Abstract
The Gerromorpha assemblages in mangroves located in the central and eastern regions of Thailand were examined, and a total of nine species belonging to six genera and three families were discovered. Four of the recorded species are new records for Thailand. Asclepios annandalei Distant, 1915 was the most common species and widely distributed throughout the study area. The most diverse genus was Xenobates, which consisted of Xenobates argentatus Andersen, 2000, Xenobates mandai Andersen, 2000, Xenobates murphyi Andersen, 2000, and Xenobates singaporensis Andersen, 2000. Three of these species are new country records. Here, we present taxonomic and ecological information of mangrove gerromorphans in the central and eastern regions of Thailand.
1. Introduction
Gerromorpha (Heteroptera) consists of eight families that occupy a wide range of habitats from rock faces of waterfalls down to open oceans [1,2]. Five families of these gerromorphans are known to inhabit marine and brackish habitats, including mangroves, intertidal zones, coastal shorelines, and open oceans [1,3]. In Southeast Asia, Gerridae from marine systems consist of approximately 23 species within three genera [4]. Ten species of Stenobates, two species of Asclepios and a single species of Rheumatometroides occur in mangroves, coastal marshes and seashores [4,5,6]. Approximately 10 species of Halobates occur close to shores and open oceans [7]. A few species of Hebrus (Hebridae) have been reported from an intertidal zone of mangroves [4]. Hermatobatidae consists of three species within genus Hermatobates that are restricted to intertidal zones throughout the Subregion [1,4]. Two species representing one genus of Mesoveliidae (Nereivelia) were found underneath logs of mangroves in Singapore and Thailand [8]. Veliidae is composed of three genera that occur in marine habitats in Southeast Asia [1,4]. Approximately thirty species of Halovelia and Haloveloides are commonly found in the intertidal zone, and twenty species of Xenobates inhabit the water surface in mangrove forests [4].
Mangroves in Thailand are located on both the Andaman Sea and the Gulf of Thailand. More specifically, the majority of large mangroves are distributed throughout the Andaman Sea in the southern region of Thailand, whereas mangroves in the Gulf of Thailand are fragmented and scattered from the eastern region toward the southern region [9]. The mangrove ecosystem represents a complex link between freshwater and saltwater [10]. Taxonomy of plants in mangroves is considered important research since they are the main components of the ecosystem [10]. Research on insects in mangrove forest ecosystems has focused on terrestrial insects, especially pests and beneficial insects of mangroves themselves, or attractive insects [11]. However, our knowledge about the species richness of aquatic insects in mangrove ecosystems, especially in Thailand, is still very scarce [12]. Most research on aquatic insects in Thai mangroves is the result of taxonomic studies on certain groups [6,13,14] or ecological research on faunal recovery [12]. Therefore, this is the first study on aquatic insects in Thailand, which provides a better understanding of species richness and distribution of marine insects. This valuable information can be further employed for various dimensions of research in mangroves of Thailand or Southeast Asia.
2. Materials and Methods
To determine the species richness of gerromorphans in mangroves in the central and eastern regions of Thailand, a faunistic survey was conducted from 2018 to 2020 (Figure 1, Table 1). In total, twenty-five separated mangroves distributed through the Gulf of Thailand in the central and eastern regions were sampled (Figure 1 and Figure 2, Table 1). At each site, two mesohabitats were recognized, which were water surface and water margin of the mangrove. Eight spots of each mesohabitat were sampled for a gerromorphan fauna in each mangrove. Specimens were collected using an aquatic D-net, although the specific sampling technique differed among mesohabitats [15]. At the water margin, insects associated with emergent and submerged vegetation were collected by sweeping the net back and forth across the margin. Insects were collected from the water surface using an aquatic D-net. Collecting was continued until no recognizably new morphospecies were collected in two consecutive samples at each mesohabitat. In each sampling regime, when the D-net was up to one-third full, the organic material was transferred to a white pan, specimens were removed with soft forceps, placed into containers with 80% ethyl alcohol, and labelled.
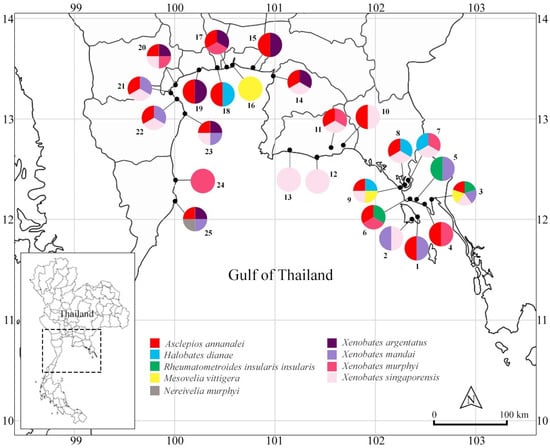
Figure 1.
Map showing species richness and distribution of gerromorphans in mangroves of central and eastern regions, Thailand. Species are color-coded. Numbers refer to collecting sites (see Table 1).

Table 1.
Collection data of samples in this study.

Figure 2.
Photos of representative collecting sites in this study: (A) Ban Tha Rani (collecting site 3), (B) Phra Chedi Klang Nam (collecting site 13), (C) Chulachomklao Fort (collecting site 16), and (D) Pranburi Forest Park (collecting site 24).
