six groups depending on the layer of their dwelling: epigeobionts, herpetobionts, herpeto-chortobionts, chortobionts, dendrobionts, and dendro-tamnobionts (Figure 2) [1,5,26,27,28,30]. The group of herpetobionts dominates in terms of number of species and abundance (13 species and 43.3% of all collected material). All species and subspecies belong to the Lygaeidae family: Rhyparochromus pini, Trapezonotus arenarius, Peritrechus nubilus, Drymus brunneus, D. sylvaticus, Plinthisus longicollis, P. reyi, Stygnocoris sabulosus, Philomyrmex insignis, Eremocoris plebejus plebejus, Eremocoris fenestratus, E. podagricus, and E. abietis. The most numerous herpetobiont species in this family are D. brunneus (4.8% of all collected material) and E. fenestratus (7.1%). According to Figure 2, the smallest population of herpetobiont species is registered in the stone–lichen pine forest (40% of all species in the biotope), and the largest population in the wet herb and birch pine forest (86.9%). With an increase in the moisture content of pine forests, the abundance of herpetobiont species is slightly changed (70.4–75.1%), as is also observed in the secondary type of forest–mountain birch forest (73%).
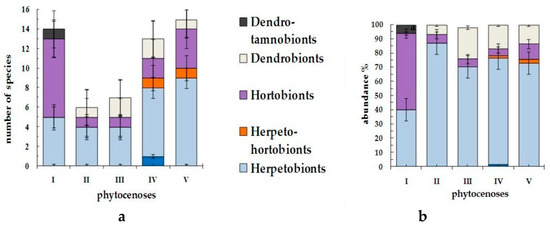
Figure 2.
Ecological structure of true bug complexes in litter of forest phytocenoses of the Kazakh Upland according to the basis position of species among vegetation tiers by, (a): number of species and (b): abundance.
The following group subject to number of species and population is the group of chortobionts (eight species and 26.7% of all collected material: Nabis punctatus punctatus (Nabidae), Deraeocoris punctulatus, Lygocoris contaminates, Lygus gemellatus (Miridae), Pterotmetus staphyliniformis (Lygaeidae), Corizus hyoscyami (Rhopalidae), Dolycoris baccarum, and Eurydema ventralis (Pentatomidae). The number of chortobiont species D. baccarum exceeds those of other species (4.6% of all collected material). The largest population of chortobiont species is registered in the stone–lichen pine forest (54% of all species in biotope). In other types of studied pine forests, the population of chortobionts is significantly small (4.9–6.5%). In mountain birch forest, the abundance of chortobionts reaches 10.8%.
The group of herpeto-chortobionts (1.1% of all collected material) is represented by two species of small population belonging to the Lygaeidae family: Ligyrocoris sylvestris and Emblethis denticollis. They are registered in wet pine forest IV and mountain birch forest V. The group of epigeobionts (0.5% of all collected material) is represented by the species of Saldula saltatoria (Saldidae) in conditions of the wettest pine forest IV.
Dendrobiont species (12.9% of all collected material) were found in all types of forests, except stone–lichen pine forest and represented by the species Elasmucha grisea (Acanthosomatidae) and Lygocoris contaminatus (Miridae). In the group of dendro-tamnobionts (0.5% of all collected material), species of Psallus anticus (Miridae) are only found in cenoses of stone–lichen pine forest.
Thus, the distribution of true bugs, along the tiers of vegetation in biocenoses, remained more or less the same and typical for all studied forests. However, we can observe the peculiar complex of ecological groups in the driest conditions of stone–lichen pine forest.
Registered species of hemipterous insects were subdivided into three groups according to their preferable localities, reflecting different soil-water contents: mesophiles, mesoxerophiles, and xerophiles [1,27,28,29,30] (Figure 3).
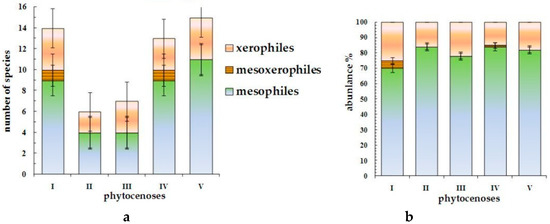
Figure 3.
