Photophore Morphogenesis and Extraocular Encephalopsin Expression during the Embryogenesis of Smalleye Pygmy Shark (Squaliolus aliae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Photophore Morphology and Development
2.3. Encephalopsin Immunodetection
2.4. Statistical Analyses
3. Results
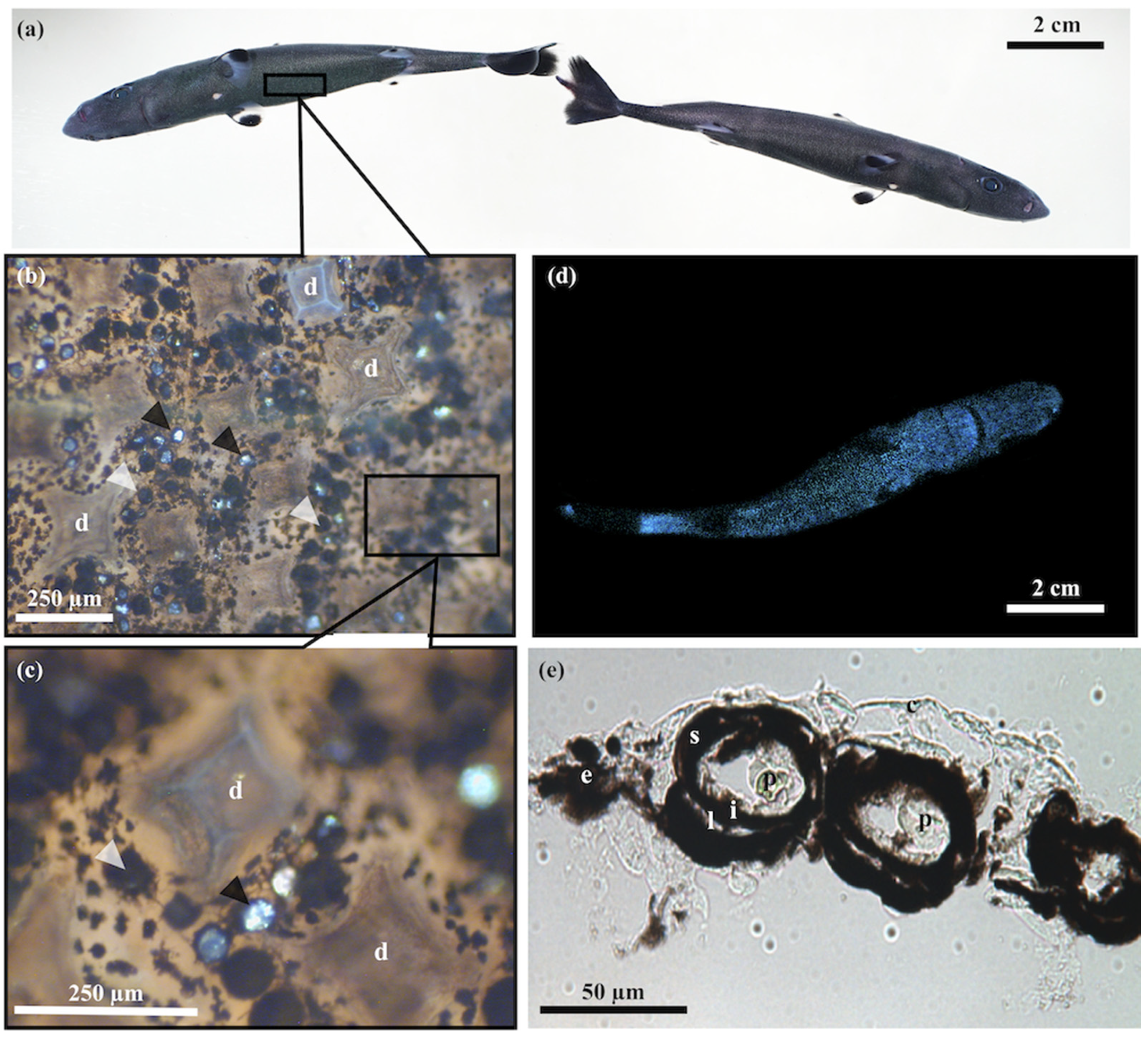
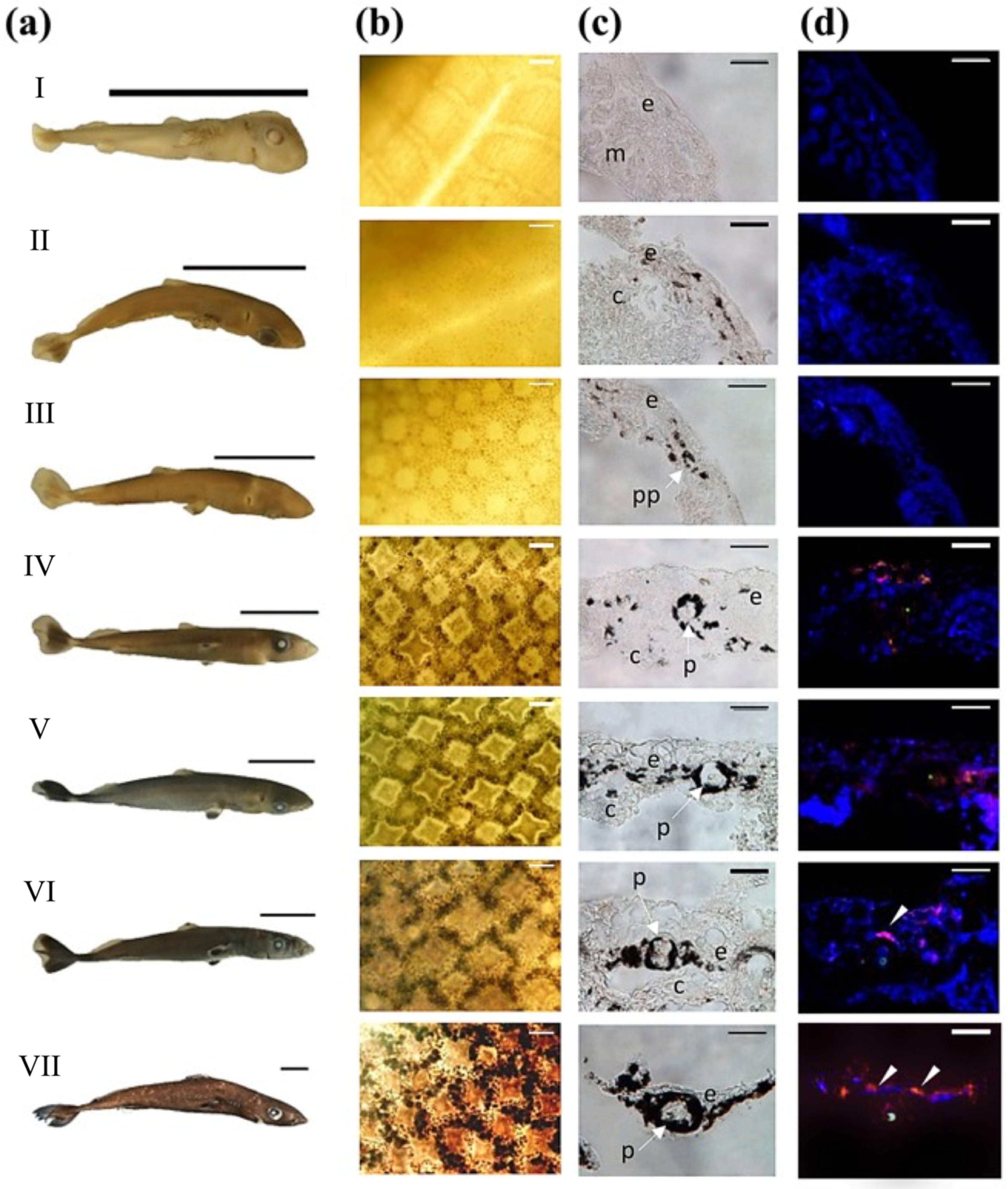
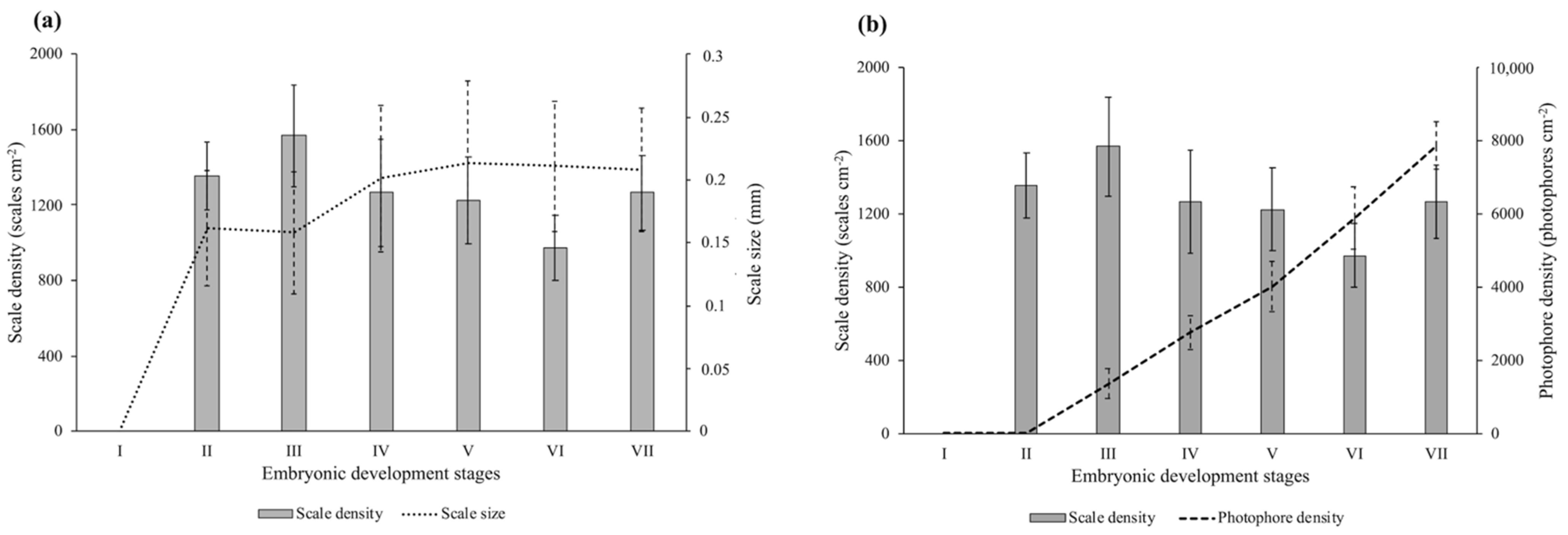
3.1. Skin Morphometry
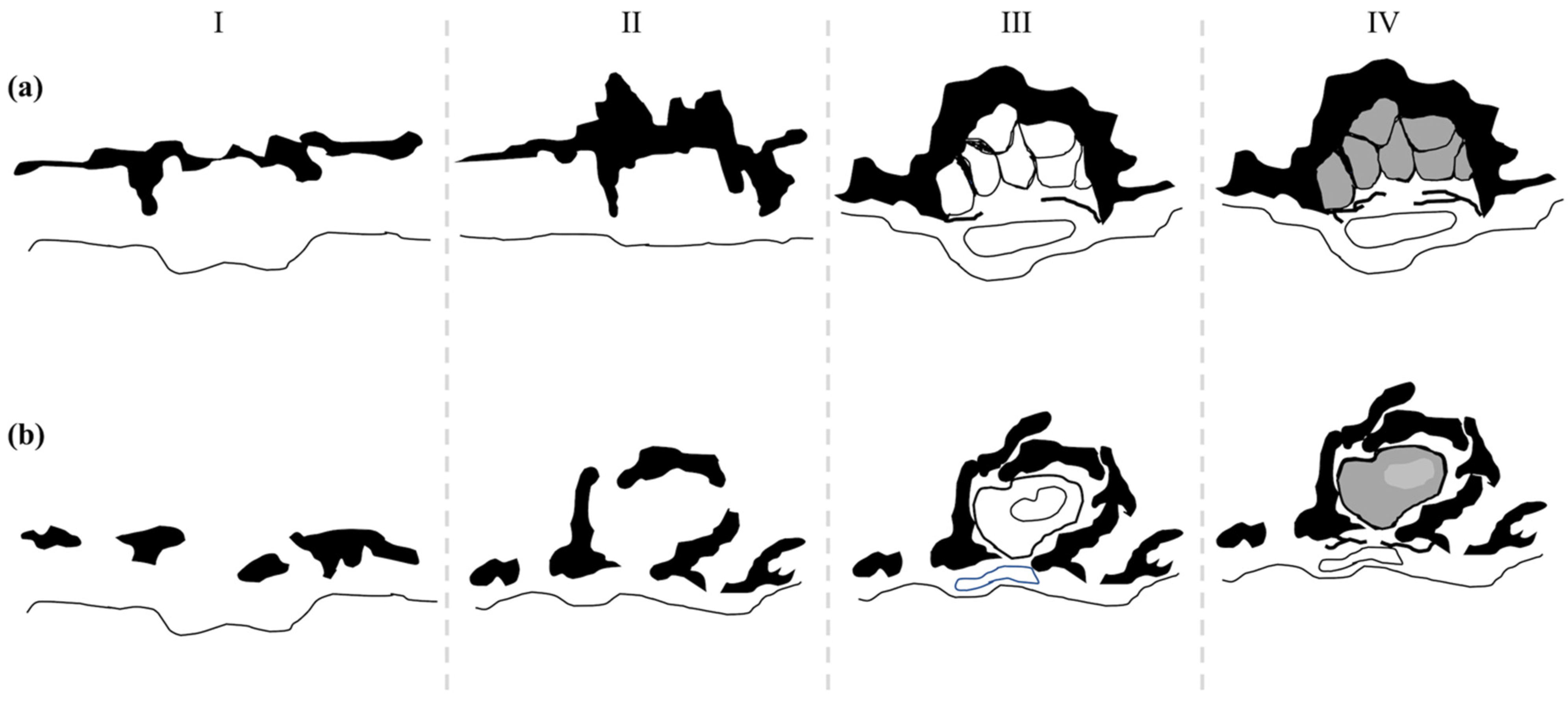
3.2. Photophore Histology and Development
3.3. Encephalopsin Associated-Expression Pattern
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haddock, S.H.D.; Moline, M.A.; Case, J.F. Bioluminescence in the sea. Ann. Rev. Mar. Sci. 2010, 2, 443–493. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Haddock, S.H.D. Quantification of bioluminescence from the surface to the deep sea demonstrates it predominance as an ecological trait. Sci. Rep. 2017, 7, 45750. [Google Scholar] [CrossRef] [PubMed]
- Young, R.E. Oceanic bioluminescence: An overview of general functions. Bull. Mar. Sci. 1983, 33, 829–845. [Google Scholar]
- Hastings, J.W. Bioluminescence. Cell Physiol. 1995, 1995, 665–681. [Google Scholar] [CrossRef]
- Harvey, E.N. Stimulation by adrenalin of the luminescence of deep-sea fish. Zoologica 1931, 12, 67–69. [Google Scholar] [CrossRef]
- Baguet, F.; Marechal, G. The stimulation of isolated photophores (Argyropelecus) by epinephrine and norepinephrine. Comp. Biochem. Physiol. C 1978, 60, 137–143. [Google Scholar] [CrossRef]
- Baguet, F.; Christophe, B. Adrenergic stimulation of isolated photophores of Maurolicus muelleri. Comp. Biochem. Physiol. C 1983, 75, 79–84. [Google Scholar]