Morphological terminology largely follows Polhemus & Polhemus [6,16], Andersen [14], Andersen & Cheng [7], and Yang & Murphy [8]. Dried male specimens of each genus were placed under a Leica EZ4W stereomicroscope coupled with the LAS EZ program to obtain images. Images were then prepared with Photoshop CS5 (Adobe Systems Inc., San Jose, CA, USA). Specimens were deposited in the Entomology Museum, the Department of Entomology, Kasetsart University, Bangkok, Thailand.
Principle Component Analysis (PCA) was used to reveal species richness patterns of Gerromorpha in mangroves in the Central and Eastern regions based on the presence/absence data. PCA compresses richness variance into component axes to show the species richness relationships among communities. This analysis was performed using PC-ORD software 5.0 [17].
3. Results
Nine species representing six genera and three families of Gerromorpha were collected from 25 mangroves in Central and Eastern regions (Table 1 and Table 2). The most species-rich family was Veliidae with four species (Table 2). In addition, four new country records were discovered during this study. The preferred mesohabitats of each genus in this study were shown in Figure 3. Halobates was commonly found in the open water of estuaries. Asclepios and Rheumatometroides were collected in shallow sections of mangroves near estuaries. Mesovelia and Nereivelia were collected from margins of mangroves along the river, but Nereivelia occupied mangroves closer to the estuary. Xenobates species were found along the shorelines of mangrove streams and inside mangrove forests.
FAMILY GERRIDAE
This family consists of 75 genera worldwide that occupy a wide range of habitats [4,16,18]. More specifically, each genus has a specific preferred habitat [1], ranging from rock faces of waterfalls to open oceans [1]. Nevertheless, only five genera are considered marine gerrids: Asclepios, Halobates, Stenobates, Rheumatometroides, and Rheumatobates [3,4]. These genera, except Rheumatobates, have been reported from Southeast Asia [3,4]. In Thailand, six species representing these four genera were recorded from the Southern Region [6,7,12].
Genus Asclepios Distant, 1915
Members of this genus are small (3.0–4.0 mm body size) and yellowish brown with dark patterns on the head, thorax, and abdomen [7]. Asclepios species are commonly found in coastal areas associated with mangroves [5,7]. This genus is in the subfamily Halobatinae and distributed in East and Southeast Asia [7]. Three species of Asclepios have been described, nevertheless only two of them have been reported from mangroves in Southeast Asia [7]. Specifically, Asclepios apicalis (Esaki, 1924) has been found in Vietnam [7]. In Thailand, Asclepios annandalei Distant, 1915 has been previously collected from Phuket Island [5].
Diagnosis: This species can be distinguished from Halobates dianae Zettel, 2001 by the smaller body (3.0–4.0 mm) and yellowish brown pronotum and thoracic pleura, whereas the latter species is larger (3.2–6.5 mm body size) with mainly dark pronotum and thoracic pleura. Furthermore, this species can be distinguished from Rheumatometroides insularis insularis (J. Polhemus & Cheng, 1982) by the middle femur distinctively longer than the middle tibia, whereas the latter species has the middle femora clearly shorter than the middle tibia [20].
Discussion: Asclepios annandalei was the most common gerrid in the study. This species was collected from mangroves in the Central (i.e., Phetchaburi, Samut Prakan, Samut Songkhram provinces) and Eastern (i.e., Chantaburi, Chon Buri, Rayong, Trat provinces) regions. This species was collected with Halobates dianae at Laem Ward, Chantaburi Province. Furthermore, this species occurred with Rheumatometroides insularis insularis in mangroves of the Eastern Region (Figure 1).
Material collected: Bangkok: collecting site 18; Chantaburi Province: collecting sites 8, 9; Phetchaburi Province: collecting sites 21, 22, 23; Prachaup Khiri Khan Province: collecting site 25; Rayong Province: collecting sites 10, 11, 12; Samut Prakarn Province: collecting sites 15, 17; Samut Sakhon Province: collecting site 19; Samut Songkram Province: collecting site 20; Trat Province: collecting sites 1, 3, 4, 6.
Genus Halobates Eschscholtz, 1822
Members of this genus are approximately 3.2–6.6 mm in length and dark in color with some small brown markings [7]. This genus is in the subfamily Halobatinae and mainly distributed in the Indo-Pacific Ocean [7], with the exceptional case of Halobates micans Eschscholtz, 1822, which is a cosmopolitan species [7]. There are 46 species of this genus throughout the world [7]. In Southeast Asia, eleven species were reported, whereas only five species were recorded from Thailand, from the southern region [7,12].
Diagnosis: This species can be distinguished from Asclepios annandalei and Rheumatometroides insularis insularis by a larger body (2.7–6.5 mm) with mainly dark pronotum and thoracic pleura, whereas the latter species are smaller (3.0–4.0 mm body size) with yellowish brown pronotum and thoracic pleura.
Discussion: Specimens of Halobates dianae were collected in Chantaburi Province of the Eastern region (Figure 1). The species was collected from open sections of estuaries associated with large intact mangrove forests. Previously, it has been known only from the Philippines [18] and represents a new country record for Thailand. Within this study, Halobates dianae was collected syntopically with Asclepios annandalei and Xenobates species.
Material collected: Chantaburi Province: collecting sites 8, 9.