Ecological structure of true bug complexes in a litter of forest phytocenoses of the Kazakh Upland according to objective attitude toward soil moisture: (a)—number of species and (b)—abundance.
It was discovered that mesophiles prevail at average values of temperature and soil moisture (56.7% of all registered species). This group includes all registered species of the Miridae, Rhopalidae, Acanthosomatidae, and Pentatomidae families and the majority of Lygaeidae species. Each biocenosis included no less than nine species linked to moderate soil moisture conditions, and in pine forests II and III, the number of mesophilic species is minimal. True bug mesophiles include the majority of definite herpetobiont species—Drymus brunneus, D. sylvaticus, and Eremocoris plebejus, among others. The abundance of mesophiles is more than 80% everywhere, except the driest conditions of dry pine forest I. Hygromesophilic Saldula saltatoria (Saldidae) dwells only in conditions of wet pine forest IV.
3.3. Comparative Analysis of the Similarities of True Bug Assemblages
For hemipterous insects, the highest species diversity (Margalef’s index) was observed in composition of herpetobium as calculated for stone–lichen pine forest (DMg = 5), herb moss and fern pine forest (DMg = 4.8), and mountain birch forest (DMg = 5.2). These are the extreme points in the ecological serial of biocenoses I–IV in relation to the moisture regime.
The highest similarity of biocenoses to species composition is between herb and birch pine forest, located in the northern slopes, and herb moss and fern pine forest, located in the valley, with a coefficient of faunistic similarity of Kj = 0.36. In biocenoses with different edificators moss and fern pine forest and mountain birch forest, Kj = 0.4. According to the Table 3, the faunistic similarity of complexes of hemipterous insects is registered in neighboring biocenoses in ecological series I–IV.

Table 3.
Coefficients of similarity of complexes of true bugs as aboveground inhabitants of pine forests and derived phytocenosis of the Kazakh Upland (Central Kazakhstan).
The similarity of biocenoses to an abundance of complexes of hemipterous insects, in general, is a repetition of the abovementioned ratio and gives a clear reason through confirmation. For stations of pine forests, the priority is defined, as follows, according to the coefficient of the common specific abundance of species: complexes of herb and birch pine forest and herb moss and fern pine forest are most similar (Kn = 52.7), and complexes of stone–lichen pine forest and herb moss and fern pine forest are less equal (Kn = 5.2).
An estimation of general biotopic similarity showed that the highest value of the total Vainstein’s coefficient is registered for complexes of hemipterous insects of herb and birch pine forest and herb moss and fern pine forest (Kv = 0.19). Further, in the course of decrease in biotopic similarity, the following pairs are formed: herb moss and fern pine forest and mountain birch forest (Kv = 0.14), and herb and stone bramble pine forest and herb moss and fern pine forest (Kv = 0.10); herb and birch pine forest and mountain birch forest (Kv = 0.10) and herb and stone bramble pine forest and mountain birch forest (Kv = 0.08); and stone–lichen pine forest and mountain birch forest (Kv = 0.07) (Table 3).
4. Discussion
Natural modifications of environment and planting in the result of weathering of granite rocks of the eastern part of the Kazakh Upland form the genetic series of peculiar stations for species of Heteroptera. In the ecological series of biocenoses I–IV, confinement of species to definite types of forests is traced, which defines the differences and similarities of the heteropterofauna of the litter.
The number of Heteroptera in the litter was low in all biocenoses. The pine forest engaging the floodland of the stream is distinguished by a higher number of true bugs in herpetobium.
Complexes of litter hemipterous insects are the most diverse in limiting conditions of the ecological series of associations of Pinus sylvestris, less moist-assured localities (stone–lichen pine forest), and moderately moist-assured localities with stable moisture regime (herb moss and fern pine forest). We believe that this is related to the wide ecological range of Heteroptera complexes in these biocenoses [40]. Moreover, hemipterous insect complexes show a rich range of herpetobiums of derivative types of forest–mountain birch forest as the result of the development to pre-climax community [41,42].
