Abstract
Although sharks have a fundamental role in maintaining the balance of aquatic ecosystems, exerting a great influence on lower levels of the food chain, their populations are declining worldwide due, to a large extent, to overfishing. Of the 64 species registered in Ecuador, from January to December 2019, 19 species were recorded in Manta from the 15,455 captured individuals, with the family Carcharhinidae being the most abundant in the catches (69.4%), and the most abundant species was Prionace glauca (57.9%). Regarding threatened species, such as Carcharhinus longimanus, Sphyrna lewini, and Sphyrna zygaena, a greater presence of immature specimens was observed in landings. However, information on the composition and biological aspects of shark species in the Ecuadorian Pacific is very scarce. Therefore, research on the characteristics of life history (age, growth, and maturity) are of utmost importance for the stock assessments that are being exploited, especially in developing countries, where this information is lacking, causing inadequate management of fishery resources.
1. Introduction
Sharks have a fundamental role in maintaining the balance of aquatic ecosystems because, being at higher trophic levels, they can exert a great influence on lower levels [1]. However, they are currently declining worldwide due to overfishing, habitat degradation, climate change, and pollution [2,3,4,5]. This decrease is intensified by their biological characteristics, such as slow growth, late maturity, and few offspring [6]. Regional information on shark catches is essential to be able to know the patterns of catches at a global level [7]. Likewise, research on life history characteristics (age, growth, and maturity) is very important for the stock assessments that are being developed [8]. However, in developing countries this information is very scarce [9,10]. This lack of information can lead to the use of information from other regions, causing an inadequate management of fishery resources [11,12].
According to the FAO [13], in South America from 1950 to 2020, 4,133,991 live-weight tons of cartilaginous fish were reported. In Peru, González–Pestana et al. [14] reported that 6099 tons (t) of sharks were landed per year from 1950 to 2010. The most abundant species in Peruvian waters were Prionace glauca, Isurus oxyrinchus, and Sphyrna zygaena. In the Ecuadorian Pacific, Jacquet et al. [15] estimated that 7000 tons of sharks were landed per year from 1979 to 2004. About 119 species of cartilaginous fish have been recorded in Ecuador, of which 64 correspond to sharks [16]. The most frequently landed shark species in the Ecuadorian Pacific are Alopias pelagicus, P. glauca and Carcharhinus falciformis [17]. The main ports where sharks are landed in Ecuador are Manta, Santa Rosa, Esmeraldas, Anconcito, Puerto López and Puerto Bolívar. Manta is the port with the highest number of shark landings [18]. In addition, during the dry season (April–November), this port presents the highest number of shark catches [17]. Information on the composition of species and some biological aspects in the Ecuadorian Pacific is very scarce and dates back more than 10 years [17,18,19,20,21]. Therefore, the objective of this study is to update the information on the species composition, seasonality, size structures, sexual proportion, morphometric relationships, and sexual maturity size in Ecuadorian waters.
2. Materials and Methods
From January to December of 2019, field trips were conducted to the “Playita mía” pier in Manta (0°56′59″ S, 80°42′34″ W). The visits were daily throughout the year with the objective of having a good sampling effort. Sharks were landed gutted with all fins, and were accurately identified at a species level by using the guide of Martínez–Ortiz and García–Domínguez [22]. The landed organisms were sexed and measured with a measuring tape graduated in centimeters (cm). The measurements taken were total length (TL), precaudal length (PCL), and interdorsal length (IL). In males, clasper length (CL) was measured from the point of rotation to the tip of the copulatory apparatus. In addition, clasper characteristics such as rotation, noncalcification, semicalcification, total calcification, rhipidion aperture, and absence or presence of sperm were recorded [23,24,25].
Like other studies [26,27,28], weight was estimated from TL by using the following potential equation,
where W is the weight, a is the intercept and b the slope. The values of these parameters for each shark species recorded in this study were obtained from previous ones as shown in Table 1.
For the adjustment of a logistic model to the binomial maturity data (0, immature; 1, mature), categories 0, non-calcified, and 1, semi-calcified, were grouped as immature and category 2, calcified, as mature. Maturity size for males was estimated by using the following equation [29],
where Pmax is the maximum proportion of mature specimens, l50 and l95 correspond to the length at which 50% and 95% of individuals have reached sexual maturity, respectively.
The inflexion point was estimated by using the following equation [30],
where a is the inflexion point, CLmax and CLmin are the maximum and minimum clasper lengths, respectively. The inflexion point was only estimated when the data were adjusted to the logistic function [31,32].
The lengths of the most abundant species (n ≥ 20) were plotted in frequency histograms. If the data met the normality and homoscedasticity assumptions, the student’s t-test was performed, otherwise, the Mann–Whitney U-test was performed, to know whether there were differences between average lengths of sexes. The chi-square test () was also performed to determine whether the sexual ratio was significantly different with respect to the expected 1:1 ratio [33].
All analyses and graphs were performed in the statistical environment R [34] by using the AquaticLifeHistory [35,36], cowplot [37], and tidyverse [38] packages.

Table 1.
Parameters used to estimate the weight from the length of the sharks landed in the Ecuadorian Pacific.
Table 1.
Parameters used to estimate the weight from the length of the sharks landed in the Ecuadorian Pacific.
| Species | a | b | Source |
|---|---|---|---|
| Alopias pelagicus | 4.61 × 10−5 | 2.494 | [39] |
| Alopias superciliosus | 1.02 × 10−5 | 2.78 | [40] |
| Carcharhinus falciformis | 2.92 × 10−6 | 3.15 | [41] |
| Carcharhinu longimanus | 1.66 × 10−5 | 2.891 | [42] |
| Carcharhinus leucas | 2.71 × 10−6 | 3.20 | [43] |
| Carcharhinus limbatus | 2.512 × 10−9 | 3.1253 | [44] |
| Carcharhinus obscurus | 1.2334 × 10−5 | 2.855 | [45] |
| Carcharhinus galapagensis | 5.7 × 10−6 | 3.0283 | [46] |
| Isurus oxyrinchus | 1.1 × 10−5 | 2.95 | [47] |
| Sphyrna zygaena | 1.6 × 10−6 | 3.20 | [48] |
| Sphyrna lewini | 3.99 × 10−6 | 3.03 | [49] |
| Prionace glauca | 3.1841 × 10−6 | 3.13 | [50] |
| Mustelus lunulatus | 2 × 10−6 | 3.1538 | Briones-Mendoza unpubl. Data |
| Galeocerdo cuvier | 1.41 × 10−6 | 3.24 | [51] |
| Triaenodon obesus | 1.8 × 10−6 | 3.344 | [52] |
| Squatina californica | 7.81 × 10−9 | 3.02 | [53] |
| Ginglymostoma cirratum | 9.006 × 10−6 | 2.911 | [54] |
| Pseudocarcharias kamoharai | 9.0843 × 10−3 | 1.3455 | [55] |
3. Results
3.1. Composition of Catches
A total of 15,455 sharks (5508 males, 7788 females, and 2159 non-sexed) were recorded during 2019 in Manta (Table 2). Of the total number of sharks recorded, 3690 individuals (1739 males, 1934 females, and 17 unsexed) were measured, with an estimated biomass of 197.9 t (Table 3). The landed specimens were composed of nine families and 19 species. The most abundant families in number were Carcharhinidae (69.4%), Alopiidae (23%) and Sphyrnidae (4.9%). The most abundant species recorded during the sampling were P. glauca (57.9%), A. pelagicus (20.3%) and C. falciformis (10.7%) (Figure 1). During the 12 months of sampling, a greater abundance of sharks occurred during the dry season, representing 60.8% of the total caught sharks (Figure 2).

Table 2.
Sharks registered monthly in number during the year 2019 in Manta.

Table 3.
Descriptive statistics of shark species measured during the year 2019 in Manta. The meaning of the abbreviations of the categories of the Red List of Threatened Species of the International Union for the Conservation of Nature (IUCN): least concern (LC), near threatened (NT), vulnerable (VU), endangered (EN) and critically endangered (CR).