Genus Rheumatometroides Hungerford & Matsuda, 1958
Members of this genus are small (2.7–4.0 mm body size) and dark with dorsal yellowish markings on head, thorax, and abdomen [6]. This genus is in the subfamily Trepobatinae and distributed from Malaysia to Australia [4]. This marine genus consists of seven described species that mostly occur in shallow sections of mangroves [4,6]. In Southeast Asia, Rheumatometroides insularis insularis has been reported from mangroves in Malaysia and Singapore [6,20].
Diagnosis: This species can be distinguished from Asclepios annandalei by the middle femora clearly shorter than the middle tibia, whereas the latter species has middle femora distinctively longer than the middle tibia [20]. Furthermore, this species can be distinguished from Halobates dianae by the smaller body (2.7–4.0 mm) and yellowish brown pronotum and thoracic pleura, whereas the latter species is larger (3.2–6.5 mm body size) with a dark pronotum and thoracic pleura.
Discussion: This species was collected from mangroves in Trat Province, the Eastern Region (Figure 1). Based on our field observations, it commonly occurred in large numbers in open areas of channels in mangrove forests. In addition, this species occurred syntopically with several species of Xenobates in the Eastern Region.
Material collected: Trat Province: collecting sites 3, 5, 6.
FAMILY MESOVELIIDAE
This small family consists of 46 species representing 12 genera and 2 subfamilies [1,23]. Interestingly, these semiaquatic bugs have been collected from a wide range of habitats, including the forest floor in tropical forests, margins of freshwater habitats, and coastal shores [23,24]. Darwinivelia, Mesovelia, Nereivelia, and Speovelia are the only genera that contain marine species [13]. In Southeast Asia, one widely distributed genus, Mesovelia, has been recorded [24]. Additionally, two other endemic genera, Nereivelia and Cryptovelia, are known from Indonesia, Malaysia, and Thailand [8,24].
Genus Mesovelia Mulsant & Rey, 1852
Members of this genus are elongated (2.0–3.5 mm body size) and yellowish with brown markings [8]. Generally, they live on aquatic vegetation and are able to walk on the water surface [1]. This genus belongs to subfamily Mesoveliinae, which contains approximately 28 species [1,8,25,26]. Furthermore, this genus is widely distributed throughout the world, including Africa, Asia, Australia, and Europe [16,27]. In Southeast Asia, Mesovelia horvathi Lundblad, 1933 and Mesovelia vittigera Horváth, 1895 are two common species recorded from various aquatic habitats [8,15,16,27]. They were collected from streams, ponds, rice paddies, peatswamps, and blacklight traps in Thailand [12,15]. To distinguish the species of Thai Mesovelia, male specimens are needed for diagnostic genitalic features [25].
Diagnosis: This species can be distinguished from Nereivelia murphyi J. Polhemus & D. Polhemus, 1989 by a larger and more slender body (2.0–3.5 mm), whereas the latter has a smaller and stouter body (1.7–2.2 mm) [8,13].
Discussion: In this study, this species was collected from a small fragmented mangrove in Samut Prakarn Province in the Central Region, and in the large intact mangroves in Chantaburi and Trat provinces in the Eastern Region. This species has been reported from Africa, Asia, Australia, and Europe [27]. Although this species commonly inhabits freshwater habitats, it was also collected from brackish waters in Southeast Asia [8,12]
Material collected: Chantaburi Province: collecting site 9; Samut Prakarn Province: collecting site 16; Trat Province: collecting site 3.
Genus Nereivelia J. Polhemus & D. Polhemus, 1989
This genus is robust (1.7–2.2 mm body size) and yellowish brown without brown patterns [13]. Two species of this genus are reported from Southeast Asia [8]. Decayed logs in the intertidal zone of mangroves represent their preferred microhabitat [8].
Diagnosis: This species can be distinguished from Mesovelia vittigera by a smaller and stouter body (1.7–2.2 mm body size), whereas the latter has a larger and slender body (2.0–3.5 mm) [8,13].
Discussion: A single male of Nereivelia murphyi was collected in this study. This rare species has only been reported from Singapore and Thailand [8,13]. The holotype was collected in a log in a river associated with mangroves on the Andaman Seaside in Ranong Province, Southern Thailand [8].
Material collected: Prachaup Khiri Khan Province: collecting site 25.
FAMILY VELIIDAE
This family is the most diverse family of Gerromorpha with 57 genera distributed throughout the world [1,16]. Most veliids inhabit the margins of fresh water habitats (i.e., ponds, streams) and are associated with vegetation [29]. Five genera extend their habitats to marine systems: Trochopus, Husseyella, Xenobates, Halovelia, and Haloveloides [3]. Three of these genera (i.e., Xenobates, Halovelia, and Haloveloides) have been reported from Southeast Asia [4]. In Thailand, species of Halovelia and Haloveloides were reported only from intertidal zones, whereas species of Xenobates are only known from mangroves [12,30,31].
Genus Xenobates Esaki, 1927
Members of this genus are very small (1.45–1.80 mm body size) and dark with some brown markings [14]. This genus belongs to the subfamily Haloveliinae and contains twenty-one species distributed from the Oriental to the Australian regions [14]. They are commonly found at tidal channels of mangrove forests [14]. In Southeast Asia, six species were reported from Singapore, Malaysia, and Thailand [14]. In Thailand, Xenobates argentatus Andersen, 2000 is the only species recorded from the Southern Region [12,14].