- Anctil, M. Cholinergic and monoaminergic mechanisms associated with control of bioluminescence in the ctenophore Mnemiopsis leidyi. J. Exp. Biol. 1985, 119, 225–238. [Google Scholar] [CrossRef]
- Awad, E.W.; Anctil, M. Identification of beta-like adrenoceptors associated with bioluminescence in the sea pansy Renilla koellikeri. J. Exp. Biol. 1993, 177, 181–200. [Google Scholar] [CrossRef]
- Mallefet, J.; Duchatelet, L.; Hermans, C.; Baguet, F. Luminescence control of Stomiidae photophores. Acta Histochem. 2019, 121, 7–15. [Google Scholar] [CrossRef]
- De Bremaeker, N.; Mallefet, J. Luminescence control in brittlestar Amphipholis squamata: Effect of cholinergic drugs. Comp. Biochem. Physiol. C 1996, 115, 75–82. [Google Scholar] [CrossRef]
- Dewael, Y.; Mallefet, J. Effect of cholinergic drugs on the luminescence control of the ophiuroid Amphiura filiformis. Echinoderm Res. 2003, 2001, 181. [Google Scholar]
- Mallefet, J.; Barker, M.; Byrne, M.; O’Hara, T. First study of bioluminescence in Ophionereis. In Echinoderms: München; Heinzeller, T., Nebelsick, J.H., Eds.; Balkema, Taylor & Francis Group: London, UK, 2004; pp. 299–304. [Google Scholar]
- Krönström, J.; Holmgren, S.; Baguet, F.; Salpietro, L.; Mallefet, J. Nitric oxide in control of luminescence from hatchetfish (Argyropelecus hemigymnus) photophores. J. Exp. Biol. 2005, 208, 2951–2961. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krönström, J.; Dupont, S.; Mallefet, J.; Thorndyke, M.; Holmgren, S. Serotonin and nitric oxide interaction in the control of bioluminescence in northern krill, Meganyctiphanes norvegica (M. Sars). J. Exp. Biol. 2007, 210, 3179–3187. [Google Scholar] [CrossRef]
- Krönström, J.; Mallefet, J. Evidence for a widespread involvement of NO in control of photogenesis in bioluminescent fish. Acta Zool. 2009, 91, 474–483. [Google Scholar] [CrossRef]
