Species Composition of Aquatic (Nepomorpha) and Semiaquatic (Gerromorpha) Heteroptera (Insecta: Hemiptera) in Kaeng Krachan National Park, Phetchaburi Province, Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Measurement of Physical Characters of Sampling Sites
2.3. Sampling and Identification of Aquatic and Semiaquatic Heteroptera
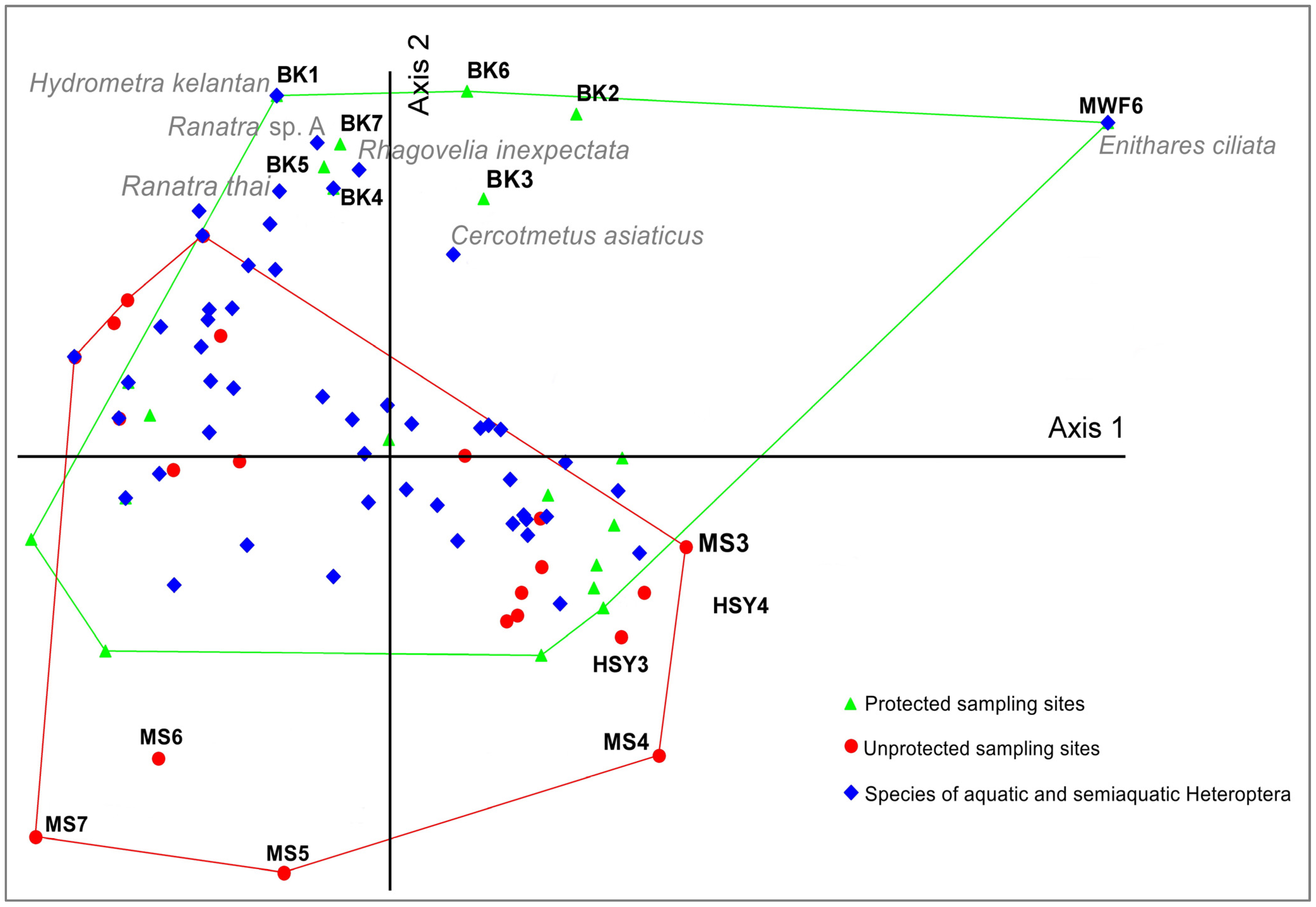
2.4. Data Analysis
3. Results
4. Discussion
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of National Park and Wildlife, DNP. Kaeng Krachan National Park. National Park, Wildlife and Plant Conservation Department. 2007. Available online: http://www.dnp.go.th/index_eng.asp (accessed on 23 December 2012).
- UNESCO World Heritage Convention. Asia and Pacific. 2021. Available online: http://whc.unesco.org/en/list/1461/documents (accessed on 26 July 2021).
- Dobias, R.T. The Shell Guide to the National Parks of Thailand; Shell Company of Thailand: Bangkok, Thailand, 1982. [Google Scholar]
- Wildlife Conservation Society, WCS. “Kaeng Krachan”. The Wildlife Conservation Society. 2012. Available online: http://www.wcsthailand.org/english/landscapekkfc-main (accessed on 23 December 2012).
- ICEM. Thailand National Report on Protected Areas and Development. Review of Protected Areas and Development in the Lower Mekong River Region, Thailand. 2003. Available online: https://portals.iucn.org/library/sites/library/files/documents/2003-106-3.pdf (accessed on 21 May 2012).
