Shading Gradients Drive Differential Responses of Leaf Traits in an Early Community Germinated by Forest Topsoil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Treatment
2.2. Sampling and Measurement
2.3. Data Analysis
3. Results
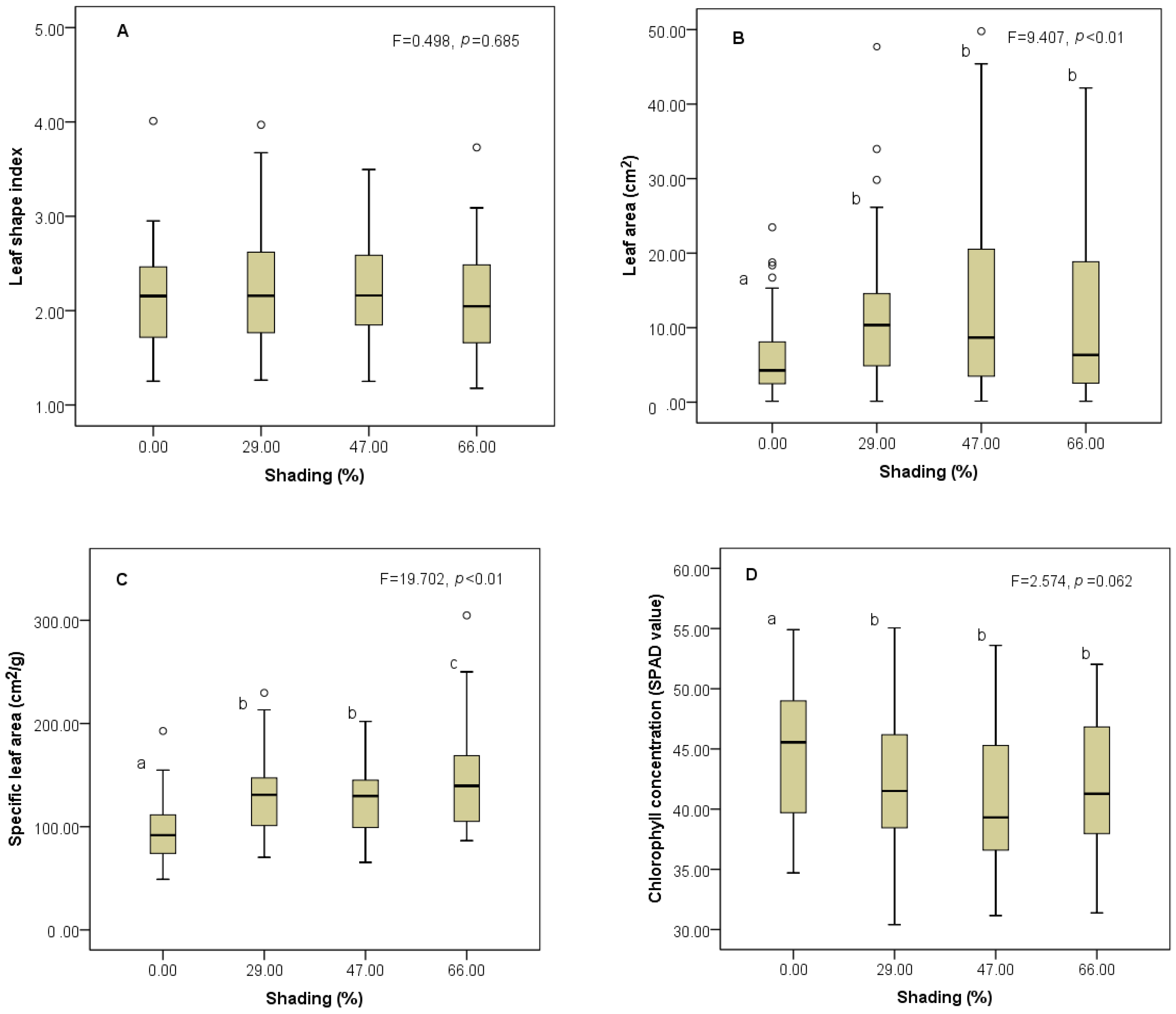
3.1. Responses of Trait Mean to Shading Gradients
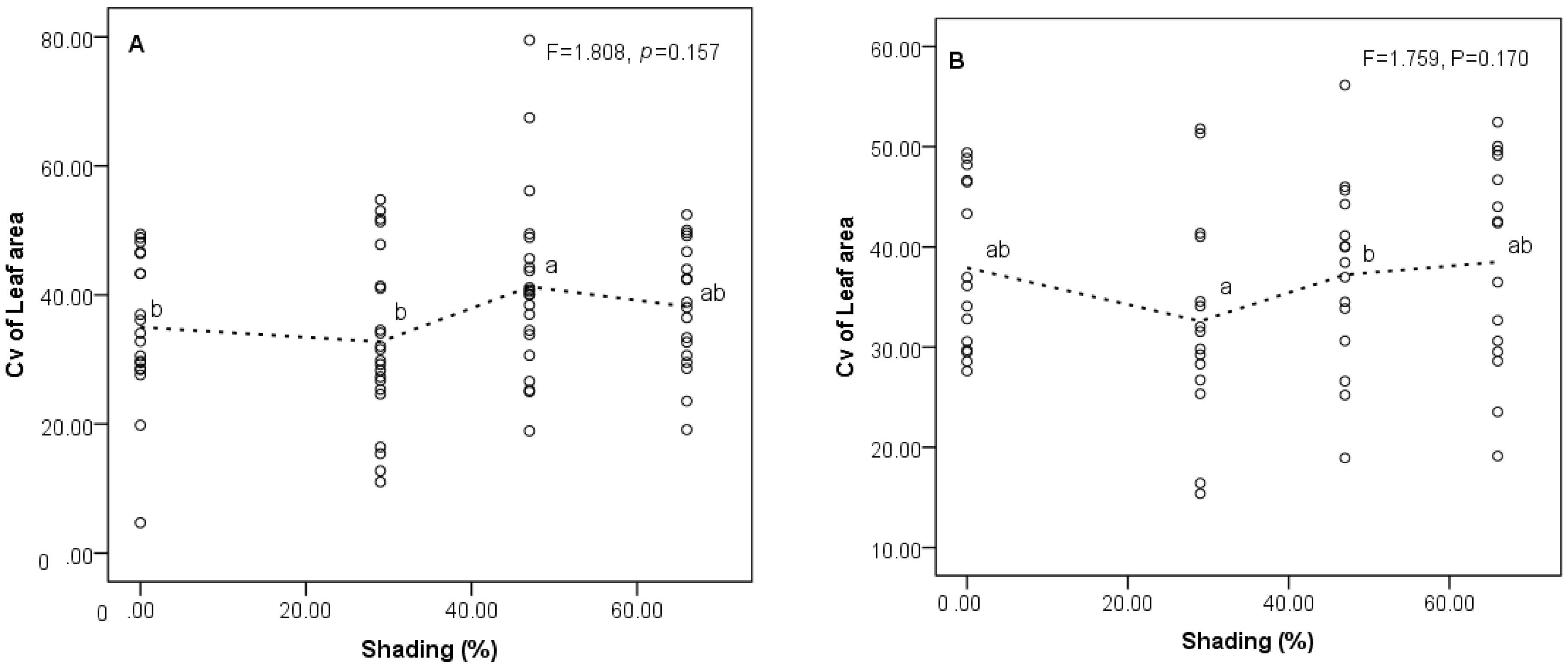
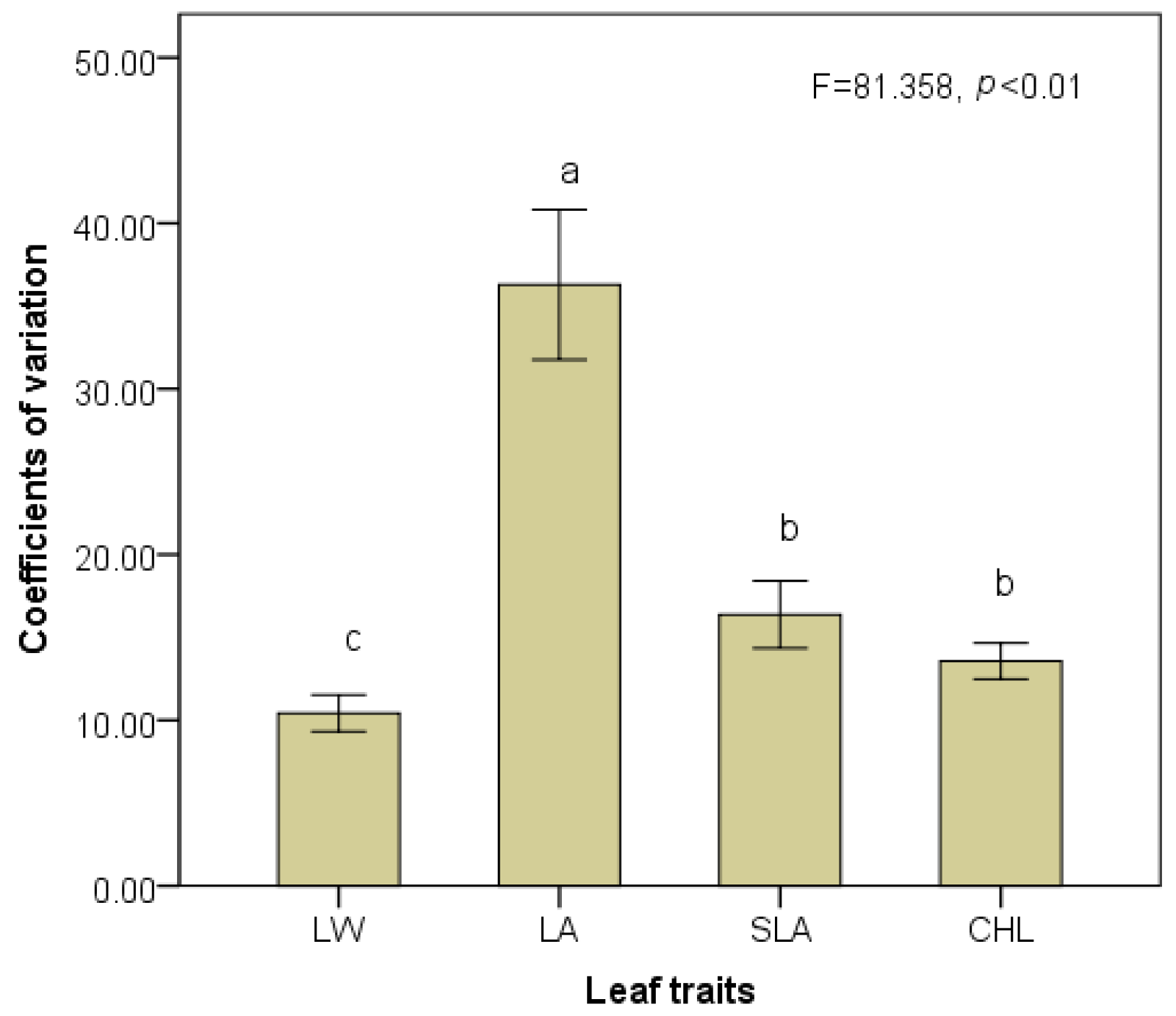
3.2. Responses of Trait Variability to Shading Gradients
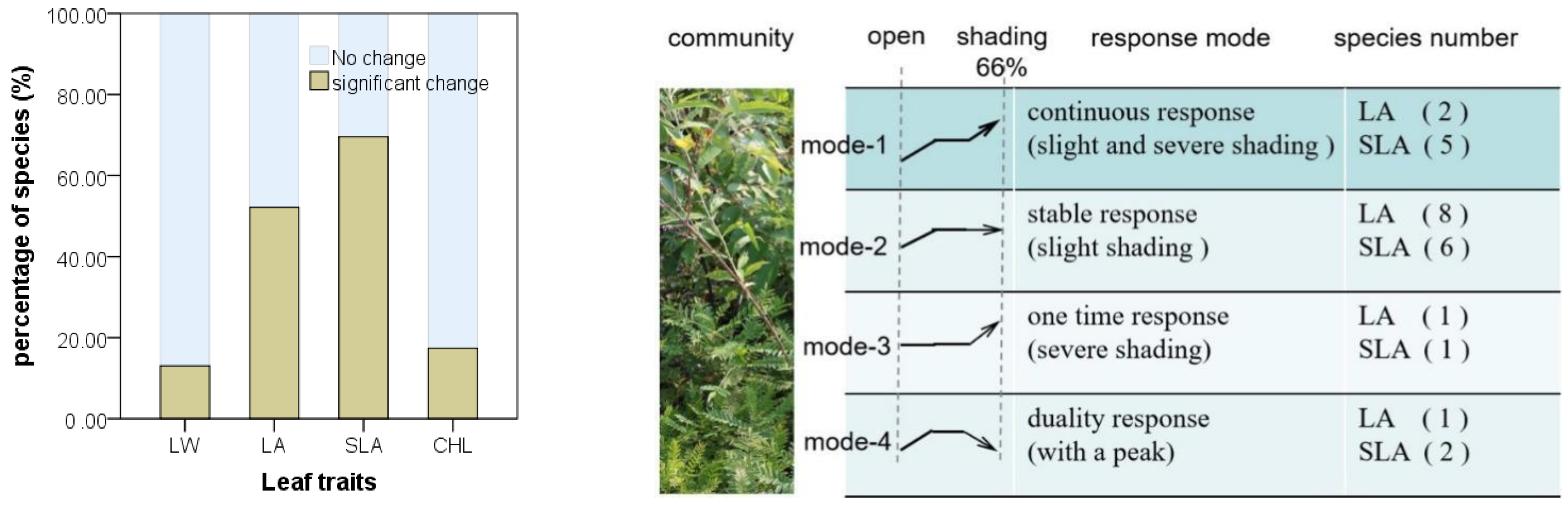
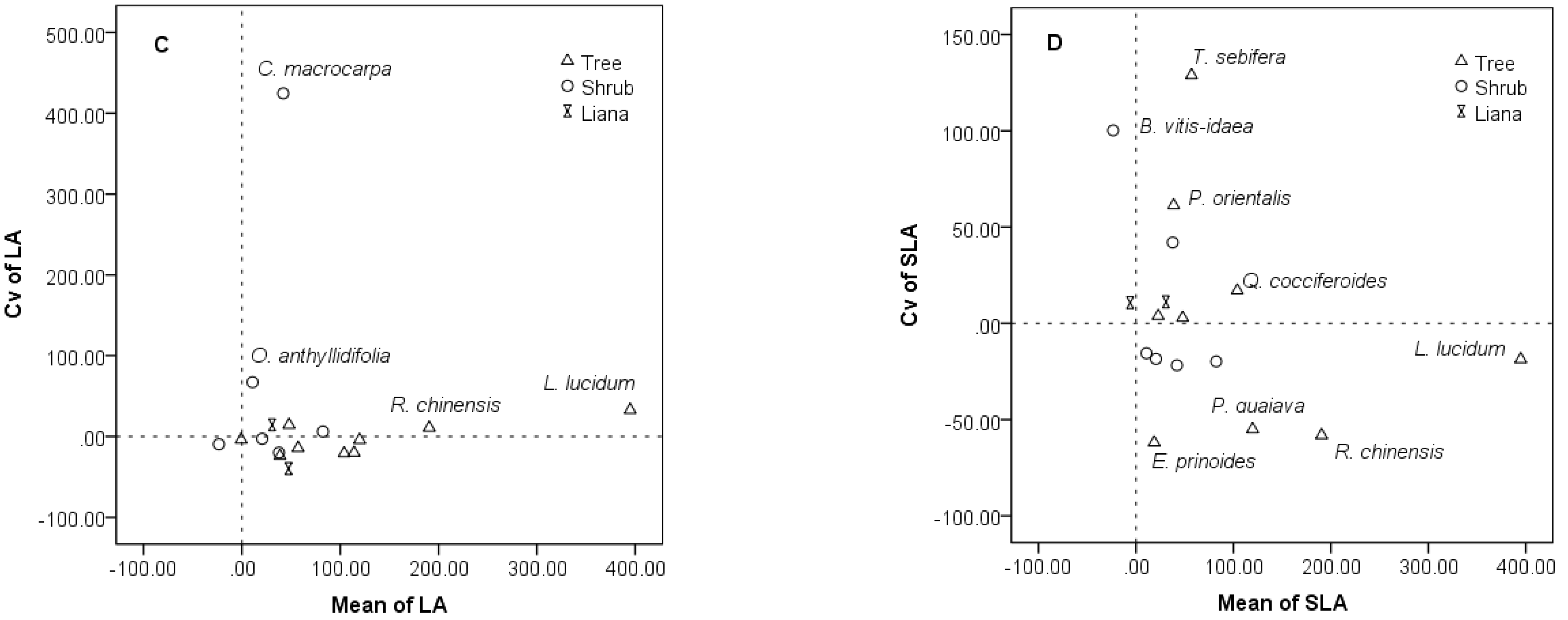
3.3. Species-Specific Changes of Leaf Traits
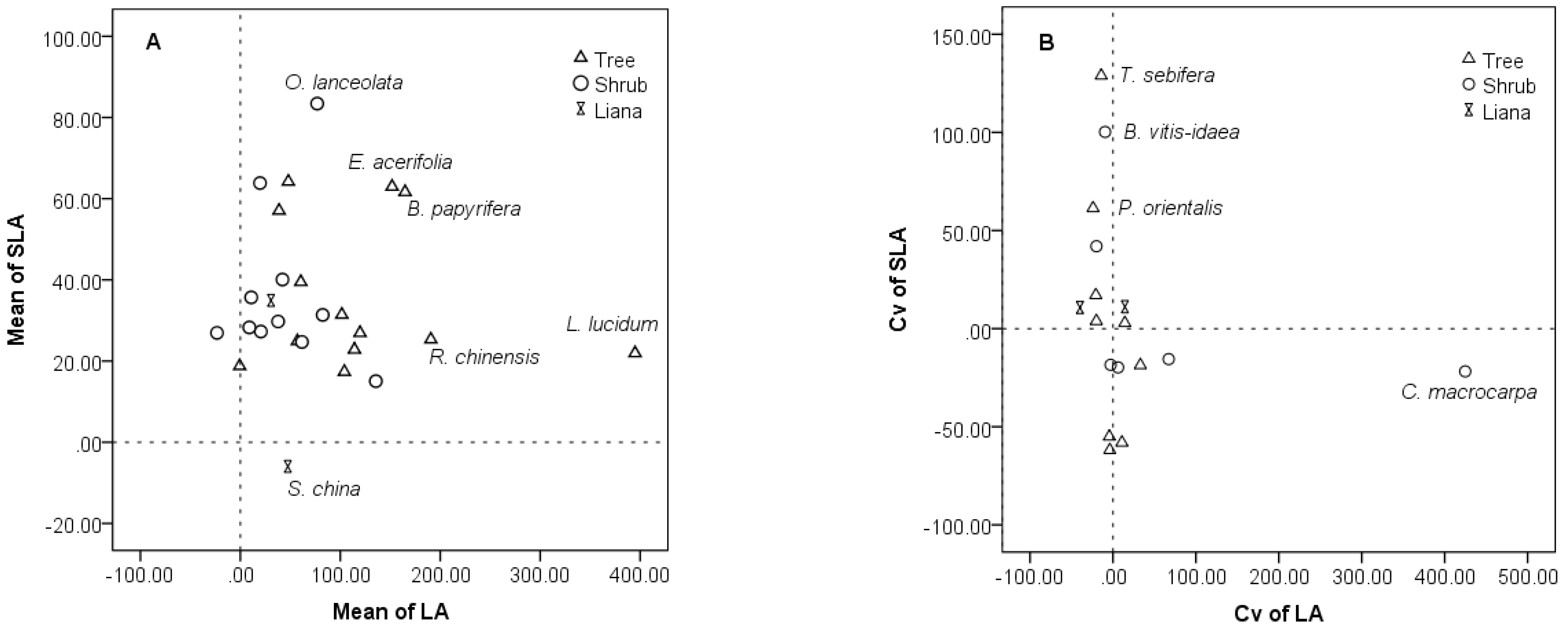
3.4. Intraspecific and Interspecific Variations in the Restoration Community
4. Discussion
4.1. Changes of Trait Mean along Shading Gradients
4.2. Changes of Trait Variability among Populations in Different Shading Treatments
4.3. Inter- and Intra-Specific Variation in the Community
4.4. Implication for Management of Restoration Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Global Forest Resources Assessment 2020: Main Report; FAO: Rome, Italy, 2020; ISBN 9789251329740. [Google Scholar]
- Matricardi, E.A.T.; Skole, D.L.; Costa, O.B.; Pedlowski, M.A.; Samek, J.H.; Miguel, E.P. Long-term forest degradation surpasses deforestation in the Brazilian Amazon. Science 2020, 369, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Rokich, D.P.; Dixon, K.W.; Sivasithamparam, K.; Meney, K.A. Topsoil handling and storage effects on woodland restoration in western Australia. Restor. Ecol. 2010, 8, 196–208. [Google Scholar] [CrossRef]
- Douterlungne, D.; Martinez, G.M.C.; Badano, E.I.; Cano, J.A.F.; Rivas, J.D.F. Restoring oak forests on bare ground using topsoil translocation. Ecol. Eng. 2018, 120, 76–84. [Google Scholar] [CrossRef]
