Abstract
Climate change and invasive species are critical factors affecting native land snail diversity. In South America, the introduced Giant African Snail (Lissachatina fulica) has spread significantly in recent decades into the habitat of the threatened native giant snails of the genus Megalobulimus. We applied species distribution modeling (SDM), using the maximum entropy method (Maxent) and environmental niche analysis, to understand the ecological relationships between these species in a climate change scenario. We compiled a dataset of occurrences of L. fulica and 10 Megalobulimus species in South America and predicted the distribution of the species in current and future scenarios (2040–2060). We found that L. fulica has a broader environmental niche and potential distribution than the South American Megalobulimus species. The distribution of six Megalobulimus species will have their suitable areas decreased, whereas the distribution of the invasive species L. fulica will not change significantly in the near future. A correlation between the spread of L. fulica and the decline of native Megalobulimus species in South America was found due to habitat alteration from climate change, but this relationship does not seem to be related to a robust competitive interaction between the invasive and native species.
1. Introduction
Climate change is one of the main drivers of global biodiversity change, along with habitat destruction and anthropogenic pressures. Some effects on biodiversity include changes in distribution, abundance, and phenology, increasing the extinction risk for some species [1]. A rapid and remarkable change is predicted for this century, which will affect all levels of biodiversity, from individuals to biomes [2,3]. It is now well established from various studies that climate change will likely be one of the main factors affecting species extinction in the 21st century [4,5].
Along with climate change, invasive species are also a significant problem affecting biodiversity changes. Invasive species usually compete for resources with native species [6] and can modify communities [7]. The interactions between native and invasive species are varied [8], whereas niche overlap might be high, and competition may lead to a decline, or even extinction, of native populations [6,9,10]. Invasive species can also negatively impact local and national economies and might cause health and social problems [11,12,13]. Climate change can also further intensify these effects by facilitating the spread and establishment of invasive species [14].
The Giant African Snail, Lissachatina fulica (Bowdich, 1822), is considered one of the world’s most invasive species, with considerable known negative impacts. This species is native to East Africa [15], and it was introduced in South America in the 1980s as a substitute for the escargot Cornu aspersum (Müller, 1774) as a human food item [16]. Since then, the species has rapidly spread across the continent, possibly still currently invading some areas [17,18,19]. This species can act as an intermediate host of several parasites, such as Angiostrongylus cantonensis (Chen, 1935), which causes eosinophilic meningitis in humans [20,21]. Lissachatina fulica has a generalist diet [22] and an outstanding reproductive potential; one snail can lay clutches of up to 400 eggs, with an annual production of 1200 eggs [23]. Predation by L. fulica on invasive veronicellid slugs has also been reported in Hawaii [24], indicating that it can also directly affect the native fauna, combined with its other biological characteristics, aside from the possible indirect effects of competition for resources.
One of the main characteristic groups of land snails in South America is the giant snail of the genus Megalobulimus (Miller, 1878). The genus has more than 80 described species [25,26,27], one of the region’s most speciose genera. It is endemic to South America, with some introductions to the Caribbean Islands [25,28,29]. They are nocturnal, burying themselves in the litter during the day, or during periods of hibernation or aestivation [25,30,31]. Megalobulimus activity is very seasonal [32,33], occurring in low densities [34], and they also have low reproductive potential, with an annual production of up to nine eggs [33]. Due to life history traits, some species are considered threatened [34,35,36].
More recently, with the increasing availability of climatological and species occurrences data, it became possible to evaluate the potential effect of invasive species on native varieties on macroecological scales—such as the case of L. fulica and the Megalobulimus snails. At the same time, further developments of niche and species distribution modeling (SDM) allow researchers to test ecological processes on large spatial scales [37,38]. SDMs can be used to test hypotheses regarding the conservation of native species, the management of invasive species [39], or the detection of areas that can be potentially invaded [39,40]. SDM analyses can also show how species distributions change over time [41].
In this context, we evaluated the potential effect of L. fulica on the distribution of the native species of the genus Megalobulimus in South America using a climate change scenario. For this, we assembled a comprehensive compilation of occurrences records for the invasive species and 10 native Megalobulimus species and explored these using SDM and ecological niche analyses. We expected that climate change would increase the potential effects of L. fulica on the future distributional ranges for the native Megalobulimus species.
2. Materials and Methods
2.1. Occurrence Datasets
The records of L. fulica were obtained through access to online databases and literature searches. We used the following online databases to search for occurrences of the target species: Species Link [42], Portal da Biodiversidade Reflora [43], GBIF [44], and iNaturalist [45]. The literature search was made in the primary collection of the Web of Science [46] using “Achatina fulica”, “A. fulica”, “Lissachatina fulica”, “L. fulica”, “Caramujo-Gigante-Africano”, and “Giant African Snail” as keywords. We obtained 2493 occurrences of the Giant African Snail in South America (Figure 1; Table S1).
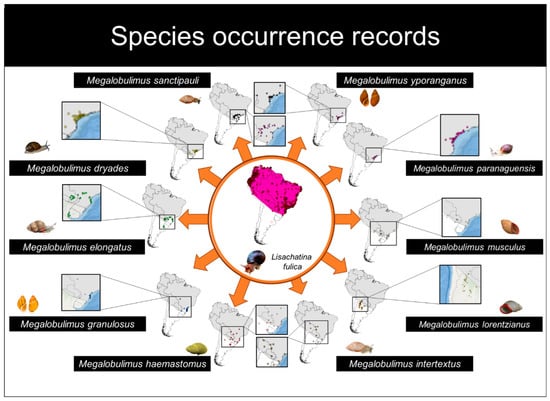
Figure 1.