- Nieser, N.; Chen, P.P. The water bugs (Hemiptera: Nepomorpha and Gerromorpha) of Vanuatu. Tijdschr. Entomol. 2005, 148, 307–327. [Google Scholar] [CrossRef]
- Chen, P.P.; Nieser, N.; Zettel, H. The Aquatic and Semiaquatic Bugs (Heteroptera: Nepomorpha and Gerromorpha) of Malaysia; Brill: Leiden, The Netherland; Boston, MA, USA, 2021; Volume 5, p. 546. [Google Scholar]
- Polhemus, J.T.; Polhemus, D.A. Global diversity of true bugs (Heteroptera; Insecta) in freshwater. Hydrobiologia 2008, 595, 379–391. [Google Scholar] [CrossRef]
- Henry, T.J. Biodiversity of Heteroptera. Insect biodiversity. Sci. Soc. 2017, 1, 279–335. [Google Scholar]
- Stys, P.; Jansson, A. Checklist of recent family–group names of Nepomorpha (Heteroptera) of the world. Acta Entomol. Fenn. 1988, 50, 44. [Google Scholar]
- Miyamoto, S. Gerridae of Thailand and North Borneo taken by the joint Thai–Japanese Biological Expedition 1961–62. Environ. Nat. Resour. J. 1967, 5, 217–257. [Google Scholar]
- Nieser, N. An illustrated key to the families of Nepomorpha in Thailand. Amemboa 1996, 1, 4–9. [Google Scholar]
- Nieser, N. A new species of Ranatra from Thailand. Insecta: Heteroptera: Nepidae. Ann. Naturhist. Mus. Wien. 1997, 99, 79–82. [Google Scholar]
- Nieser, N. Introduction to the Notonectidae (Nepomorpha) of Thailand. Amemboa 1998, 2, 10–14. [Google Scholar]
- Papáček, M.; Zettel, H. Revision of the Oriental genus Idiotrephes (Heteroptera: Nepomorpha: Helotrephidae). Eur. J. Entomol. 2000, 97, 201–211. [Google Scholar] [CrossRef]
- Sites, R.W.; Polhemus, J.T. A new species of Telmatotrephes (Heteroptera: Nepidae) from Thailand, with distributional notes on congeners. Aquat. Insects 2000, 23, 333–340. [Google Scholar] [CrossRef]
- Sites, R.W.; Polhemus, J.T. Distribution of Helotrephidae (Heteroptera) in Thailand. J. N. Y. Entomol. Soc. 2001, 109, 372–391. [Google Scholar] [CrossRef]
- Sites, R.W.; Polhemus, J.T. Two new species of Hydrometra latreille (Heteroptera: Hydrometridae) from Thailand. Proc. Entomol. Soc. Wash. 2003, 105, 138–143. [Google Scholar]
- Zettel, H. Nieserius gen. n., a new genus of the subfamily Hyrcaninae (Heteroptera: Hebridae) from Thailand, Laos, and Nepal, with the first known subaquatic species of Gerromorpha. Aquat. Insects 1999, 21, 39–52. [Google Scholar] [CrossRef]
- Vitheepradit, A.; Sites, R.W.; Zettel, H.; Man, Y.C. Review of the Hydrometridae (Heteroptera) of Thailand, with distribution records. Nat. Hist. Bull. Siam Soc. 2003, 51, 197–223. [Google Scholar]
- Vitheepradit, A.; Sites, S.W. A review of Ptilomera (Heteroptera: Gerridae) in Thailand, with descriptions of three new species. Ann. Entomol. Soc. Am. 2007, 100, 139–151. [Google Scholar] [CrossRef]
- Vitheepradit, A. The Semiaquatic Heteroptera (Gerromorpha) of Thailand: Faunistics, Biogeography and Phylogeography. Ph.D. Dissertation, University of Missouri, Columbia, MO, USA, 2008. [Google Scholar]
- Vitheepradit, A.; Sites, R.W. A review of Eotrechus Kirkaldy (Hemiptera: Heteroptera: Gerridae) of Thailand with descriptions of three new species. Zootaxa 2007, 1478, 19. [Google Scholar]
- Sites, R.W.; Vitheepradit, A. Heleocoris (Heteroptera: Naucoridae: Laccocorinae) of Thailand, with description of a new species. Zootaxa 2011, 2736, 16. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, P.; Bu, W. Contribution to the knowledge on the Oriental genus Perittopus Fieber, 1861 (Hemiptera: Heteroptera: Veliidae) with descriptions of four new species from China and Thailand. Zootaxa 2013, 3616, 31–48. [Google Scholar] [CrossRef]
- Vitheepradit, A. A new species of Namtokocoris Sites (Hemiptera: Naucoridae) from Thailand. Zootaxa 2017, 4320, 592–596. [Google Scholar] [CrossRef]
- Cook, J.L.; Sites, R.W.; Vitheepradit, A. The Pleidae (Hemiptera, Heteroptera) of Thailand, with the descriptions of two new species and a discussion of species from Southeast Asia. ZooKeys 2020, 973, 35. [Google Scholar] [CrossRef]
- Zettel, H.; Laciny, A. A second species of Pleciogonus (Hemiptera, Heteroptera, Gerridae) from Thailand. Linz. Boil. Beitr. 2021, 53, 445–449. [Google Scholar]