Thus, a number of similarities of complexes of litter true bugs in the biocenoses II–IV, IV–V, III–IV, II–V, III–V, I–V, II–III, and I–II are formed, where the proximity of the heteropterofauna of native pine forests and derivative forest–mountain birch forest type is disclosed. The hydrothermal regime of litter in herb and birch pine forests with less warming and desiccation of soil approaches that of valley pine forests. The formation of close ecological conditions is also evident in the composition of tree, grass, shrub, and moss layers [16,20]. Ecological conditions of the secondary birch forest are the most similar to conditions of herb and birch pine forest according to its distribution over the relief. Such similarity of litter true bugs in biocenoses might partly be explained by species migration from neighboring localities, because the composition of ecological groups of Heteroptera, with regard to the moist factor, is defined with the nature of neighboring biotope and presence of ecological corridors in the network of forest faunistic complexes [43].
In each biocenoses, the largest number of species is related to Lygaeidae (17 species, or 56.7% of the total number of detected species). The majority of their representatives dwell in the ground layer. They compose dominating taxonomic and ecological groups (nuclei) of studied herpetobionts. The second highest number of species is represented by the Miridae family (five species or 16.6% of the total number of species), which includes dwellers of grassy and tree layers.
The number of D. brunneus species exceeds the number of other species in all types of forests, with the exception of stone–lichen pine forest. This species is most abundantly encountered in plant detritus of herb and birch as well as herb moss and fern pine forests, where the numbers reach 2.3 ± 0.3 and 2.9 ± 0.4 sp/m2, respectively. In the litter of herb and birch pine and valley pine forests, the share of background species of D. brunneus exceeds 50%. In wet herb-moss-and-fern pine forest, it is the only dominating species among hemipterous insects in litter.
In derivative mountain birch forest, D. brunneus is also superdominant. However, more favorable lighting conditions and warmer soil in these localities evidently restrict the optimum zone for D. brunneus. Due to this, the share of species here is decreased by up to 24%, becoming lower than that of other more thermophilic forms. In dry plantings, D. brunneus was sporadic. It is worth emphasizing that D. brunneus tends to be found in dry plantings in the Buzuluk Pine Forest surrounded by steppes on the East European Plain [44,45].
It is less abundant in pure breed forest stands. Our observations indicate the sensitivity of this species to the nature of litter and its composition and moisture content, and it tends toward plantings of mixed type. These features of the species are also noted by other researchers [46]. The eurybiontness of species is described in studies of the heterophterofauna of forest steppe [47,48,49].
Phytophagy (polyphagy) prevails among true bugs in all types of studied forest biocenoses. True herpetobionts and herperto-chortobionts include 15 species (50%), all remaining species, being in forest litter during diapause or moving along the soil surface, using it as a temporary station [50,51,52]. The predominant species (excluding Dolycoris baccarum and Elasmucha grisea) are also mainly typical inhabitants of the litter.
We believe that the domination and subdomination of chortobiont Dolycoris baccarum (Pentatomidae) in the herpetobium of separate forest biocenoses may be explained by the specific seasonal cycle and seasonal adaptations (such as summer diapauses) of species in litter [48,53,54,55].
Therefore, regular registration of typical hortobionts and dendrobionts in the litter over a long time and throughout the growing season can be considered as a result of diurnal or seasonal migrations of these species [50].
Regarding the prevalence of species related to conditions of moderate moisture, most of the superdominant and dominant (Drymus brunneus, Eremocoris plebejus plebejus, Dolycoris baccarum, and Eremocoris fenestratus) belong to the grass–forest and forest–meadow ecological complex of species, except Plinthisus longicollis (rocky desert group) and Elasmucha grisea (deciduous forest group) [1,5].
In the studied forests, a significant proportion of litter true bugs are known as typical forest species. For example, the baseline species Drymus brunneus is typical for northern forests [56] and is encountered in the subzones of northern and far-north taiga [38,57].