- Claes, J.M.; Mallefet, J. Hormonal control of luminescence from lantern shark (Etmopterus spinax) photophores. J. Exp. Biol. 2009, 212, 3684–3692. [Google Scholar] [CrossRef]
- Duchatelet, L.; Delroisse, J.; Pinte, N.; Sato, K.; Ho, H.-S.; Mallefet, J. Adrenocorticotropic hormone and cyclic adenosine monophosphate are involved in the control of shark bioluminescence. Photochem. Photobiol. 2019, 96, 37–45. [Google Scholar] [CrossRef]
- Duchatelet, L.; Claes, J.M.; Delroisse, J.; Flammang, P.; Mallefet, J. Glow on sharks: State of the art on bioluminescence research. Oceans 2021, 2, 822–842. [Google Scholar] [CrossRef]
- Duchatelet, L.; Delroisse, J.; Mallefet, J. Bioluminescence in lanternsharks: Insight from hormone receptor localization. Gen. Comp. Endocrinol. 2020, 294, 113488. [Google Scholar] [CrossRef]
- Schnitzler, C.E.; Pang, K.; Powers, M.L.; Reitzel, A.M.; Ryan, J.F.; Simmons, D.; Tada, T.; Park, M.; Gupta, J.; Brooks, S.Y. Genomic organization, evolution, and expression of photoprotein and opsin genes in Mnemiopsis leidyi: A new view of ctenophore photocytes. BMC Biol. 2012, 10, 107. [Google Scholar] [CrossRef]
- Delroisse, J.; Ullrich-Lüter, E.; Ortega-Martinez, O.; Dupont, S.; Arnone, M.-I.; Mallefet, J.; Flammang, P. High opsin diversity in a non-visual infaunal brittle star. BMC Genom. 2014, 15, 1035. [Google Scholar] [CrossRef] [PubMed]
- Delroisse, J.; Ullrich-Lüter, E.; Blaue, S.; Eeckhaut, I.; Flammang, P.; Mallefet, J. Fine structure of the luminous spines and luciferase detection in the brittle star Amphiura filiformis. Zool. Anz. 2017, 269, 1–12. [Google Scholar] [CrossRef]