- Andersen, N.M.; Weir, T.A. Australian Water Bugs: Their Biology and Identification (Hemiptera–Heteroptera, Gerromorpha and Nepomorpha); Apollo Books: Melbourne, Australia, 2004; p. 341. [Google Scholar]
- Dias-Silva, K.; Cabette, H.S.R.; Juen, L.; De Marco, P., Jr. The influence of habitat integrity and physical–chemical water variables on the structure of aquatic and semiaquatic Heteroptera. Zool 2010, 27, 918–930. [Google Scholar]
- Odum, E.P.; Finn, J.T.; Franz, E.H. Perturbation theory and the subsidy-stress gradient. Biosci 1979, 29, 349–352. [Google Scholar] [CrossRef]
- Buss, D.F.; Baptista, D.F.; Nessimian, J.L.; Egler, M. Substrate specificity, environmental degradation and disturbance structuring macroinvertebrate assemblages in neotropical streams. Hydrobiologia 2004, 518, 179–188. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. Trophic relationships of macroinvertebrates. In Methods in Stream Ecology, Volume 1; Academic Press: London, UK, 2017; pp. 413–433. [Google Scholar]
- Vieira, T.B.; Dias-Silva, K.; Pacífico, E.D.S. Effects of riparian vegetation integrity on fish and Heteroptera communities. Appl. Ecol. Environ. Res. 2015, 13, 53–65. [Google Scholar]
- Cunha, E.J.; de Assis Montag, L.F.; Juen, L. Oil palm crops effects on environmental integrity of Amazonian streams and Heteropteran (Hemiptera) species diversity. Ecol. Indic. 2015, 52, 422–429. [Google Scholar] [CrossRef]
- Dias–Silva, K.; Brasil, L.S.; Juen, L.; Cabette, H.S.R.; Costa, C.C.; Freitas, P.V.; De Marco, P. Influence of local variables and landscape metrics on Gerromorpha (Insecta: Heteroptera) assemblages in Savanna streams, Brazil. Neotrop. Entomol. 2020, 49, 191–202. [Google Scholar] [CrossRef]
- Guterres, A.P.M.; Cunha, E.J.; Juen, L. Tolerant semiaquatic bugs species (Heteroptera: Gerromorpha) are associated to pasture and conventional logging in the Eastern Amazon. J. Insect Conserv. 2021, 25, 555–567. [Google Scholar] [CrossRef]
- Brasil, L.S.; Luiza-Andrade, A.; Calvão, L.B.; Dias-Silva, K.; Faria, A.P.J.; Shimano, Y.; Oliveira-Junior, J.M.B. Aquatic insects and their environmental predictors: A scientometric study focused on environmental monitoring in lotic environmental. Environ. Monit. Assess. 2020, 192, 10. [Google Scholar] [CrossRef]
- Bonada, N.; Prat, N.; Resh, V.H.; Statzner, B. Developments in aquatic insect biomonitoring: A comparative analysis of recent approaches. Annu. Rev. Entomol. 2006, 51, 495–523. [Google Scholar] [CrossRef]
- Skern, M.; Zweimuller, I.; Schiemer, K. Aquatic Heteroptera as indicators for terrestrialisation of floodplain habitats. Limnologica 2010, 40, 241–250. [Google Scholar] [CrossRef]
- Jansson, A. Micronectinae (Heteroptera, Corixidae) as indicator of water quality in Lake Vesijarvi, Southern Finland, during the period of 1976–1986. Biol. Res. Univ. Jyvask. 1987, 10, 119–128. [Google Scholar]
- Savage, A.A. The distribution of Corixidae in relation to the water quality of British lakes: A monitoring model. Freshw. Forum. 1994, 4, 32–661. [Google Scholar]
- Dias-Silva, K.; Vieira, T.B.; de Matos, T.P.; Juen, L.; Simiao-Ferreira, J.; Hughes, R.M.; Junior, P.D.M. Measuring stream habitat conditions: Can remote sensing substitute for field data? Sci. Total Environ. 2021, 788, 147617. [Google Scholar] [CrossRef]
- Spence, J.R.; Andersen, N.M. Semiaquatic bugs (Gerromorpha). In Heteroptera of Economic Importance; CRC Press: Boca Raton, FL, USA, 2000; pp. 601–606. [Google Scholar]
- Nakthong, L.; Vitheepradit, A.; Sites, R.W. Key to the species of Eotrechinae (Hemiptera: Heteroptera: Gerridae) of Thailand and review of the fauna of the Phetchabun Mountain Range. Zootaxa 2014, 3860, 47–63. [Google Scholar] [CrossRef]
- Raruanysong, S.; Vitheepradit, A.; Sites, R.W. Key to the species of Ptilomerinae (Hemiptera: Heteroptera: Gerridae) of Thailand and review of the fauna of the Tennaserim Mountain Range. Zootaxa 2014, 3852, 101–117. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi (Version 1.6) (Computer Software). 2021. Available online: http://www.jamovi.org (accessed on 17 May 2022).