Occurrence records and spatial distribution maps of the ten species of Megalobulimus and Lissachatina fulica in South America. The gray minimum convex polygon of L. fulica is represented in the maps of the native species.
For the genus Megalobulimus, the records were obtained from the selected literature and validated by specialists, as records from the online databases have doubtful identifications [47]. Throughout the literature, we used occurrences from species with at least 10 occurrence records of the following 10 species: Megalobulimus dryades (Fontenelle, Simone, and Cavallari, 2021); Megalobulimus elongatus (Bequaert, 1948); Megalobulimus granulosus (Rang, 1831); Megalobulimus haemastomus (Scopoli, 1786); Megalobulimus intertextus (Pilsbry, 1895); Megalobulimus lorentzianus (Doering, 1876); Megalobulimus musculus (Bequaert, 1948); Megalobulimus paranaguensis (Pilsbry and Ihering, 1900); Megalobulimus yporanganus (Ihering and Pilsbry, 1901); and Megalobulimus sanctipauli (Ihering and Pilsbry, 1900) (Figure 1; Table S2). Across these 10 species, we obtained 250 records from five countries (Brazil, Argentina, Uruguay, Paraguay, and Bolivia) in 4 different South American biomes (Atlantic Rainforest, Cerrado, Pampas, and Chaco) (Figure 1; Table S2).
We created a dataset containing geographical coordinate information (in decimal degrees) and species names. After completing the datasets, we reduced spatial sampling bias by thinning occurrence records to a minimum distance of at least 4 km between them [48].
2.2. Environmental Data
Environmental data for current and future climatic conditions were obtained from WorldClim 2.0 [49] at a spatial resolution of 4 km. We used the scenario CSM2-MR with shared socioeconomic pathway 126 (ssp126) for 2040–2060. This scenario predicts average global warming of 2 °C until 2100 [50]. The 19 raw bioclimatic variables were used in our modeling experiment (Table 1). The variables of the current scenario were standardized and submitted to principal component analysis (PCA). Then, we used the first six principal components responsible for approximately 95% of the variations as environmental predictors (Table 1). This approach was used to prevent multicollinearity between the bioclimatic variables [51,52]. For the 2040–2060 scenario, we projected the linear coefficients of the current principal components into the future to generate a correspondence between scenarios, and then we applied PCA to generate PCs for that scenario.

Table 1.
Summary of the PCA from which the principal components (PC) used as new environmental layers were generated. Each cell value represents the individual loadings of each variable in each of the PCs. The PCs, along with their individual and accumulated proportions, are also shown.
2.3. Modeling Procedures
Validating the absences of the Megalobulimus species is complex because they have low-density populations, and because the individuals remain buried in the soil [25,30]. Nonetheless, true absences are a general issue affecting the prediction of species ranges. Moreover, most species analyzed here have a minimum number of occurrences. For these reasons, the potential distribution of species was estimated using the maximum entropy algorithm (Maxent), with the automatic linear and quadratic resources activated and all other parameters set as default [53]. Maxent is relatively robust for species with few occurrences [54]. We used the receiver operating characteristic curve (ROC) threshold to transform species suitability into binary maps balancing omission and commission errors. We used the Jaccard index to evaluate our models, using the models produced with observed data as a base [55]. This index varies between 0 and 1, with values close to 1 indicating better goodness of fit and a correct preview [55]. Jaccard values around 0.5 indicate a random distribution of data.
We used a Student t-test for dependent samples to evaluate the overlap between the L. fulica and Megalobulimus species in current and future scenarios. We also used a Student t-test to assess whether there was a difference in the geographical distribution in both scenarios. We overlapped the resulting maps to evaluate the changes between the current and 2040–2060 scenarios. In this approach, the grid was divided into (1) areas with no prediction; (2) areas suitable only in the current scenario; (3) areas suitable only in the 2040–2060 scenario; (4) areas suitable in both scenarios (stable areas). Additionally, we calculated the expected species richness projected in the current and future scenarios, summing the number of species in a grid cell in the current and future scenarios. The analyses were run in the R 3.6.6 environment [56], using the script of the ENMTML package [57] (File S3). Differences between treatments were considered when p < 0.05.
2.4. Bioclimatic Niche Analysis
We used Broennimann et al.’s [38] method to evaluate the environmental niche of each species. We used the PCA-env approach to consider all the areas occupied by the species. We compared the environmental conditions available for native species with the environmental conditions of invasive species. With these comparisons, we calculated the niche overlap using D metrics [58], with values ranging from 0 (no overlap between niches) to 1 (complete overlap between niches). We then created a randomization routine to test the hypothesis of niche overlap based on niche similarity, following the method of Warren et al. [59]. The niche similarity test compares whether the niche overlap of the range of the native species randomly distributed over its background keeps the range of invasive species unchanged (1→2), and then the reciprocal comparison is made (1←2). Moreover, niche unfilling and stability metrics were also calculated. Stability is the proportion of kernel densities in one species distribution overlapping with the other species distribution, whereas unfilling is the proportion of kernel distribution of one distribution located in conditions other than the further distribution. These variables ranged from 0 to 1 [60]. The script of the environmental niche analysis can be found in File S4.
3. Results
The Jaccard value for L. fulica was 0.755, and for Megalobulimus species, the values varied between 0.444 and 0.933, with a mean value of 0.725 (S.D. = 0.158). Eight species of Megalobulimus had Jaccard values higher than 0.7 (Table 2). We observed in the current scenario that most of South America is suitable for L. fulica, except for the majority of Argentina, and Uruguay, where few areas are suitable in the north of both countries, and the northern Chilean regions (Figure 2). An island of suitability was found in western Patagonia (Figure 2). Megalobulimus species had smaller suitable areas when compared with L. fulica, and most of them had significant overlapping distribution with the invasive species, except for M. lorentzianus (Figure 2).

