Eight New Records of Siphonophores (Cnidaria: Hydrozoa) in Korean Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Morphological Analysis
2.3. DNA Extraction, Amplification, and Sequencing
2.4. Sequence Analysis
3. Results
3.1. Abyla haeckeli (Lens and van Riemsdijk, 1908)
3.1.1. Synonymy
3.1.2. Material Examined
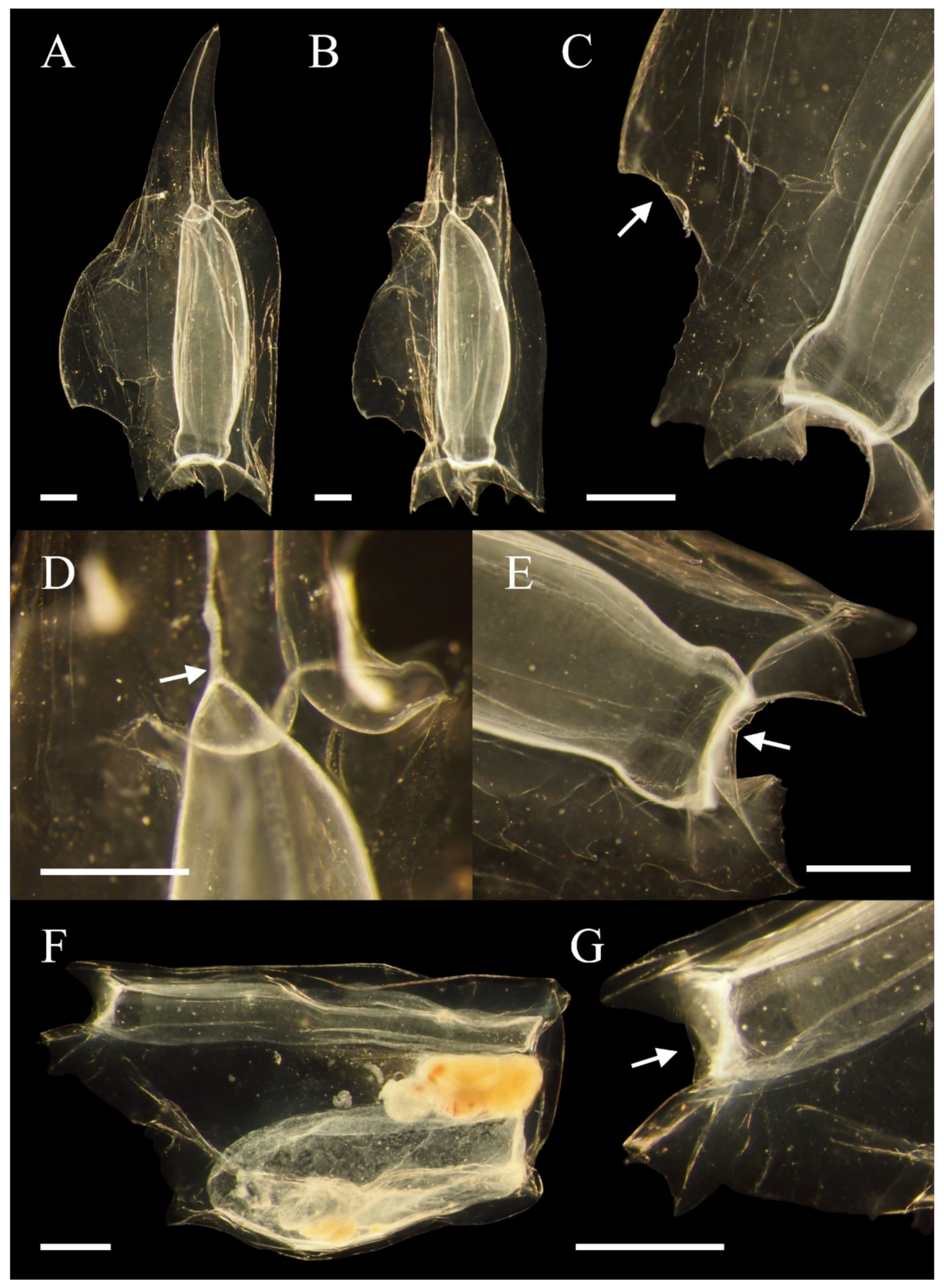
3.1.3. Descriptions
3.1.4. Remarks
3.1.5. Distribution
3.2. Ceratocymba leuckartii (Huxley, 1859)
3.2.1. Synonymy
3.2.2. Material Examined
3.2.3. Descriptions
3.2.4. Remarks
3.2.5. Distribution
3.3. Bassia bassensis (Quoy and Gaimard, 1833)
3.3.1. Synonymy
3.3.2. Material Examined
3.3.3. Descriptions
3.3.4. Remarks
3.3.5. Distribution
3.4. Dimophyes arctica (Chun, 1897)
3.4.1. Synonymy
3.4.2. Material Examined
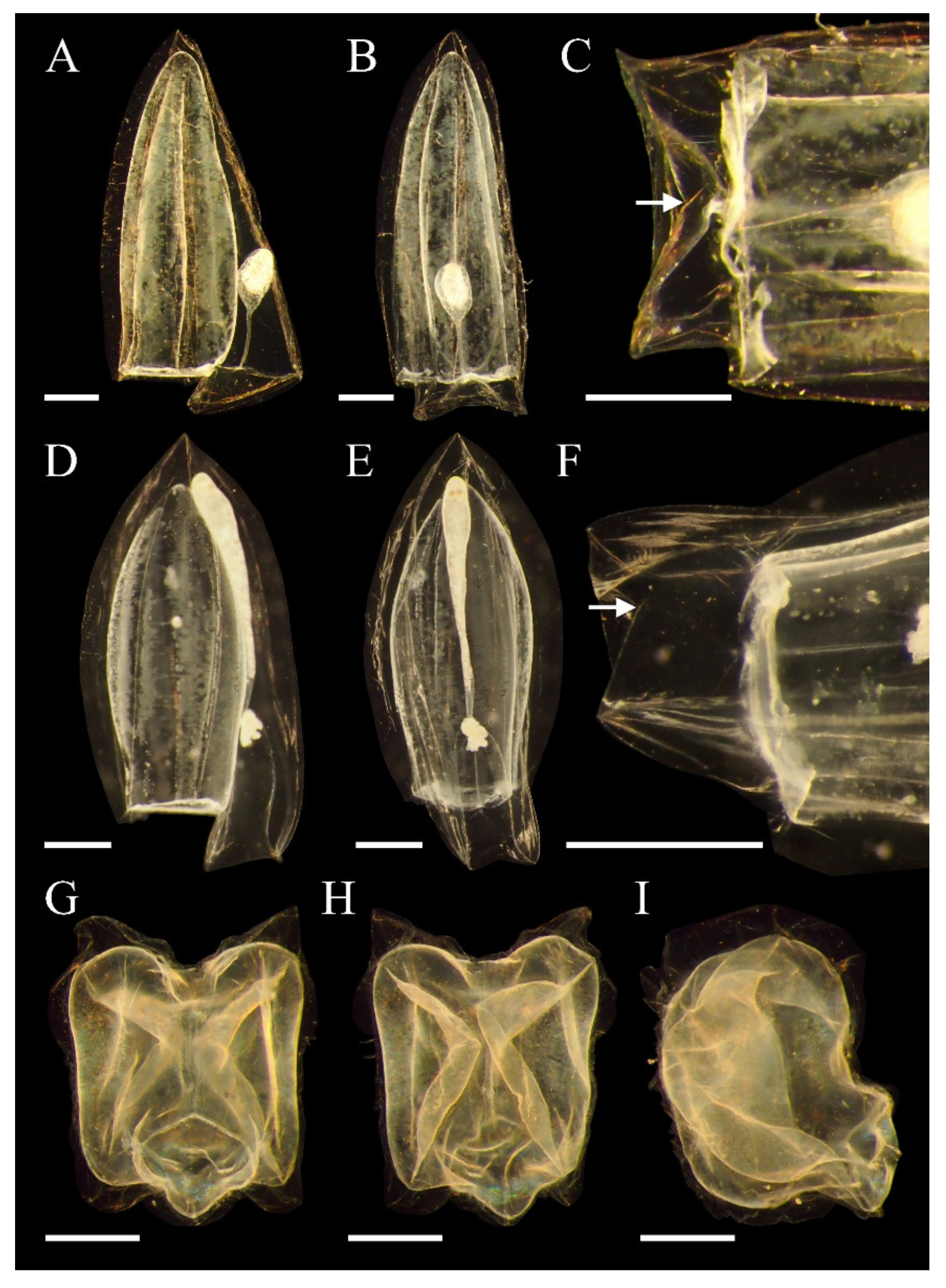
3.4.3. Descriptions
3.4.4. Remarks
3.4.5. Distribution
3.5. Lensia subtilis (Chun, 1886)
3.5.1. Synonymy
3.5.2. Material Examined
3.5.3. Descriptions
3.5.4. Remarks
3.5.5. Distribution
3.6. Lensia subtiloides (Lens and van Riemsdijk, 1908)
3.6.1. Synonymy
3.6.2. Material Examined
3.6.3. Descriptions
3.6.4. Remarks
3.6.5. Distribution
3.7. Muggiaea atlantica (Cunningham, 1892)
3.7.1. Synonymy
3.7.2. Material Examined
3.7.3. Descriptions
3.7.4. Remarks
3.7.5. Distribution
3.8. Nanomia bijuga (Delle Chiaje, 1844)
3.8.1. Synonymy
3.8.2. Material Examined
3.8.3. Descriptions
3.8.4. Remarks
3.8.5. Distribution
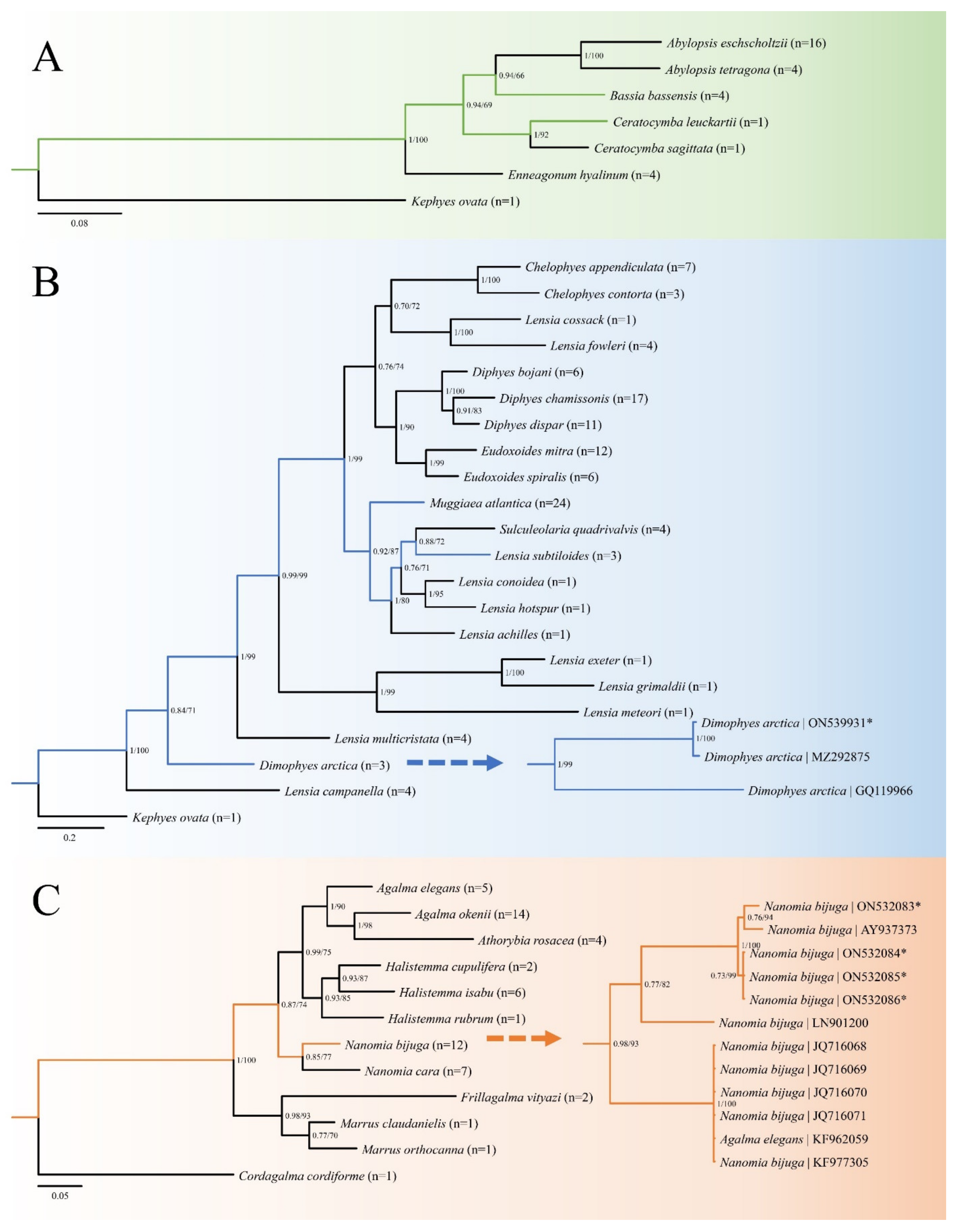
3.9. Molecular Phylogenetic Analysis and Genetic Diversity
4. Discussion
4.1. Morphological Analysis and Distribution
4.2. Molecular Analysis
4.3. Siphonophores in Korean Waters
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pugh, P. The vertical distribution of the siphonophores collected during the SOND cruise, 1965. J. Mar. Biol. Assoc. U. K. 1974, 54, 25–90. [Google Scholar] [CrossRef]
- Palma, S.; Retamal, M.C.; Silva, N.; Silva, C. Horizontal and vertical distributions of siphonophores in relation to oceanographic conditions in Chilean Patagonian fjords. Sci. Mar. 2014, 78, 339–351. [Google Scholar] [CrossRef]