- McCune, B.; Mefford, M.J. PC–ORD. Multivariate Analysis of Ecological Data, Version 6; MjM Software: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Niemi, G.J.; McDonald, M.E. Application of ecological indicators. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 9–111. [Google Scholar] [CrossRef]
- Rosenberg, D.M.; Resh, V.H. Introduction to Freshwater Biomonitoring and Benthic Macroinvertebrates; Freshwater Biomonitoring and Benthic Macroinvertebrates; Chapman and Hall: New York, NY, USA, 1993; p. 488. [Google Scholar]
- Yoshimura, M. Effects of forest disturbances on aquatic insect assemblages. Entomol. Sci. 2012, 15, 145–154. [Google Scholar] [CrossRef]
- Andersen, N.H.; Wallace, J.B. Habitat, life history, and behavioral adaptations of aquatic insects. In An Introduction to the Aquatic Insects of North America, 2nd ed.; Kendall/Hunt Publishing: Dubuque, IA, USA, 1984; pp. 38–58. [Google Scholar]
- Erman, N.A. The use of riparian systems by aquatic insects. In California Riparian Systems: Ecology, Conservation, and Productive Management; University of California Press: Berkeley, CA, USA, 1984; pp. 177–1982. [Google Scholar]
- Hellawell, J.M. Biological Indicators of Freshwater Pollution and Environmental Management. In Pollution Monitoring Series; Melanby, K., Ed.; Elsevier: Amsterdam, The Netherlands, 1986; p. 546. [Google Scholar]
- Metcalfe, J.L. Biological water quality assessment of running waters based on macroinvertebrate communities. History and present status in Europe. Environ. Pollut. 1989, 60, 101–139. [Google Scholar] [CrossRef]
- Wright, J.F.; Furse, M.T.; Armitage, P.D.; Moss, D. New procedures for identifying running-water sites subject to environmental stress and for evaluating sites for conservation, based on the macroinvertebrate fauna. Arch. Hydrobiol. 1993, 127, 319–326. [Google Scholar] [CrossRef]
- Pinel–Alloul, B.; Méthot, G.; Lapierre, L.; Willsie, A. Macrobenthic community as a biological indicator of ecological and toxicological factors in Lake Saint-Francois (Quebec). Environ. Pollut. 1996, 91, 65–87. [Google Scholar] [CrossRef]
- Nedeau, E.J.; Merritt, R.W.; Kaufman, M.G. The effect of an industrial effluent on an urban stream benthic community: Water quality vs. habitat quality. Environ. Pollut. 2003, 123, 13. [Google Scholar] [CrossRef]
- Polhemus, D.A. Conservation of aquatic insects: Worldwide crisis or localized threats? Am. Zool. 1993, 33, 588–598. [Google Scholar] [CrossRef]
- Wesner, J.S. Seasonal variation in the trophic structure of a spatial prey subsidy linking aquatic and terrestrial food webs: Adult aquatic insects. Oikos 2010, 119, 170–179. [Google Scholar] [CrossRef]
- Hoang, D.H.; Bae, Y.J. Aquatic insect diversity in a tropical Vietnamese stream in comparison with that in a temperate Korean stream. Limnology 2006, 7, 45–55. [Google Scholar] [CrossRef]
- Maneechan, W.; Prommi, T.O. Diversity and distribution of aquatic insects in streams of the Mae Klong Watershed, Western Thailand. J. Entomol. 2015, 2015, 912451. [Google Scholar] [CrossRef]
- Sundar, S.; Silva, D.P.; de Oliveira Roque, F.; Simião-Ferreira, J.; Heino, J. Predicting climate effects on aquatic true bugs in a tropical biodiversity hotspot. J. Insect Conserv. 2021, 25, 229–241. [Google Scholar] [CrossRef]
- Pramual, P.; Wongpakam, K. Seasonal variation of black fly (Diptera: Simuliidae) species diversity and community structure in tropical streams of Thailand. Entomol. Sci. 2010, 13, 17–28. [Google Scholar] [CrossRef]
- Thamsenanupap, P.; Seetapan, K.; Prommi, T. Caddisflies (Trichoptera, Insecta) as bioindicator of water quality assessment in a small stream in northern Thailand. Sains Malays. 2021, 50, 655–665. [Google Scholar]
- Kim, D.G.; Yoon, T.J.; Baek, M.J.; Bae, Y.J. Impact of rainfall intensity on benthic macroinvertebrate communities in a mountain stream under the East Asian monsoon climate. J. Freshw. Ecol. 2018, 33, 489–501. [Google Scholar] [CrossRef]
- Mekong River Commission. Understanding the Mekong River’s Hydrological Conditions: A Brief Commentary Note on the “Monitoring the Quantity of Water Flowing Through the Upper Mekong Basin Under Natural (Unimpeded) Conditions” study by Alan Basist and Claude Williams. Vientiane, MRC Secretariat. 2020. Available online: https://www.mrcmekong.org/resource/ajg4w9 (accessed on 29 April 2022).
- Mekong River Commission. Situation Report on Dry Season Hydrological Conditions in the Lower Mekong River Basin: November 2020–May 2021. Vientiane: MRC Secretariat. 2021. Available online: https://www.mrcmekong.org/assets/Publications/Dry-season-situation-report-Nov-2020-to-May-2021.pdf (accessed on 29 April 2022).