Table 2.
Descriptive data of the species distribution models for each species.
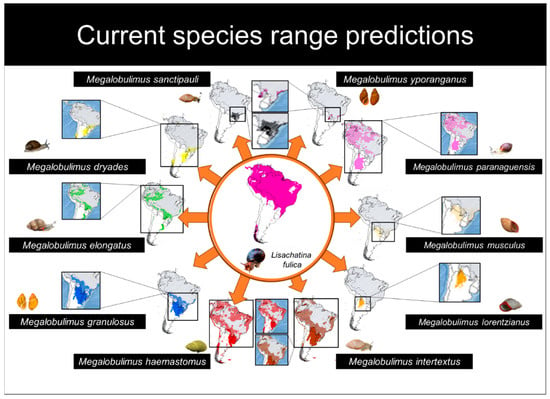
Figure 2.
Predicted distribution maps for the current scenario of the ten species of Megalobulimus and Lissachatina fulica. The native species are represented by the following colors: M. sanctipauli (black), M. dryades (yellow), M. elongatus (green), M. granulosus (blue), M. haemastomus (red), M. intertextus (brown), M. lorentzianus (orange), M. musculus (beige), M. paranaguensis (pink), and M. yporanganus (purple). The lines on the maps for each species correspond to the distribution of L. fulica.
For future scenarios, our results indicate a decrease in suitable areas for L. fulica and six Megalobulimus species (M. dryades, M. haemastomus, M. paranaguensis, M. sanctipauli, M. yporanganus, and M. intertextus), while we observed an increase in suitable areas for four species (M. elongatus, M. granulosus, M. lorentzianus, and M. musculus) (Table 2; Figure 3). There were no significant differences found between present and future scenarios in distribution areas (t = 0.489; d.f. = 10; p = 0.635) or between overlapping distribution areas between L. fulica and Megalobulimus species (t = 1.932; d.f. = 9; p = 0.085).
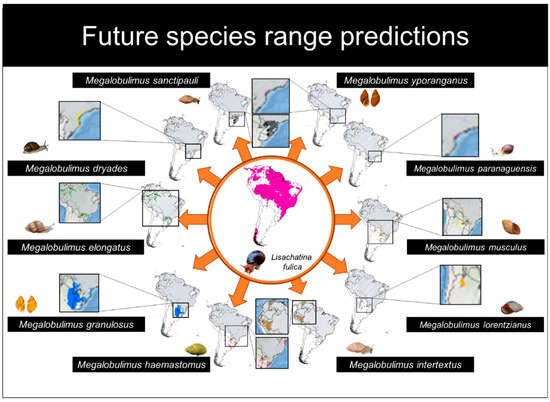
Figure 3.
Predicted distribution maps for the future scenario (2040–2060) of the ten species of Megalobulimus and Lissachatina fulica. The native species are represented by the following colors: M. sanctipauli (black), M. dryades (yellow), M. elongatus (green), M. granulosus (blue), M. haemastomus (red), M. intertextus (brown), M. lorentzianus (orange), M. musculus (beige), M. paranaguensis (pink), and M. yporanganus (purple). The lines on the maps for each species correspond to the distribution of L. fulica.
With the overlap between current and future distributions for each species, we observed that the most suitable predicted areas were climatically stable (Figure 4). Lissachatina fulica showed large stable areas where distribution remained unchanged between current and future predictions (Figure 4). Megalobulimus elongatus, M. granulosus, and M. lorentzianus showed large suitable areas predicted for the future, indicating an increase in their distribution, whereas M. dyades, M. haemastomus, M. paranaguensis, M. sanctipauli, and M. yporanganus showed larger suitable areas in the current scenario, indicating a trend to decrease their distribution in the future (Table 2; Figure 4).
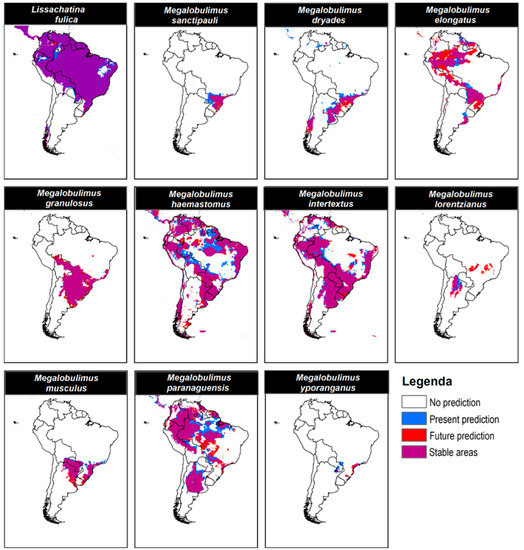
Figure 4.
Maps of overlapping potential distribution between current and future (2040–2060) scenarios indicate stable areas (purple), predicted areas under the current scenario (blue), and predicted areas only in future scenarios (red). White regions were predicted to be unsuitable.
The modeled species richness of Megalobulimus in the current scenario was high in the Brazilian southern and southeastern coastal plain and the frontier between Brazil, Argentina, and Paraguay, with a small connection among the areas in these countries (Figure 5). There is a trend to reduce the richness and a loss of connection between both regions in the future (Figure 5). The expected richness reduces in the interior of Brazil, without migration to the south of the continent (Figure 5). These richest areas continue to overlap with the L. fulica distribution in both scenarios (Figure 5).
