Distribution, Biogeography and Characteristics of the Threatened and Data-Deficient Flora in the Southwest Australian Floristic Region
Abstract
1. Introduction
- Are threatened and data-deficient flora evenly distributed across plant lineages and across the landscape?
- Do spatial and biogeographic patterns of threatened and data-deficient flora reflect those of the flora as a whole, or are they associated with evolutionary history or threats, such as macro-scale levels of land transformation, which is a threat commonly cited as an overwhelming contributor to extinction risk [35,36]?
- Are centres of high occurrence of data-deficient flora the same or a subset of those of the threatened flora, indicating that the conservation needs of data-deficient flora may at least partly be met by mitigating threats to natural populations of threatened flora?
2. Materials and Methods
2.1. Data Sources
2.2. Data Analyses
3. Results
3.1. Characteristics of the Conservation-Listed Flora
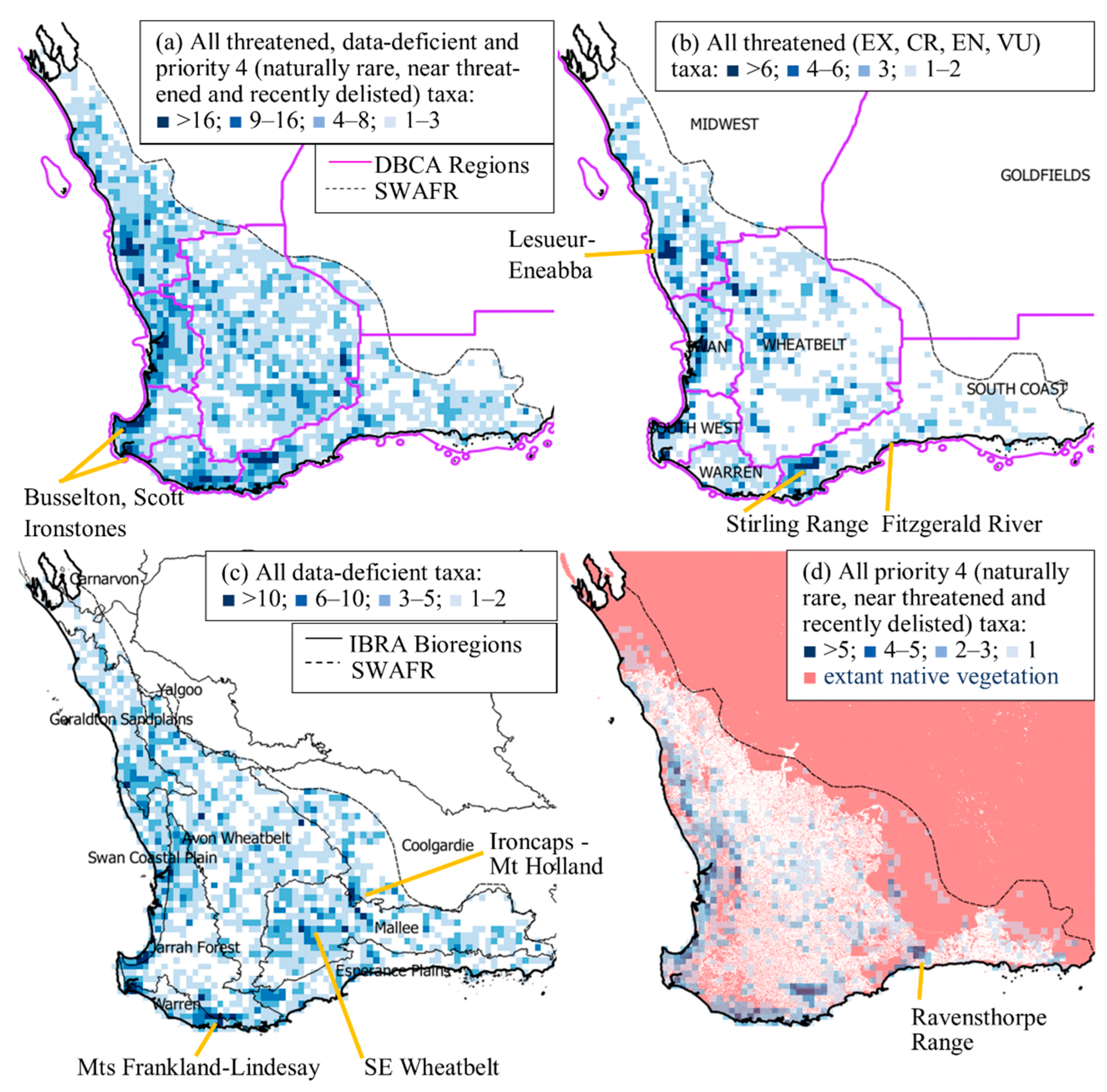
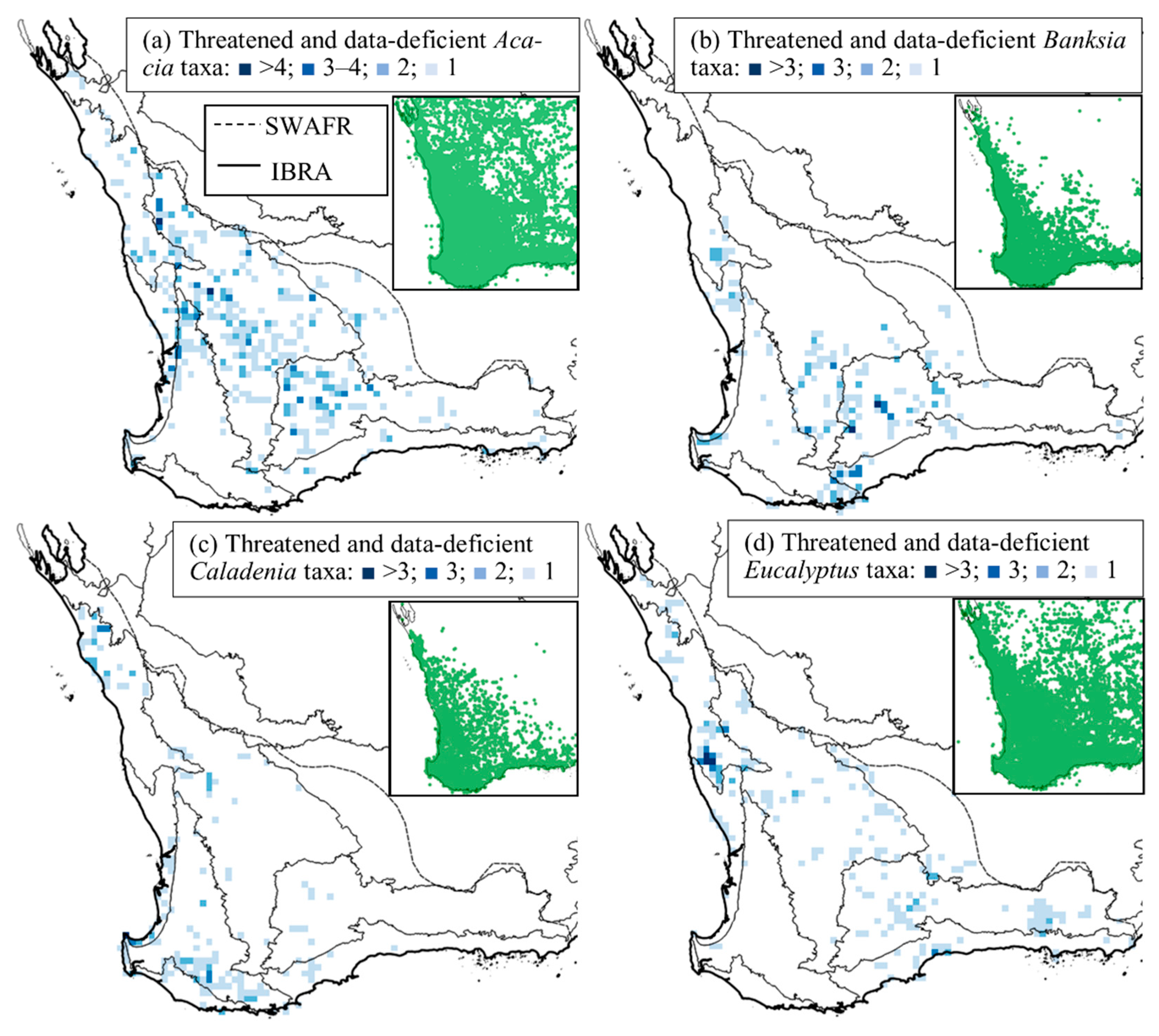
3.2. Spatial Distribution of Conservation-Listed Flora
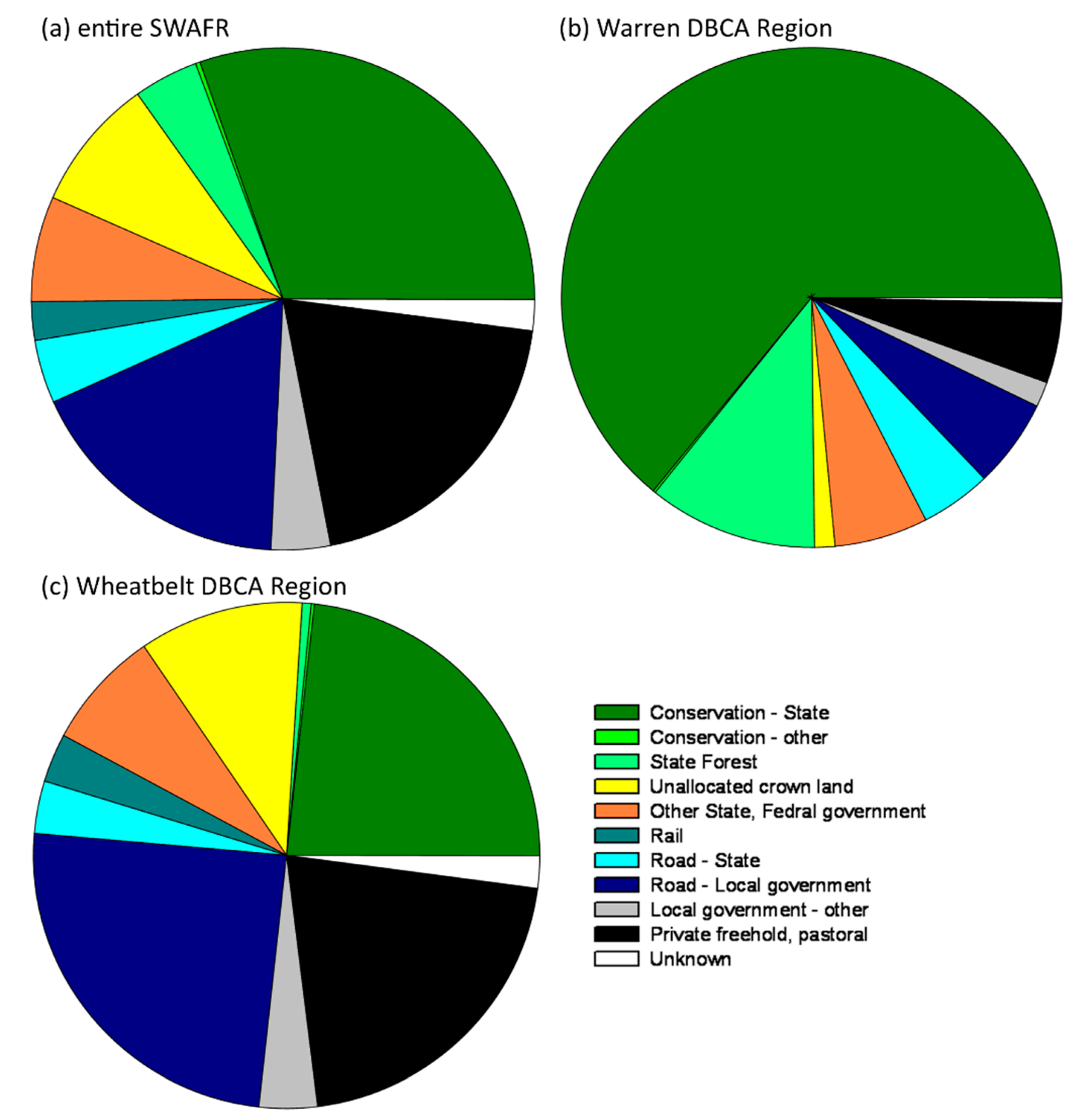
3.3. Administrative Regions and Tenure of Conservation-Listed Flora