- Bracken-Grissom, H.D.; DeLeo, D.M.; Porter, M.L.; Iwanicki, T.; Sickles, J.; Frank, T.M. Light organ photosensitivity in deep-sea shrimp may suggest a novel role in counterillumination. Sci. Rep. 2020, 10, 4485. [Google Scholar] [CrossRef] [PubMed]
- Duchatelet, L.; Sugihara, T.; Delroisse, J.; Koyanagi, M.; Rezsohazy, R.; Terakita, A.; Mallefet, J. From extraocular photoreception to pigment movement regulation: A new control mechanism of the lanternshark luminescence. Sci. Rep. 2020, 10, 10195. [Google Scholar] [CrossRef] [PubMed]
- Johann, L.; der Organe, I.V. Über eigentümliche epitheliale Gebilde (Leuchtorgane) bei Spinax niger Aus dem zoologischen Institut der Universität Rostock. Von. Z. Wiss. Zool. 1899, 66, 136. [Google Scholar]
- Ohshima, H. Some Observations on the Luminous Organs of Fishes; Imperial University of Tokyo: Tokyo, Japan, 1911; Volume 27, pp. 1–25. [Google Scholar]
- Renwart, M.; Delroisse, J.; Claes, J.M.; Mallefet, J. Ultrastructure organization of lantern shark (Etmopterus spinax) photophores. Zoomorphology 2014, 133, 405–416. [Google Scholar] [CrossRef]
- Renwart, M.; Delroisse, J.; Flammang, P.; Claes, J.M.; Mallefet, J. Cytological changes during luminescence production in lanternshark (Etmopterus spinax Linnaeus, 1758) photophores. Zoomorphology 2015, 134, 107–116. [Google Scholar] [CrossRef]
- Claes, J.M.; Mallefet, J. Early development of bioluminescence suggests camouflage by counter-illumination in the velvet belly lantern shark Etmopterus spinax (Squaloidea: Etmopteridae). J. Fish Biol. 2008, 73, 1337–1350. [Google Scholar] [CrossRef]
- Duchatelet, L.; Claes, J.M.; Mallefet, J. Embryonic expression of encephalopsin supports bioluminescence perception in lanternshark photophores. Mar. Biol. 2019, 166, 21. [Google Scholar] [CrossRef]