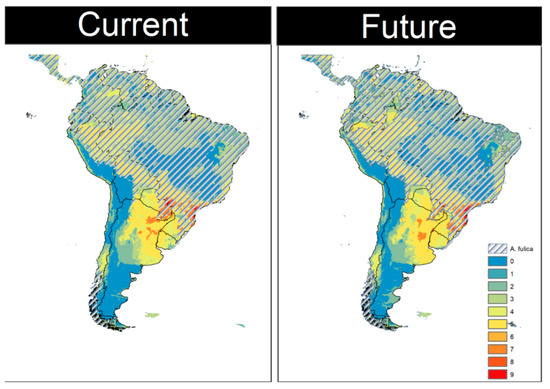
Figure 5.
Potential species richness distribution of Megalobulimus species in the current and future scenarios. Locations with low species richness are shown in blue, whereas locations with high species richness are shown in red. The potential distribution of Lissachatina fulica in both scenarios is hatched.
Lissachatina fulica and the native species had distinct niches (Figure 6; Table 3) and slight overlaps (D < 0.05; Table 3). Niche similarity tests indicated that overlap values between L. fulica and M. lorentzianus, and L. fulica and M. intertextus, were no different from a null expectation (Table 3). The invasive species occupies a more prominent and extensive climatic space than the Megalobulimus species (Figure 6). A great unfilling (<0.56) of Megalobulimus species can be detected in all cases, whereas L. fulica had small unfilling values (>0.05), except for M. lorentzianus (=0.558). Lissachatina fulica had great stability values (<0.95) for almost all Megalobulimus species, except for M. lorentzianus (=0.442).
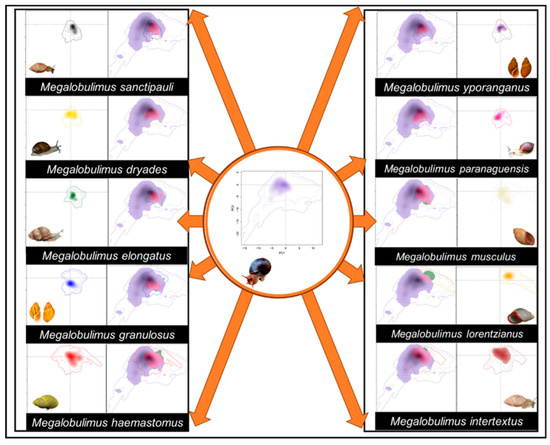
Figure 6.
Broennimann et al. [38] PCA-env environmental niche analysis between Lissachatina fulica and 10 Megalobulimus species. The solid and dashed lines represent 50% and 100% of the available environmental conditions for each species, respectively. The following colors represent the species: L. fulica (purple), M. sanctipauli (black), M. dryades (yellow), M. elongatus (green), M. granulosus (blue), M. haemastomus (red), M. intertextus (brown), M. lorentzianus (orange), M. musculus (beige), M. paranaguensis (pink), and M. yporanganus (purple). In the niche overlap analysis, the stability is pink, the unfilling of Megalobulimus species in the L. fulica environmental niche is purple, and the unfilling of L. fulica in the Megalobulimus environmental niche is green.

Table 3.
Results of niche overlap comparison between Lissachatina fulica and Megalobulimus species. Significant p-values of the similarity test are shown in bold.
4. Discussion
Here, we showed that L. fulica has a broader environmental niche and potential distribution than the South American native Megalobulimus species, whose environmental niches are narrower than the invasive species niche. Our results agree with previous studies suggesting that L. fulica has high environmental tolerance and prefers warmer environments [19,23,34,61,62], making their niches wider in tropical regions. Although L. fulica has small genetic variability where it was introduced, as in South America [63], its generalist behavior allows the species to occupy a wide distribution. On the other hand, Megalobulimus species occur in more restricted geographical distributions [25,35,36,64,65] and have more specific habits [31,32,33,34,63,66], which makes their niche breadth more specialized.
Our models in the current scenario for L. fulica matched previous predictions made for South America [19,67,68], with susceptible areas identified in Guyana, French Guiana, Suriname, Peru, Venezuela, Ecuador, and Colombia. At the same time, regions in Chile and Uruguay appear least vulnerable to invasion, except for the area of southern Chile. The suitable areas found in different SDMs for L. fulica in southern Chile seem to be an artifact of methodology caused by the occurrence of this species in other northern Andes areas, such as Ecuador and Peru. The main difference is that most Amazon Rainforests are considered suitable for L. fulica. Although the models cannot be directly compared [53], parallel interpretation of the output can help identify areas susceptible to L. fulica invasion [19]. This can indicate that the current distribution of L. fulica is still underestimated, and that new occurrences may still be revealed in many other areas.
In South America, few countries have specific legislation and control policies for the Giant African Snail [17,19]. We suggest that control and management policies should be created and implemented, especially in the northern countries of the continent. Besides the wide distribution in South America, our results show that the distribution of L. fulica did not significantly differ between the current and future scenarios tested, indicating that the spread of the species could stabilize in the near future.
Native land snails are among the most threatened groups globally, with the highest extinction rates reported [69]. Furthermore, this number is undoubtedly underestimated [70]. In South America, this scenario is similar to other regions, with the conservation status of most land snails inadequate [35,71,72]. The distribution of land snails in South America suffers from knowledge gaps [26,73,74], especially concerning taxonomic identity (Linnean shortfall) and spatial distribution (Wallacean shortfall). Nonetheless, SDMs are essential tools to access the distribution of these “data deficient” groups [36,65], allowing for inclusion in broad-scale conservation studies. Integrating information on the basic biology and ecology of land snails is essential to better inform such conservation efforts and the interpretation of the SDMs. Given this scenario, future studies in the areas highlighted in our analysis may add important information about species distribution and their natural history.