A dweller in the litterfall of leafy forests, Drymus sylvaticus is encountered at the southern border of medium taiga. Ligyrocoris sylvestris is widely spread in the taiga zone, forest–tundra, and in Polar and Northern Urals [58].
Rhyparochromus pini and Philomyrmex insignis were found in the litter of pine forest of Northern Kazakhstan, in the forest zone of Siberia [59,60,61].
Eremocoris plebejus plebejus is a usual litter inhabitant of the lichen pine forests of Northern Kazakhstan, pine–birch forest of the forest zone of the European part of Russia [5,58]. Eremocoris abietis dwells in the litter of the pine forests of Northern Kazakhstan [5]. The species is widespread in subzones of medium, northern, far northern taiga, and in forest–tundra, prevailing in conifer forests. It is encountered in mixed and leafy forests to the south in the taiga zone [61,62].
Eremocoris podagricus dwells mainly in forests, on limestone, or in sand soils. We distinguished the singular occurrence of E. podagricus in the mountain birch forest of the Kazakh Upland, and this species was not previously registered in the territory of Kazakhstan.
The species and subspecies of open area, subarid, and arid habitats, Nabis punctatus punctatus, Trapezonotus arenarius, Emblethis denticollis, and Plinthisus reyi, etc., under the canopy of forests, are evidence of the regional peculiarities of these cenoses, such as a wide range of environmental conditions [46,48].
The majority of discovered true bug species are widely spread: their ranges include Transpalearctic, Holarctic, and Western, with the belt boreal–subtropic group prevailing. Transpalearctic boreal–subtropic ranges are peculiar for 40.8% of species, with Transeurasian boreal–subboreal accounting for 7.4%. Holarctic ranges of species comprise 14.8% (among them, 75% of boreal–subtropic species). The species with a Western Palearctic type of range comprise 14.8%: superatlantic boreal–subtropic 7.4%; and Panatlantic boreal–subtropic 7.4%. Around 14.8% of species are related to a Western boreal–subtropic type of range; with 3.7% to Western–Sharp continental, southern; and 3.7% to extratropic pancontinental.
The faunistic complexes of litter true bugs in the studied forests specifically show, in fact, that the majority of species are distinguished by wide boreal–subtropic distribution and large longitudinal continuity of ranges—Transpalearctic, with more species of more narrow Transeurasian, Western, and Continental types of range.
5. Conclusions
According to the results of soil zoological studies inside the herpetobium of intrazonal native pine forests in the Karkaraly mountain range in the eastern part of the Kazakh Upland and the derivative forest–mountain birch forest type, 30 species and subspecies of hemipterous insects belonging to 22 genera and 8 families were discovered. Fourteen species and subspecies (ten representatives of the Lygaeidae) are registered in Central Kazakhstan for the first time. The species Eremocoris podagricus is reported from Kazakhstan for the first time.
The boreal mesophilic Drymus brunneus (Lygaeidae family), dominant in most investigated forest biotopes, are actually eurytopic here. In the arid steppe zone, formation of specific hemipterous insect complexes of the litter of pine forests and typical forest species comprise a significant proportion of true bugs (more than 26.7%). The majority has Transpalearctic boreal–subtropic ranges. The presence of a significant share (20%) of species of open spaces in forest cenoses and eurybiont species are evidence of the subtility and violation of the integrity of forests in the Kazakh Upland.