- Seigel, A.L. Revision of the Dalatiid shark genus Squaliolus: Anatomy, systematics, ecology. Copeia 1978, 1978, 602–614. [Google Scholar] [CrossRef]
- Claes, J.M.; Ho, H.-C.; Mallefet, J. Control of luminescence from pygmy shark (Squaliolus aliae) photophores. J. Exp. Biol. 2012, 215, 1691–1699. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delroisse, J.; Duchatelet, L.; Flammang, P.; Mallefet, J. Photophore distribution and enzymatic diversity within the photogenic integument of the cookie-cutter shark Isistius brasiliensis (Chondrichthyes: Dalatiidae). Front. Mar. Sci. 2021, 8, 627045. [Google Scholar] [CrossRef]
- Mallefet, J.; Stevens, D.W.; Duchatelet, L. Bioluminescence of the largest luminous vertebrate, the kitefin shark, Dalatias licha: First insights and comparative aspects. Front. Mar. Sci. 2021, 8, 633582. [Google Scholar] [CrossRef]
- Duchatelet, L.; Marion, R.; Mallefet, J. A third luminous shark family: Confirmation of luminescence ability for Zameus squamulosus (Squaliformes; Somniosidae). Photochem. Photobiol. 2021, 97, 739–744. [Google Scholar] [CrossRef]
- Lourtie, A.; Duchatelet, L.; Straube, N.; Puozzo, N.; Grace, M.A.; Naylor, G.J.P.; Delroisse, J. Placoid scales in bioluminescent sharks: Scaling their evolution using morphology and elemental composition. Front. Mar. Sci. 2022, 9, 908237. [Google Scholar] [CrossRef]
- Compagno, L.J.V.; Dando, M.; Fowler, S. Sharks of the World; Harper Collins: London, UK, 2004. [Google Scholar]
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef]
- Martin, U.; Mallefet, J. The diet of deep-water sharks. Deep Sea Res. I 2022, 103898, in press. [Google Scholar] [CrossRef]