The genus Megalobulimus is probably one of the groups most highly threatened by many factors, such as anthropogenic pressures and climate change [34,35,36]. In this respect, our results reinforce that this group is of great concern, as six species have their suitable areas reduced. Moreover, an expected distributional shift to higher latitudes due to climate change [36,75,76] was not detected, with a loss of connection between populations (Figure 5), especially in south and southeastern Brazil and the frontiers between Brazil, Argentina, and Paraguay. This situation is expected, since land snails, in general, are a group with low dispersal abilities [34,77]. Thus, our results show a decline in Megalobulimus species that needs to be monitored in more detail to prevent the loss of future local populations and species extinctions.
Our results indicate that the suitable areas for the Megalobulimus species, which occur in areas that overlap with L. fulica (e.g., M. dryades, M. paranaguensis, M. yporanganus), will decrease. At the same time, species with smaller or almost no overlap (e.g., M. elongatus and M. lorentzianus) will see an increase in their suitable areas in South America. Moreover, there were no changes in overlapping areas between L. fulica and Megalobulimus species in current and future scenarios. In general, invasive species become established, and native species usually decline due to habitat modifications by humans [12]. Although the presence in protected areas is reported in some cases [78,79,80], the abundance of L. fulica tends to decline from disturbed habitats at the forest margin toward the interior of the natural forest [81,82]. Thus, the occurrence of invasive species can be used as an indicator of environmental conditions becoming unsuitable for native species.
It is speculated in the literature that the decline in some native populations might be caused by invasive achatinid species [83,84,85]. However, no clear cases in which competition between native and invasive achatinids has been demonstrated [34,85,86], and there is little evidence of competitive exclusion in land snail communities [87,88]. Invasive land snails occur preferentially in strongly modified environments and are abundant in deforested habitats [82], and changes in community composition are not primarily caused by invasive species [12,89]. Some behavioral changes of L. fulica in the presence of other snails can be detected [24,86], but no negative direct quantitative effect of this invasive species on native species is reported. Therefore, as far as we know, there is no negative immediate direct quantitative impact of this invasive species. Although the possibility of competition between invasive and native land snails cannot be excluded, it is speculated that it might be limited, since most land snails are generalists, but adverse effects may lead to decline or extinction in the long term [12,80,81]. The lack of data regarding the interaction effects of the invasive L. fulica on the native species requires long-term field studies to better understand these relationships.
5. Conclusions
We observed that L. fulica has a broader niche and potential distribution compared with the native Megalobulimus species, where niches are narrower than for invasive species. We found that the distribution of L. fulica will probably not change significantly in the near future, whereas some Megalobulimus species will decrease their suitable areas. Our results indicate a correlation between the spread of L. fulica and the decline of native Megalobulimus species due to climate change, but this relationship does not seem to have a strong competitive interaction. Thus, the occurrence of invasive species can indicate environmental conditions becoming unsuitable for native species. Through SDMs and environmental niche analysis, we identified areas of potential distribution of invasive and native species and the niche relationship between them. This application provides essential and promising information for non-model and lesser-known organisms, such as giant land snails. This knowledge is beneficial to governmental authorities for the development of actions to control L. fulica populations, and at the same time, establish baseline data for integrated conservation actions to preserve the threatened native malacofauna diversity, focusing on the preservation of their natural habitats.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14060467/s1, Table S1: Occurrence points of L. fulica; Table S2: Occurrence points of Megalobulimus species; File S3: Script of SDM; File S4: Environmental niche analysis of L. fulica and Megalobulimus species.
Author Contributions
Conceptualization, D.d.P.S. and M.S.M.; methodology, D.d.P.S., D.P.L.-J., B.V. and J.C.P.-O.; formal analysis, W.S.T., D.d.P.S., B.V. and M.S.M.; data curation, W.S.T. and M.S.M.; writing—original draft preparation, W.S.T., D.d.P.S. and M.S.M.; writing—review and editing, W.S.T., D.d.P.S., B.V., D.P.L.-J., J.C.P.-O. and M.S.M.; supervision, D.d.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
M.S.M. received a grant from the São Paulo Research Foundation (FAPESP), processes 2011/20917-8, 2013/00670-6 and 2017/01081-5. D.d.P.S. was supported by productivity grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Proc. Number: 304494/2019-4). D.P.L.-J. was supported by productivity grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Proc. Number: 305923/2020-0).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks are due to Mark Stevens (South Australian Museum), for the English review and improvement of this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vale, M.M.; Alves, M.A.S.; Lorini, M.L. Mudanças climáticas: Desafios e oportunidades para a conservação da biodiversidade brasileira. Oecol. Bras. 2009, 13, 518–535. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Evol. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Cordellier, M.; Pfenninger, A.; Streit, B.; Pfenninger, M. Assessing the effects of climate change on the distribution of pulmonate freshwater snail biodiversity. Mar. Biol. 2012, 159, 2519–2531. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; Ferreira-de-Siqueira, M.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Bøhn, T.; Amundsen, P.A.; Sparrow, A. Competitive exclusion after invasion? Biol. Invasions 2007, 10, 359–368. [Google Scholar] [CrossRef]