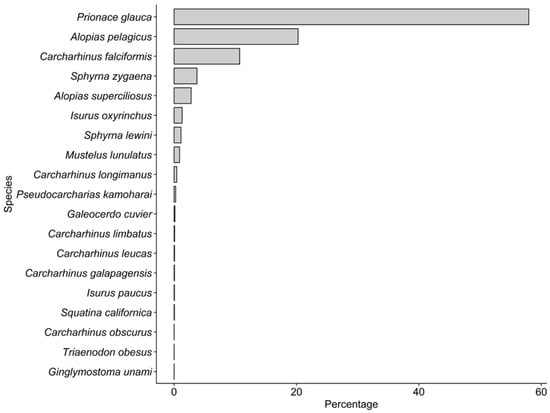
Figure 1.
Composition of landed shark species in Manta during the year 2019.
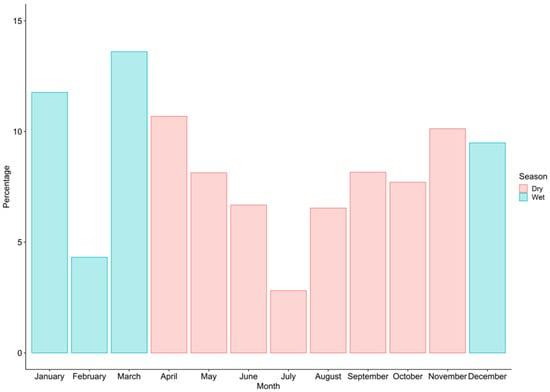
Figure 2.
Catches of sharks by the artisanal fishing fleet in Manta from January to December 2019.
3.2. Composition of Sizes, Sexual Proportion, Morphometric Relations and Maturity Size
3.2.1. Carcharhinus falciformis
A total of 1656 C. falciformis were recorded, of which 887 were females (54%), 693 were males (42%), and 76 were unsexed individuals (5%). Females were significantly more abundant than males (p < 1.058 × 10–6), but in February, May, June, July, September, November, and December the sex ratios were not significantly different from parity (Table 4a). The sizes of the females fluctuated between 61 and 246 cm TL (mean ± S.E. = 180.35 ± 1.99), whereas the males had lengths between 66 and 272 cm TL (mean ± S.E. = 176.46 ± 1.83) (Figure 3a). Females were significantly larger than males (Mann–Whitney U-test, U = 68,223.5, p = 0.015). A strong correlation was found between TL and PCL for combined sexes (TL = 1.3135PCL + 4.9085, n = 734, R2 = 0.99, p < 2.2 × 10−16), females (TL = 1.3185PCL + 4.2645, n = 350, R2 = 0.99, p < 2.2 × 10−16), and males (TL = 1.3082PCL + 5.5774, n = 384, R2 = 0.99, p < 2.2 × 10−16).

Table 4.
Monthly sex ratios of the main species landed in the Ecuadorian Pacific.
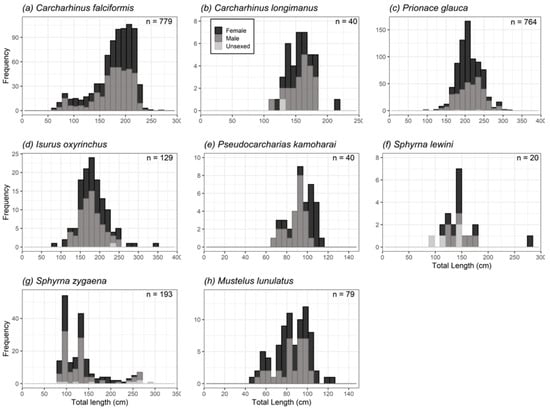
Figure 3.
Composition of sizes of shark species (n ≥ 20) landed in Manta: (a) Carcharhinus falciformis; (b) Carcharhinus longimanus; (c) Prionace glauca; (d) Isurus oxyrinchus; (e) Pseudocarcharias kamoharai; (f) Sphyrna lewini; (g) Sphyrna zygaena; (h) Mustelus lunulatus.
The claspers of 361 C. falciformis were measured, of which 153 were not calcified (66–186 cm TL and 1–15 cm CL), 21 were semicalcified (168–195 cm TL and 11–21 cm CL), and 187 were calcified (164–272 cm TL and 16–33 cm CL) (Figure 4a). The estimates of L50 and L95 for males were 182.38 cm TL ± 1.07 S.E. and 196.29 cm TL ± 2.99 S.E., respectively (Figure 5a). The inflexion point was estimated at 188.2 cm TL.
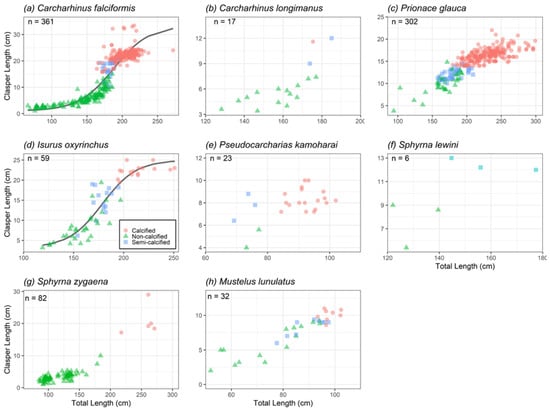
Figure 4.
Relationship between the total length and the clasper length of the most abundant species: (a) Carcharhinus falciformis; (b) Carcharhinus longimanus; (c) Prionace glauca; (d) Isurus oxyrinchus; (e) Pseudocarcharias kamoharai; (f) Sphyrna lewini; (g) Sphyrna zygaena; (h) Mustelus lunulatus.
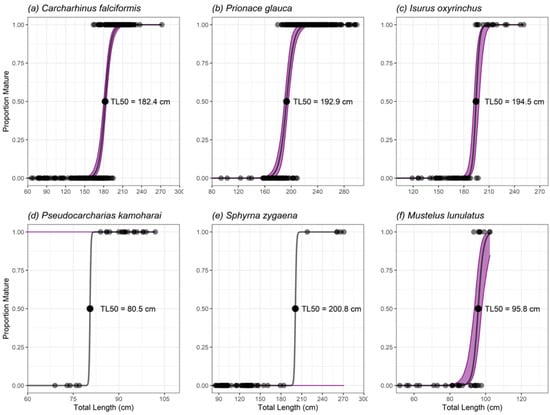
Figure 5.
Length at maturity of male individuals of the main shark species landed in Manta: (a) Carcharhinus falciformis; (b) Prionace glauca; (c) Isurus oxyrinchus; (d) Pseudocarcharias kamoharai; (e) Sphyrna zygaena; (f) Mustelus lunulatus. The black dots in the middle of the curve correspond to the length at which 50% of individuals have reached sexual maturity (TL50). The black dots at the top and bottom correspond to mature and immature individuals, respectively. The purple shaded areas represent the 95% bootstrapped confidence intervals.
3.2.2. Carcharhinus longimanus
A total of 67 C. longimanus were reported, which were composed of 37 females (55%), 29 males (43%), and 1 non-sexed individual (1%). There were no differences in the sex ratio ( = 0.96, p = 0.32). This parity pattern was maintained during all months (Table 4b). Females had a size range of 117–215 cm TL (mean ± S.E. = 154.10 ± 5.43), males 112–185 cm TL (mean ± S.E. = 159.36 ± 4.10), and the unsexed individual measured 130 cm TL (Figure 3b). No significant differences were found in mean lengths between sexes (Student’s t-test, t = −0.78, p = 0.43). A significant correlation was observed between TL and PCL for combined sexes (TL = 1.3602PCL + 4.6311, n = 29, R2 = 0.99, p < 2.2 × 10–16).
A total of 17 C. longimanus claspers were examined, of which 14 were not calcified (128–177 cm TL and 3–7 cm CL), two were semicalcified (174–185 cm TL and 9–12 cm CL) and one was calcified (175 cm TL and 12 cm CL) (Figure 4b).
3.2.3. Prionace glauca
A total of 8956 P. glauca were reported, which comprised 4786 females (53%), 2798 males (31%), and 1372 unsexed individuals (15%). Females were significantly more abundant than males, with a sex ratio of 1M:1.7F ( = 52 1.7, p < 2.2 × 10–16); however, in February, May, June, July, and August there were no differences found in sex ratios (Table 4c). The lengths of the females ranged between 130 and 314 cm TL (mean ± S.E. = 207.93 ± 1.35), those of males were between 94 and 299 cm TL (mean ± S.E. = 214.36 ± 1.82), whereas the unsexed individuals were 177–233 cm TL (mean ± S.E. = 205.40 ± 28) (Figure 3c). Significant differences were observed between sexes (Mann–Whitney U-test, U = 82,685.5, p = 0.00037). The relationship between TL and PCL for combined sexes was significant (TL = 1.2759 PCL + 8.2472, n = 762, R2 = 0.90, p < 2.2 × 10–16), females (TL = 1.2532PCL + 10.933, n = 417, R2 = 0.88, p < 2.2 × 10–16) and males (TL = 1.2883PCL + 7.3407, n = 345, R2 = 0.93, p < 2.2 × 10–16).