Xenobates argentatus Andersen, 2000: 280–281 [14].
Diagnosis: This species can be distinguished from other species of Thai congeners by the middle femora with short hairs, whereas the other Thai congeners have the middle femora with a row of long hairs.
Discussion: This species has previously been collected from mangroves in Southern Thailand [12,14]. In this study, it is the most wide-spread species of Xenobates, which were collected from Chon Buri, Prachuap Khiri Khan, Phetchaburi, Samut Prakan, Samut Sakhon, and Samut Songkhram provinces (Figure 1). This species co-occurred with Xenobates murphyi Andersen, 2000 and Xenobates singaporensis Andersen, 2000 at different collecting sites in Central and Eastern regions.
Material collected: Chon Buri Province: collecting site 14; Prachuap Khiri Khan Province: collecting site 25; Phetchaburi Province: collecting site 23; Samut Prakan Province: collecting sites 15, 17; Samut Sakhon Province: collecting site 19; Samut Songkram Province: collecting site 20.
Diagnosis: This species can be distinguished from Xenobates argentatus by the middle femora with a row of long hairs, whereas the latter species has the middle femora with short hairs. It can also be distinguished from Xenobates singaporensis by males with an unmodified sternum VII, whereas males of the latter species have a deep depression on sternum VII. This species can be distinguished from Xenobates murphyi by antennal segments II and III with long hairs anteriorly, whereas the latter species has antennal segments II and III with short hairs anteriorly.
Discussion: This species has been previously recorded from Singapore [14]. Our results represent a new country record for Thailand. In this study, this species was collected from Bangkok, Phetchaburi, and Trat provinces (Figure 1). Specifically, it was the most common species on Kho Chang Island, Trat Province. This species was collected with Xenobates singaporensis at several mangrove sites.
Material collected: Bangkok: collecting site 18; Phetchaburi Province: collecting sites 21, 22, 23; Trat Province: collecting sites 1, 2, 3, 5.
Xenobates murphyi Andersen, 2000: 277–278 [14].
Diagnosis: This species can be distinguished from Xenobates argentatus by the middle femora with a row of long hairs, whereas the latter species has the middle femora with short hairs. It can be distinguished from Xenobates singaporensis by males with an unmodified sternum VII, whereas males of the latter species have a deep depression on sternum VII. This species can be distinguished from Xenobates mandai by antennal segments II and III with short hairs anteriorly, whereas the latter species has antennal segment II and III with long hairs anteriorly.
Discussion: This species has been previously recorded from Indonesia, Malaysia, Philippines and Singapore [14]. Our findings represent a new country record of this species in Thailand. In this study, it was widely distributed throughout the study area (Figure 1). It occurred syntopically with Xenobates argentatus at several mangrove forests.
Material collected: Chantaburi Province: collecting site 7; Prachuap Khiri Khan Province: collecting sites 24, 25; Rayong Province: collecting site 11; Samut Prakan Province: collecting site 17; Samut Songkram Province: collecting site 20; Trat Province: collecting sites 4, 6.
Xenobates singaporensis Andersen, 2000: 275–277 [14].
Diagnosis: This species can be distinguished from Xenobates argentatus by the middle femora with a row of long hairs, whereas the latter species has the middle femora with short hairs. This species can be distinguished from Xenobates mandai and Xenobates murphyi by males with a deep depression on sternum VII, whereas males of the latter species have an unmodified sternum VII.
Discussion:Xenobates singaporensis has been previously recorded from Singapore [14], while our findings represent a new country record of this species in Thailand. In this study, it was widely distributed in investigated mangroves (Figure 1). This species occurred syntopically with Xenobates argentatus and Xenobates murphyi.
Material collected: Chantaburi Province: collecting sites 7, 8, 9; Chon Buri Province: collecting site 14; Phetchaburi Province: collecting sites 21, 22, 23; Rayong Province: collecting sites 10, 11, 12, 13; Samut Songkram Province: collecting site 20; Trat Province: collecting sites 2, 3.

Table 2.
Checklist of Gerromorpha taxa found in this study.
Table 2.
Checklist of Gerromorpha taxa found in this study.
| Family | Species | Central Region | Eastern Region |
|---|---|---|---|
| Gerridae | Asclepios annandalei Distant, 1915 | X | X |
| Halobates dianae Zettel, 2001 * | X | ||
| Rheumatometroides insularis insularis (J. Polhemus & Cheng, 1982) | X | ||
| Mesoveliidae | Mesovelia vittigera Horváth, 1895 | X | X |
| Nereivelia murphyi J. Polhemus & D. Polhemus, 1989 | X | ||
| Veliidae | Xenobates argentatus Andersen, 2000 | X | X |
| Xenobates mandai Andersen, 2000 * | X | X | |
| Xenobates murphyi Andersen, 2000 * | X | X | |
| Xenobates singaporensis Andersen, 2000 * | X | X |
* = new country record.

Figure 3.
Preferred mesohabitats of marine Gerromorpha in mangroves of central and eastern regions.