- Parker, I.M.; Simberloff, D.; Lonsdale, W.M.; Goodell, K.; Wonham, M.; Kareiva, P.M.; Williamson, M.H.; Holle, B.; Moyle, P.B.; Byers, J.E. Impact: Toward a framework for understanding the ecological effects of invaders. Biol. Invasions 1999, 1, 3–19. [Google Scholar] [CrossRef]
- Mooney, H.A.; Cleland, E.E. The evolutionary impact of invasive species. Proc. Natl. Acad. Sci. USA 2001, 98, 5446–5451. [Google Scholar] [CrossRef]
- Novacek, M.J.; Cleland, E.E. The current biodiversity extinction event: Scenarios for mitigation and recovery. Proc. Natl. Acad. Sci. USA 2001, 98, 5466–5470. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with invasive-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Nurinsiyah, A.S.; Hausdorf, B. Listing, impact assessment and prioritization of introduced land snail and slug species in Indonesia. J. Molluscan Stud. 2019, 85, 92–102. [Google Scholar] [CrossRef]
- Adelino, J.R.P.; Heringer, G.; Diagne, C.; Courchamp, F.; Faria, L.B.; Zenni, R.D. The economic costs of biological invasions in Brazil: A first assessment. NeoBiota 2021, 67, 349–374. [Google Scholar] [CrossRef]
- Bellard, C.; Jeschke, J.M.; Leroy, B.; Mace, G.M. Insights from modeling studies on how climate change affects invasive alien species geography. Ecol. Evol. 2018, 8, 5688–5700. [Google Scholar] [CrossRef] [PubMed]
- Bequaert, J.C. Studies in the Achatininae, a group of African land snails. Bull. Mus. Comp. Zool. 1950, 105, 1–216. [Google Scholar]
- Teles, H.M.S.; Vaz, J.F.; Fontes, R.L.; Domingos, M.F. Registro de Achatina fulica Bowdich, 1822 (Mollusca, Gastropoda) no Brasil: Caramujo hospedeiro intermediário da angiostrongilíase. Rev. Saúde Pública 1997, 31, 310–312. [Google Scholar] [CrossRef]
- Thiengo, S.; Faraco, F.A.; Salgado, N.C.; Cowie, R.H.; Fernandez, M.A. Rapid spread of an invasive snail in South America: The giant African snail, Achatina fulica, in Brazil. Biol. Invasions 2007, 9, 693–702. [Google Scholar] [CrossRef]
- Thiengo, S.C.; Simões, R.O.; Fernandes, M.A.; Maldonado, A. Angiostrongylus cantonensis and Rat Lungworm disease in Brazil. Hawai’i J. Med. Public Health 2013, 72, 18–22. [Google Scholar]
- Vogler, R.E.; Beltramino, A.A.; Sede, M.M.; Gutiérrez-Gregoric, D.E.; Núñez, V.; Rumi, A. The giant African snail, Achatina fulica (Gastropoda: Achatinidae): Using bioclimatic models to identify South American areas susceptible to invasion. Am. Malac. Bull. 2013, 31, 39–50. [Google Scholar] [CrossRef]
- Caldeira, R.L.; Mendonça, C.L.G.F.; Goveia, C.O.; Lenzi, H.L.; Graeff-Texteira, C.; Lima, W.S.; Mota, E.M.; Pecora, I.L.; Medeiros, A.M.Z.; Carvalho, O.S. First record of molluscs naturally infected with Angiostrongylus cantonensis (Chen, 1935) (Nematoda: Metastrongylidae) in Brazil. Memórias Inst. Oswaldo Cruz 2007, 102, 887–889. [Google Scholar] [CrossRef]
- Morassutti, A.L.; Thiengo, S.C.; Fernandez, M.; Sawanyawisuth, K.; Graeff-Teixeira, C. Eosinophilic meningitis caused by Angiostrongylus cantonensis: An emergent disease in Brazil. Mem. Inst. Oswaldo Cruz 2014, 109, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, F.S.; Peso-Aguiar, M.C.; Assunção-Albuquerque, M.J.T. Distribution, feeding behavior and control strategies of the exotic land snail Achatina fulica (Gastropoda: Pulmonata) in the northeast of Brazil. Braz. J. Biol. 2008, 68, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Rault, S.K.; Baker, G.M. Achatina fulica Bowdich and other Achatinidae as pests in tropical agriculture. In Molluscs as Crop Pests; Baker, G.M., Ed.; CAB International: New York, NY, USA, 2002; pp. 55–114. [Google Scholar]
- Meyer, W.M., III; Hayes, K.A.; Meyer, A.L. Giant African snail, Achatina fulica, as a snail predator. Am. Malacol. Bull. 2008, 24, 117–119. [Google Scholar] [CrossRef]
- Bequaert, J.C. Monograph of the Strophocheilidae, a neotropical family of terrestrial mollusks. Bull. Mus. Comp. Zool. 1948, 100, 1–210. [Google Scholar]
- Simone, L.R.L. Land and Freshwater Molluscs of Brazil; EGB/Fapesp: São Paulo, Brazil, 2006; pp. 1–390. [Google Scholar]
- Borda, V.; Ramirez, R. Re-characterization of the Red-lip Megalobulimus (Gastropoda: Strophocheilidae) from Peru with description of a new species. Zoologia 2013, 30, 675–691. [Google Scholar] [CrossRef]
- Chase, R.; Robinson, D.G. The uncertain history of land snails on Barbados: Implications for conservation. Malacologia 2001, 43, 33–57. [Google Scholar]
- Rosenberg, G.; Muratov, I. Status Report on the Terrestrial Mollusca of Jamaica. Proc. Acad. Nat. Sci. Phila. 2005, 155, 117–161. [Google Scholar] [CrossRef]
- Scott, M.I.H. Estudio anatómico del Borus Strophocheilus lorentzianus (Doer.) (Mol. Pulm.). Rev. Mus. Plata 1939, 7, 217–278. [Google Scholar]
- Miranda, M.S.; Correia, L.V.B.; Pecora, I.L. Activity and reproduction in Megalobulimus paranaguensis (Gastropoda, Eupulmonata): Implications for conservation in captivity for a South American land snail. J. Nat. Hist. 2020, 54, 435–443. [Google Scholar] [CrossRef]
- Miranda, M.S.; Fontenelle, J.H. Population dynamics of Megalobulimus paranaguensis in the Brazilian southeast coast. Zoologia 2015, 32, 463–468. [Google Scholar] [CrossRef]
- Fontenelle, J.H.; Miranda, M.S. Aspects of biology of Megalobulimus paranaguensis (Gastropoda, Acavoidea) in the coastal plain of the Brazilian southeast. Iheringia Ser. Zool. 2017, 107, e2017004. [Google Scholar] [CrossRef]
- Miranda, M.S.; Fontenelle, J.H.; Pecora, I.L. Population structure of a native and an invasive species of snail in an urban fragment of Atlantic Rainforest. J. Nat. Hist. 2015, 49, 19–35. [Google Scholar] [CrossRef]
- Santos, S.B.; Miyahira, I.C.; Mansur, M.C.D. Freshwater and terrestrial molluscs in Brasil: Current status of knowledge and conservation. Tentacle 2013, 21, 40–42. [Google Scholar]
- Beltramino, A.A.; Vogler, R.E.; Gutiérrez-Gregoric, D.E.; Rumi, A. Impact of climate change on the distribution of a giant land snail from South America: Predicting future trends for setting conservation priorities on native malacofauna. Clim. Change 2015, 131, 621–633. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Araújo, M.B.; Guisan, A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Devictor, V.; Clavel, J.; Julliard, R.; Lavergne, S.; Mouillot, D.; Thuiller, W.; Venail, P.; Villéger, S.; Mouquet, N. Defining and measuring ecological specialization. J. Appl. Ecol. 2010, 47, 15–25. [Google Scholar] [CrossRef]
- SpeciesLink. Available online: https://splink.cria.org.br/ (accessed on 1 February 2021).