Thirty-eight (12.58%) of the 302 P. glauca claspers analyzed were not calcified (94 to 193 cm TL and 4 to 15 cm CL), 37 were semicalcified (158 to 209 cm TL and 10 to 14 cm CL) and 227 were fully calcified (180–299 cm TL and 14–22 cm CL) (Figure 4c). The L50 and L95 for males were 192.88 cm TL ± 1.52 S.E. and 208.92 cm TL ± 2.71 S.E., respectively (Figure 5b). The inflexion point was not estimated because the data did not fit the logistics function.
3.2.4. Isurus oxyrinchus
A total of 203 I. oxyrinchus were sampled, being 94 females (46.3%), 93 males (45.8%), and 16 unsexed individuals (7.9%). The general sex ratio was not significantly different from the expected ratio 1:1 ( = 0. 005, p = 0.94), and the same result was observed for all sampling months (Table 4d). Female sizes ranged from 83 to 341 cm TL (mean ± S.E. = 187.74 ± 5.56), those of males were from 119 to 251 cm TL (mean ± S.E. = 178.14 ± 3.45) and the unsexed individual measured 230 cm TL (Figure 3d). There were no significant differences between male and female mean sizes of I. oxyrinchus (Mann–Whitney U-test, U = 1757.5, p = 0.17). A significant correlation was found for combined sexes between TL and PCL (TL = 1.2432PCL + 2.3802, n = 49, R2 = 0.98, p < 2.2 × 10–16).
Fifty-nine I. oxyrinchus claspers were examined, of which 29 were not calcified (119–197 cm TL and 3–19 cm CL), 14 were semicalcified (154–195 cm TL and 6–19 cm CL), and 16 were fully calcified (194–251 cm TL and 19–25 cm CL) (Figure 4d). The estimations of L50 and L95 for males were 194.52 cm TL ± 1.85 S.E. and 200.67 cm TL ± 3.76 S.E., respectively (Figure 5c). The inflexion point was estimated at 178.82 cm TL.
3.2.5. Pseudocarcharias kamoharai
A total of 40 P. kamoharai were recorded, of which 17 were females (42.5%) and 23 were males (57.5%). The sex ratio was not significantly different from the expected 1:1 ratio ( = 0.9, p = 0.34). Females had sizes of 73–114 cm TL (mean ± S.E. = 100.35 ± 2.98), and males were 69–102 cm TL (mean ± S.E. = 88.61 ± 1.91) (Figure 3e). The average length of females was significantly longer than that of males (t-test, t = 3.46, p = 0.0013). The relationship between TL and PCL was significant for combined sexes (TL = 1.2835PCL + 1.5187, n = 38, R2 = 0.98, p < 2.2 × 10–16), for females (TL = 1.2736PCL + 2.8587, n = 16, R2 = 0.99, p < 1.1 × 10–15) and males (TL = 1.2232PCL + 5.2416, n = 22, R2 = 0.98, p < 2.2 × 10–16).
3.2.6. Sphyrna lewini
A total of 174 S. lewini were sampled, composed of 45 females (26%), 27 males (16%) and 102 unsexed individuals (59%). Females were more abundant than males ( = 4.5, p = 0.03). Regarding the months, only in March was the sex ratio significantly different from the expected 1:1 ratio (Table 4e). Females had lengths ranging from 117 to 276 cm TL (mean ± S.E. = 156.12 ± 16.06), males from 122 to 177 cm TL (mean ± S.E. = 147.05 ± 7.41), and the unsexed individuals from 92 to 150 cm TL (mean ± S.E. = 124.30 ± 13.35) (Figure 3f). There were no differences between sex sizes (Mann–Whitney U-test, U = 31.5, p = 0.95). The relationship between TL and PCL for combined sexes was significant (TL = 1.4335PCL −1.1776, n = 9, R2 = 0.98, p < 8.2 × 10–8).
Six immature males were recorded, of which three were not calcified (122–140 cm TL and 5–9 cm CL) and three were semicalcified (145–177 cm TL and 12–13 cm CL) (Figure 4f).
3.2.7. Sphyrna zygaena
During field trips, 179 (31%) of the 577 individuals of S. zygaena were females, 181 males (31.4%), and 217 were unsexed individuals (37.6%). No differences in sex ratios were observed ( = 0.01, p = 0.91), except for the months of January, March, and May, when this parameter was different from parity (Table 4f). Females had a size range of 82–251 cm TL (mean ± S.E. = 130.63 ± 4.22), males 85–272 cm TL (mean ± S.E. = 130.66 ± 4.82), and unsexed individuals 83–287 cm TL (mean ± S.E. = 196.90 ± 26.92) (Figure 3g). There were no significant differences between the lengths of females and males (Mann–Whitney U-test, U = 3953.05, p = 0.46). A significant relationship was found between TL and PCL for combined sexes (TL = 1.3654PCL + 2.3627, n = 180, R2 = 0.99, p < 2.2 × 10–16).
3.2.8. Mustelus lunulatus
A total of 140 M. lunulatus were recorded, composed of 88 females (63%) and 52 males (37%). The general sex ratio was significantly different from parity ( = 9.3, p = 0.002), and it differed significantly from expected during February to May and August to November (Table 4g). The size range of the females was 49–123 cm TL (mean ± S.E. = 85.03 ± 2.66) and that of males was 52–102 cm TL (mean ± S.E. = 84.42 ± 2.42) (Figure 3h). No significant differences were observed between the mean lengths of females and males (Student’s t-test, t = 0.16, p = 0.86). The relationship between TL and PCL was significant for combined sexes (TL = 1.2313PCL + 0.4659, n = 73, R2 = 0.98, p < 2.2 × 10–16).
3.2.9. Family Alopiidae
The biological aspects of A. pelagicus and A. superciliosus were previously published by Briones–Mendoza et al. (see [56]).
4. Discussion
4.1. Composition of Catches
Approximately 64 shark species have been reported in Ecuador [16]. Between 2003 and 2006, Martínez–Ortíz et al. [17] documented 34 species of sharks in Manta. However, only 19 species were recorded in this study. This could be possibly due to the fact that the sampling in this study was only for one year, whereas that of Martínez–Ortíz et al. covered 3 years, although a possible loss of diversity as a result of increased fishing effort should not be discarded [7,57,58]. Sixteen years ago, Martínez–Ortíz et al. reported that the dominant species in landings in Manta was A. pelagicus, representing the 36% of the total species landed. However, in this study a decrease of 15.7% was observed, and Prionace glauca has become the dominant species in landings in Manta, with an increase of 33.9% (Figure 6). It is possible that these changes are due to overfishing and differences in the life history characteristics of both species. For example, P. glauca has an average of 30 offspring per litter [59], whereas A. pelagicus has only 2 offspring per litter [39]. C. falciformis remains in third place. However, it has suffered a decrease of 4.8%. In the case of vulnerable species, such as S. zygaena [60], and a critically endangered species, such as S. lewini [61], a decrease in landings is reported [17] (Figure 6). It is possible that this was related to the implementation of Ministerial Agreement 116, which allows Ecuadorian artisanal vessels to hold on board as bycatch a maximum of five hammerhead shark (juveniles up to 150 cm LT), which must have their fins attached to the body [62]. The season of greatest shark landings was the dry season (April–November). This could be due to 2 reasons, according to Martínez–Ortíz et al. [17]. First, the different type of fishing material and depth at which the hooks operate during the dry and rainy (December–March) seasons. Second, directed fishing at sharks due to the low abundance of target species during the dry season.