- Munro, C.; Siebert, S.; Zapata, F.; Howison, M.; Damian-Serrano, A.; Church, S.H.; Goetz, F.E.; Pugh, P.R.; Haddock, S.H.; Dunn, C.W. Improved phylogenetic resolution within Siphonophora (Cnidaria) with implications for trait evolution. Mol. Phylogenet. Evol. 2018, 127, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Chuan, C.H.; Venmathi Maran, B.A.; Yap, T.K.; Cheong, K.C.; Syed Hussein, M.A.; Saleh, E. New Records of Cubozoan and Scyphozoan Jellyfish from Sabah Waters, Malaysia. Diversity 2021, 13, 420. [Google Scholar] [CrossRef]
- Marambio, M.; Canepa, A.; Lòpez, L.; Gauci, A.A.; Gueroun, S.K.; Zampardi, S.; Boero, F.; Yahia, O.K.-D.; Yahia, M.N.D.; Fuentes, V. Unfolding jellyfish bloom dynamics along the Mediterranean basin by transnational citizen science initiatives. Diversity 2021, 13, 274. [Google Scholar] [CrossRef]
- Dunn, C.W.; Pugh, P.R.; Haddock, S.H. Molecular phylogenetics of the Siphonophora (Cnidaria), with implications for the evolution of functional specialization. Syst. Biol. 2005, 54, 916–935. [Google Scholar] [CrossRef]
- Pugh, P. (Ed.) Siphonophorae. South Atlantic Zooplankton; Backhuys Publishers: Leiden, The Netherlands, 1999; Volume 1, pp. 467–511. [Google Scholar]
- Mapstone, G.M. Global diversity and review of Siphonophorae (Cnidaria: Hydrozoa). PLoS ONE 2014, 9, e87737. [Google Scholar] [CrossRef]
- Totton, A.K.; Bargmann, H.E. A Synopsis of the Siphonophora; British Museum (Natural History): London, UK, 1965. [Google Scholar]
- Totton, A.K. Siphonophora of the Indian Ocean together with systematic and biological notes on related specimens from other oceans. Discov. Rep. 1954, 27, 1–162. [Google Scholar]
- Kirkpatrick, P.; Pugh, P. Siphonophores and Velellids: Keys and Notes for the Identification of the Species; Brill Archive: Leiden, The Netherlands, 1984; Volume 29. [Google Scholar]
- Mapstone, G.M. Siphonophora (Cnidaria, Hydrozoa) of Canadian Pacific Waters; NRC Research Press: Ottawa, ON, Canada, 2009. [Google Scholar]
- Pugh, P. The taxonomic status of the genus Moseria (Siphonophora, Physonectae). Zootaxa 2006, 1343, 1–42. [Google Scholar] [CrossRef]
- Nishiyama, E.Y.; Araujo, E.M.; Oliveira, O.M. Species of Lensia (Cnidaria: Hydrozoa: Siphonophorae) from southeastern Brazilian waters. Zoologia 2016, 33, e20160030. [Google Scholar] [CrossRef][Green Version]