- Polhemus, D.A.; Polhemus, J.T. The Aphelocheirinae of tropical Asia (Heteroptera: Naucoridae). Department of Zoology, National University of Singapore. Raffles. Bull. Zool. 1988, 36, 167–300. [Google Scholar]
- Sites, R.W.; Willig, M.R. Microhabitat associations of three sympatric species of Naucoridae (Insecta: Hemiptera). Environ. Entomol. 1991, 20, 127–134. [Google Scholar] [CrossRef]
- Chester, E.T.; Robson, B.J. Drought refuges, spatial scale and recolonisation by invertebrates in non–perennial streams. Freshw. Biol. 2011, 56, 2094–2104. [Google Scholar] [CrossRef]
- Boersma, K.S.; Bogan, M.T.; Henrichs, B.A.; Lytle, D.A. Invertebrate assemblages of pools in arid–land streams have high functional redundancy and are resistant to severe drying. Freshw. Biol. 2013, 59, 491–501. [Google Scholar] [CrossRef]
- Herbst, D.B.; Cooper, S.D.; Medhurst, R.B.; Wiseman, S.W.; Hunsaker, C.T. Drought ecohydrology alters the structure and function of benthic invertebrate communities in mountain streams. Freshw. Biol. 2019, 39, 10401–10417. [Google Scholar] [CrossRef]
- Lake, P.S. Ecological effects of perturbation by drought in flowing waters. Freshw. Biol. 2003, 48, 1161–1172. [Google Scholar] [CrossRef]
- Saffarinia, P.; Anderson, K.E.; Herbst, D.B. Effects of experimental multi-season drought on abundance, richness, and beta diversity patterns in perennially flowing stream insect communities. Hydrobiologia 2022, 849, 879–897. [Google Scholar] [CrossRef]
- Andersen, N.M. The semiaquatic bugs (Hemiptera, Gerromorpha). In Phylogeny, Adaptations, Biogeography and Classification; Brill: Leiden, The Netherland, 1982; p. 455. [Google Scholar]
- Andersen, N.M. Classification, phylogeny, and zoogeography of the pond skater genus Gerris Fabricius (Hemiptera: Gerridae). Can. J. Zool. 1993, 71, 2473–2508. [Google Scholar] [CrossRef]
- Taylor, S.J.; Mcpherson, J.E. Gerromorpha (Hemiptera: Heteroptera) in Southern Illinois: Species assemblages and habitats. Gt. Lakes Entomol. 2006, 39, 26. [Google Scholar]
- Yang, C.M.; Lua, H.K.; Yeo, K.L. Semiaquatic bug (Heteroptera: Gerromorpha) fauna in the nature reserves of Singapore. Gard Bull. 1997, 49, 313–319. [Google Scholar]
- Yule, C.M.; Sen, Y.H. Freshwater Invertebrates of the Malaysian Region; Academy of Sciences Malaysia: Kuala Lumpur, Malaysia, 2004; pp. 457–490. [Google Scholar]
- Zettel, H. Five new species of Perittopus Fieber, 1861 (Hemiptera: Veliidae) fron Southeast Asia. Raffles Bull. Zol. 2001, 49, 109–120. [Google Scholar]
- Chen, P.P.; Nieser, N.A. Taxonomic Revision of the Oriental Water Strider Genus Metrocoris Mayr (Hemiptera, Gerridae). Part I, II Zoological Museum. Steenstrupia 1993, 19, 1–43, 45–82. [Google Scholar]
- Chen, P.P.; Zettel, H. A taxonomic revision of the Oriental water strider genus Ventidius Distant (Hemiptera, Gerromorpha, Gerridae). Tijdschr. Entomol. 1998, 141, 137–208. [Google Scholar] [CrossRef]
- Vitheepradit, A. The Aquatic and Semiaquatic Heteroptera of the Phu Pan and Mountain Ranges of Thailand. Master’s Thesis, University of Missouri, Columbia, MO, USA, 2000. [Google Scholar]