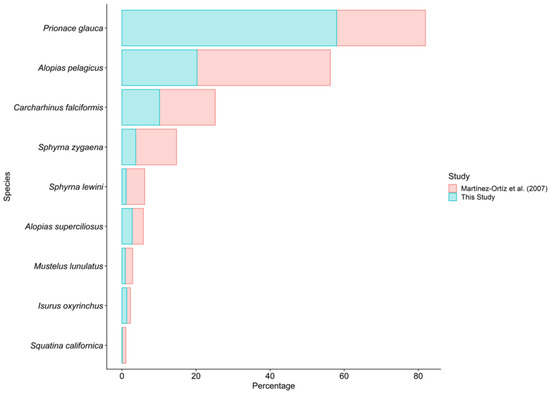
Figure 6.
Comparison of changes in landings of the shark species most frequently landed in Manta [17].
In this study, two demersal species (Squatina californica and M. lunulatus) and 17 pelagic species were reported. This could be due to the fact that in Manta the dominant fishing gear is the pelagic longline, representing 82% of the landings [63]. There are two very well-defined fishing seasons in Ecuador. The first goes from May to November and is characterized by directed fishing for tuna, swordfish, and billfish, where fishermen use circle hooks number 36 and 38. The second is distinguished by directed fishing for Coryphaena hippurus that goes from December to April and uses J hooks number 4 and 5. Artisanal vessels that use pelagic longlines generally fish between 20 and 70 nm off the coast [64]. This study does not provide detailed information regarding the gear and fishing method used, which must be well understood before developing a fishery management strategy [26]. Therefore, in future research, this information should be better documented to improve fishery-management strategies.
4.2. Composition of Sizes, Sexual Proportion, Morphometric Relations and Maturity Size
4.2.1. Carcharhinus falciformis
The size range (61–272 cm TL) reported in this study was lower than that recorded in Manta, between 2003 and 2006 (61–309 cm TL) [17], in Campeche Bank, between 1985 and 1989 (65–314 cm TL) [65], and that the maximum size recorded in the annotated checklist of the chondrichthyan fishes (350 cm TL) by Weigmann (2016) [66]. However, the size range of this study closely resembles the one reported in the central–western Pacific in 2014 (65–271 cm TL) [11]. The sex ratio was skewed toward females, which coincides with what was reported by Hoyos–Padilla et al. [67] and Varghese et al. [68]. However, it differs from other studies [17,69,70], which found no difference in abundance between females and males. The size at maturity for males was 182.38 cm TL, which was very similar to that recorded in the central–western Pacific (183 cm TL) [11], in the Mexican South Pacific (180 cm TL) [69] and on the west coast of Baja California Sur (182 cm TL) [67]. However, the size at maturity seems to be bigger in the eastern Indian Ocean (207.6 cm TL) [70], in the eastern Arabian Sea (218.98 cm TL) [68], and in Campeche Bank (225 cm TL) [65]. These results suggest that males of C. falciformis reach sexual maturity at a smaller size in the Pacific Ocean. The estimated inflexion point in the eastern Indian Ocean was at 196.9 cm TL [70], which appears to be above the one reported by this study for the Ecuadorian Pacific (188.5 cm TL).
4.2.2. Carcharhinus longimanus
The maximum length recorded in this study (215 cm TL) for C. longimanus was lower than other lengths reported in previous studies [71,72,73,74] and than that documented in the annotated checklist of the chondrichthyan fishes (350 cm TL) [66]. As reported in other papers [42,74], no significant differences in sex ratio were found in this study. In the western Central Pacific Ocean, D’Alberto et al. [75] reported that the smallest mature male of C. longimanus averages 190 cm TL, whereas the larger immature one was 195 cm TL. In the western North Pacific Ocean, Joung et al. [42] found that the smallest mature male averaged 172 cm TL, whereas the larger immature averaged 202 cm TL. In this study, only one mature individual was found, which averages 175 cm TL, and the largest immature male measured 185 cm TL. It was estimated that 94% of C. longimanus examined males had not reached sexual maturity, whereas all females were immature, taking as a reference the maturity length estimated by D’Alberto et al. (224 cm TL) [75]. Due to this fact, it is possible that females of C. longimanus approach coastal areas to give birth to their young [76], which would facilitate the capture of juvenile specimens [77]. Therefore, these results suggest possible breeding areas for C. longimanus in Ecuadorian waters.
4.2.3. Prionace glauca
The maximum size of P. glauca (314 cm TL) is similar to the one reported by other studies [78,79,80,81] and less than that recorded globally (383.5 cm TL) [66]. However, the minimum size was larger than that documented in Mexican waters [77,82]. This is likely due to the breeding areas found in the Mexican Pacific [83]. The sex ratio was skewed toward females in this study, which is consistent with data reported by Cruz–Ramírez et al. [82]. However, Carrera–Fernández et al. [77] found a biased proportion toward males, whereas other studies found no difference in abundance between females and males [78,84]. The average maturity size was 192.88 cm TL, which is similar to that recorded in the southeastern Pacific Ocean (190.3 cm TL) [84], in the Ecuadorian Pacific (187.1 cm TL) [78], although compared to northeastern Brazil, higher values have been reported in this study (225 cm TL) [81]. However, da Silva et al. [85], in one review, found no difference in the mean size at maturity of the studies among oceans.
4.2.4. Isurus oxyrinchus
This present study reported a greater size range (83–341 cm TL) than the ones performed in eastern Indonesia (130.8–310 cm TL) [86], in the eastern Arabian Sea (97–269 cm TL) [68], and in the southeastern Pacific Ocean (75.5–240 cm TL) [84]. However, it was lower than the northwest Pacific (80–375 cm TL) [47] and the maximum size reported globally (445 cm TL) [66]. No differences in sex ratio from expected were found, which is consistent with other studies [68,84,87,88], although other ones have shown bias toward males [89] and females [90]. The size at maturity for males was estimated at 194.52 cm TL, which is similar to that reported in New South Wales, Australia (195 cm TL) [87] and South Africa (194–206 cm TL) [91]. However, in the northwest Pacific, a larger length at maturity (210.2 cm TL) was recorded [47], whereas in eastern Indonesia (185.7 cm TL) [86], on the southwest coast of Baja California (180 cm TL) [88] and in the southeastern Pacific Ocean (180.2 cm TL) the sizes at maturity were smaller than those reported in this present study for the Ecuadorian Pacific. The estimated inflexion point in this study (178.82 cm TL) was higher than that of eastern Indonesia (164.8 cm TL) [86].
4.2.5. Pseudocarcharias kamoharai
In the Ecuadorian Pacific, between 2003 and 2009, a maximum length of 113 cm TL was reported [92], which coincides with the value reported in this present study (114 cm TL). However, it was lower than the recorded length in the southwest Atlantic Ocean (122 cm TL) [93] and that the maximum size worldwide (122 cm TL) [66]. In this study, the sex ratio was not significantly different from the expected 1:1, which differs from some studies that found a sex ratio skewed towards females [86,92,93] and males [94,95]. The sexual maturity size recorded for the Ecuadorian Pacific was 80.52 cm TL, which is quite similar to the ones registered in the same area between 2003 and 2009 (78.9 cm TL) [92] and in the southwest Atlantic Ocean, between 2005 and 2007 (80 cm TL) [93]. However, the size at maturity of this study was higher than the one reported in eastern Indonesia (72.5 cm TL) [86] and lower than that recorded in the eastern tropical Atlantic (89.4 cm TL) [96].
4.2.6. Sphyrna lewini
The maximum length recorded in this study (276 cm TL) was smaller than that observed in the Ecuadorian Pacific, between 2003 and 2009 (310 cm TL) [97], in the Gulf of California (363 cm TL) [98], in Indonesian waters (316.8 cm TL) [99], in northeastern Brazil (321 cm TL) [100], and all over the world (430 cm TL) [66]. It is possible that this is due to the fact that in Ecuador only the incidental capture of a maximum of five individuals with a size less than 150 cm TL is allowed, according to Ministerial Agreement 116 [62]. However, in this study five specimens of S. lewini exceeding 150 cm TL were reported. The sex ratio was significantly different from parity, which coincides with that reported in Ecuadorian Pacific, where a sex ratio biased toward females was recorded [97], but differs from that reported in Indonesian waters [99], where they found no differences in sex ratio. All males reported in this study were immature, as they did not have fully calcified claspers. Taking as a reference the size at maturity for females reported by Estupiñán–Montaño et al. in the Ecuadorian Pacific (219.4 cm TL) [97], 88.9% (n = 8) of the females reported in this study were immature, whereas only 11.1% (n = 1) were mature. These results coincide with those documented by Estupiñán–Montaño et al. [97], who also found a greater presence of immature specimens in landings, suggesting possible breeding areas.