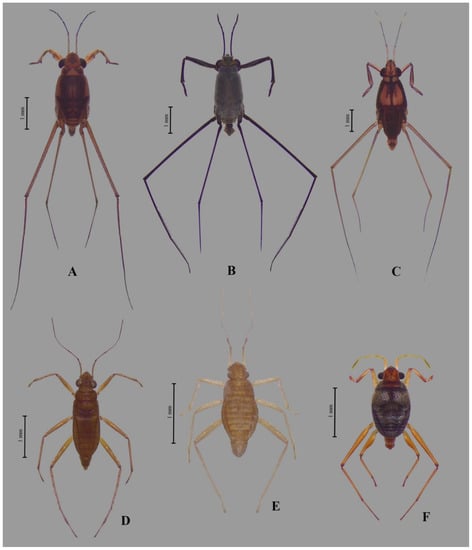
Figure 4.
Habitus of a male of each genus collected for the 25 mangroves in Thailand: (A) Asclepios annandalei, (B) Halobates dianae, (C) Rheumatometroides insularis insularis, (D) Mesovelia vittigera, (E) Nereivelia murphyi, and (F) Xenobates mandai.
Principle Component Analysis (PCA) showed little distinct grouping consistent with geographic expectations based on the presence/absence data of species richness of Gerromorpha from 25 mangroves in the eastern and central regions (Figure 5). Most of the collecting sites of both regions were aligned together in the middle of axes 1 and 2. Nevertheless, two collecting sites from Chantaburi Province (e.g., collecting sites 8, 9) and three collecting sites from Trat Province (e.g., collecting sites 3, 5, 6) were arranged in the parameter of the eastern region group. Meanwhile, collecting site 20 in Phetchaburi Province and a collecting site 25 in Prachaup Khiri Khan Province were separated from other collecting sites in the central region group.
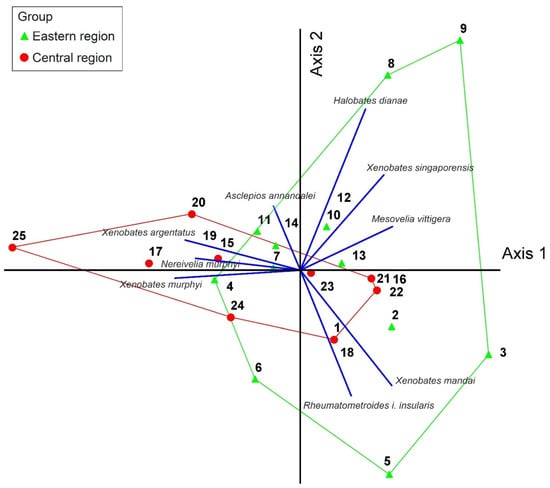
Figure 5.
Principle component analysis (PCA) on species richness of Gerromorpha of each collecting site. The first and second PC axes explain 24.370% (eigenvalue: 2.193) and 16.909% (eigenvalue: 1.522) of the variation in the data set, respectively. Numbers refer to collecting sites (see Table 1).
4. Discussion
Species of Asclepios are commonly found in coastal areas associated with mangroves [7]. In Malaysia and Singapore, Asclepios annandalei is relatively rare and only collected in mangroves [20]. In this study, this species was common and distributed throughout the study area. Based on field observations, members of this species are normally found in a pair, where a male is riding a female without genital contact (a mate guarding behavior) in shaded areas. A large number of individuals was found skating against currents in tidal streams of mangroves and an irrigation canal associated with a mangrove plantation at high tide in several collecting sites located in the Central Region. A large population of this species was observed in a small fragmented mangrove at Bangtaboon, Phetchaburi Province. Thorough examination of specimens revealed the two morphological forms of this species. The first form represents majority of specimens collected and perfectly matches the description of Asclepios annandalei. The second form was found only at three collecting sites in the Central Region. These two forms can be distinguished in the following manner. The first form has a solid large dark pattern on the meso- and metathorax, whereas the second form has a thin dark stripe on the meso- and metathorax. Additionally, males of the first form have a distinct large tooth in the middle of the profemur, whereas males of the second form have a small tooth in the middle of the profemur. Male genital structures of the two forms are similar to each other. This phenomenon was observed in a previous project in populations in the mangroves in the Andaman Sea [12]. Representatives of both forms will be sent for identification confirmation by the experts. Additionally, specimens of these two forms were kept frozen for further molecular analyses.
Members of Halobates were commonly found near shores of marine habitats, nevertheless some species of this genus occur in the open oceans [7]. In this study, Halobates dianae was collected from the open water of estuaries associated with large intact mangrove forests in the eastern region (Figure 3). This species was previously collected from the open water of a sea and a river associated with mangroves in the Philippines [21]. A large population of adults and nymphs was observed swiftly moving in a zigzag pattern on the water surface of the open section of a mangrove lined river in this study. Species of Rheumatometroides mostly occur in shallow sections of mangroves [4,6] (Figure 3). In this study, individuals commonly occurred in large numbers in shaded areas of channels in mangrove forests, similar to the mesohabitat used by Asclepios. Specimens of Mesovelia vittigera have been commonly collected from streams, ponds, rice paddies, peat swamps, and blacklight traps in Thailand [12,15]. Although this species commonly inhabits freshwater habitats, it has also been collected from brackish waters in Southeast Asia [8,12,32]. In this study, the species was collected from water margins of mangroves. Species of Nereivelia generally hide in crevices of logs filled with air bubbles at the margin of mangrove streams during high tide and come out searching for food during low tide [8,13]. Due to the cryptic behavior and preferred habitat, Nereivelia murphyi is rarely collected in Thailand [8,13]. In this study, a single male was captured on a mud flat next to a river in mangrove in Khlong Khao Daeng, Prachaup Khiri Khan Province, Central Region (Figure 3). This species was previously collected at Ranong Province on the Andaman Sea [13]. Therefore, this cryptic species predictably occurs in mangroves of both sides of the peninsula of Thailand. Members of Xenobates are commonly found at tidal channels of mangrove forests [14]. In this study, adults and nymphs were commonly found around mangrove trees and boardwalk poles in mangrove forest during high tide and in mud puddles during low tide (Figure 3). Xenobates argentatus was distributed throughout the study area, while the other congeners were restricted to a certain region or even a single province.