- Geller, J.; Meyer, C.; Parker, M.; Hawk, H. Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Mol. Ecol. Resour. 2013, 13, 851–861. [Google Scholar] [CrossRef]
- Schuchert, P. DNA barcoding of some Pandeidae species (Cnidaria, Hydrozoa, Anthoathecata). Rev. Suisse Zool. 2018, 125, 101–127. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Simmons, M.P.; Ochoterena, H. Gaps as characters in sequence-based phylogenetic analyses. Syst. Biol. 2000, 49, 369–381. [Google Scholar] [CrossRef]
- Young, N.D.; Healy, J. GapCoder automates the use of indel characters in phylogenetic analysis. BMC Bioinform. 2003, 4, 6. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Yamaoka, K.; Nakagawa, T.; Uno, T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 1978, 6, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Gascuel, O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Sears, M. Notes on Siphonophores: A Revision of the Abylinae; Museum of Comparative Zoology: Cambridge, MA, USA, 1953. [Google Scholar]
- Bedot, M. Les Siphonophores de la Baie d’Amboine: Étude Suivie d’une Revision de la Famille des Agalmidae. Rev. Suisse Zool. 1896, 3, 367–414. [Google Scholar] [CrossRef]
- Lens, A.D.; van Riemsdijk, M.T.A.A. The Siphonophora of the Siboga Expedition; Late EJ Brill: Leiden, The Netherlands, 1908; Volume 9. [Google Scholar]
- Alvarino, A. Siphonophores of the Pacific with a Review of the World Distribution; University of California Press: Berkeley, CA, USA, 1971. [Google Scholar]
- Li, K.Z.; Yin, J.Q.; Huang, L.M.; Song, X.Y. Comparison of siphonophore distributions during the southwest and northeast monsoons on the northwest continental shelf of the South China Sea. J. Plankton Res. 2012, 34, 636–641. [Google Scholar] [CrossRef]
- Schiariti, A.; Dutto, M.S.; Morandini, A.C.; Nagata, R.M.; Pereyra, D.Y.; Tapia, F.A.P.; Briz, L.D.; Genzano, G. An overview of the Medusozoa from the Southwestern Atlantic. In Plankton Ecology of the Southwestern Atlantic; Springer: Berlin/Heidelberg, Germany, 2018; pp. 413–449. [Google Scholar]
- Alvariño, A. Distribution of siphonophores in the regions adjacent to the Suez and Panama Canals. Fish. Bull. 1974, 72, 527–546. [Google Scholar]