Author Contributions
Data curation, G.T.K., A.B.Y. and N.M.D.; investigation, V.S.A. and N.P.S.; methodology, V.S.A. and A.B.M.; Project administration, V.S.A., G.T.K., A.B.Y. and N.M.D.; resources, A.B.M. and M.T.K.; software, M.T.K. and A.K.A.; visualization, N.P.S.; writing—original draft, V.S.A.; writing—review and editing, N.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors are most grateful to I.M. Kerzhner (Zoological Institute of the Russian Academy of Sciences) and R.B. Asanova (Institute of Zoology Republic of Kazakhstan) for their help in identifying hemiptera.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Esenbekova, P.A. Bugs (Heteroptera) of Kazakhstan; Nur-Print: Almaty, Kazakhstan, 2013; 349p. (In Russian) [Google Scholar]
- Meirmanov, E. Eurydema wilkinsi Dist. (Hemiptera, Pentatomidae) as a pest of cabbage in the Kyzyl-Orda region. Entomol. Rev. 1959, 38, 111–118. (In Russian) [Google Scholar]
- Meyrmanov, E. Central Asian cabbage bug (Eurydema maracandica Osh.) and measures to combat it. Uzb. Biol. J. 1962, 5, 29–34. (In Russian) [Google Scholar]
- Matesova, G.Y.; Mityaev, I.D.; Yukhnevich, L.A. Insects and Mites–Pests of Fruit and Berry Crops of Kazakhstan; AN KazSSR: Alma-Ata, Kazakhstan, 1962; pp. 3–46. (In Russian) [Google Scholar]
- Asanova, R.B.; Iskakov, B.V. Harmful and Useful Hemiptera (Heteroptera) of Kazakhstan; Key. Kainar: Alma-Ata, Kazakhstan, 1977; 204p. (In Russian) [Google Scholar]
- Mityaev, I.D.; Kazenas, V.A.; Kastcheev, V.A. History, condition and prospects of entomology in Kazakhstan. Proc. Inst. Zool. 2005, 49, 70–84. (In Russian) [Google Scholar]
- Iskakov, B.V. On the fauna of soil hemiptera (Heteroptera) of Northern Kazakhstan. Izv. AN KazSSR 1976, 2, 10–13. (In Russian) [Google Scholar]
- Esenbekova, P.A.; Zlatanov, B.V. To the fauna of epigeobiont hemiptera (Hemiptera-Heteroptera) of the mountains and sub-mountain plains of the Northern Tien Shan. In Proceedings of the Scientific and Practical Conference Conservation of Biodiversity of Ecosystems of Mountain Territories of Kazakhstan, Almaty, Kazakhstan, 7–8 April 2006; pp. 125–130. (In Russian). [Google Scholar]
- Esenbekova, P.A.; Kenzhegaliev, A.M. Soil Hemiptera (Heteroptera) of Kazakhstan. Soil Sci. Agrochem. 2009, 4, 63–68. (In Russian) [Google Scholar]
- Slavchenko, N.P. Dependence of the formation of complexes of hemiptera (Heteroptera) litter on the conditions of forest growth. In Problems of Ecology of the Animal World of Central Kazakhstan; Karaganda State University: Karaganda, Kazakhstan, 1981; pp. 124–129. (In Russian) [Google Scholar]
- Slavchenko, N.P. Soil mesofauna of forest biocenoses of the Karkaraly mountain massif (Central Kazakhstan melkosopochnik). In Problems of Soil Zoology; Radyanske Zakarpatya: Kiev, Ukraine, 1981; pp. 202–203. (In Russian) [Google Scholar]
- Slawtschenko, N.P. Bodenentomophege des Karkaralinsker Naturparks (Kasachisches Hochland). In Verhandlungen des XII Internationalen Symposiums über Entomofaunistik in Mitteleuropa: Materials (Kiev, 25–30 September 1988); Naukova Dumka: Kiev, Ukraine, 1991; p. 151. [Google Scholar]
- Bekenov, A.B.; Yerzhanov, N.T.; Kapitonov, V.I.; Savchenko, N.P.; Berber, A.P.; Isenov, H.A.; Abukenova, V.S. Rare and Endangered Animals of the Kazakh Melkosopochnik; Pavlodar State University: Pavlodar, Kazakhstan, 2004; pp. 21–55. (In Russian) [Google Scholar]
- Asanova, R.B. Hemiptera of Kazakhstan. Infraoder Pentatomomorpha; Institute of Zoology and Gene Pool of Animals of NAS RK: Almaty, Kazakhstan, 1996; Chapter 1, 2; p. 312. (In Russian) [Google Scholar]
- Pavlov, N.V. Principles of formation of Flora, abbreviations and denotations. In Flora of Kazakhstan; Academy of Sciences of the Kazakh SSR: Alma-Ata, Kazakhstan, 1956; Volume 1, pp. 30–32. (In Russian) [Google Scholar]
- Gorchakovsky, P.L. Forest Oases of the Kazakh Upland; Nauka: Moscow, Russia, 1987; pp. 33–71. (In Russian) [Google Scholar]
- FAO-UNESCO Soil Map of the World. FAO-Unesco Soil Tnap of the World. VIII. North and Central Asia. Unesco, Paris. 1978. Available online: https://www.fao.org/soils-portal/soil-survey/soil-maps-and-databases/faounesco-soil-map-of-the-world/en/ (accessed on 10 May 2022).