Although gerromorphans are adapted to live on water surfaces or at margins of aquatic systems, they display a wide variety of habitat preferences [3]. In general, members of marine Gerridae and Veliidae occur on the water surface [3], whereas marine Hydrometridae are found at the banks of mangrove rivers [33]. In this study, each group of Gerromorpha clearly showed the mesohabitat preference pattern throughout the study area (Figure 3). Members of Halobates (Gerridae) occurred in the open water of mangrove streams or the shorelines where strong waves and turbulence are present, whereas members of Asclepios (Gerridae) and Rheumatometroides (Gerridae) were found only in shaded areas on the side of mangrove streams characterized by presence of low wave and turbulence (Figure 3). Species of Xenobates (Veliidae) were found near mangrove trees or boardwalk poles away from the shorelines, where they are protected from water movement (Figure 3). Members of Mesoveliidae were collected only from the marginal sections of mangroves (Figure 3). Although there is no clear explanation for the habitat preference of aquatic insects in mangroves, salinity, waves, temperature, predation capability and food availability were suggested as key factors that influence the distribution of semiaquatic gerromorphans in mangroves [34]. The effect of anthropogenic disturbance on insect diversity has received significant attention during the past decade [35,36,37]. For example, mangroves associated with a higher level of human activities, such as agriculture, urbanization, and tourism, have lower Gerromorpha species richness (e.g., collecting sites 12, 13, 16, 24) in this study. On the other hand, the remote mangroves, with low accessibility were characterized by a higher species richness among the studied insects (e.g., collecting sites 3, 9, 23, 25) (Figure 2A–D).
The biogeographic patterns of Gerromorpha from mangroves in the Central and Eastern regions were not clearly displayed based on the PCA results. This could have been influenced by the distribution patterns of gerromorphans recorded in this study. Most of Gerromorpha species are widely distributed in the study area, such as members of Xenobates, the most speciose genus in this study [14], whereas Nereivelia murphyi and Halobates dianae are two endemic species that have been reported from specific areas [8,21]. The restricted distribution of Nereivelia murphyi and Halobates dianae could have resulted in clear separation of collecting sites 8, 9, and 25 from other collecting sites of the same region (Figure 5). More specifically, Nereivelia murphyi was collected only from collecting site 25 in Prachaup Khiri Khan Province, Central Region, whereas Halobates dianae was found at collecting sites 8 and 9 in Chantaburi Province, Eastern Region. Therefore, the clear biogeographic patterns in mangroves may not be displayed based on Gerromorpha in this study due to their wide distribution.
Although the Thai government has been strongly protecting the mangrove areas from deforestation and habitat degradation, the rate of habitat destruction still occurs to an excessive degree [10]. Furthermore, land alteration for agricultural purposes in mangrove areas in Thailand is still taking place at a rapid speed [38]. Human disturbances (e.g., oil spills, sewage, garbage dumps, and pesticide drainage) have been reported to reduce the diversity of animals in Thai mangroves [39]. Thailand is located in Southeast Asia, where the fauna of marine insects is highly diverse [14,33]. Nevertheless, the taxonomic knowledge on aquatic and semiaquatic insects in mangroves of Thailand is still in its beginnings. The results of this study provide fundamental knowledge of the Thai mangrove ecosystem, which is currently undergoing extensive anthropogenic disturbance. Species in this fragile environment may be at risk of exaptation or extinction, and studies such as this are needed to record and monitor species presence and composition for the future.
5. Conclusions
The species of Gerromorpha in mangroves in the central and eastern regions of Thailand were documented for the first time. Although this study focused on mangrove aquatic insects in the small study area in Thailand, nine species of gerromorphans were discovered with four new country records. However, the larger part of remaining Thai mangrove fauna is still unexplored. We suggest including physiochemical water properties and anthropogenic pressures in future research, which might provide a better understanding of the relationship between aquatic insects and land use adjacent to mangroves.
Author Contributions
Conceptualization, A.V. and L.-a.N.; methodology, A.V. and L.-a.N.; software, A.V.; validation, L.-a.N. and A.V.; investigation, L.-a.N.; resources, A.V. and L.-a.N.; specimen curation, A.V. and L.-a.N.; data curation, L.-a.N. and A.V.; writing—original draft preparation, L.-a.N. and A.V.; writing—review and editing, A.V.; funding acquisition, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Kasetsart University Research and Development Institute, KURDI (Grant No. K-S (D) 40.58, P-Y (D) 140.60, and FF (KU) 14.64) and the Thailand Science Research and Innovation (RSA6280047).
Institutional Review Board Statement
This research was approved by the Institutional Animal Care and Use Committee, Faculty of Agriculture, Kasetsart University, Thailand, under Project number ACKU59-AGR-009.