- Portal da Biodiversidade Reflora. Available online: https://portaldabiodiversidade.icmbio.gov.br/portal/ (accessed on 1 February 2021).
- Global Biodiversity Information Facility (GBIF). Available online: https://www.gbif.org (accessed on 1 February 2021).
- iNaturalist. Available online: https://www.inaturalist.org/ (accessed on 1 February 2021).
- Web of Science. Available online: www.webofknowledge.com (accessed on 1 February 2021).
- Miranda, M.S.; (University of Campinas, Campinas, SP, Brazil). Personal communication, 2021.
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- WorldClim 2.0. Available online: http://www.worldclim.org (accessed on 1 February 2021).
- Meinshausen, M.; Nicholls, Z.R.J.; Lewis, J.; Gidden, M.J.; Vogel, E.; Freund, M.; Beyerle, U.; Gessner, C.; Nauels, A.; Bauer, N.; et al. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Model Dev. 2020, 13, 3571–3605. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- De Marco, P.; Nóbrega, C.C. Evaluating collinearity effects on species distribution models: An approach based on virtual species simulation. PLoS ONE 2018, 13, e0202403. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Leroy, B.; Delsol, R.; Hugueny, B.; Meynard, C.N.; Barhoumi, C.; Barbet-Massin, M.; Bellard, C. Without quality presence–absence data, discrimination metrics such as TSS can be misleading measures of model performance. J. Biogeogr. 2018, 45, 1994–2002. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.6.6.; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 February 2020).
- Andrade, A.F.A.; Velazco, S.J.E.; Júnior, P.M. ENMTML: An R package for a straightforward construction of complex ecological niche models. Environ. Model. Softw. 2020, 125, 104615. [Google Scholar] [CrossRef]
- Schoener, T.W. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 1970, 51, 408–418. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef]
- Albuquerque, F.S.; Peso-Aguiar, M.C.; Assunção-Albuquerque, M.J.T.; Gálvez, L. Do climate variables and human density affect Achatina fulica (Bowditch) (Gastropoda: Pulmonata) shell length, total weight and condition factor? Braz. J. Biol. 2009, 69, 879–885. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patiño-Montoya, A.; Giraldo, A.; Tidon, R. Variation in the population density of the giant African snail (Lissachatina fulica) in the Neotropical region. Caldasia 2022, 44, 1–21. [Google Scholar] [CrossRef]
- Fontanilla, I.K.C.; Maria, I.M.P.S.; Garcia, J.R.M.; Ghate, H.; Naggs, F.; Wade, C. Restricted Genetic Variation in Populations of Achatina (Lissachatina) fulica outside of East Africa and the Indian Ocean Islands Points to the Indian Ocean Islands as the Earliest Known Common Source. PLoS ONE 2014, 9, e105151. [Google Scholar] [CrossRef] [PubMed]
- Beltramino, A.A. Distribution of Megalobulimus sanctipauli (Ihering & Pilsbry, 1900) (Gastropoda: Megalobulimidae) in South America. CheckList 2013, 9, 469–471. [Google Scholar] [CrossRef]
- Beltramino, A.A. Distribución histórica y área de distribución potencial del megamolusco terrestre Megalobulimus lorentzianus (Doering, 1876) (Gastropoda: Pulmonata) em América del Sur. Boletín Asoc. Argent. Malacol. 2014, 4, 10–13. [Google Scholar]
- Miranda, M.S.; Correia, L.V.B. Food choice in Megalobulimus paranaguensis (Gastropoda, Eupulmonata). Stud. Neotrop. Fauna Environ. 2021, 56, 108–111. [Google Scholar] [CrossRef]
- Borrero, F.J.; Breure, A.S.H.; Christensen, C.; Correoso, M.; Mogollón-Ávila, V. Into the Andes: Three new introductions of Lissachatina fulica (Gastropoda, Achatinidae) and its potential distribution in South America. Tentacle 2009, 17, 6–8. [Google Scholar]
- Correoso, M.; Coello, M. Modelación y distribución de Lissachatina fulica (Gastropoda: Achatinidae) en Ecuador. Potenciales impactos ambientales y sanitarios. Rev. Geoespacial 2009, 6, 79–90. [Google Scholar]
- Lydeard, C.; Cowie, R.H.; Ponder, W.F.; Bogan, A.E.; Bouchet, P.; Clark, S.A.; Cummings, K.S.; Frest, T.J.; Gargominy, O.; Herbert, D.G.; et al. The global decline of nonmarine mollusks. Bioscience 2004, 54, 321–330. [Google Scholar] [CrossRef]