- Gudochkin, M.V.; Chaban, P.S. Forests of Kazakhstan; Part. 1; Kazgosizdat: Alma-Ata, Kazakhstan, 1958; pp. 98–108. (In Russian) [Google Scholar]
- Gribanov, L.N. Classification of types on mountain island pine forests of the Central Kazakh upland and Kalbinsky ridge. Bull. Agric. Sci. 1965, 2, 9–15. (In Russian) [Google Scholar]
- Kupriyanov, A.N. Synopsis of the Flora of the Kazakh Upland; Academic Publishing Geo: Novosibirsk, Russia, 2020; 423p. [Google Scholar]
- Abdulina, S.A. List of Vascular Plants of Kazakhstan; Zhane Phytointroduction Institute: Almaty, Kazakhstan, 1999; 187p. [Google Scholar]
- The World Flora Oline. Available online: http://www.worldfloraonline.org/ (accessed on 30 June 2022).
- Gilarov, M.S. Taking censuses of larger soil invertebrates (mesofauna). In Methods of Soil Zoological Studies; Gilarov, M.S., Ed.; Nauka: Moscow, Russia, 1975; pp. 12–29. (In Russian) [Google Scholar]
- Tikhomirova, A.L. Taking censuses of soil surface dwelling invertabrates. In Methods of Soil Zoological Studies; Gilarov, M.S., Ed.; Nauka: Moscow, Russia, 1975; pp. 73–85. (In Russian) [Google Scholar]
- Putchkov, V.G. The main trophic groups of herbivorous hemiptera insects and the change in the nature of their nutrition during development. Zool. J. 1956, 35, 32–40. (In Russian) [Google Scholar]
- Kerzhner, I.M.; Yachevskii, T.L. Hemiptera order (Heteroptera)—Hemiptera or bugs. In Key of the Insects of the European Part of the USSR; Bey-Bienko, G.Y., Ed.; Nauka: Moscow, Russia; Leningrad, Russia, 1964; Volume 1, pp. 655–845. (In Russian) [Google Scholar]
- Vinokurov, N.N. Hemiptera Insects (Heteroptera) of Yakutia; Nauka: Leningrad, Russia, 1979; 232p. (In Russian) [Google Scholar]
- Putchkov, V.G. Hemiptera of the family Rhopalidae (Heteroptera) of the fauna of the USSR. In Keys of the Fauna of the USSR, Published by the Zoological Institute of the Academy of Sciences of the USSR; Nauka: Leningrad, Russia, 1986; Volume 146, 132p. (In Russian) [Google Scholar]
- Vinokurov, N.N.; Kanyukova, E.V. Hemiptera Insects (Heteroptera) of Siberia; Nauka: Novosibirsk, Russia, 1995; 238p. (In Russian) [Google Scholar]
- Esenbekova, P.A.; Temreshev, I.I.; Nurushev, M.Z. Ecological groups of Hemipteran fauna (Hemiptera, Heteroptera) of the Republic of Kazakhstan. Bull. Omsk St. Agr. Univ. 2015, 1, 45–50. (In Russian) [Google Scholar]
- Renkonen, O. Statistisch-okologische Untersuchunden uber die terrestishe Kafterwelt der finischen Bruchmoore. Ann. Zool. Soc. Zool. -Bot. Fenn. Vanamo 1938, 6, 1–231. [Google Scholar]
- Lebedeva, N.V.; Krivolutsky, D.A. Biological diversity and methods of assessment. In Geography and Monitoring of Biodiversity; Kasimov, N.S., Romanova, E.P., Tishkov, A.A., Eds.; Scientific and Educational-Methodical Center: Moscow, Russia, 2002; pp. 8–76. (In Russian) [Google Scholar]
- Chernov, Y.I. Basic synecological characteristics of soil invertebrates and methods of their analysis. In Methods of Soil Zoological studies; Gilarov, M.S., Ed.; Nauka: Moscow, Russia, 1975; pp. 160–216. (In Russian) [Google Scholar]