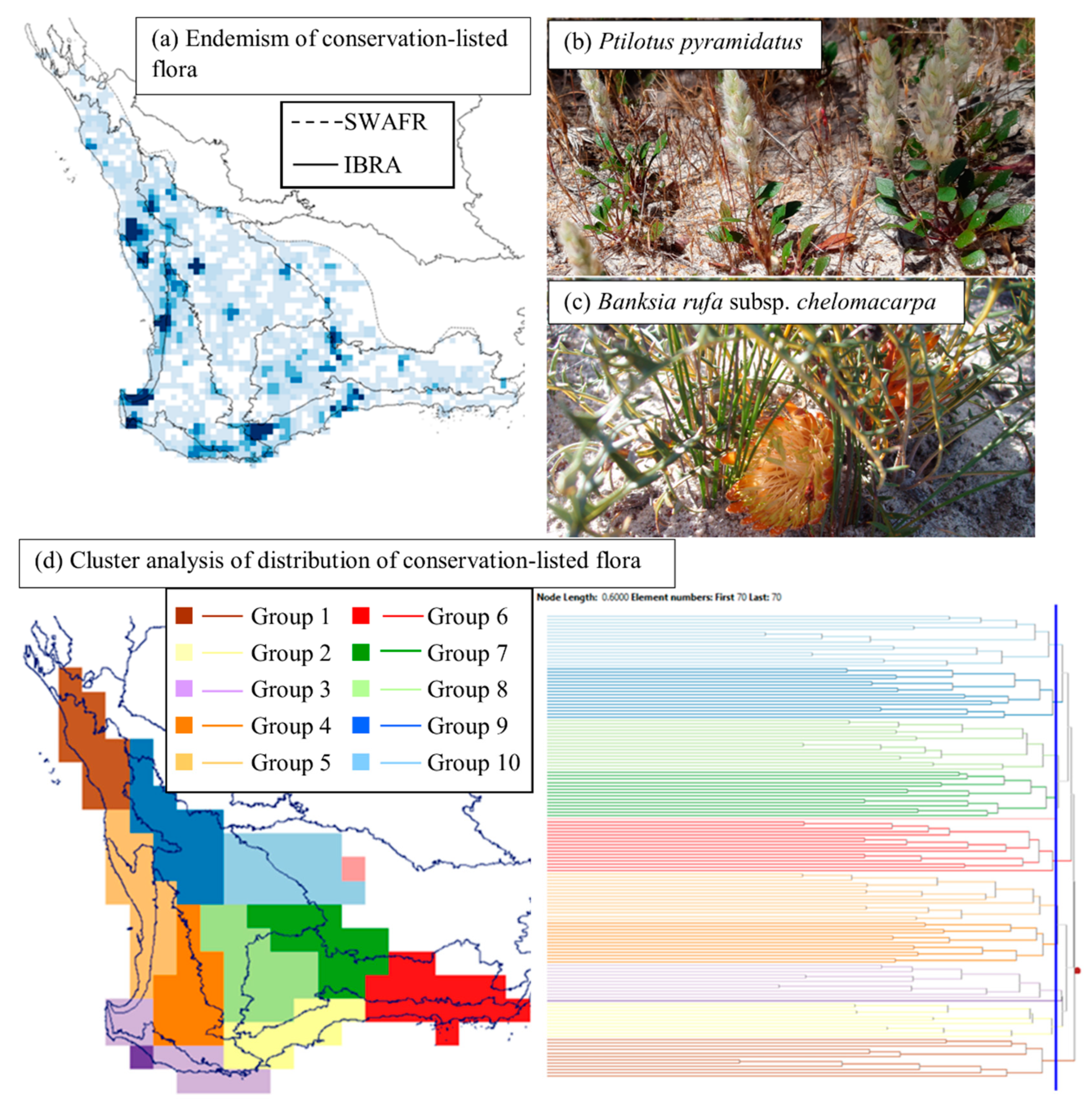
3.4. Endemism and Biogeographic Patterns of Conservation-Listed Flora
4. Discussion
4.1. Biogeographic Patterns—Hotspots for Conservation-Listed Flora
4.2. The Elephant in the Room—The Data-Deficient Flora
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nic Lughadha, E.; Bachman, S.P.; Leão, T.C.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.A.; Gâteblé, G.; et al. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species; Version 2021-3; IUCN: Gland, Switzerland, 2021; Available online: https://www.iucnredlist.org (accessed on 1 June 2022).