- Zhou, Q.Q.; Li, F.; Cai, X.A.; Rao, X.Q.; Zhou, L.X.; Liu, Z.F.; Lin, Y.B.; Fu, S.L. Survivorship of plant species from soil seedbank after translocation from subtropical natural forests to plantation forests. For. Ecol. Manag. 2019, 432, 741–747. [Google Scholar] [CrossRef]
- Zhao, G.J.; Shen, Y.X.; Liu, W.Y.; Tan, B.L.; Li, Z.J.; Wang, Q.H. Quantifying the effect of shading and watering on seed germination in translocated forest topsoil at a subtropical karst of China. For. Ecol. Manag. 2020, 459, 117811. [Google Scholar] [CrossRef]
- Poorter, L. Growth responses of 15 rain-forest tree species to a light gradient: The relative importance of morphological and physiological traits. Funct. Ecol. 1999, 13, 396–410. [Google Scholar] [CrossRef]
- Lin, J.; Harcombe, P.A.; Fulton, M.R.; Hall, R.W. Sapling growth and survivorship as a function of light in a mesic forest of southeast Texas, USA. Oecologia 2002, 132, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, C.; Oikawa, S.; Hikosaka, K. Seasonal change in light partitioning among coexisting species of different functional groups along elevation gradient in subalpine moorlands. New Phytol. 2014, 204, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Melcher, P.J.; Liu, W.H.; Middleton, E.; Pardee, T. How strong is intracanopy leaf plasticity in temperate deciduous trees? Am. J. Bot. 2006, 93, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Legner, N.; Fleck, S.; Leuschner, C. Within-canopy variation in photosynthetic capacity, SLA and foliar N in temperate broad-leaved trees with contrasting shade tolerance. Trees 2014, 28, 263–280. [Google Scholar] [CrossRef]
- Sasso, S.A.Z.; Marchese, J.A.; Moura, A.P.C.; Gil, B.V.; Perboni, A.T.; Donazzolo, J.; Oliveira, F.L.R.; Sant’Anna-Santos, B.F.; Rohr, A.; Danner, M.A. Shade and sapling size influence restoration of Araucaria angustifolia. J. For. Res. 2020, 32, 1833–1841. [Google Scholar] [CrossRef]