- Kerzhner, I.M.; Josifov, M. Family Miridae. In Catalogue of the Heteroptera of the Palaearctic Region, 3; Aukema, B., Rieger, C., Eds.; The Netherlands Entomological Society: Amsterdam, The Netherlands, 1999; pp. 1–576. [Google Scholar]
- Göllner-Scheiding, U. Family Acanthosomatidae. In Catalogue of the Heteroptera of the Palaearctic Region, 5; Aukema, B., Rieger, C., Eds.; The Netherlands Entomological Society: Amsterdam, The Netherlands, 2006; pp. 166–181. [Google Scholar]
- Rider, D.A. Family Pentatomidae. In Catalogue of the Heteroptera of the Palaearctic Region, 5; Aukema, B., Rieger, C., Eds.; The Netherlands Entomological Society: Amsterdam, The Netherlands, 2006; pp. 233–384. [Google Scholar]
- Aukema, B.; Rieger, C.; Rabitsch, W. (Eds.) Catalogue of the Heteroptera of the Palaearctic Region, 6; Suppliment; The Netherlands Entomological Society: Amsterdam, The Netherlands, 2013; 629p. [Google Scholar]
- Vinokurov, N.N.; Kanyukova, E.V.; Golub, V.B. Catalogue of the Heteroptera of Asian Part of Russia; Nauka: Novosibirsk, Russia, 2010; 317p. (In Russian) [Google Scholar]
- Emeljanov, A.F. Proposals on the classification and nomenclature of areals. Entomol. Rev. 1974, 53, 11–26. (In Russian) [Google Scholar]
- Gilarov, M.S. Species, population and biocenosis. Russ. Ornithol. J. 2015, 24, 247–259. (In Russian) [Google Scholar]
- Nosova, L.M.; Fomicheva, L.I.; Shapechenkova, V.L. Insect complexes and features of interaction of phytophages with plants in pine crops. News Acad. Sci. USSR 1984, 5, 687–701. (In Russian) [Google Scholar]
- Nosova, L.M.; Tikhonova, E.V.; Leonova, N.B. The effects of tree edifiers on biological diversity of forest ecosystems. For. Sci. 2005, 4, 40–48. (In Russian) [Google Scholar]
- Kondratieva, A.M.; Golub, V.B. On the study of the fauna of hemipterous insects of coastal areas of reservoirs of the Usmansky Bor (Voronezh region). Vestnic Mordovian Univ. 2009, 1, 32–35. (In Russian) [Google Scholar]
- Shiperovich, V.Y. The fauna of soils and forest stands in various types of forest in the reserve “Buzuluk pine forest”. Zool. Zhurnal 1939, 18, 196–210. (In Russian) [Google Scholar]
- Kirichenko, A.N. Review of the hemipterous insect of the areas of middle and lower reaches of the Ural River and the Volga-Ural interfluve. In Proceedings of the ZIN of the USSR Academy of Sciences; Steinberg, D.M., Ed.; Publishing House of the USSR Academy of Sciences: Moscow, Russia; Leningradб, Russia, 1954; Volume XVI, pp. 285–320. (In Russian). [Google Scholar]
- Galich, D.E. Lygaeidae (Heteroptera) of the Tyumen region. Sci. Bull. Belgorod State Univ. 2014, 3, 53–57. (In Russian) [Google Scholar]
- Vinokurov, N.N.; Golub, V.B.; Zinovieva, A.N. On Hemiptera of the family Lygaeidae (Heteroptera) of Bashkortostan. Scientific Foundation “Biologist”. Mon. Sci. J. 2015, 1, 28–31. (In Russian) [Google Scholar]