- Rengarajan, K. Distribution of siphonophores along the west coast of India and the Laccadive Sea. J. Mar. Biol. Assoc. India 1975, 17, 56–72. [Google Scholar]
- Huxley, T.H. The Oceanic Hydrozoa; A Description of the Calycophoridae and Physophoridae Observed during the Voyage of HMS “Rattlesnake” in the Years 1846–1850; Edward Bowditch: London, UK, 1859. [Google Scholar]
- Daniel, R.; Daniel, A. On the siphonophores of the Bay of Bengal. I. Madras Coast. J. Mar. Biol. Assoc. India 1963, 5, 185–220. [Google Scholar]
- Casanova, B. Spatial and structural evolution on the euphausiid populations from the Antartic to the gulf of Aden. Investig. Pesq. 1980, 44, 377–394. [Google Scholar]
- Pagès, F.; Gili, J.-M.; Bouillon, J. Medusae (Hydrozoa, Scyphozoa, Cubozoa) of the Benguela Current (southeastern Atlantic). Sci. Mar. 1992, 56, 1–64. [Google Scholar]
- Alvariño, A. Antarctic Siphonophores from Plankton Samples of the United States Antarctic Research Program: Eltanin Cruises for Spring, Summer, Fall, and Winter (Cruises 3–5, 8–23, 25–28, 30, 35, and 38); American Geophysical Union: Washington, DC, USA, 1990; Volume 49. [Google Scholar]
- Quoy, J.; Gaimard, J. Zoophytes. Voyage de l’Astrolabe. Zoologie 1833, 4, 1–390. [Google Scholar]
- Bigelow, H.B. The Siphonophorae; Cambridge Museum: Cambridge, UK, 1911; Volume 38. [Google Scholar]
- Totton, A.K. Siphonophora; British Museum: London, UK, 1932. [Google Scholar]
- Mills, C.E.; Marques, A.C.; Migotto, A.E.; Calder, D.R.; Hand, C.; Rees, J.; Haddock, S.; Dunn, C.; Pugh, P. Hydrozoa: Polyps, hydromedusae, and siphonophora. In The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon; Carlton, J.T., Ed.; University of California Press: Berkeley, CA, USA, 2007; pp. 118–167. [Google Scholar]
- Chun, C. Die Siphonophoren der Plankton-Expedition; Lipsius & Tischer: Kiel, Germany, 1897. [Google Scholar]
- Bigelow, H.B. Medusae and Siphonophorae Collected by the US Fisheries Steamer “Albatross” in the Northwestern Pacific, 1906; US Government Printing Office: Washington, DC, USA, 1913; Volume 44.