- Williams, L.J.; Cavender-Bares, J.; Paquette, A.; Messier, C.; Reich, P.B. Light mediates the relationship between community diversity and trait plasticity in functionally and phylogenetically diverse tree mixtures. J. Ecol. 2020, 108, 1617–1634. [Google Scholar] [CrossRef]
- Pagès, J.P.; Pache, G.; Joud, D.; Magnan, N.; Michalet, R. Direct and indirect effects of shade on four forest tree seedlings in the french alps. Ecology 2003, 84, 2741–2750. [Google Scholar] [CrossRef]
- Semchenko, M.; Lepik, M.; Götzenberger, L.; Zobel, K. Positive effect of shade on plant growth: Amelioration of stress or active regulation of growth rate? J. Ecol. 2012, 100, 459–466. [Google Scholar] [CrossRef]
- Callaway, R.M. Positive Interactions and Interdependence in Plant Communities; Springer: Dordrecht, The Netherlands, 2007; pp. 23–48. [Google Scholar]
- Chang-Yang, C.-H.; Needham, J.; Lu, C.-L.; Hsieh, C.-F.; Sun, I.-F.; McMahon, S.M. Closing the life cycle of forest trees: The difficult dynamics of seedling-to-sapling transitions in a subtropical rain forest. J. Ecol. 2021, 109, 2705–2716. [Google Scholar] [CrossRef]
- Jefferson, L.V.; Pennacchio, M. The impact of shade on establishment of shrubs adapted to the high light irradiation of semi-arid environments. J. Arid Environ. 2005, 63, 706–716. [Google Scholar] [CrossRef]
- López, R.P.; Squeo, F.A.; Gutiérrez, J.R. Differential effect of shade, water and soil type on emergence and early survival of three dominant species of the Atacama Desert. Austral Ecol. 2016, 41, 428–436. [Google Scholar] [CrossRef]
- Lusk, C.H.; Reich, P.B.; Montgomery, R.A.; Ackerly, D.D.; Cavender-Bares, J. Why are evergreen leaves so contrary about shade? Trends Ecol. Evol. 2008, 23, 299–303. [Google Scholar] [CrossRef]
- Sánchez-Gómez, D.; Zavala, M.A.; Valladares, F. Functional traits and plasticity linked to seedlings’ performance under shade and drought in Mediterranean woody species. Ann. For. Sci. 2008, 65, 311. [Google Scholar] [CrossRef] [Green Version]