4.2.7. Sphyrna zygaena
As mentioned before, it is forbidden to catch specimens of hammerhead sharks greater than 150 cm TL in Ecuador. However, this study recorded 38 specimens of S. zygaena with sizes exceeding 150 cm TL. Therefore, a greater vigilance and the application of stricter laws are needed [101,102]. Regarding the sex ratio, no significant differences from expected were found, disagreeing with what was reported in the Ecuadorian Pacific between 2003 and 2006, where the sex ratio was biased toward males [17], whereas another study carried out between 2007 and 2012 in the same region registered bias toward females [103]. The size at maturity for males in this study (200.81 cm TL) was larger than that reported in the Gulf of California, between 1995 and 2000 (193.7 cm TL) [48], but smaller than the one reported in the Ecuadorian Pacific between 2003 and 2006 (215 cm TL) [104] and between 2007 and 2012 (263.7 cm TL) [103]. According to the size at maturity of this study, 89.80% of the landed male individuals were immature, whereas 97.67% of the females had not reached sexual maturity, taking as a reference the maturity size estimated by López–Martínez et al. (239.3 cm TL) [103]. These results are consistent with other studies conducted in the Ecuadorian Pacific [18,21], where most of the individuals landed were immature. The concordances in the results of these studies seem to be associated with the fact that S. zygaena remains in coastal areas during the first years of life [76,105] and, therefore, is more susceptible to capture.
4.2.8. Mustelus lunulatus
The size range reported in this work (49–123 cm TL) was very similar to that documented in the Colombian Pacific in 2001 (50–125 cm TL) [106], but in the Ecuadorian Pacific the size range was wider in 2013 (41.4–135 cm TL) [107] and was also less than the maximum length recorded by Weigmann (2016) in the annotated checklist of the chondrichthyan fishes (175 cm TL) [66]. Females were significantly more abundant than males, disagreeing with what was documented in Colombia [106] and Ecuador [107], where the sexual proportion was no different from parity. The length at maturity for males (95.93 cm TL) was similar to that reported in the Gulf of California (91.5 cm TL) [108] and in the Ecuadorian Pacific (97.2 cm TL) [107]. Of the total number of males examined, 75% were immature. As for females, 82% had not reached sexual maturity, according to the maturity size estimated by Pérez–Jiménez and Sosa–Nishizaki (103.2 cm TL) [108]. These results coincide with those reported by other studies [106,107,108], which also recorded a greater number of immature individuals.
In this study, a decrease in the relative abundance of some species of great commercial interest such as A. pelagicus, C. falciformis, S. zygaena, and S. lewini was found. Nevertheless, research on life history characteristics (age, growth, and maturity) of sharks in the Ecuadorian Pacific is very scarce and outdated. This information is essential to carry out stock assessments that are being exploited. Therefore, it is recommended to carry out studies to fill the information gaps that exist in this area and thereby improve the management of sharks in Ecuador.
Author Contributions
J.B.-M.: conceptualization, data curation, formal analysis investigation, methodology, software, writing-original draft, writing-review and editing. D.M.: conceptualization, investigation, methodology, supervision, visualization, writing-original draft, writing-review and editing, software. P.C.-P.: investigation, methodology, supervision, visualization, writing-original draft, writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the fishermen of Playita Mía for having facilitated their landings for this study, the students of the Facultad Ciencias del Mar of the Universidad Laica Eloy Alfaro de Manabí for their support in the field trips, and José Alió for reviewing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bornatowski, H.; Navia, A.F.; Braga, R.R.; Abilhoa, V.; Corrêa, M.F.M. Ecological importance of sharks and rays in a structural foodweb analysis in southern Brazil. ICES J. Mar. Sci. 2014, 71, 1586–1592. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Pacoureau, N.; Rigby, C.L.; Pollom, R.A.; Jabado, R.W.; Ebert, D.A.; Finucci, B.; Pollock, C.M.; Cheok, J.; Derrick, D.H. Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 2021, 31, 4773–4787.e4778. [Google Scholar] [CrossRef] [PubMed]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P. Half a century of global decline in oceanic sharks and rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, M.F.; Porcu, C.; Bellodi, A.; Cannas, R.; Cau, A.; Cuccu, D.; Mulas, A.; Follesa, M.C. Temporal dynamics of demersal chondrichthyan species in the central western Mediterranean Sea: The case study in Sardinia Island. Fish. Res. 2017, 193, 81–94. [Google Scholar] [CrossRef]
- Simpfendorfer, C.; Heupel, M.; White, W.; Dulvy, N. The importance of research and public opinion to conservation management of sharks and rays: A synthesis. Mar. Freshw. Res. 2011, 62, 518–527. [Google Scholar] [CrossRef]
- Arunrugstichai, S.; True, J.; White, W. Catch composition and aspects of the biology of sharks caught by Thai commercial fisheries in the Andaman Sea. J. Fish Biol. 2018, 92, 1487–1504. [Google Scholar] [CrossRef]
- King, J.; McFarlane, G. Marine fish life history strategies: Applications to fishery management. Fish. Manag. Ecol. 2003, 10, 249–264. [Google Scholar] [CrossRef]
- Hacohen-Domené, A.; Polanco-Vásquez, F.; Estupiñan-Montaño, C.; Graham, R.T. Description and characterization of the artisanal elasmobranch fishery on Guatemala’s Caribbean coast. PLoS ONE 2020, 15, e0227797. [Google Scholar] [CrossRef] [PubMed]
- Tyabji, Z.; Wagh, T.; Patankar, V.; Jabado, R.W.; Sutaria, D. Catch composition and life history characteristics of sharks and rays (Elasmobranchii) landed in the Andaman and Nicobar Islands, India. PLoS ONE 2020, 15, e0231069. [Google Scholar] [CrossRef]
- Grant, M.I.; Smart, J.J.; White, W.T.; Chin, A.; Baje, L.; Simpfendorfer, C.A. Life history characteristics of the silky shark Carcharhinus falciformis from the central west Pacific. Mar. Freshw. Res. 2018, 69, 562–573. [Google Scholar] [CrossRef]
- Smart, J.; Chin, A.; Tobin, A.; Simpfendorfer, C.; White, W. Age and growth of the common blacktip shark Carcharhinus limbatus from Indonesia, incorporating an improved approach to comparing regional population growth rates. Afr. J. Mar. Sci. 2015, 37, 177–188. [Google Scholar] [CrossRef]
- FAO FishstatJ. FishStatJ-Software for Fishery and Aquaculture Statistical Time Series; FAO FishstatJ: Rome, Italy, 2020. [Google Scholar]
- Gonzalez-Pestana, A.; Kouri, C.; Velez-Zuazo, X. Shark fisheries in the Southeast Pacific: A 61-year analysis from Peru. F1000Research 2014, 3, 164. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, J.; Alava, J.J.; Pramod, G.; Henderson, S.; Zeller, D. In hot soup: Sharks captured in Ecuador’s waters. Environ. Sci. 2008, 5, 269–283. [Google Scholar] [CrossRef]