Data Availability Statement
Data are available from the corresponding author upon request.
Acknowledgments
We thank Sajeemat Attawanno (Kasetsart University) and Prabseuk Sritipsak (Kasetsart University) for their assistance in the field. We are grateful to Boonsatien Boonsoong (Kasetsart University) for his analysis assistance. We extend our gratitude to the Department of National Parks, Wildlife and Plant Conservation for permission and logistic support to conduct the fieldwork. We would also like to thank the Department of Entomology, Faculty of Agriculture, Kasetsart University, for the facility support. Lastly, we thank Michael L. Ferro (Clemson University), the editors, and the reviewers for their critical comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andersen, N.M. The Semiaquatic Bugs (Hemiptera, Gerromorpha); Entomonograph; Brill: Klampenborg, Denmark, 1982; Volume 3, p. 445. [Google Scholar]
- Vitheepradit, A.; Sites, R.W. A review of Eotrechus Kirkaldy (Hemiptera: Heteroptera: Gerridae) of Thailand with descriptions of three new species. Zootaxa 2007, 1478, 1–19. [Google Scholar]
- Andersen, N.M.; Polhemus, J.T. Water-strider (Hemiptera, Gerridae, Veliidae). In Marine Insects; Cheng, L., Ed.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1976; pp. 187–224. [Google Scholar]
- Chen, P.P.; Nieser, N.; Zettel, H. The Aquatic and Semi-Aquatic Bugs (Heteroptera: Nepomorpha & Gerromorpha) of Malesia; Brill: Lei-den-Boston, The Netherlands, 2005; Volume 5, p. 546. [Google Scholar]
- Andersen, N.M.; Foster, W.A. Sea skaters of India, Sri Lanka and the Maldives, with a new species and a revised key to Indian Ocean species of Halobates and Asclepios (Hemiptera, Gerridae). J. Nat. Hist. 1992, 26, 533–553. [Google Scholar] [CrossRef]
- Polhemus, J.T.; Polhemus, D.A. The Trepobatinae (Hemiptera, Gerridae) of New Guinea and surrounding regions, with a review of the World fauna. Part 4. The marine Tribe Stenobatini. Entomol. Scand. 1996, 27, 279–346. [Google Scholar] [CrossRef]
- Andersen, N.M.; Cheng, L. The marine insect Halobates (Heteroptera: Gerridae): Biology, adaptations, distribution, and phylogeny. Oceanography and marine biology. Annu. Rev. 2004, 42, 119–180. [Google Scholar]
- Yang, C.M.; Murphy, D. Guide to the aquatic Heteroptera of Singapore and Peninsular Malaysia. 6. Mesoveliidae, with description of a new Nereivelia species from Singapore. Raffles Bull. Zool. 2011, 59, 53–60. [Google Scholar]
- Pumijumnong, N. Mangrove forests in Thailand. In Mangrove Ecosystems of Asia; Springer: New York, NY, USA, 2014; pp. 61–79. [Google Scholar]
- Faridah-Hunum, I.; Latiff, A.; Hakeem, K.R.; Ozturk, M. Mangrove Ecosystems of Asia: Status, Challenges and Management Strategies; Springer: New York, NY, USA, 2014; pp. 81–92. [Google Scholar]
- Veenakumari, K.; Prashanth, M. A note on the entomofauna of mangrove associates in the Andaman Island (Indian Ocean: India). J. Nat. Hist. 2009, 43, 807–823. [Google Scholar] [CrossRef]
- Sites, R.W.; Vitheepradit, A. Recovery of the freshwater lentic insect fauna in Thailand following the tsunami of 2004. Raffles Bull. Zool. 2010, 58, 329–348. [Google Scholar]
- Polhemus, J.T.; Polhemus, D.A. The new mesoveliid genus and two new species of Hebrus (Heteroptera: Mseveliidae, Hebridae) from intertidal habitats in South-east Asia mangrove swamps. Raffles Bull. Zool. 1989, 37, 73–82. [Google Scholar]
- Andersen, N.M. The marine Haloveliinae (Hemiptera: Veliidae) of Singapore, Malaysia and Thailand, with six new species of Xenobates Esaki. Raffles Bull. Zool. 2000, 48, 273–292. [Google Scholar]
- Vitheepradit, A. The Semiaquatic Heteroptera (Gerromorpha) of Thailand: Faunistics, Biogeography, and Phylogeography. Ph.D. Thesis, University of Missouri, Columbia, MO, USA, 2008. [Google Scholar]
- Andersen, N.M.; Weir, T.A. Mesoveliidae, Hebridae, and Hydrometridae of Australia (Hemiptera: Heteroptera: Gerromorpha), with a reanalysis of the phylogeny of semi-aquatic bugs. Invertebr. Syst. 2004, 18, 467–522. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC–ORD Multivariate Analysis of Ecological Data, Version 6; MjM Software Design: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Chen, P.P.; Zettel, H. Key to the genera and subgenera of Gerridae (Gerromorpha) of Thailand and adjacent countries, with a check-list of species known from Thailand. Amemboa 1998, 2, 24–41. [Google Scholar]