- Park, N.; Yeom, J.; Jeong, R.; Lee, W. Novel attempt at discrimination of a bullet-shaped siphonophore (Family Diphyidae) using matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-ToF MS). Sci. Rep. 2021, 11, 19077. [Google Scholar] [CrossRef]
- Stepanjants, S.; Dianov, M. The computer approach to the study of the morphological and biological peculiarities of siphonophora Dimophyes arctica (Chun, 1897). Data bases and computer graphics in zoological investigations. Tr. Zool. Inst. Akad. Nauk. SSSR 1997, 269, 154–165. [Google Scholar]
- Grossmann, M.M.; Lindsay, D.J. Diversity and distribution of the Siphonophora (Cnidaria) in Sagami Bay, Japan, and their association with tropical and subarctic water masses. J. Oceanogr. 2013, 69, 395–411. [Google Scholar] [CrossRef]
- Hosia, A.; Båmstedt, U. Seasonal abundance and vertical distribution of siphonophores in western Norwegian fjords. J. Plankton Res. 2008, 30, 951–962. [Google Scholar] [CrossRef]
- Gao, S. The vertical distribution of the Medusae, Pteropoda, Heteropoda and Thaliacea in the East China Sea. Stud. Mar. Sin 1990, 31, 83–92. [Google Scholar]
- Will, J.F. Horae Tergestinae Oder Beschreibung und Anatomie der im Herbste 1843 bei Triest Beobachteten Akalephen; Voss: Hamburg, Germany, 1844. [Google Scholar]
- Chun, C. Über Bau und Entwickelung der Siphonophoren. Sitz. Königlich Preuss. Akad. Wiss. 1886, 1886, 681–688. [Google Scholar]
- Margulis, R.Y. Distribution of Siphonophores of the genus Lensia (suborder Calycophorae) in the Atlantic. Okeanologiya 1971, 11, 80–84. [Google Scholar]
- Daniel, R. Siphonophora from the Indian Ocean; Zoological Survey of India: Kolkata, India, 1974; Volume 15. [Google Scholar]
- Cunningham, J. On a species of siphonophore observed at Plymouth. J. Mar. Biol. Assoc. U. K. 1892, 2, 212–215. [Google Scholar] [CrossRef][Green Version]
- Daniel, R. Fauna of India and the Adjacent Countries. Coelenterata: Hydrozoa, Siphonophora; Zoological Survey of India: Kolkata, India, 1985. [Google Scholar]
- Delle Chiaje, S. Animali Senza Vertebre del Regno di Napoli. Descrizione e Notomia Degli Animali Invertebrati Della Sicilia Citeriore Osservati Vivi Negli Anni 1822–1830; C. Batelli: Naples, Italy, 1844; Volume 8. [Google Scholar]
- Haeckel, E. Ueber Arbeitstheilung in Natur-und Menschenleben: Vortrag; Charisius: Berlin, Germany, 1869. [Google Scholar]