- Humphreys, A.M.; Govaerts, R.; Ficinski, S.Z.; Nic Lughadha, E.; Vorontsova, M.S. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat. Ecol. Evol. 2019, 3, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, J.J.; Hui, C.; Castillo, M.L.; Iriondo, J.M.; Keet, J.-H.; Khapugin, A.A.; Médail, F.; Rejmánek, M.; Theron, G.; Yannelli, F.A.; et al. Recent anthropogenic plant extinctions differ in biodiversity hotspots and coldspots. Curr. Biol. 2019, 29, 2912–2918.e2. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Türe, C.; Böcük, H. Distribution patterns of threatened endemic plants in Turkey: A quantitative approach for conservation. J. Nat. Conserv. 2010, 18, 296–303. [Google Scholar] [CrossRef]
- Juiling, S.; Leon, S.K.; Jumian, J.; Tsen, S.; Lee, Y.L.; Khoo, E.; Sugau, J.B.; Nilus, R.; Pereira, J.T. Conservation assessment and spatial distribution of endemic orchids in Sabah, Borneo. Nat. Conserv. Res. 2020, 5 (Suppl. S1), 136–144. [Google Scholar] [CrossRef]
- Sousa-Baena, M.S.; Garcia, L.C.; Peterson, A.T. Knowledge behind conservation status decisions: Data basis for “Data Deficient” Brazilian plant species. Biol. Conserv. 2014, 173, 80–89. [Google Scholar] [CrossRef]
- IUCN Standards and Petitions Subcommittee. Guidelines for Using the IUCN Red List Categories and Criteria; Version 13; IUCN: Gland, Switzerland, 2017; Available online: http://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 5 June 2019).
- Le Breton, T.D.; Zimmer, H.C.; Gallagher, R.V.; Cox, M.; Allen, S.; Auld, T.D. Using IUCN criteria to perform rapid assessments of at-risk taxa. Biodivers. Conserv. 2019, 28, 863–883. [Google Scholar] [CrossRef]
- Gioia, P.; Hopper, S.D. A new phytogeographic map for the Southwest Australian Floristic Region after an exceptional decade of collection and discovery. Bot. J. Linn. Soc. 2017, 184, 1–15. [Google Scholar] [CrossRef]
- Cowling, R.; Witkowski, E.; Milewski, A.; Newbey, K. Taxonomic, edaphic and biological aspects of narrow plant endemism on matched sites in Mediterranean South Africa and Australia. J. Biogeogr. 1994, 21, 651–664. [Google Scholar] [CrossRef]
- Gosper, C.R.; Yates, C.J.; Prober, S.M.; Parsons, B.C. Contrasting changes in vegetation structure and diversity with time since fire in two Australian Mediterranean-climate plant communities. Austral. Ecol. 2012, 37, 164–174. [Google Scholar] [CrossRef]
- Gallagher, R.V. Correlates of range size variation in the Australian seed-plant flora. J. Biogeogr. 2016, 43, 1287–1298. [Google Scholar] [CrossRef]
- Gibson, N.; Prober, S.; Meissner, R.; van Leeuwen, S. Implications of high species turnover on the south-western Australian sandplains. PLoS ONE 2017, 12, e0172977. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.J.; Robinson, T.; Wardell-Johnson, G.W.; Keppel, G.; Hopper, S.D.; Schut, A.G.T.; Byrne, M. High species diversity and turnover in granite inselberg floras highlight the need for a conservation strategy protecting many outcrops. Ecol. Evol. 2019, 9, 7660–7675. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C. One biodiversity hotspot to rule them all: Southwestern Australia—An extraordinary evolutionary centre for plant functional and taxonomic diversity. J. R. Soc. West Aust. 2021, 104, 91–122. [Google Scholar]
- Hopper, S.D. OCBIL theory: Towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant Soil 2009, 322, 49–86. [Google Scholar] [CrossRef]
- Cowling, R.M.; Potts, A.J.; Bradshaw, P.L.; Colville, J.; Arianoutsou, M.; Ferrier, S.; Forest, F.; Fyllas, N.M.; Hopper, S.D.; Ojeda, F.; et al. Variation in plant diversity in mediterranean-climate ecosystems: The role of climatic and topographical stability. J. Biogeogr. 2015, 42, 552–564. [Google Scholar] [CrossRef]
- Gosper, C.R.; Coates, D.J.; Hopper, S.D.; Byrne, M.; Yates, C.J. The role of landscape history in the distribution and conservation of threatened flora in the Southwest Australian Floristic Region. Biol. J. Linn. Soc. 2021, 133, 394–410. [Google Scholar] [CrossRef]
- Gosper, C.R.; Kinloch, J.; Coates, D.J.; Byrne, M.; Pitt, G.; Yates, C.J. Differential exposure and susceptibility to threats based on evolutionary history: How OCBIL theory informs flora conservation. Biol. J. Linn. Soc. 2021, 133, 373–393. [Google Scholar] [CrossRef]