- Distant, W.L. A few undescribed Rhynchota. Ann. Mag. Nat. 1915, 15, 503–507. [Google Scholar] [CrossRef][Green Version]
- Cheng, L.; Yang, C.M.; Andersen, N.M. Guide to the aquatic Heteroptera of Singapore and Peninsular Malaysia. I. Gerridae and Hermatobatidae. Raffles Bull. Zool. 2001, 49, 129–148. [Google Scholar]
- Zettel, H. Halobates dianae (Heteroptera: Gerridae), a new sea skater from the Philippines. Linz. Boil. Beitr. 2001, 33, 1097–1102. [Google Scholar]
- Polhemus, J.T.; Cheng, L. Notes on marine water-striders with descriptions of new species. Pac. Insects 1982, 24, 219–227. [Google Scholar]
- Damgaard, J.; Moreira, F.F.F.; Hayashi, M.; Weir, T.A.; Zettel, H. Molecular phylogeny of the pond treaders (Insecta: Hemiptera: Heteroptera: Mesoveliidae), discussion of the fossil record and a checklist of species assigned to the family. Insects Syst. Evol. 2012, 43, 175–212. [Google Scholar] [CrossRef]
- Andersen, N.M.; Polhemus, J.T. Four new genera of Mesoveliidae (Hemiptera, Gerromorpha) and the phylogeny and classification of the family. Insects Syst. Evol. 1980, 11, 369–392. [Google Scholar] [CrossRef]
- Andersen, N.M.; Polhemus, D.A. A new genus of terrestrial Mesoveliidae from the Seychelles (Hemiptera, Gerromorpha). J. N. Y. Entomol. Soc. 2003, 111, 12–21. [Google Scholar] [CrossRef]
- Floriano, C.F.B.; Moreira, F.F.F.; Bispo, P.C.; Morales, L.; Molano-Rendon, F. A new species of Mesovelia (Heteroptera: Gerromorpha: Mesoveliidae) from South America, with identification key and note on Colombian species. Zootaxa 2016, 4175, 345–352. [Google Scholar] [CrossRef]
- Polhemus, J.T.; Polhemus, D.A. The genus Mesovelia Mulsant & Rey in New Guinea (Heteroptera: Mesoveliidae). J. N. Y. Entomolo. Soc. 2000, 108, 205–230. [Google Scholar]
- Horváth, G. Hemipteres nouveaux de I’Europe et des pays limitrophes. Revue Entomol. 1895, 14, 152–165. [Google Scholar]
- Hecher, C. Key to the genera of Veliidae (Gerromorpha) of Thailand and adjacent countries, with a check-list of genera and species known from Thailand. Amemboa 1998, 2, 3–7. [Google Scholar]
- Andersen, N.M. The coral bugs, genus Halovelia Bergroth (Hemiptera, Veliidae). I. History, classification, and taxonomy of species except the H. Malaya-group. Entomol. Scand. 1989, 20, 75–120. [Google Scholar] [CrossRef]
- Andersen, N.M. Cladistic Biogeography of Marine Waterstrider (Insecta, Hemiptera) in the Indo-Pacific. Aust. Syst. Bot. 1991, 4, 151–163. [Google Scholar]
- Zettel, H.; Tran, A.D. First inventory of the water bugs (Heteroptera: Nepomorpha: Gerromorpha) of Langkawi Island, Kedah, Malaysia. Raffles Bull. Zool. 2009, 57, 279–295. [Google Scholar]
- Andersen, N.M. The evolution of marine insects: Phylogenetic, ecological and geographical aspects of species diversity in marine water striders. Ecography 1999, 22, 98–111. [Google Scholar] [CrossRef]
- Conjard, S.; Garrouste, R.; Gustave, S.D.; Gros, O. Mangrove semiaquatic bugs (Hemiptera: Gerridea) from Guadeloupe in Lesser Antilles: First records and new data on species distribution. Aquat. Insects 2021, 42, 239–246. [Google Scholar] [CrossRef]
- Ramírez, A.; Engman, A.; Rosas, K.G.; Perez-Reyes, O.; Martinó-Cardona, D.M. Urban impacts on tropical island streams: Some key aspects influencing ecosystem response. Urban Ecosyst. 2012, 15, 315–325. [Google Scholar] [CrossRef]
- Dolný, A.; Harabiš, F.; Bárta, D.; Lhota, S.; Drozd, P. Aquatic insects indicate terrestrial habitat degradation: Changes in taxonomical structure and functional diversity of dragonflies in tropical rainforest of East Kalimantan. Trop. Zool. 2012, 25, 141–157. [Google Scholar] [CrossRef]
- Hepp, L.U.; Restello, R.M.; Milesi, S.V.; Biasi, C.; Molozzi, J. Distribution of aquatic insects in urban headwater streams. Acta Limnol. Bras. 2013, 25, 1–9. [Google Scholar] [CrossRef]
- Khemnark, C. Ecology and Management of Mangrove Restoration and Regeneration in East and Southeast Asia. In Proceedings of the IV Ecotone, Surat Thani, Thailand, 18–22 January 1995; Amarin Co. Ltd.: Bangkok, Thailand, 1995; p. 339. [Google Scholar]
- Aksornkoae, S.; Paphavasit, N.; Wattayakorn, G. Mangroves of Thailand: Present status of conservation, use and management. In The Economic and Environmental Values of Mangrove Forests and Their Present State of Conservation in the South-East Asia/Pacific Regions; Clough, B.F., Ed.; International Society for Mangrove Ecosystems: Okinawa, Japan, 1993; pp. 83–133. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).