- Lusk, C.H.; Laughlin, D.C. Regeneration patterns, environmental filtering and tree species coexistence in a temperate forest. New Phytol. 2017, 213, 657–668. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.P.; Sun, J.; Wang, M.T.; Lyu, M.; Niklas, K.J.; Michaletz, S.T.; Zhong, Q.L.; Cheng, D.L. The leaf economics spectrum constrains phenotypic plasticity across a light gradient. Front. Plant Sci. 2020, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Daz, S.; Cabido, M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Valencia, R.; Ackerly, D.D. Functional traits and niche-based tree community assembly in an Amazonian forest. Science 2008, 322, 580–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balachowski, J.A.; Volaire, F.A. Implications of plant functional traits and drought survival strategies for ecological restoration. J. Appl. Ecol. 2018, 55, 631–640. [Google Scholar] [CrossRef]
- Bergholz, K.; Kober, K.; Jeltsch, F.; Schmidt, K.; Weiss, L. Trait means or variance—What determines plant species’ local and regional occurrence in fragmented dry grasslands? Ecol. Evol. 2021, 11, 3357–3365. [Google Scholar] [CrossRef]
- Umaña, M.N.; Swenson, N.G.; Marchand, P.; Cao, M.; Lin, L.X.; Zhang, C.C. Relating leaf traits to seedling performance in a tropical forest: Building a hierarchical functional framework. Ecology 2021, 102, e03385. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, M.; Swenson, N.G. Why functional traits do not predict tree demographic rates. Trends Ecol. Evol. 2018, 33, 326–336. [Google Scholar] [CrossRef]
- Zeldin, J.; Lichtenberger, T.M.; Foxx, A.J.; Williams, E.W.; Kramer, A.T. Intraspecific functional trait structure of restoration-relevant species: Implications for restoration seed sourcing. J. Appl. Ecol. 2020, 57, 864–874. [Google Scholar] [CrossRef]