- Reif, W.E. Functions of scales and photophores in mesopelagic luminescent sharks. Acta Zool. 1985, 66, 111–118. [Google Scholar] [CrossRef]
- Claes, J.M.; Mallefet, J. Functional physiology of lantern shark (Etmopterus spinax) luminescent pattern: Differential hormonal regulation of luminous zones. J. Exp. Biol. 2010, 213, 1852–1858. [Google Scholar] [CrossRef]
- Claes, J.M.; Mallefet, J. Comparative control of luminescence in sharks: New insights from the slendertail lanternshark (Etmopterus molleri). J. Exp. Mar. Biol. Ecol. 2015, 467, 87–94. [Google Scholar] [CrossRef]
- Claes, J.M.; Sato, K.; Mallefet, J. Morphology and control of photogenic structures in a rare dwarf pelagic lantern shark (Etmopterus splendidus). J. Exp. Mar. Biol. Ecol. 2011, 406, 1–5. [Google Scholar] [CrossRef]
- Claes, J.M.; Nilsson, D.-E.; Straube, N.; Collin, S.P.; Mallefet, J. Iso-luminance counterillumination drove bioluminescent shark radiation. Sci. Rep. 2014, 4, 4328. [Google Scholar] [CrossRef] [PubMed]
- Mesinger, A.F.; Case, J.F. Bioluminescence maintenance in juvenile Porichthys notatus. Biol. Bull. 1991, 181, 181–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mallefet, J.; Shimomura, O. Presence of coelenterazine in mesopelagic fishes from the Strait of Messina. Mar. Biol. 1995, 124, 381–385. [Google Scholar] [CrossRef]
- Duchatelet, L.; Hermans, C.; Duhamel, G.; Cherel, Y.; Guinet, C.; Mallefet, J. Coelenterazine detection in five myctophid species from the Kerguelen Plateau. In Proceedings of the Second Symposium on Kerguelen Plateau: Marine Ecosystem and Fisheries; Meunier, F.J., Ed.; Australian Antarctic Division: Kingston, TAS, Australia, 2019; pp. 31–41. [Google Scholar]