- Svyatoduh, N.Y.; Golub, V.B. Ecological complexes of true bugs (Hemiptera: Heteroptera) as above-ground inhabitants of Tellerman oakery (Voronezh Province, Russia). News St. Petersburg For. Acad. 2015, 211, 105–118. (In Russian) [Google Scholar]
- Vinokurov, N.N.; Rudoi, V.V. To the fauna of terrestrial bugs (Heteroptera: Cimicomorpha, Pentatomomorpha) of Altai Krai (Russia). Russ. Entomol. 2022, 31, 1–9. [Google Scholar] [CrossRef]
- Kerzhner, I.M. Fauna of the USSR. Proboscis Insects; V. XIII. I. 2.Hemiptera of the Nabidae Family. New Series, 124. AN USSR, Zoological Institute; Nauka: Leningrad, Russia, 1981; 327p. (In Russian) [Google Scholar]
- Redei, D.; Harmat, B.; Hufnagel, L. Ecology of acalipta spesies occurring in Hungary (Insecte: Heteroptera: Tingidae): Data to the knowledge on the ground-living Heteroptera of Hungary, № 3. Appl. Ecol. Environ. Res. 2004, 2, 73–91. [Google Scholar] [CrossRef]
- Vinokurov, N.N. Bugs of the genus Suldula V.D., 1914 (Heteroptera, Saldidae) in Russian and adjacent countries. Euroasian Entomol. J. 2004, 3, 101–118. (In Russian) [Google Scholar]
- Perepelitsa, L.V. The role of the photoperiod in the development of Dolycoris baccarum. Bull. Union Sci. Res. Inst. Plant Prot. 1971, 21, 11–13. (In Russian) [Google Scholar]
- Saulich, A.K.; Musolin, D.L. Diapause in the seasonal cycle of stink bugs (Heteroptera, Pentatomidae) from the temperate zone. Entomol. Rev. 2012, 92, 1–26. (In Russian) [Google Scholar] [CrossRef]
- Saulich, A.K.; Musolin, D.L. Seasonal cycles of Pentatomoidea. Invasive stink bugs and related species (Pentatomoidea). In Biology, Higher Systematics, Semiochemistry, and Management; McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 543–585. [Google Scholar]
- Kirichenko, A.N. True bugs (Hemiptera-Heteroptera). In Animal World of the USSR; Pavlovsky, E.N., Ed.; USSR Academy of Sciences Press: Moscow, Russia; Leningrad, Russia, 1953; Volume 4, pp. 486–505. (In Russian) [Google Scholar]
- Kozminykh, V.O. True bugs (Heteroptera: Lygaeidae) of the Middle Urals (with resulting data to the total Urals fauna). Eversmannia 2019, 59–60, 10–39. (In Russian) [Google Scholar]
- Zinovyeva, A.N. The seed bugs (Heteroptera, Lygaeidae) of the North-East of European part of Russia. Entomol. Rev. 2021, 100, 97–110. [Google Scholar] [CrossRef]
- Nemkov, V.A. Entomofauna of Steppe Cis-Urals (the History of Formation and Study of Composition, Changes, Safety); Universitetskaya kniga: Moscow, Russia, 2011; 316p. (In Russian) [Google Scholar]
- Vinokurov, N.N. Annotated catalogue of the true bugs (Heteroptera) of Yakutia. Zoosystematica Rossica. Suppl. 2020, 3, 3–203. (In Russian) [Google Scholar] [CrossRef]
- Neimorovets, V.V. Spesies of the genus Eremocoris Fieber (Heteroptera, Lygaeidae) from Russia and adjacent countries. Entomol. Rev. 2002, 81, 666–683. (In Russian) [Google Scholar]
- Esenbekova, P.A. Zoogeographic features of hemiptera of Kazakhstan. Tethys Entomol. Res. 2010, 19, 59–78. (In Russian) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).