- Zirbel, C.R.; Brudvig, L.A. Trait-environment interactions affect plant establishment success during restoration. Ecology 2020, 101, e02971. [Google Scholar] [CrossRef] [Green Version]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef] [Green Version]
- Burton, J.I.; Perakis, S.S.; Mckenzie, S.C.; Lawrence, C.E.; Puettmann, K.J. Intraspecific variability and reaction norms of forest understorey plant species traits. Funct. Ecol. 2017, 31, 1881–1893. [Google Scholar] [CrossRef] [Green Version]
- Roches, S.D.; Post, D.M.; Turley, N.E.; Bailey, J.K.; Hendry, A.P.; Kinnison, M.T.; Schweitzer, J.A.; Palkovacs, E.P. The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2018, 2, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Candeias, M.; Fraterrigo, J. Trait coordination and environmental filters shape functional trait distributions of forest understory herbs. Ecol. Evol. 2020, 10, 14098–14112. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Prentice, I.C.; Wright, I.J.; Evans, B.J.; Togashi, H.F.; Caddy-Retalic, S.; McInerney, F.A.; Sparrow, B.; Leitch, E.; Lowe, A.J. Components of leaf-trait variation along environmental gradients. New Phytol. 2020, 228, 82–94. [Google Scholar] [CrossRef]
- Mello, F.N.A.; Estrada-Villegas, S.; DeFilippis, D.M.; Schnitzer, S.A. Can functional traits explain plant coexistence? A case study with tropical lianas and trees. Diversity 2020, 12, 397. [Google Scholar] [CrossRef]
- Di Biase, L.; Fattorini, S.; Cutini, M.; Bricca, A. The role of inter- and intraspecific variations in grassland plant functional traits along an elevational gradient in a Mediterranean mountain area. Plants 2021, 10, 359. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Roumet, C.; Cruz, P.; Blanchard, A.; Autran, P.; Garnier, E. Evidence for a ‘plant community economics spectrum’ driven by nutrient and water limitations in a Mediterranean rangeland of southern France. J. Ecol. 2012, 100, 1315–1327. [Google Scholar] [CrossRef] [Green Version]