- Coates, D.J.; Atkins, K.A. Priority setting and the conservation of Western Australia’s diverse and highly endemic flora. Biol. Conserv. 2001, 97, 251–263. [Google Scholar] [CrossRef]
- Silcock, J.L.; Fensham, R.J. Using evidence of decline and extinction risk to identify priority regions, habitats and threats for plant conservation in Australia. Aust. J. Bot. 2018, 66, 541–555. [Google Scholar] [CrossRef]
- Brummitt, N.A.; Bachman, S.P.; Griffiths-Lee, J.; Lutz, M.; Moat, J.F.; Farjon, A.; Donaldson, J.S.; Hilton-Taylor, C.; Meagher, T.R.; Albuquerque, S.; et al. Green plants in the red: A baseline global assessment for the IUCN sampled red list index for Plants. PLoS ONE 2015, 10, e0135152. [Google Scholar] [CrossRef] [PubMed]
- Monks, L.; Barrett, S.; Beecham, B.; Byrne, M.; Chant, A.; Coates, D.; Cochrane, J.A.; Crawford, A.; Dillon, R.; Yates, C. Recovery of threatened plant species and their habitats in the biodiversity hotspot of the Southwest Australian Floristic Region. Plant Divers. 2019, 41, 59–74. [Google Scholar] [CrossRef]
- Hopper, S.D.; van Leeuwen, S.; Brown, A.P.; Patrick, S.J. Western Australia’s Endangered Flora; Department of Conservation and Land Management: Perth, Australia, 1990. [Google Scholar]
- Coates, D.J.; Atkins, K.A. Threatened flora of Western Australia: A focus for conservation outside reserves. In Conservation Outside Nature Reserves; Hale, P., Lamb, D., Eds.; University of Queensland: Brisbane, Australia, 1997; pp. 432–441. [Google Scholar]
- Wege, J.A.; Thiele, K.R.; Shepherd, K.A.; Butcher, R.; Macfarlane, T.D.; Coates, D.J. Strategic taxonomy in a biodiverse landscape: A novel approach to maximizing conservation outcomes for rare and poorly known flora. Biodivers. Conserv. 2015, 24, 17–32. [Google Scholar] [CrossRef]
- Parsons, B.C.; Gosper, C.R. Contemporary fire regimes in a fragmented and an unfragmented landscape: Implications for vegetation structure and persistence of the fire-sensitive malleefowl. Int. J. Wildland Fire 2011, 20, 184–194. [Google Scholar] [CrossRef][Green Version]
- Gosper, C.R.; Prober, S.M.; Yates, C.J. Estimating fire interval bounds using vital attributes: Implications of uncertainty and among-population variability. Ecol. Appl. 2013, 23, 924–935. [Google Scholar] [CrossRef]
- Tulloch, A.I.T.; Pichancourt, J.-B.; Gosper, C.R.; Sanders, A.; Chadès, I. Fire management strategies to maintain species population processes in a fragmented landscape of fire-interval extremes. Ecol. Appl. 2016, 26, 2175–2189. [Google Scholar] [CrossRef]
- Gibson, N.; Yates, C.J.; Dillon, R. Plant communities of the ironstone ranges of South Western Australia: Hotspots for plant diversity and mineral deposits. Biodivers. Conserv. 2010, 19, 3951–3962. [Google Scholar] [CrossRef]
- Hopper, S.D.; Silveira, F.A.O.; Fiedler, P.L. Biodiversity hotspots and Ocbil theory. Plant Soil 2016, 403, 167–216. [Google Scholar] [CrossRef]
- Franks, A.J.; Yates, C.J.; Hobbs, R.J. Defining plant functional groups to guide rare plant management. Plant Ecol. 2009, 204, 207–216. [Google Scholar] [CrossRef]
- Burgman, M.A.; Keith, D.; Hopper, S.D.; Widyatmoko, D.; Drill, C. Threat syndromes and conservation of the Australian flora. Biol. Conserv. 2007, 134, 73–82. [Google Scholar] [CrossRef]
- Fensham, R.J.; Laffineur, B.; Collingwood, T.D.; Beech, E.; Bell, S.; Hopper, S.D.; Phillips, G.; Rivers, M.C.; Walsh, N.; White, M. Rarity or decline: Key concepts for the Red List of Australian eucalypts. Biol. Conserv. 2020, 243, 108455. [Google Scholar] [CrossRef]
- Western Australian Herbarium. Florabase—The Western Australian Flora; Department of Biodiversity, Conservation and Attractions: Perth, Australia. Available online: https://florabase.dpaw.wa.gov.au/ (accessed on 22 April 2022).
- Department of Biodiversity, Conservation and Attractions (DBCA). Threatened and Priority Flora (DBCA-036). Available online: https://catalogue.data.wa.gov.au/dataset/threatened-and-priority-flora (accessed on 22 March 2019).
- Smith, M.G.; Jones, A. Threatened and Priority Flora List; Department of Biodiversity, Conservation and Attractions: Perth, Australia, 5 December 2018. Available online: https://www.dpaw.wa.gov.au/plants-and-animals/threatened-species-and-communities/threatened-plants (accessed on 12 April 2022).