- Dolný, A.; Ožana, S.; Burda, M.; Harabiš, F. Effects of landscape patterns and their changes to species richness, species composition, and the conservation value of Odonates (insecta). Insects 2021, 12, 478. [Google Scholar] [CrossRef]
- Danehy, R.J.; Bilby, R.E.; Justice, T.E.; Lester, G.T.; Jones, J.E.; Haddadi, S.S.; Merritt, G.D. Biological Diversity Responses to Flood Disturbance and Forest Management in Small, Forested Watersheds. Water 2021, 13, 2793. [Google Scholar] [CrossRef]
- Polhemus, J.T.; Polhemus, D.A. Revision of the genus Hydrometra Latereille in Indochina and the western Malay Archipelago (Heteroptera: Hydrometridae). Bishop Museum Occasional Papers 1995, 43, 9–72. [Google Scholar]
- Lansbery, I. A review of Oriental species of Ranatra Fabricius (Hemiptera–Heteroptera: Nepidae). Trans. Ent. Soc. Lond. 1972, 124, 287–341. [Google Scholar] [CrossRef]
- Lansbery, I. A review of genus Cercotmetus Amyot & Serville, 1843 (Hemiptera–Heteroptera: Nepidae). Tijdschr. Entomol. 1973, 116, 83–106. [Google Scholar]
- Ometo, J.P.H.; Martinelli, L.A.; Ballester, M.V.; Gessner, A.; Krusche, A.V.; Victoria, R.L.; Williams, M. Effects of land use on water chemistry and macroinvertebrates in two streams of the Piracicaba river basin, southeast Brazil. Freshw. Biol. 2000, 44, 327–337. [Google Scholar] [CrossRef]
- Hepp, L.U.; Santos, S. Benthic communities of streams related to different land uses in a hydrographic basin in southern Brazil. Environ. Monit. Assess. 2009, 157, 305–318. [Google Scholar] [CrossRef]
- Hepp, L.U.; Milesi, S.V.; Biasi, C.; Restello, R.M. Effects of agricultural and urban impacts on macroinvertebrates assemblages in streams (Rio Grande do Sul, Brazil). Zoologia 2010, 27, 106–113. [Google Scholar] [CrossRef]
- Nessimian, J.L.; Venticinque, E.M.; Zuanon, J.; De Marco, P., Jr.; Gordo, M.; Fidelis, L. Land use, habitat integrity, and aquatic insect assemblages in Central Amazonian streams. Hydrobiologia 2008, 614, 117–131. [Google Scholar] [CrossRef]
- Batalla Salvarrey, A.V.; Kotzian, C.B.; Spies, M.R.; Braun, B. The influence of natural and anthropic environmental variables on the structure and spatial distribution along longitudinal gradient of macroinvertebrate communities in southern Brazilian streams. J. Insect Sci. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Lock, K.; Adriaens, T.; Van De Meutter, F.; Goethals, P. Effect of water quality on waterbugs (Hemiptera: Gerromorpha & Nepomorpha) in Flanders (Belgium): Results from a large-scale field survey. Ann. Limnol. 2013, 49, 121–128. [Google Scholar]
- Ishadi, N.A.M.; Rawi, C.S.M.; Ahmad, A.H.; Abdul, N.H. The influence of heavy metals and water parameters on the composition and abundance of water bugs (Insecta: Hemiptera) in the Kerian River Basin, Perak, Malaysia. Trop. Life Sci. Res. 2014, 25, 61. [Google Scholar]
- Brasil, L.S.; Giehl, N.F.D.S.; Batista, J.D.; De Resende, B.O.; Cabette, H.S.R. Aquatic insects in organic and inorganic habitats in the streams on the Central Brazilian savanna. Rev. Colomb. Entomol. 2014, 43, 286–291. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Leveque, C.; Sullivan, C.A. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Sundar, S.; Heino, J.; Roque, F.D.O.; Simaika, J.P.; Melo, A.S.; Tonkin, J.D.; Nogueira, D.G.; Silva, D.P. Conservation of freshwater macroinvertebrate biodiversity in tropical regions. Aquat. Conserv. 2020, 30, 1238–1250. [Google Scholar] [CrossRef]