- Niu, K.; Zhang, S.; Lechowicz, M.J. Harsh environmental regimes increase the functional significance of intraspecific variation in plant communities. Funct. Ecol. 2020, 34, 1666–1677. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Merilä, J. Heritable variation and evolution under favourable and unfavourable conditions. Trends Ecol. Evol. 1999, 14, 96–101. [Google Scholar] [CrossRef]
- Valladares, F.; Gianoli, E.; Gómez, J.M. Ecological limits to plant phenotypic plasticity. New Phytol. 2007, 176, 749–763. [Google Scholar] [CrossRef]
- Keenan, T.F.; Niinemets, Ü. Global leaf trait estimates biased due to plasticity in the shade. Nat. Plants 2016, 3, 16201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerband, A.C.; Funk, J.L.; Barton, K.E. Intraspecific trait variation in plants: A renewed focus on its role in ecological processes. Ann. Bot. 2021, 127, 397–410. [Google Scholar] [CrossRef]
- Hofhansl, F.; Chacón-Madrigal, E.; Brännström, Å.; Dieckmann, U.; Franklin, O. Mechanisms driving plant functional trait variation in a tropical forest. Ecol. Evol. 2021, 11, 3856–3870. [Google Scholar] [CrossRef]
- Buchanan, S.; Isaac, M.E.; Van den Meersche, K.; Martin, A.R. Functional traits of coffee along a shade and fertility gradient in coffee agroforestry systems. Agrofor. Syst. 2019, 93, 1261–1273. [Google Scholar] [CrossRef]
- Callaway, R.M.; Pennings, S.C.; Richards, C.L. Phenotypic plasticity and interactions among plants. Ecology 2003, 84, 1115–1128. [Google Scholar] [CrossRef] [Green Version]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T.L. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef] [Green Version]