- Régnier, C.; Fontaine, B.; Bouchet, P. Not knowing, not recording, not listing: Numerous unnoticed mollusk extinctions. Conserv. Biol. 2009, 23, 1214–1221. [Google Scholar] [CrossRef]
- Ramírez, R.; Paredes, C.; Arenas, J. Moluscos del Perú. Rev. Biol. Trop. 2003, 51, 225–284. [Google Scholar]
- Gutiérrez-Gregoric, D.E.; Núñez, V.; Vogler, R.E.; Beltramino, A.A.; Rumi, A. Gasterópodos terrestres de la provincia de Misiones, Argentina. Rev. Biol. Trop. 2013, 61, 1759–1768. [Google Scholar] [CrossRef]
- Simone, L.R.L. Mollusca terrestres. In Biodiversidade do Estado de São Paulo: Uma Síntese do Conhecimento ao Final do Século XX. Invertebrados Terrestres; Brandão, C.R.F., Cancello, E.M., Eds.; FAPESP: São Paulo, Brazil, 1999; Volume 5, pp. 3–8. [Google Scholar]
- Salvador, R.B. Land snail diversity in Brazil. Strombus 2019, 25, 10–20. [Google Scholar]
- Foden, W.; Mace, G.; Vié, J.C.; Ângulo, A.; Butchart, S.; DeVantier, L.; Dublin, H.; Gutsche, A.; Stuart, S.; Turak, E. Species susceptibility to climate change impacts. In The 2008 Review of the IUCN Red List of Threatened Species; Vié, J.C., Hilton-Taylor, C., Stuart, S.N., Eds.; IUCN: Gland, Switzerland, 2008; pp. 1–11. [Google Scholar]
- Johnson, D.M.; Büntgen, U.; Frank, D.C.; Kausrud, K.; Haynes, K.J.; Liebhold, A.M.; Esper, J.; Stenseth, N.C. Climatic warming disrupts recurrent Alpine insect outbreaks. Proc. Natl. Acad. Sci. USA 2010, 107, 20576–20581. [Google Scholar] [CrossRef]
- Barker, G.M. The Biology of Terrestrial Molluscs; CABI: Wallingford, New Zealand, 2001; pp. 1–560. [Google Scholar]
- Fischer, M.L.; Colley, E. Espécie invasora em reservas naturais: Caracterização da população de Achatina fulica Bowdich, 1822 (Mollusca—Achatinidae) na Ilha Rasa, Guaraqueçaba, Paraná, Brasil. Biota Neotrop. 2005, 5, 127–144. [Google Scholar] [CrossRef]
- Fischer, M.L.; Simião, M.; Colley, E.; Zenni, R.D.; Silva, D.A.T.; Latoski, N. O caramujo exótico invasor na vegetação nativa em Morretes, PR: Diagnóstico da população de Achatina fulica Bowdich, 1822 em um fragmento de Floresta Ombrófila Densa aluvial. Biota Neotrop. 2006, 6, 1–5. [Google Scholar] [CrossRef]
- Eston, M.R.; Menezes, G.V.; Antunes, A.Z.; Santos, A.S.R.; Santos, A.M.R. Espécie invasora em unidade de conservação: Achatina fulica (Bowdich, 1822) no Parque Estadual Carlos Botelho, Sete Barras, SP, Brasil. Rev. Inst. Florest. 2006, 18, 173–179. [Google Scholar]
- Lake, P.S.; O’Dowd, D.J. Red crabs in rain forest, Christmas Island: Biotic resistance to invasion by an exotic snail. Oikos 1991, 62, 25–29. [Google Scholar] [CrossRef]
- Nurinsiyah, A.S.; Fauzia, H.; Hennig, C.; Hausdorf, B. Native and introduced land snail species as ecological indicators in different land use types in Java. Ecol. Indic. 2016, 70, 557–565. [Google Scholar] [CrossRef]
- Cowie, R.H. Decline and homogenization of Pacific faunas: The land snails of American Samoa. Biol. Conserv. 2001, 99, 207–222. [Google Scholar] [CrossRef]
- Cowie, R.H. Invasive non-marine molluscs in the islands of the tropical and subtropical Pacific: A review. Am. Malacol. Bull. 2005, 20, 95–103. [Google Scholar]
- Colley, E.; Fischer, M.L. Avaliação dos problemas enfrentados no manejo do caramujo gigante africano Achatina fulica (Gastropoda: Pulmonata) no Brasil. Zoologia 2009, 26, 674–683. [Google Scholar] [CrossRef]
- Miranda, M.S.; Pecora, I.L. Conservation implications of behavioural interactions between the Giant African Snail and a Native Brazilian species. Ethol. Ecol. Evol. 2017, 29, 209–217. [Google Scholar] [CrossRef]
- Barker, G.M.; Mayhill, P.C. Patterns of diversity and habitat relationships in terrestrial mollusc communities of the Pukeamaru Ecological District, northeastern New Zealand. J. Biogeogr. 1999, 26, 215–238. [Google Scholar] [CrossRef]
- Chiba, S.; Cowie, R.H. Evolution and extinction of land snails on oceanic islands. Ann. Rev. Ecol. Evol. Syst. 2016, 47, 123–141. [Google Scholar] [CrossRef]
- O’Loughlin, L.S.; Green, P.T. The secondary invasion of giant African land snail has little impact on litter or seedling dynamics in rainforest. Austral Ecol. 2017, 42, 819–830. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).