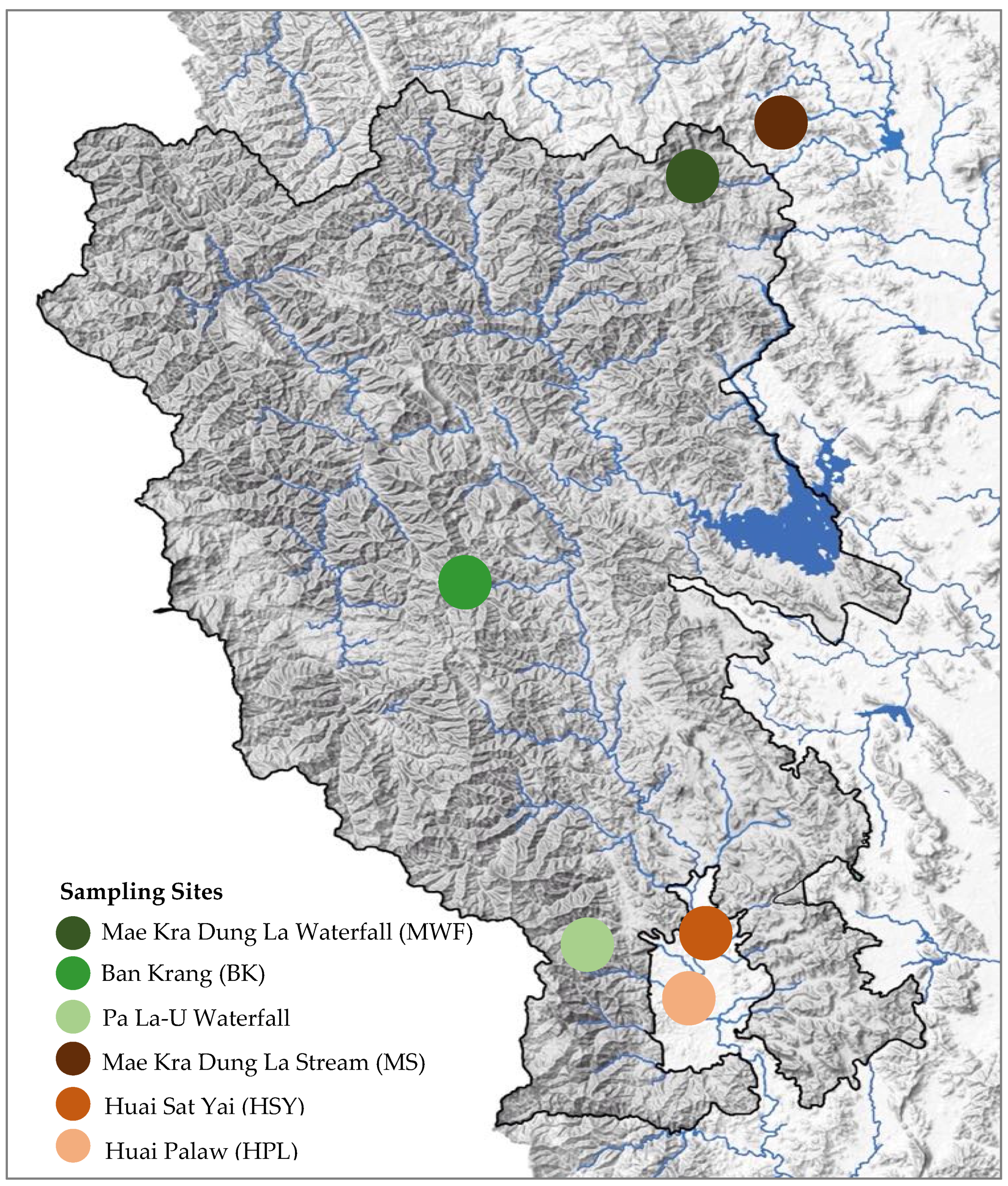

| Sampling Sites | GPS Coordinates | Stream Width (m) Average (Min-Max) | Depth (m) Average (Min-Max) | Riparian Width (m) | Marginal Vegetation | Substrate Types in Stream | |||
|---|---|---|---|---|---|---|---|---|---|
| Boulder | Cobble | Gravel | Sand | ||||||
| MWF * | 13°11.175′ N 099°32.472′ E (335 m) | 5.78 (2.90–11.50) | 0.16 (0.09–0.31) | >30 | √ | √ | √ | ||
| BK * | 12°48.144′ N 099°25.640′ E (386 m) | 3.12 (2.00–4.80) | 0.19 (0.05–0.37) | >30 | √ | √ | |||
| PWF * | 12°45.250′ N 099°78.848′ E (232 m) | 7.36 (1.40–4.23) | 0.20 (0.04–0.45) | >30 | √ | √ | √ | ||
| MS | 13°12.182′ N 099°32.500′ E (350 m) | 3.16 (1.56–4.45) | 0.26 (0.07–0.44) | 5–30 | √ | ||||
| HSY | 12°30.832′ N 099°34.151′ E (176 m) | 7.80 (3.25–12.00) | 0.33 (0.10–0.70) | 1–5 | √ | √ | |||
| HPL | 12°32.017′ N 099°29.940′ E (199 m) | 9.37 (1.72–12.26) | 0.21 (0.10–0.46) | 1–5 | √ | ||||
| Species | Sites | |||||
|---|---|---|---|---|---|---|
| MWF * | BK * | PWF * | MS | HSY | HPL | |
| Nepomorpha | ||||||
| Aphelocheiridae | ||||||
| Aphelocheirus (M.) asiaticus (Hoberlandt & Stys) | x | x | ||||
| Aphelocheirus (A.) grik Polhemus & Polhemus | x | x | ||||
| Helotrephidae | ||||||
| Helotrephes otoeis Nieser & Chen | x | x | ||||
| Hydrotrephes jani Zettel | x | x | ||||
| Tiphotrephes indicus (Distant) | x | x | x | |||
| Micronectidae | ||||||
| Micronecta quadristrigata Breddin | x | x | ||||
| Naucoridae | ||||||
| Gestroiella limnocoroides Montandon | x | x | ||||
| Gestroiella siamensis Polhemus, Polhemus & Sites | x | x | ||||
| Heleolaccocoris ovatus (Montandon) | x | x | x | |||
| Heleolaccocoris strabus (Montandon) | x | |||||
| Naucoris scutellaris (Stål) | x | |||||
| Nepidae | ||||||
| Cercotmetus asiaticus Amyot & Serville | x | x | x | |||
| Ranatra thai Lansbury | x | x | ||||
| Ranatra sp. A | x | |||||
| Notonectidae | ||||||
| Anisops nigrolineatus (Lundblad) | x | x | ||||
| Aphelonecta gavini (Lunsbury) | x | x | ||||
| Enithares ciliata (Fabricius) | x | |||||
| Nychia sappho Kirkaldy | x | x | ||||
| Gerromorpha | ||||||
| Gerridae | ||||||
| Amemboa armata Polhemus & Andersen | x | x | x | x | ||
| Amemboa cristata Polhemus & Andersen | x | x | x | x | ||
| Limnogonus nitidus (Mayr) | x | x | x | x | ||
| Limnometra matsudai (Miyamoto) | x | x | ||||
| Metrocoris acutus Chen & Nieser | x | |||||
| Metrocoris borneensis Polhemus | x | |||||
| Metrocoris malayensis Chen & Nieser | x | |||||
| Metrocoris nigrofasciatus Distant | x | |||||
| Metrocoris nigrofascioides (Chen & Nieser) | x | x | ||||
| Metrocoris sp. A | x | x | ||||
| Metrocoris sp. B | x | |||||
| Onychotrechus esakii Andersen | x | |||||
| Pleciogonus wongsirii Chen, Nieser & Wattanachaiyingchareon | x | x | ||||
| Ptilomera jariyae Vitheepradit & Sites | x | x | x | |||
| Ptilomera tigrina Uhler | x | x | x | x | x | x |
| Rhagadotarsus kraepelini Breddin | x | |||||
| Rheumatogonus intermedius Hungerford | x | x | ||||