- Laffan, S.W.; Lubarsky, E.; Rosauer, D.F. Biodiverse: A tool for the spatial analysis of biological and other diversity. Ecography 2010, 33, 643–647. [Google Scholar] [CrossRef]
- Fremout, T.; Thomas, E.; Gaisberger, H.; Van Meerbeek, K.; Muenchow, J.; Briers, S.; Gutierrez-Miranda, C.E.; Marcelo-Peña, J.L.; Kindt, R.; Atkinson, R.; et al. Mapping tree species vulnerability to multiple threats as a guide to restoration and conservation of tropical dry forests. Glob. Change Biol. 2020, 26, 3552–3568. [Google Scholar] [CrossRef] [PubMed]
- Wege, J.A.; Shepherd, K.A. 50 years of botanical discovery: A golden anniversary edition of Nuytsia, the journal of the Western Australian Herbarium. Nuytsia 2020, 31, 1–7. [Google Scholar]
- Fitzpatrick, M.C.; Gove, A.D.; Sanders, N.J.; Dunn, R.R. Climate change, plant migration, and range collapse in a global biodiversity hotspot: The Banksia (Proteaceae) of Western Australia. Glob. Change Biol. 2008, 14, 1337–1352. [Google Scholar] [CrossRef]
- Phillips, R.D.; Backhouse, G.; Brown, A.P.; Hopper, S.D. Biogeography of Caladenia (Orchidaceae), with special reference to the South-west Australian Floristic Region. Aust. J. Bot. 2009, 57, 259–275. [Google Scholar] [CrossRef]
- Barrett, S.; Yates, C.J. Risks to a mountain summit ecosystem with endemic biota in southwestern Australia. Austral Ecol. 2015, 40, 423–432. [Google Scholar] [CrossRef]
- Shearer, B.L.; Crane, C.E.; Barrett, S.; Cochrane, A. Phytophthora cinnamomi invasion, a major threatening process to conservation of flora diversity in the South-west Botanical Province of Western Australia. Aust. J. Bot. 2007, 55, 225–238. [Google Scholar] [CrossRef]
- Gosper, C.R.; Miller, B.P.; Gallagher, R.V.; Kinloch, J.; van Dongen, R.; Adams, E.; Barrett, S.; Cochrane, A.; Comer, S.; McCaw, L.; et al. Mapping risk to plant populations from short fire intervals via relationships between maturation period and environmental productivity. Plant Ecol. 2022. [Google Scholar] [CrossRef]
- Lambers, H.; Albornoz, F.; Kotula, L.; Laliberté, E.; Ranathunge, K.; Teste, F.P.; Zemunik, G. How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant Soil 2018, 424, 11–33. [Google Scholar] [CrossRef]
- Storey, J.; Brown, A.; Tiong, G.; Cootes, J. Orchid conservation initiatives in Western Australia: The Adopt an Orchid Project (ADORP). J. Hardy Orchid Soc. 2013, 10, 95–103. [Google Scholar]
- Roberts, D.L.; Taylor, L.; Joppa, L.N. Threatened or Data Deficient: Assessing the conservation status of poorly known species. Divers. Distrib. 2016, 22, 558–565. [Google Scholar] [CrossRef]
- Prober, S.M.; Smith, F.P. Enhancing biodiversity persistence in intensively used agricultural landscapes: A synthesis of 30 years of research in the Western Australian wheatbelt. Agr. Ecosyst. Environ. 2009, 132, 173–191. [Google Scholar] [CrossRef]
- Evans, M.C. Deforestation in Australia: Drivers, trends and policy responses. Pac. Conserv. Biol. 2016, 22, 130–150. [Google Scholar] [CrossRef]
- Gosper, C.R.; Yates, C.J.; Prober, S.M.; Williams, M.R. Fire does not facilitate invasion by alien annual grasses in an infertile Australian agricultural landscape. Biol. Invasions 2011, 13, 533–544. [Google Scholar] [CrossRef]
- Llorens, T.M.; Yates, C.J.; Byrne, M.; Nistelberger, H.M.; Williams, M.R.; Coates, D.J. Complex interactions between remnant shape and the mating system strongly influence reproductive output and progeny performance in fragmented populations of a bird-pollinated shrub. Biol. Conserv. 2013, 164, 129–139. [Google Scholar] [CrossRef]
- Raiter, K.G.; Prober, S.M.; Hobbs, R.J.; Possingham, H.P. Lines in the sand: Quantifying the cumulative development footprint in the world’s largest remaining temperate woodland. Landsc. Ecol. 2017, 32, 1969–1986. [Google Scholar] [CrossRef]
- Gibson, N. Flora and vegetation of the eastern Goldfields ranges: Part 7. Middle and South Ironcap, Digger Rock and Hatter Hill. J. R. Soc. West Aust. 2004, 87, 49–62. [Google Scholar]