- Calle-Morán, M.D.; Béarez, P. Updated checklist of marine cartilaginous fishes from continental and insular Ecuador (Tropical Eastern Pacific Ocean). Cybium Rev. Int. Ichtyol. 2020, 44, 239–250. [Google Scholar]
- Martínez-Ortíz, J.; Galván-Magaña, F.; Carrera-Fernández, M.; Mendoza-Intriago, D.; Estupiñán-Montaño, C.; Cedeño-Figueroa, L. Abundancia estacional de tiburones desembarcados en Manta-Ecuador. In Tiburones en el Ecuador: Casos de Estudio; Martínez-Ortíz, J., Galván-Magaña, F., Eds.; EPESPO-PMRC: Manta, Ecuador, 2007; pp. 9–27. [Google Scholar]
- Coello, D.; Herrera, M. Desembarque de tiburones en las pesquerías artesanales del Ecuador durante el 2012. Rev. Científica Cienc. Nat. Ambient. 2018, 12, 1–8. [Google Scholar]
- Aguilar, F.; Revelo, W.; Coello, D.; Cajas, J.; Ruíz, W.; Díaz, M.; Moreno, J. Desembarques Artesanales de Tiburones y Rayas en los Principales Puertos Pesqueros del Ecuador Durante 2006; Informe Interno; Instituto Nacional de Pesca: Guayaquil, Ecuador, 2007. [Google Scholar]
- Ruiz, W.; Díaz, M. Desembarques Artesanales de Tiburones y Rayas en los Principales Puertos Pesqueros del Ecuador Durante 2007; Informe Final de Tiburón; Instituto Nacional de Pesca: Guayaquil, Ecuador, 2007. [Google Scholar]
- Herrera, M.; Coello, D.; Cajas, J. Desembarques y aspectos biológicos de Elasmobranquios en las pesquerías artesanales del Ecuador durante 2011. Boletín Científico Técnico 2012, 22, 1–8. [Google Scholar]
- Martínez-Ortiz, J.; García-Domínguez, M. Guía de Campo. Condrictios del Ecuador. Quimeras, Tiburones y Rayas; Ministerio de Agricultura, Ganadería, Acuacultura y Pesca (MAGAP)/ViceMinisterio de Acuacultura y Pesca (VMAP)/Subsecretaria de Recursos Pesqueros (SRP): Guayaquil, Ecuador, 2013. [Google Scholar]
- Mejía-Falla, P.; Navia, A.; Cortés, E. Reproductive variables of Urotrygon rogersi (Batoidea: Urotrygonidae): A species with a triannual reproductive cycle in the eastern tropical Pacific Ocean. J. Fish Biol. 2012, 80, 1246–1266. [Google Scholar] [CrossRef] [PubMed]
- White, W. Catch composition and reproductive biology of whaler sharks (Carcharhiniformes: Carcharhinidae) caught by fisheries in Indonesia. J. Fish Biol. 2007, 71, 1512–1540. [Google Scholar] [CrossRef]
- Clark, E.; von Schmidt, K. Sharks of the central Gulf coast of Florida. Bull. Mar. Sci. 1965, 15, 13–83. [Google Scholar]
- White, W.T.; Baje, L.; Appleyard, S.A.; Chin, A.; Smart, J.J.; Simpfendorfer, C.A. Shark longline fishery of Papua New Guinea: Size and species composition and spatial variation of the catches. Mar. Freshw. Res. 2019, 71, 627–640. [Google Scholar] [CrossRef]
- White, W.; Baje, L.; Simpfendorfer, C.; Appleyard, S.; Chin, A.; Sabub, B.; Rochel, E.; Naylor, G. Elasmobranch bycatch in the demersal prawn trawl fishery in the Gulf of Papua, Papua New Guinea. Sci. Rep. 2019, 9, 9254. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, S.; White, W.; Vieira, S.; Sabub, B. Artisanal shark fishing in Milne Bay Province, Papua New Guinea: Biomass estimation from genetically identified shark and ray fins. Sci. Rep. 2018, 8, 6693. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.I. Reproduction in fisheries science. In Reproductive Biology and Phylogeny of Chondrichthyes: Sharks Batoids Chimaeras; Routledge: London, UK, 2005; Volume 3, pp. 81–128. [Google Scholar]
- Piner, K.R.; Hamel, O.S.; Menkel, J.L.; Wallace, J.R.; Hutchinson, C.E. Age validation of canary rockfish (Sebastes pinniger) from off the Oregon coast (USA) using the bomb radiocarbon method. Can. J. Fish. Aquat. Sci. 2005, 62, 1060–1066. [Google Scholar] [CrossRef]
- White, W.T.; Giles, J.; Potter, I.C. Data on the bycatch fishery and reproductive biology of mobulid rays (Myliobatiformes) in Indonesia. Fish. Res. 2006, 82, 65–73. [Google Scholar] [CrossRef]
- White, W.; Dharmadi. Species and size compositions and reproductive biology of rays (Chondrichthyes, Batoidea) caught in target and non-target fisheries in eastern Indonesia. J. Fish Biol. 2007, 70, 1809–1837. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Smart, J.J.; Chin, A.; Tobin, A.J.; Simpfendorfer, C.A. Multimodel approaches in shark and ray growth studies: Strengths, weaknesses and the future. Fish Fish. 2016, 17, 955–971. [Google Scholar] [CrossRef]
- Smart, J. AquaticLifeHistory: Fisheries Life History Analysis Using Contemporary Methods. 2019. Available online: https://github.com/jonathansmart/AquaticLifeHistory (accessed on 12 January 2022).
- Wilke, C.O.; Wickham, H.; Wilke, M.C.O. Package ‘Cowplot’—Streamlined Plot Theme Plot Annot. ‘Ggplot2. 2019. Available online: https://cran.r-project.org/web/packages/cowplot/index.html (accessed on 12 January 2022).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Liu, K.-M.; Chen, C.-T.; Liao, T.-H.; Joung, S.-J. Age, growth, and reproduction of the pelagic thresher shark, Alopias pelagicus in the Northwestern Pacific. Copeia 1999, 1999, 68–74. [Google Scholar] [CrossRef]
- Liu, K.-M.; Chiang, P.-J.; Chen, C.-T. Age and growth estimates of the bigeye thresher shark, Alopias superciliosus, in northeastern Taiwan waters. Fish. Bull. 1998, 96, 482–491. [Google Scholar]
- Joung, S.-J.; Chen, C.-T.; Lee, H.-H.; Liu, K.-M. Age, growth, and reproduction of silky sharks, Carcharhinus falciformis, in northeastern Taiwan waters. Fish. Res. 2008, 90, 78–85. [Google Scholar] [CrossRef]
- Joung, S.-J.; Chen, N.-F.; Hsu, H.-H.; Liu, K.-M. Estimates of life history parameters of the oceanic whitetip shark, Carcharhinus longimanus, in the Western North Pacific Ocean. Mar. Biol. Res. 2016, 12, 758–768. [Google Scholar] [CrossRef]
- Branstetter, S.; Stiles, R. Age and growth estimates of the bull shark, Carcharhinus leucas, from the northern Gulf of Mexico. Environ. Biol. Fishes 1987, 20, 169–181. [Google Scholar] [CrossRef]
- Castro, J.I. Biology of the blacktip shark, Carcharhinus limbatus, off the southeastern United States. Bull. Mar. Sci. 1996, 59, 508–522. [Google Scholar]
- Simpfendorfer, C.A.; Unsworth, P. Gill-net mesh selectivity of dusky sharks (Carcharhinus obscurus) and whiskery sharks (Furgaleus macki) from south-western Australia. Mar. Freshw. Res. 1998, 49, 713–718. [Google Scholar] [CrossRef]
- Wetherbee, B.M.; Crow, G.L.; Lowe, C.G. Biology of the Galapagos shark, Carcharhinus galapagensis, in Hawai’i. Environ. Biol. Fishes 1996, 45, 299–310. [Google Scholar] [CrossRef]
- Joung, S.-J.; Hsu, H.-H. Reproduction and embryonic development of the shortfin mako, Isurus oxyrinchus Rafinesque, 1810, in the northwestern Pacific. Zool. Stud. 2005, 44, 487. [Google Scholar]
- Nava Nava, P.; Márquez-Farías, J.F. Talla de madurez del tiburón martillo, Sphyrna zygaena, capturado en el Golfo de California. Hidrobiológica 2014, 24, 129–135. [Google Scholar]
- Stevens, J.; Lyle, J. Biology of three hammerhead sharks (Eusphyra blochii, Sphyrna mokarran and S. lewini) from northern Australia. Mar. Freshw. Res. 1989, 40, 129–146. [Google Scholar] [CrossRef]