| Rheumatagonus vietnamensis Zettel & Chen | x | x | ||||
| Ventidius modulatus Lundblad | x | x | ||||
| Ventidius pulai Cheng | x | x | ||||
| Hebridae | ||||||
| Hebrus longisetosus Zettel | x | x | ||||
| Hyrcanus saxatilis Andersen | x | |||||
| Hydrometridae | ||||||
| Hydrometra annamana Hungerford & Evans | x | x | x | |||
| Hydrometra greeni Kirkaldy | x | x | ||||
| Hydrometra kelantan Polhemus & Polhemus | x | |||||
| Hydrometra longicapitis Torre-Bueno | x | x | x | |||
| Hydrometra orientalis Lundblad | x | |||||
| Mesoveliidae | ||||||
| Mesovelia horvathi Lundblad | x | x | x | x | x | |
| Mesovelia vittigera (Horváth) | x | x | x | x | ||
| Veliidae | ||||||
| Microvelia douglasi Scott | x | x | x | x | x | x |
| Microvelia genitalis Lundblad | x | x | x | x | ||
| Microvelia leveillei (Lethierry) | x | x | ||||
| Microvelia sp. A | x | x | x | |||
| Microvelia sp. B | x | x | ||||
| Neoalardus typicus (Distant) | x | |||||
| Perittopus asiaticus Zettel | x | |||||
| Rhagovelia femorata Dover | x | x | x | x | x | |
| Rhagovelia inexpectata Zettel | x | |||||
| Rhagovelia sondaica Polhemus & Polhemus | x | x | ||||
| Rhagovelia sumatrensis Lundblad | x | |||||
| Strongylovelia setosa (Zettel & Tran) | x | x | x | |||
| Strongylovelia sp. A | x | x | ||||
| Species of Nepomorpha | 7 | 1 | 6 | 5 | 6 | 10 |
| Species of Gerromorpha | 17 | 13 | 27 | 15 | 12 | 15 |
| Total species | 24 | 14 | 33 | 20 | 18 | 25 |
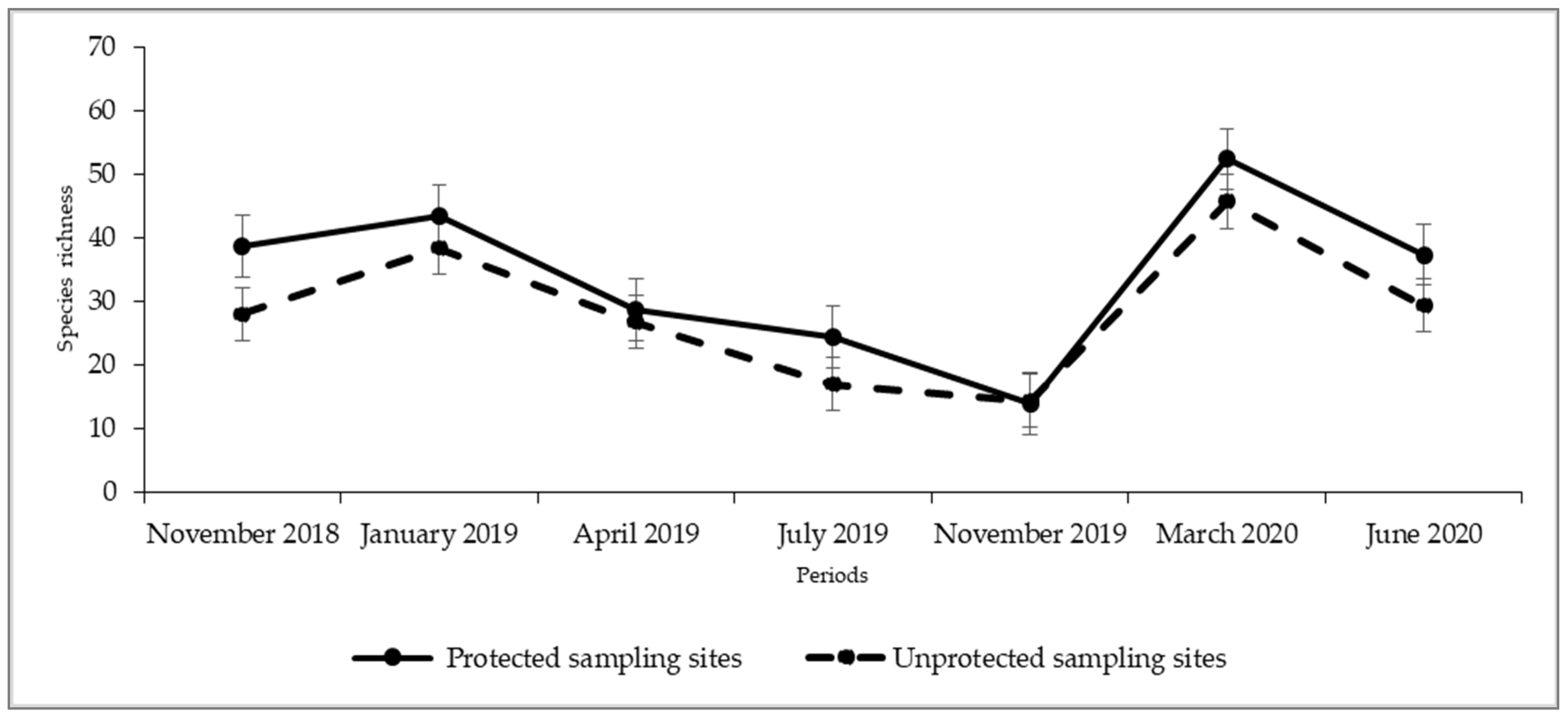
| Months | N | Mean ± SD | Student t-Test | |
|---|---|---|---|---|
| Protected Sampling Sites | Unprotected Sampling Sites | (p) * | ||
| November 2018 | 3 | 38.70 ± 4.04 | 28.00 ± 3.61 | 0.239 |
| January 2019 | 3 | 43.30 ± 9.29 | 38.30 ± 4.51 | 0.402 |
| April 2019 | 3 | 28.70 ± 4.16 | 26.70 ± 7.09 | 0.197 |
| July 2019 | 3 | 24.30 ± 3.79 | 17.00 ± 6.56 | 0.19 |
| November 2019 | 3 | 14.00 ± 6.24 | 14.30 ± 4.51 | 0.944 |
| March 2020 | 3 | 52.30 ± 4.93 | 45.70 ± 8.96 | 0.109 |
| June 2020 | 3 | 37.30 ± 5.69 | 29.39 ± 4.73 | 0.134 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attawanno, S.; Vitheepradit, A. Species Composition of Aquatic (Nepomorpha) and Semiaquatic (Gerromorpha) Heteroptera (Insecta: Hemiptera) in Kaeng Krachan National Park, Phetchaburi Province, Thailand. Diversity 2022, 14, 462. https://doi.org/10.3390/d14060462
Attawanno S, Vitheepradit A. Species Composition of Aquatic (Nepomorpha) and Semiaquatic (Gerromorpha) Heteroptera (Insecta: Hemiptera) in Kaeng Krachan National Park, Phetchaburi Province, Thailand. Diversity. 2022; 14(6):462. https://doi.org/10.3390/d14060462
Chicago/Turabian StyleAttawanno, Sajeemat, and Akekawat Vitheepradit. 2022. "Species Composition of Aquatic (Nepomorpha) and Semiaquatic (Gerromorpha) Heteroptera (Insecta: Hemiptera) in Kaeng Krachan National Park, Phetchaburi Province, Thailand" Diversity 14, no. 6: 462. https://doi.org/10.3390/d14060462
APA StyleAttawanno, S., & Vitheepradit, A. (2022). Species Composition of Aquatic (Nepomorpha) and Semiaquatic (Gerromorpha) Heteroptera (Insecta: Hemiptera) in Kaeng Krachan National Park, Phetchaburi Province, Thailand. Diversity, 14(6), 462. https://doi.org/10.3390/d14060462