- Metschnikoff, E. Studien über die Entwicklung der Medusen und Siphonophoren. Z. Wiss. Zool. 1874, 24, 15–83. [Google Scholar]
- Claus, C. Über Halistemma tergestinum n. sp. Nebst Bemerkungen über den Feinern Bau der Physophoriden; Alfred Hölder: Vienna, Austria, 1878. [Google Scholar]
- Fewkes, J.W. The Siphonophores. Am. Nat. 1883, 17, 833–845. [Google Scholar] [CrossRef]
- Agassiz, A.; Murray, J. Reports on the Scientific Results of the Expedition to the Tropical Pacific, in Charge of Alexander Agassiz, by the US Fish Commission Steamer “Albatross” from August, 1899, to March, 1900, Commander Jefferson F. Moser, USN, Commanding: With Remarks on the Deep-sea Deposits. Preliminary Report and List of Stations. I; Museum of Comparative Zoology: Cambridge, MA, USA, 1902. [Google Scholar]
- Bouillon, J.; Medel, M.D.; Pagès, F.; Gili, J.M.; Boero, F.; Gravili, C. Fauna of the Mediterranean hydrozoa. Sci. Mar. 2004, 68, 5–438. [Google Scholar] [CrossRef]
- Dunn, C.W.; Wagner, G.P. The evolution of colony-level development in the Siphonophora (Cnidaria: Hydrozoa). Dev. Genes Evol. 2006, 216, 743–754. [Google Scholar] [CrossRef]
- Lindsay, D.J. A checklist of midwater cnidarians and ctenophores from Sagami Bay–species sampled during submersible surveys from 1993–2004. Bull. Plankton Soc. Jpn. 2006, 53, 104–110. [Google Scholar]
- Pugh, P.R. The distribution of siphonophores in a transect across the North Atlantic Ocean at 32 N. J. Exp. Mar. Biol. Ecol. 1975, 20, 77–97. [Google Scholar] [CrossRef]
- Bouillon, J.; Boero, F. The hydrozoa: A new classification in the ligth of old knowledge. Thalass. Salentina 2000, 24, 3–45. [Google Scholar]
- Zheng, L.; He, J.; Lin, Y.; Cao, W.; Zhang, W. 16S rRNA is a better choice than COI for DNA barcoding hydrozoans in the coastal waters of China. Acta Oceanol. Sin. 2014, 33, 55–76. [Google Scholar] [CrossRef]
- Park, N.; Prudkovsky, A.A.; Lee, W. Integrated taxonomy for halistemma species from the northwest pacific ocean. Water 2020, 12, 3283. [Google Scholar] [CrossRef]
- Park, J.H.; Song, J.I. Two new records of hydromedusae (Cnidaria: Hydrozoa) in Korea. Anim. Syst. Evol. Divers. 2004, 20, 31–37. [Google Scholar]