- Kohler, N.E.; Casey, J.G.; Turner, P.A. Length-weight relationships for 13 species of sharks from the western North Atlantic. Fish. Bull. 1995, 93, 412–418. [Google Scholar]
- Branstetter, S.; Musick, J.A.; Colvocoresses, J.A. A Comparison Of The Age And Growth Of The Tiger Shark, Galeocerdo cuvieri, From Off Virginia And From The Northwestern Gulf-Of-Mexico. Fish. Bull. 1987, 85, 269–279. [Google Scholar]
- Kulbicki, M.; Guillemot, N.; Amand, M. A general approach to length-weight relationships for New Caledonian lagoon fishes. Cybium 2005, 29, 235–252. [Google Scholar]
- Williams, C.M.; Williams, J.P.; Claisse, J.T.; Pondella, D.J.; Domeier, M.L.; Zahn, L.A. Morphometric relationships of marine fishes common to central California and the southern California bight. Bull. S. Calif. Acad. Sci. 2013, 112, 217–227. [Google Scholar] [CrossRef][Green Version]
- Castro, J.I. The biology of the nurse shark, Ginglymostoma cirratum, off the Florida east coast and the Bahama Islands. Environ. Biol. Fishes 2000, 58, 1–22. [Google Scholar] [CrossRef]
- Ariz, J.; de Molina, A.D.; Santana, J. Length-weight relationships, conversion factors and analyses of sex-ratio, by length-range, for several species of pelagic sharks caught in experimental cruises on board Spanish longliners in the South Western Indian Ocean during 2005. In Proceedings of the Third Session of the IOTC Working Party on Ecosystems and Bycatch (Previously the Working Party on Bycatch), Victoria, Seychelles, 18 September 2017; 2007; pp. 11–13. [Google Scholar]
- Briones-Mendoza, J.; Carrasco-Puig, P.; Toala-Franco, D. Reproductive biology aspects of Alopias pelagicus and A. superciliosus (Lamniformes: Alopiidae) in the Ecuadorian Pacific. Neotrop. Ichthyol. 2021, 19, 1–19. [Google Scholar] [CrossRef]
- Stevens, J.; Bonfil, R.; Dulvy, N.K.; Walker, P. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Jennings, S.; Kaiser, M.J. The effects of fishing on marine ecosystems. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 34, pp. 201–352. [Google Scholar]
- Nakano, H.; Stevens, J.D. The biology and ecology of the blue shark, Prionace glauca. Sharks Open Ocean Biol. Fish. Conserv. 2008, 1, 140–151. [Google Scholar]
- Rigby, C.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Herman, K.; Jabado, R.; Liu, K.; Marshall, A.; Pacoureau, N. Sphyrna zygaena. The IUCN Red List of Threatened Species 2019. Technical Report. 2019. [Google Scholar]
- Rigby, C.; Dulvy, N.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.; Herman, K.; Jabado, R.; Liu, K. Sphyrna lewini. The IUCN Red List of Threatened Species. e.T39385A2918526. 2019. [Google Scholar]
- MAGAP. MAGAP Aplica Medidas de Conservación para Tiburones Martillos en el Ecuador; MAGAP: La Troncal, Ecuador, 2013. [Google Scholar]
- Martínez-Ortiz, J.; Aires-da-Silva, A.M.; Lennert-Cody, C.E.; Maunder, M.N. The Ecuadorian artisanal fishery for large pelagics: Species composition and spatio-temporal dynamics. PLoS ONE 2015, 10, e0135136. [Google Scholar] [CrossRef]
- Rosas-Luis, R.; Loor-Andrade, P.; Carrera-Fernández, M.; Pincay-Espinoza, J.; Vinces-Ortega, C.; Chompoy-Salazar, L. Cephalopod species in the diet of large pelagic fish (sharks and billfishes) in Ecuadorian waters. Fish. Res. 2016, 173, 159–168. [Google Scholar] [CrossRef]
- Bonfil, R.; Mena, R.; De Anda, D. Biological paramenters of commercially exploited silky sharks, Carcharhinus falciformis, from the Campeche Bank, Mexico. NOAA Technol. Rep. NMFS 1993, 115, 73–86. [Google Scholar]
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Padilla, E.M.; Ceballos-Vázquez, B.P.; Galván-Magaña, F. Reproductive biology of the silky shark Carcharhinus falciformis (Chondrichthyes: Carcharhinidae) off the west coast of Baja California Sur, Mexico. Aqua Int. J. Ichthyol 2012, 18, 15–24. [Google Scholar]
- Varghese, S.P.; Gulati, D.; Unnikrishnan, N.; Ayoob, A. Biological aspects of silky shark Carcharhinus falciformis in the eastern Arabian Sea. J. Mar. Biol. Assoc. UK 2016, 96, 1437–1447. [Google Scholar] [CrossRef]
- Galván-Tirado, C.; Galvan-Magaña, F.; Ochoa-Báez, R. Reproductive biology of the silky shark Carcharhinus falciformis in the southern Mexican Pacific. J. Mar. Biol. Assoc. UK 2015, 95, 561–567. [Google Scholar] [CrossRef]
- Hall, N.; Bartron, C.; White, W.; Potter, I. Biology of the silky shark Carcharhinus falciformis (Carcharhinidae) in the eastern Indian Ocean, including an approach to estimating age when timing of parturition is not well defined. J. Fish Biol. 2012, 80, 1320–1341. [Google Scholar] [CrossRef] [PubMed]
- Lessa, R.; Santana, F.M.; Paglerani, R. Age, growth and stock structure of the oceanic whitetip shark, Carcharhinus longimanus, from the southwestern equatorial Atlantic. Fish. Res. 1999, 42, 21–30. [Google Scholar] [CrossRef]
- Seki, T.; Taniuchi, T.; Nakano, H.; Shimizu, M. Age, growth and reproduction of the oceanic whitetip shark from the Pacific Ocean. Fish. Sci. 1998, 64, 14–20. [Google Scholar] [CrossRef][Green Version]
- Coelho, R.; Hazin, F.H.; Rego, M.; Tambourgi, M.; Oliveira, P.; Travassos, P.; Carvalho, F.; Burgess, G. Notes on the reproduction of the oceanic whitetip shark, Carcharhinus longimanus, in the southwestern Equatorial Atlantic ocean. Collect. Vol. Sci. Pap. ICCAT 2009, 64, 1734–1740. [Google Scholar]
- Tambourgi, M.R.d.S.; Hazin, F.H.; Oliveira, P.G.; Coelho, R.; Burgess, G.; Roque, P.C. Reproductive aspects of the oceanic whitetip shark, Carcharhinus longimanus (Elasmobranchii: Carcharhinidae), in the equatorial and southwestern Atlantic Ocean. Braz. J. Oceanogr. 2013, 61, 161–168. [Google Scholar] [CrossRef][Green Version]
- D’Alberto, B.M.; Chin, A.; Smart, J.J.; Baje, L.; White, W.T.; Simpfendorfer, C.A. Age, growth and maturity of oceanic whitetip shark (Carcharhinus longimanus) from Papua New Guinea. Mar. Freshw. Res. 2016, 68, 1118–1129. [Google Scholar] [CrossRef]
- Clarke, S.; Coelho, R.; Francis, M.; Kai, M.; Kohin, S.; Liu, K.; Simpfendorfer, C.; Tovar-Avila, J.; Rigby, C.; Smart, J. Report of the Pacific Shark Life History Expert Panel Workshop, 28–30 April 2015; Scientific Committee Eleventh Regular Session, Western and Central Pacific Fisheries Commission: Pohnpei, Federated States of Micronesia, 2015. [Google Scholar]
- Carrera-Fernández, M.; Galván-Magaña, F.; Ceballos-Vázquez, B.P. Reproductive biology of the blue shark Prionace glauca (Chondrichthyes: Carcharhinidae) off Baja California Sur, México. Aqua 2010, 16, 101–110. [Google Scholar]
- Briones-Mendoza, J.; Pincay-Espinoza, J.; Palma-Chávez, J.; Romero-Caicedo, A. Notas sobre la biología del tiburón azul Prionace glauca (Carcharhiniformes: Carcharhinidae) en aguas ecuatorianas. Rev. Mex. Biodivers. 2016, 87, 1387–1390. [Google Scholar] [CrossRef]
- Jolly, K.; Da Silva, C.; Attwood, C. Age, growth and reproductive biology of the blue shark Prionace glauca in South African waters. Afr. J. Mar. Sci. 2013, 35, 99–109. [Google Scholar] [CrossRef]
- Megalofonou, P.; Damalas, D.; De Metrio, G. Biological characteristics of blue shark, Prionace glauca, in the Mediterranean Sea. J. Mar. Biol. Assoc. UK 2009, 89, 1233–1242. [Google Scholar] [CrossRef]