- Gosper, C.R.; Fox, E.; Burbidge, A.H.; Craig, M.D.; Douglas, T.K.; Fitzsimons, J.A.; McNee, S.; Nicholls, A.O.; O’Connor, J.; Prober, S.M.; et al. Multi-century periods since fire in an intact woodland landscape favour bird species declining in an adjacent agricultural region. Biol. Conserv. 2019, 230, 82–90. [Google Scholar] [CrossRef]
- Byrne, M.; Steane, D.A.; Joseph, L.; Yeates, D.K.; Jordan, G.J.; Crayn, D.; Aplin, K.; Cantrill, D.J.; Cook, L.G.; Crisp, M.D.; et al. Decline of a biome: Evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. J. Biogeogr. 2011, 38, 1635–1656. [Google Scholar] [CrossRef]
- Nistelberger, N.; Gibson, N.; Macdonald, B.; Tapper, S.-L.; Byrne, M. Phylogeographic evidence for two mesic refugia in a biodiversity hotspot. Heredity 2014, 113, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Markey, A.; Kern, S.; Gibson, N. Floristic communities of the Ravensthorpe Range, Western Australia. Conserv. Sci. West Aust. 2012, 8, 187–239. [Google Scholar]
- Keppel, G.; Robinson, T.P.; Wardell-Johnson, G.W.; Yates, C.J.; Van Niel, K.P.; Byrne, M.; Schut, A.G.T. A low-altitude mountain range as an important refugium for two narrow endemics in the Southwest Australian Floristic Region biodiversity hotspot. Ann. Bot. 2017, 119, 289–300. [Google Scholar] [CrossRef][Green Version]
| Genus | Number of SWAFR Taxa | Number of Threatened (T) Taxa | % Genus T | Number of Data-Deficient (DD) Taxa | % Genus DD | % Genus T + DD |
|---|---|---|---|---|---|---|
| High proportional representation 1 (T + DD) | ||||||
| Drummondita | 7 | 2 | 28.6 # | 3 | 42.9 | 71.4 |
| Brachyloma | 10 | 0 | 0 | 7 | 70.0 | 70.0 |
| Babingtonia | 12 | 0 | 0 | 8 | 66.7 | 66.7 |
| Netrostylis | 6 | 0 | 0 | 4 | 66.7 | 66.7 |
| Calectasia | 14 | 2 | 14.3 # | 7 | 50 | 64.3 |
| Cyathostemon | 11 | 0 | 0 | 7 | 63.6 | 63.6 |
| Malleostemon | 16 | 0 | 0 | 10 | 62.5 | 62.5 |
| Eryngium | 8 | 0 | 0 | 5 | 62.5 | 62.5 |
| Enekbatus | 8 | 0 | 0 | 5 | 62.5 | 62.5 |
| Synaphea | 76 | 6 | 7.9 | 40 | 52.6 | 60.5 |
| High proportional representation 1 (T only) | ||||||
| Drakaea | 9 | 5 | 55.6 | 0 | 0 | 55.6 |
| Lambertia | 19 | 5 | 26.3 | 1 | 5.3 | 31.6 |
| Darwinia | 72 | 16 ^ | 22.2 | 19 | 26.4 | 48.6 |
| Androcalva | 14 | 3 | 21.4 | 3 | 21.4 | 42.9 |
| Myoporum | 10 | 2 | 20.0 | 0 | 0 | 20.0 |
| Commersonia | 12 | 2 | 16.7 | 1 | 8.3 | 25.0 |
| Trithuria | 6 | 1 | 16.7 | 0 | 0 | 16.7 |
| Tetratheca | 35 | 5 | 14.3 | 15 | 42.9 | 57.1 |
| Eremophila | 133 | 19 ^ | 14.3 | 22 | 16.5 | 30.8 |
| Acrotriche | 7 | 1 | 14.3 | 0 | 0 | 14.3 |
| High raw numbers 1 (T) | ||||||
| Acacia | 586 | 32 | 5.5 | 131 | 22.4 | 27.8 |
| Grevillea | 288 | 30 | 10.4 | 74 | 25.7 | 36.1 |
| Eucalyptus | 431 | 26 | 6.0 | 42 | 9.7 | 15.8 |
| Caladenia | 196 | 25 | 12.8 | 26 | 13.3 | 26.0 |
| Banksia | 237 | 20 | 8.4 | 45 | 19.0 | 27.4 |
| Verticordia | 161 | 16 | 9.9 | 46 | 28.6 | 38.5 |
| Daviesia | 112 | 12 | 10.7 | 15 | 13.4 | 24.1 |
| Gastrolobium | 113 | 12 | 10.6 | 27 | 23.9 | 34.5 |
| Conostylis | 80 | 9 | 11.3 | 6 | 7.5 | 18.8 |
| Stylidium | 233 | 7 | 3.0 | 67 | 28.8 | 31.8 |
| SWAFR total | 9355 | 406 | 4.3 | 1819 | 19.4 | 23.8 |
| Family | Number of SWAFR Taxa | Number of Threatened (T) Taxa | % Family T | Number of Data-Deficient (DD) Taxa | % Family DD | % Family T + DD |
|---|---|---|---|---|---|---|
| High proportional representation 1 (T + DD) | ||||||
| Elaeocarpaceae | 20 | 5 | 11.9 # | 17 | 40.5 | 52.4 |
| Dasypogonaceae | 19 | 2 | 10.5 # | 7 | 36.8 | 47.4 |
| Araliaceae | 41 | 0 | 0 | 14 | 34.1 | 34.1 |
| Gyrostemonaceae | 18 | 1 | 5.6 | 5 | 27.8 | 33.3 |
| Hemerocallidaceae | 60 | 1 | 1.7 | 19 | 31.7 | 33.3 |
| Malvaceae | 202 | 14 | 6.9 # | 52 | 25.7 | 32.7 |
| Rhamnaceae | 123 | 0 | 0 | 40 | 32.5 | 32.5 |
| Brassicaceae | 38 | 1 | 2.6 | 11 | 28.9 | 31.6 |
| Stylidiaceae | 243 | 7 | 2.9 | 69 | 28.4 | 31.3 |
| Ericaceae | 398 | 12 | 3.0 | 108 | 27.1 | 30.2 |
| High proportional representation 1 (T only) | ||||||
| Scrophulariaceae | 147 | 21 | 14.3 | 22 | 15.0 | 29.3 |
| Haemodoraceae | 137 | 12 | 8.7 | 12 | 8.7 | 17.5 |