- Park, N.; Lee, W. Four new records of family Diphyidae (Hydrozoa: Siphonophorae) in Korean waters. J. Species Res. 2020, 9, 131–146. [Google Scholar]
- Park, J.H.; Won, J.H. Two New Records of Shiphonophores (Hydrozoa: Siphonophora) in Korea. Anim. Syst. Evol. Divers. 2005, 21, 201–205. [Google Scholar]
- Park, J.H. Five new records of hydromedusae (Cnidaria: Hydrozoa) in Korea. Anim. Syst. Evol. Divers. 2007, 23, 175–181. [Google Scholar] [CrossRef][Green Version]
- Park, J.H.; Won, J.H. Three new records of marine Hydromedusae (Cnidaria: Hydrozoa) in Korea. Anim. Syst. Evol. Divers. 2004, 20, 179–184. [Google Scholar]
- Park, J.H. Two New Records of Siphonophores (Cnidaria: Hydrozoa: Siphonophora) in Korean Waters. Anim. Syst. Evol. Divers. 2010, 26, 67–70. [Google Scholar] [CrossRef]
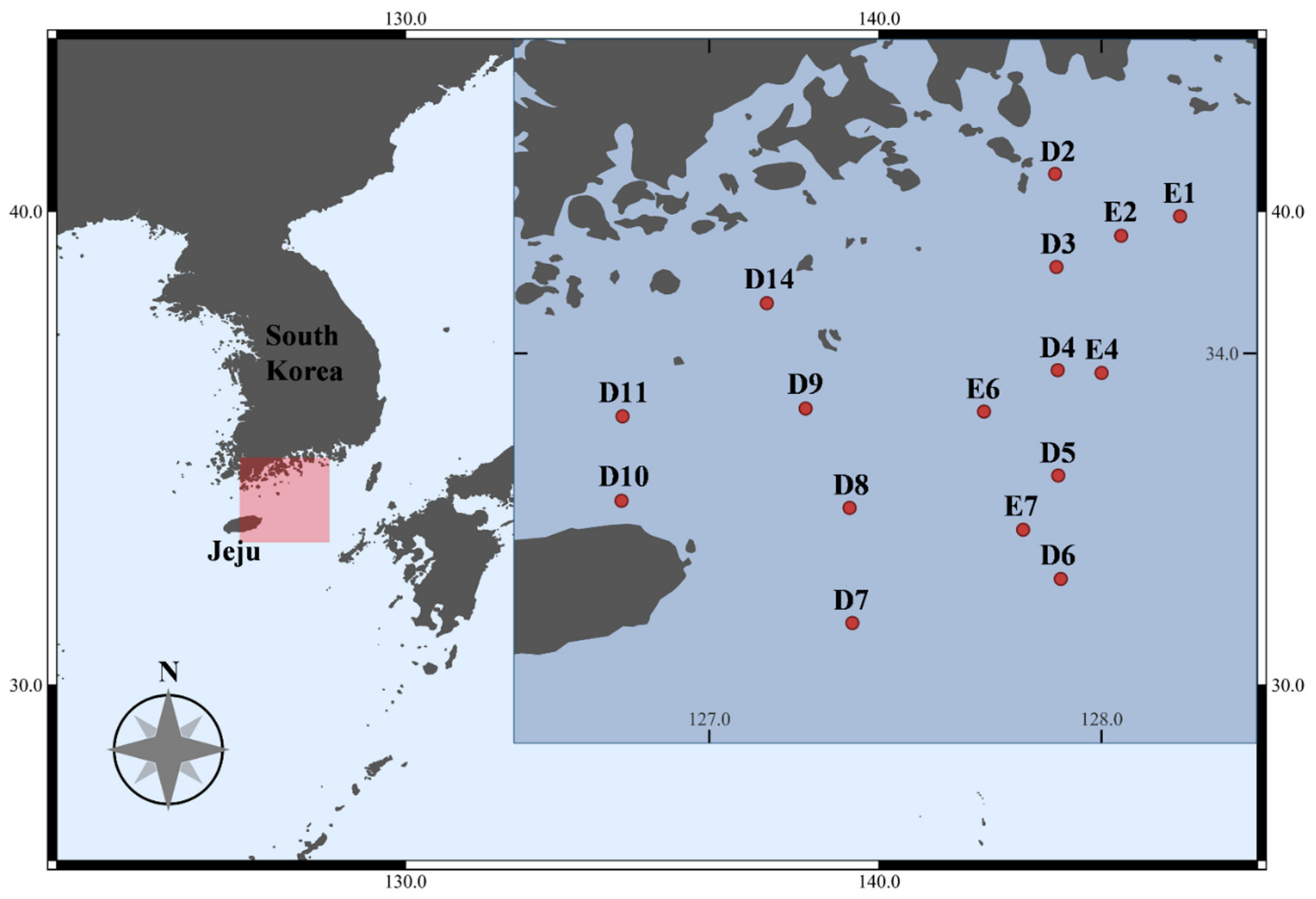

| Station | Latitude (N) | Longitude (E) | Date | Depth (m) | Temperature (°C) | Salinity |
|---|---|---|---|---|---|---|
| D2 | 34°27′28.3″ | 127°52′53.0″ | 23 April 2018 | 42 | 13.3 | 34.1 |
| D3 | 34°13′12.6″ | 127°53′5.8″ | 27 April 2018 | 65 | 14.2 | 34.2 |
| D4 | 33°57′25.7″ | 127°53′17.6″ | 27 April 2018 | 90 | 16.5 | 34.4 |
| D5 | 33°41′17.9″ | 127°53′22.7″ | 27 April 2018 | 107 | 17.9 | 34.5 |
| D6 | 33°25′27.7″ | 127°53′45.6″ | 27 April 2018 | 111 | 18.0 | 34.5 |
| D7 | 33°18′43.7″ | 127°21′52.9″ | 26 April 2018 | 133 | 18.4 | 34.3 |
| D8 | 33°36′20.6″ | 127°21′27.4″ | 26 April 2018 | 98 | 17.9 | 34.5 |
| D9 | 33°51′33.0″ | 127°14′43.8″ | 26 April 2018 | 82 | 16.8 | 34.4 |
| D10 | 33°37′25.9″ | 126°46′34.0″ | 26 April 2018 | 130 | 15.8 | 34.3 |
| D11 | 33°50′21.2″ | 126°46′43.8″ | 26 April 2018 | 95 | 15.9 | 34.4 |
| D14 | 34°7′40.7″ | 127°8′47.8″ | 23 April 2018 | 40 | 13.8 | 34.2 |
| E1 | 34°21′0.0″ | 128°11′60.0″ | 15 June 2021 | 60 | 20.0 | 33.2 |
| E2 | 34°17′60.0″ | 128°3′0.0″ | 17 June 2021 | 65 | 20.0 | 32.8 |
| E4 | 33°57′0.0″ | 128°0′0.0″ | 18 June 2021 | 80 | 21.6 | 32.6 |
| E6 | 33°51′5.0″ | 127°42′1.0″ | 17 June 2021 | 80 | 21.3 | 32.9 |
| E7 | 33°32′60.0″ | 127°47′60.0″ | 18 June 2021 | 105 | 23.2 | 32.3 |
| Suborder | Family | Subfamily | Genus | Species | References |
|---|---|---|---|---|---|