- Lessa, R.; Santana, F.M.; Hazin, F.H. Age and growth of the blue shark Prionace glauca (Linnaeus, 1758) off northeastern Brazil. Fish. Res. 2004, 66, 19–30. [Google Scholar] [CrossRef]
- Cruz-Ramírez, A.; Soriano-Velásquez, S.R.; Santana-Hernández, H.; Ramírez-Santiago, C.E.; Acal-Sánchez, D.E. Aspectos reproductivos del tiburón azul Prionace glauca capturado por la flota palangrera de mediana altura del Puerto de Manzanillo, Colima. Cienc. Pesq. 2012, 20, 39–48. [Google Scholar]
- Salomón-Aguilar, C.; Villavicencio-Garayzar, C.; Reyes-Bonilla, H. Zonas y temporadas de reproducción y crianza de tiburones en el Golfo de California: Estrategia para su conservación y manejo pesquero. Cienc. Mar. 2009, 35, 369–388. [Google Scholar] [CrossRef]
- Bustamante, C.; Bennett, M.B. Insights into the reproductive biology and fisheries of two commercially exploited species, shortfin mako (Isurus oxyrinchus) and blue shark (Prionace glauca), in the south-east Pacific Ocean. Fish. Res. 2013, 143, 174–183. [Google Scholar] [CrossRef]
- da Silva, T.E.F.; Lessa, R.; Santana, F.M. Current knowledge on biology, fishing and conservation of the blue shark (Prionace glauca). Neotrop. Biol. Conserv. 2021, 16, 71–88. [Google Scholar] [CrossRef]
- White, W.T. Biological observations on lamnoid sharks (Lamniformes) caught by fisheries in eastern Indonesia. J. Mar. Biol. Assoc. UK 2007, 87, 781–788. [Google Scholar] [CrossRef]
- Stevens, J. Observations on reproduction in the shortfin mako Isurus oxyrinchus. Copeia 1983, 126–130. [Google Scholar] [CrossRef]
- Conde-Moreno, M.; Galván-Magaña, F. Reproductive biology of the mako shark Isurus oxyrinchus on the south-western coast of Baja California, Mexico. Cybium 2006, 30, 75–83. [Google Scholar]
- Maia, A.; Queiroz, N.; Cabral, H.; Santos, A.; Correia, J. Reproductive biology and population dynamics of the shortfin mako, Isurus oxyrinchus Rafinesque, 1810, off the southwest Portuguese coast, eastern North Atlantic. J. Appl. Ichthyol. 2007, 23, 246–251. [Google Scholar] [CrossRef]
- Canani, G.; Oddone, M.C. Reproductive biology of Isurus oxyrinchus captured by the south Brazilian surface longline commercial fleet in the Southwest Atlantic Ocean, with data on CPUE and size distribution by sex. J. Northwest Atl. Fish. Sci. 2020, 51, 105–116. [Google Scholar] [CrossRef]
- Cliff, G.; Dudley, S.; Davis, B. Sharks caught in the protective gill nets off Natal, South Africa. 3. The shortfin mako shark Isurus oxyrinchus (Rafinesque). S. Afr. J. Mar. Sci. 1990, 9, 115–126. [Google Scholar] [CrossRef]
- Estupiñán-Montaño, C.; Galván-Magaña, F. First Insight into the Biological Aspects of the Crocodile Shark Pseudocarcharias kamoharai in the Eastern Pacific Ocean. Thalass. Int. J. Mar. Sci. 2021, 37, 229–233. [Google Scholar] [CrossRef]
- Oliveira, P.; Hazin, F.; Carvalho, F.; Rego, M.; Coelho, R.; Piercy, A.; Burgess, G. Reproductive biology of the crocodile shark Pseudocarcharias kamoharai. J. Fish Biol. 2010, 76, 1655–1670. [Google Scholar] [CrossRef]
- Kiszka, J.J.; Aubail, A.; Hussey, N.E.; Heithaus, M.R.; Caurant, F.; Bustamante, P. Plasticity of trophic interactions among sharks from the oceanic south-western Indian Ocean revealed by stable isotope and mercury analyses. Deep Sea Res. Part I Oceanogr. Res. Pap. 2015, 96, 49–58. [Google Scholar] [CrossRef]
- Kindong, R.; Wang, H.; Wu, F.; Dai, X.; Tian, S. Age, growth, and sexual maturity of the crocodile shark, Pseudocarcharias kamoharai, from the Eastern Atlantic Ocean. Front. Mar. Sci. 2020, 7, 857. [Google Scholar] [CrossRef]
- Wu, F.; Kindong, R.; Dai, X.; Sarr, O.; Zhu, J.; Tian, S.; Li, Y.; Nsangue, B.T. Aspects of the reproductive biology of two pelagic sharks in the eastern Atlantic Ocean. J. Fish Biol. 2020, 97, 1651–1661. [Google Scholar] [CrossRef]
- Estupiñán-Montaño, C.; Carrera-Fernández, M.; Galván-Magaña, F. Reproductive biology of the scalloped hammerhead (Sphyrna lewini) in the central-eastern Pacific Ocean. J. Mar. Biol. Assoc. UK 2021, 101, 465–470. [Google Scholar] [CrossRef]
- Torres-Huerta, A.M.; Villavicencio-Garayzar, C.; Corro-Espinosa, D. Biología reproductiva de la cornuda común Sphyrna lewini Griffith & Smith (Sphyrnidae) en el Golfo de California. Hidrobiológica 2008, 18, 227–238. [Google Scholar]
- White, W.; Bartron, C.; Potter, I. Catch composition and reproductive biology of Sphyrna lewini (Griffith & Smith) (Carcharhiniformes, Sphyrnidae) in Indonesian waters. J. Fish Biol. 2008, 72, 1675–1689. [Google Scholar]
- Hazin, F.; Fischer, A.; Broadhurst, M. Aspects of reproductive biology of the scalloped hammerhead shark, Sphyrna lewini, off northeastern Brazil. Environ. Biol. Fishes 2001, 61, 151–159. [Google Scholar] [CrossRef]
- Carr, L.A.; Stier, A.C.; Fietz, K.; Montero, I.; Gallagher, A.J.; Bruno, J.F. Illegal shark fishing in the Galápagos Marine Reserve. Mar. Policy 2013, 39, 317–321. [Google Scholar] [CrossRef]
- Alava, J.; Barragán-Paladines, M.; Denkinger, J.; Muñoz-Abril, L.; Jiménez, P.; Paladines, F.; Valle, C.; Tirapé, A.; Gaibor, N.; Calle, M. Massive Chinese fleet jeopardizes threatened shark species around the Galápagos marine reserve and waters off Ecuador: Implications for national and international fisheries policy. Int. J. Fish. Sci. Res. 2017, 1, 1001. [Google Scholar]
- López-Martínez, J.; Cabanilla-Carpio, C.; Ruiz Choez, W.; Arzola-Sotelo, E.A. Interannual variability of distribution, abundance and population dynamics of the smooth hammerhead Sphyrna zygaena (Linnaeus, 1758) in the central-southeast Pacific Ocean. J. Fish Biol. 2020, 97, 341–353. [Google Scholar] [CrossRef]
- Carrera-Fernández, M.; Martínez-Ortíz, J. Aspectos reproductivos de los tiburones martillo Sphyrna lewini (Grifftith & Smith, 1834) y S. zygaena (Linnaeus, 1758) en aguas del Ecuador. In Tiburones en el Ecuador: Casos de Estudio; Martínez-Ortíz, J., Galván-Magaña, F., Eds.; EPESPO-PMRC: Manta, Ecuador, 2007; pp. 51–56. [Google Scholar]
- Bolaño-Martínez, N. Ecología Trófica de Juveniles del Tiburón Martillo Sphyrna zygaena (Linnaeus, 1758) en Aguas Ecuatorianas; Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas: La Paz, Mexico, 2009. [Google Scholar]
- Navia, A.F.; Giraldo, A.; Mejía-Falla, P.A. Notas sobre la biología y dieta del toyo vieja (Mustelus lunulatus) en la zona central de pesca del Pacífico colombiano. Investig. Mar. 2006, 34, 217–222. [Google Scholar] [CrossRef]
- Briones-Mendoza, J.; Pincay-Espinoza, J.E.; Palma-Chávez, J.; Romero-Caicedo, A. Notas sobre la biología del tiburón mamona Mustelus lunulatus (Carcharhiniformes: Triakidae) en el Pacífico Central ecuatoriano. Rev. Biol. Mar. Oceanogr. 2018, 53, 279–284. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.C.; Sosa-Nishizaki, O. Determining reproductive parameters for population assessments of two smoothhounds (Mustelus californicus and Mustelus lunulatus) from the northern Gulf of California, Mexico. Bull. Mar. Sci. 2010, 86, 3–13. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).