- Straube, N.; Li, C.; Claes, J.M.; Corrigan, S.; Naylor, G.J.P. Molecular phylogeny of Squaliformes and first occurrence of bioluminescence in sharks. BMC Evol. Biol. 2015, 15, 162. [Google Scholar] [CrossRef] [PubMed]
- Delroisse, J.; Duchatelet, L.; Flammang, P.; Mallefet, J. De novo transcriptome analyses provide insights into opsin-based photoreception in the lanternshark Etmopterus spinax. PLoS ONE 2018, 13, e0209767. [Google Scholar] [CrossRef]
| Species (Adult) | TL Interval (mm) | Mean PDe ± s.d. (Photophores cm−²) | Replicates | Reference |
|---|---|---|---|---|
| E. spinax | 310–510 | 2883 ± 232 | n = 20 | [42] |
| E. molleri | 400–465 | 3862 ± 193 | n = 8 | [43] |
| E. splendidus | 203–235 | 4620 ± 360 | n = 3 | [44] |
| S. aliae | 143–225 | 7863 ± 636 | n = 16 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duchatelet, L.; Ho, H.-C.; Mallefet, J. Photophore Morphogenesis and Extraocular Encephalopsin Expression during the Embryogenesis of Smalleye Pygmy Shark (Squaliolus aliae). Diversity 2022, 14, 1100. https://doi.org/10.3390/d14121100
Duchatelet L, Ho H-C, Mallefet J. Photophore Morphogenesis and Extraocular Encephalopsin Expression during the Embryogenesis of Smalleye Pygmy Shark (Squaliolus aliae). Diversity. 2022; 14(12):1100. https://doi.org/10.3390/d14121100
Chicago/Turabian StyleDuchatelet, Laurent, Hsuan-Ching Ho, and Jérôme Mallefet. 2022. "Photophore Morphogenesis and Extraocular Encephalopsin Expression during the Embryogenesis of Smalleye Pygmy Shark (Squaliolus aliae)" Diversity 14, no. 12: 1100. https://doi.org/10.3390/d14121100
APA StyleDuchatelet, L., Ho, H.-C., & Mallefet, J. (2022). Photophore Morphogenesis and Extraocular Encephalopsin Expression during the Embryogenesis of Smalleye Pygmy Shark (Squaliolus aliae). Diversity, 14(12), 1100. https://doi.org/10.3390/d14121100