- Sancho-Knapik, D.; Escudero, A.; Mediavilla, S.; Scoffoni, C.; Zailaa, J.; Cavender-Bares, J.; Álvarez-Arenas, T.G.; Molins, A.; Alonso-Forn, D.; Ferrio, J.P.; et al. Deciduous and evergreen oaks show contrasting adaptive responses in leaf mass per area across environments. New Phytol. 2021, 230, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Illuminated behaviour: Phytochrome as a key regulator of light foraging and plant anti-herbivore defence. Plant Cell Environ. 2009, 32, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calcerrada, J.; Pardos, J.A.; Gil, L.; Aranda, I. Acclimation to light in seedlings of Quercus petraea (Mattuschka) Liebl. and Quercus pyrenaica Willd. planted along a forest-edge gradient. Trees 2007, 21, 45–54. [Google Scholar] [CrossRef]
- Lambers, H.; Oliveira, R.S. Physiological Plant Ecology; Springer: Cham, Switzerland, 2019; p. 37. [Google Scholar]
- Matesanz, S.; Ramírez-Aliente, J.A. A review and meta-analysis of intraspecific differences in phenotypic plasticity: Implications to forecast plant responses to climate change. Glob. Ecol. Biogeogr. 2019, 28, 1682–1694. [Google Scholar] [CrossRef]
- Valladares, F.; Wright, S.J.; Lasso, E.; Kitajima, K.; Pearcy, R.W. Plastic phenotypic response to light of 16 congeneric shrubs from a panamanian rainforest. Ecology 2000, 81, 1925–1936. [Google Scholar] [CrossRef]
- Carlucci, M.B.; Debastiani, V.J.; Pillar, V.D.; Duarte, L.D.S. Between- and within-species trait variability and the assembly of sapling communities in forest patches. J. Veg. Sci. 2015, 26, 21–31. [Google Scholar] [CrossRef]
- Anderegg, L.D.L.; Loy, X.; Markham, I.P.; Elmer, C.M.; Hovenden, M.J.; HilleRisLambers, J.; Mayfield, M.M. Aridity drives coordinated trait shifts but not decreased trait variance across the geographic range of eight Australian trees. New Phytol. 2021, 229, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.B.; Leigh, A.; Boyce, C.K.; Jones, C.S.; Niklas, K.J.; Royer, D.L.; Tsukaya, H. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 2011, 38, 535–552. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Huang, X.L.; Chen, J.Z.; Li, L.X.; Cheng, J.; Wang, S.; Liu, J.M. Plasticity of leaf traits of juglans regia l. f. luodianense liu et xu seedlings under different light conditions in karst habitats. Forests 2021, 12, 81. [Google Scholar] [CrossRef]