| Calycophorae | Abylidae | Abylinae | Abyla | Abyla haeckeli (Lens and van Riemsdijk, 1908) | This study |
| Ceratocymba | Ceratocymba leuckartii (Huxley, 1859) | This study | |||
| Abylopsinae | Abylopsis | Abylopsis eschscholtzii (Huxley, 1859) | [75] | ||
| Abylopsis tetragona (Otto, 1823) | [76] | ||||
| Bassia | Bassia bassensis (Quoy and Gaimard, 1833) | This study | |||
| Diphyidae | Diphyinae | Chelophyes | Chelophyes appendiculata (Eschscholtz, 1829) | [74] | |
| Chelophyes contorta (Lens and van Riemsdijk, 1908) | [74] | ||||
| Dimophyes | Dimophyes arctica (Chun, 1897) | This study | |||
| Diphyes | Diphyes bojani (Eschscholtz, 1825) | [77] | |||
| Diphyes chamissonis (Huxley, 1859) | [75] | ||||
| Diphyes dispar (Chamisso and Eysenhardt, 1821) | [76] | ||||
| Eudoxoides | Eudoxoides mitra (Huxley, 1859) | [74] | |||
| Eudoxoides spiralis (Bigelow, 1911) | [74] | ||||
| Lensia | Lensia subtilis (Chun, 1886) | This study | |||
| Lensia subtiloides (Lens and van Riemsdijk, 1908) | This study | ||||
| Muggiaea | Muggiaea atlantica (Cunningham, 1892) | This study | |||
| Muggiaea bargmannae (Totton, 1954) | [77] | ||||
| Cystonectae | Physaliidae | Physalia | Physalia physalis (Linnaeus, 1758) | [73] | |
| Rhizophysidae | Bathyphysa | Bathyphysa conifera (Studer, 1878) | [78] | ||
| Physonectae | Agalmatidae | Agalma | Agalma okenii (Eschscholtz, 1825) | [78] | |
| Nanomia | Nanomia bijuga (Delle Chiaje, 1844) | This study | |||
| 3 | 5 | 3 | 14 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, N.; Lee, W. Eight New Records of Siphonophores (Cnidaria: Hydrozoa) in Korean Waters. Diversity 2022, 14, 494. https://doi.org/10.3390/d14060494
Park N, Lee W. Eight New Records of Siphonophores (Cnidaria: Hydrozoa) in Korean Waters. Diversity. 2022; 14(6):494. https://doi.org/10.3390/d14060494
Chicago/Turabian StylePark, Nayeon, and Wonchoel Lee. 2022. "Eight New Records of Siphonophores (Cnidaria: Hydrozoa) in Korean Waters" Diversity 14, no. 6: 494. https://doi.org/10.3390/d14060494
APA StylePark, N., & Lee, W. (2022). Eight New Records of Siphonophores (Cnidaria: Hydrozoa) in Korean Waters. Diversity, 14(6), 494. https://doi.org/10.3390/d14060494