| Frankeniaceae | 23 | 2 | 8.7 | 4 | 17.4 | 26.1 |
| Casuarinaceae | 35 | 3 | 8.6 | 4 | 11.4 | 20.0 |
| Orchidaceae | 494 | 42 | 8.5 | 61 | 12.3 | 20.9 |
| Xyridaceae | 12 | 1 | 8.3 | 1 | 8.3 | 16.7 |
| Polygonaceae | 13 | 1 | 7.7 | 0 | 0.0 | 7.7 |
| Proteaceae | 1032 | 77 | 7.5 | 216 | 20.9 | 28.4 |
| Colchicaceae | 28 | 2 | 7.1 | 1 | 3.6 | 10.7 |
| Rutaceae | 206 | 12 | 5.8 | 41 | 19.9 | 25.7 |
| Low proportional representation 1 (T + DD) | ||||||
| Cupressaceae | 13 | 0 | 0 | 0 | 0 | 0 |
| Lauraceae | 16 | 0 | 0 | 0 | 0 | 0 |
| Loranthaceae | 17 | 0 | 0 | 0 | 0 | 0 |
| Potamogetonaceae | 14 | 0 | 0 | 0 | 0 | 0 |
| Ranunculaceae | 13 | 0 | 0 | 0 | 0 | 0 |
| Zygophyllaceae | 21 | 0 | 0 | 0 | 0 | 0 |
| Xanthorrhoeaceae | 11 | 0 | 0 | 0 | 0 | 0 |
| Campanulaceae | 33 | 0 | 0 | 1 | 3.0 | 3.0 |
| Crassulaceae | 19 | 0 | 0 | 1 | 5.3 | 5.3 |
| Convolvulaceae | 17 | 0 | 0 | 1 | 5.9 | 5.9 |
| SWAFR total | 9355 | 406 | 4.3 | 1819 | 19.4 | 23.8 |
| Criterion | Solely Listed under Criterion | Listed under Criterion and Other Criteria 1 | Total |
|---|---|---|---|
| Total A (Population size reduction) | 11 | 24 | 35 |
| A1 (past; reversible, understood, ceased) | 0 | 7 | 7 |
| A2 (past; not reversible, not understood or not ceased) | 5 | 8 | 13 |
| A3 (projected) | 5 | 5 | 10 |
| A4 (past and projected; not reversible, not understood or not ceased) | 1 | 5 | 6 |
| Total B (Geographic range and fragmentation, number of locations, decline and/or fluctuation) | 127 | 107 | 234 |
| B1 (Extent of occurrence) ab (locations or fragmentation + continuing decline) | 8 | 148 | 156 |
| B2 (Area of occupancy) ab (locations or fragmentation + continuing decline) | 18 | 153 | 171 |
| Other B1, B2 combinations | 29 | 20 | 49 |
| Total C (Small population size and decline) | 38 | 92 | 130 |
| C1 (continuing decline) | 2 | 21 | 23 |
| C2 (continuing decline, subpopulation, population fluctuation) | 35 | 79 | 114 |
| Total D (Very small or restricted population) | 100 | 57 | 157 |
| D (Mature individuals) | 61 | 58 | 119 |
| D2 (VU; restricted + plausible future threat) | 36 | 7 | 43 |
| Total E (Quantitative Analysis) | 0 | 0 | 0 |
| Status | DBCA Region | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| South Coast | Warren | South West | Wheatbelt | Swan | Goldfields | Midwest | ||
| Taxa | ||||||||
| EX | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 3 1 |
| CR | 36 | 7 | 26 | 60 | 24 | 0 | 44 | 153 1 |
| EN | 32 | 12 | 22 | 48 | 27 | 0 | 44 | 131 1 |
| VU | 45 | 12 | 13 | 53 | 30 | 2 | 31 | 116 1 |
| Total | 113 | 31 | 63 | 162 | 81 | 2 | 119 | 403 |
| Populations | ||||||||
| EX | 0 | 0 | 2 | 7 | 0 | 0 | 0 | 9 |
| CR | 393 | 23 | 183 | 362 | 279 | 0 | 340 | 1580 |
| EN | 301 | 118 | 289 | 549 | 258 | 0 | 521 | 2036 |
| VU | 375 | 152 | 109 | 555 | 426 | 5 | 400 | 2022 |
| Total | 1069 | 293 | 583 | 1473 | 963 | 5 | 1261 | 5647 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gosper, C.R.; Percy-Bower, J.M.; Byrne, M.; Llorens, T.M.; Yates, C.J. Distribution, Biogeography and Characteristics of the Threatened and Data-Deficient Flora in the Southwest Australian Floristic Region. Diversity 2022, 14, 493. https://doi.org/10.3390/d14060493
Gosper CR, Percy-Bower JM, Byrne M, Llorens TM, Yates CJ. Distribution, Biogeography and Characteristics of the Threatened and Data-Deficient Flora in the Southwest Australian Floristic Region. Diversity. 2022; 14(6):493. https://doi.org/10.3390/d14060493
Chicago/Turabian StyleGosper, Carl R., Julia M. Percy-Bower, Margaret Byrne, Tanya M. Llorens, and Colin J. Yates. 2022. "Distribution, Biogeography and Characteristics of the Threatened and Data-Deficient Flora in the Southwest Australian Floristic Region" Diversity 14, no. 6: 493. https://doi.org/10.3390/d14060493
APA StyleGosper, C. R., Percy-Bower, J. M., Byrne, M., Llorens, T. M., & Yates, C. J. (2022). Distribution, Biogeography and Characteristics of the Threatened and Data-Deficient Flora in the Southwest Australian Floristic Region. Diversity, 14(6), 493. https://doi.org/10.3390/d14060493