| Leaf Traits | Source of Variation | df | Relative Variation (%) | F | p |
|---|---|---|---|---|---|
| Leaf shape index (LW) | Intra-species | 3 | 1.58 | 0.821 | 0.488 |
| Inter-species | 19 | 98.42 | 49.981 | <0.001 | |
| Leaf area (LA) | Intra-species | 3 | 29.54 | 8.630 | <0.001 |
| Inter-species | 19 | 70.46 | 20.587 | <0.001 | |
| Specific leaf area (SLA) | Intra-species | 3 | 46.94 | 21.240 | <0.001 |
| Inter-species | 19 | 53.06 | 24.009 | <0.001 | |
| Chlorophyll concentration (CHL) | Intra-species | 3 | 7.39 | 1.595 | 0.202 |
| Inter-species | 17 | 92.61 | 19.983 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Zhao, G.; Shen, Y.; Li, Z.; Tan, B.; Zhu, H.; Wang, Q.; Fu, X. Shading Gradients Drive Differential Responses of Leaf Traits in an Early Community Germinated by Forest Topsoil. Diversity 2022, 14, 600. https://doi.org/10.3390/d14080600
Chen F, Zhao G, Shen Y, Li Z, Tan B, Zhu H, Wang Q, Fu X. Shading Gradients Drive Differential Responses of Leaf Traits in an Early Community Germinated by Forest Topsoil. Diversity. 2022; 14(8):600. https://doi.org/10.3390/d14080600
Chicago/Turabian StyleChen, Fajun, Gaojuan Zhao, Youxin Shen, Zhenjiang Li, Beilin Tan, Hong Zhu, Qinghe Wang, and Xun Fu. 2022. "Shading Gradients Drive Differential Responses of Leaf Traits in an Early Community Germinated by Forest Topsoil" Diversity 14, no. 8: 600. https://doi.org/10.3390/d14080600
APA StyleChen, F., Zhao, G., Shen, Y., Li, Z., Tan, B., Zhu, H., Wang, Q., & Fu, X. (2022). Shading Gradients Drive Differential Responses of Leaf Traits in an Early Community Germinated by Forest Topsoil. Diversity, 14(8), 600. https://doi.org/10.3390/d14080600






